Abstract
Purpose
Fatigue is the most ubiquitous side effect of cancer treatment, but its etiology remains elusive. Further investigations into cancer-related fatigue pathobiology necessitate the expanded use of animal models. This study describes the development of a murine model of radiation-induced fatigue.
Methods
Voluntary wheel running activity measured fatigue in 5–8 week-old, male C57BL/6 mice before and after γ irradiation totaling 2400 cGy (3 consecutive days × 800 cGy daily fractionated doses) to the lower abdominal areas. Three trials confirmed fatigue behavior at this dose. Anhedonia, body weight, and hemoglobin were also measured. Gastrointestinal, skeletal muscle, and bone marrow tissue samples were evaluated for signs of damage.
Results
In two validation trials, irradiated mice (trial 1, n=8; trial 2, n=8) covered less cumulative distance in kilometers post-irradiation (trial 1, mean=115.3±12.3; trial 2, mean=113.6±21.8) than sham controls (trial 1, n=5, mean=126.3±5.7, p=0.05; trial 2, n=8, mean=140.9±25.4, p=0.02). Decreased mean daily running distance and speed were observed during the last four hours of the dark cycle in irradiated mice compared to controls for two weeks post-irradiation. There were no differences in saccharin preference or hemoglobin levels between groups, no effect of changes in body weight or hemoglobin on wheel running distance, additionally, histology showed no damage to muscle, bone marrow, or gastrointestinal integrity, with the latter confirmed by ELISA.
Conclusion
We characterized a novel mouse model of fatigue caused by peripheral radiation and not associated with anemia, weight changes, or anhedonia. This model provides opportunities for detailed study of the mechanisms of radiation-induced fatigue.
Keywords: Cancer, Fatigue, Radiation Therapy, Murine Model, Radiation-Induced Fatigue
1. Introduction
Fatigue is the most distressing, costly, and ubiquitous symptom experienced by patients with cancer, especially during treatment. [1–3]. Cancer-Related Fatigue (CRF) is a complex, multidimensional condition associated with cognitive deficits, persistent tiredness unrelieved by sleep or rest, and weakness not proportional to recent activity [1, 4]. It causes disturbances in memory, mood, motivation, and attention, interfering with daily function and negatively affecting patients’ quality of life [5, 6]. CRF severity varies over the course of the day, usually worsening in the evening [7, 8]. Importantly, CRF is a key reason for decreased compliance or discontinuation of potentially life-saving cancer therapies [9–11]. However, definitive mechanisms and treatments for CRF remain elusive [12].
Both peripheral and central inflammatory pathways have important influences on CRF during and after treatment [13–15]. Our group has observed associations between inflammatory markers and intensification of fatigue in men with non-metastatic prostate cancer receiving external beam radiation therapy (EBRT) [16]. The development of CRF upon treatment initiation and persistence after treatment completion suggests that radiation therapy (RT) triggers CRF initiation. We seek to understand how the physiological responses to localized, peripheral irradiation lead to fatigue development. Clinical studies, which have predominated CRF research to date, rely mainly on patient self-report with results that may be subject to differences in patient genetics, psychological responses to cancer diagnoses/treatment, and other factors that make causation difficult to infer. However, mouse models provide researchers with an ideal system for overcoming such challenges. In addition to allowing strict control of experimental conditions, treatments, genetics, and sample collection for biochemical or histological analysis, behavioral assays can quantify aspects of subjective symptoms to assist in phenotyping the condition of interest. Therefore, a mouse model of radiation-induced fatigue would be instrumental to understanding the pathobiology underlying this debilitating condition.
Existing in vivo mouse models examining fatigue-like behavior related to cancer or cancer therapy involve the use of one or more of the following: tumorigenic mice [17], chemotherapeutics [10, 18], antigenic challenge such as lipopolysaccharide or cytokine administration [19, 20], or brain or total body irradiation [21, 22]. These models often show symptoms associated with CRF, including cognitive deficiencies, assessed by learning or memory tests [23], and depressive behaviors, such as anhedonia [20]. However, the relationship of cognitive deficits and depression, as well as other commonly associated factors, such as anemia and weight changes, to CRF as a result of peripheral irradiation is unclear. We hope to generate fatigue-like behavior in mice through peripherally localized radiation, simulating the fatigue observed in men who have received EBRT for non-metastatic prostate cancer, while explaining concomitant, and possibly confounding symptoms.
In this study, we outline the development of a murine model of radiation-induced fatigue through peripherally-targeted, fractionated irradiation. The primary outcome of fatigue development was assessed by voluntary wheel running activity (VWRA), a common measure of fatigue-like behavior in mice [17, 18]. To further characterize fatigue behavior in this model and identify alternative explanations for its presence, we measured relevant physiological and behavioral parameters. These include assessing fatigue behavior through incremental changes in VWRA speed and distance during the dark phase (waking hours) of the day, because mice do not use running wheels for a significant amount of time during the light phase [24, 25]. Additionally, anemia, weight changes, changes in reward-seeking behavior (hedonia) that is indicative of depressive behavior, and damage to gastrointestinal tissues, skeletal muscle, and bone marrow were evaluated. We present a unique, reproducible model of radiation-induced fatigue with the potential to further investigate the etiology of fatigue and serve as a preclinical assay for testing potential CRF therapeutics.
2. Materials & Methods
2.1 Ethical considerations
This study was approved by the National Heart, Lung, and Blood Institute Animal Care and Use Committee (NHLBI ACUC # H-0288). All investigators taking part in animal handling and measurement of study outcomes were properly trained by the National Institutes of Health (NIH) Office of Animal Care and Use and the NHLBI Murine Phenotyping Core. All aspects of animal testing, housing, and environmental conditions used in this study were in compliance with The Guide for the Care and Use of Laboratory Animals [26].
2.2 Housing and Experimental Animals
Five week old male C57BL/6 mice (16.0–24.8 grams) were purchased from Charles River Laboratories (Frederick, MD) and tattooed on their tails upon arrival for identification. Mice were assigned to individual polyethylene cages (Innovive Inc., product code: M-BTM-AD, San Diego, CA), which were kept in the NHLBI Murine Phenotyping Core animal facility rooms at 21–23°C, 45–50% relative humidity, and on a 12:12 hour light-dark cycle with the light phase beginning at 6am and dark phase at 6pm. Mice received ad libitum access to water and chow (NIH Rodent Diet 31, Bethesda, MD). Bi-daily checks were performed and documented to ensure good general health status of the mice, and food and water availability. Mice were housed individually for the full duration of the trial.
To mitigate stress due to travel, tattooing, or adjustment to new surroundings, 24 hours was allowed for recovery. After this initial 24-hour acclimation period, each mouse was handled gently for a period of three minutes for three consecutive days to decrease stress during procedures. Each mouse was weighed daily during this handling period, and weekly thereafter, to ensure they were in good overall health and to characterize any relationship between body weight and fatigue behavior.
2.3 Running Wheel Acclimation
Following completion of handling, mice were introduced to individual voluntary wheel running cages (Lafayette Instrument Company, # 80820, Lafayette, IN) equipped with compatible electronic counters (Lafayette Single Mouse Activity Wheel Counter, # 86061, Lafayette, IN) for continuous recording of the number of wheel revolutions and distance (in meters) traveled for the entire length of the experiment. Wheel running activity was recorded for five to seven days during acclimation to identify any outliers. On day 10 of the experiment, mice were divided randomly into either a sham control or an irradiated group.
2.4 Experimental Procedures
The development of this radiation-induced fatigue mouse model was conducted in two sequential phases. The first phase was finding the minimum radiation dose capable of producing fatigue behavior without signs of morbidity, debilitation, or mortality. This phase involved two pilot trials where animals’ response to different γ radiation dosages was monitored. In the first of these pilot trials, mice received sham irradiation (n=3), or 100, 200, 300, or 400 cGy (n=4 mice/dose) per day for 3 successive days. In pilot trial 2, mice were assigned to sham irradiation (n=3), 600 cGy (n=4) or 800 cGy/day (n=4) for 3 days.
The goal of the second phase of the study was to validate the selected radiation dose that generated the fatigue behavior in mice in the first phase without evident toxicity or morbidity. Through two independent trials in the second phase of the study, we hoped to characterize and confirm the fatigue behavior generated by the selected radiation dose. Both of study phases and their trials are outlined in detail in Table 1.
Table 1.
Study timeline, trials, and procedures.
| Baseline / Running Wheel Acclimation |
Irradiation / Sham Treatment (3 days) |
Week 1 | Week 2 | Study End |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VWRA Measurement | ||||||||||
| PHASE 1 | D1–D2 | D3–D5 | D6–D12 | D10 | D13–D15 | D18 | D24 | D31- | D31 | |
| Pilot Trial 1 (N=19) |
Mice Arrival & Tail Tattoo |
Handling & Body Weight |
Wheel Acclimation |
Group Allocation |
sham (n=3), 100, 200, 300, & 400 cGy (n=4/dose) |
Body Weight #2 |
Body Weight #3 |
Body Weight #4 |
||
| Pilot Trial 2 (N=11) |
sham (n=3), 600 & 800 cGy (n=4/dose) |
Body Weight #2 |
Body Weight #3 |
Body Weight #4 |
||||||
| PHASE 2 | Mice Arrival & Tail Tattoo |
Handling & Body Weight |
D6–D8 | Group Allocation |
D13–D15 | D22 | D24–26 | D30 | D34–D36 | D36 |
| Validation Trial 1 (N=13) |
Baseline Saccharin Test |
sham (n=5), 800 cGy (n=8) |
Body Weight #2 |
Saccharin Test #2 |
Body Weight #3 |
Saccharin Test #3 |
||||
| D8 | D13–D15 | D18 | D25 | D31 | D31 | |||||
| Validation Trial 2 (N=16) |
Baseline Hgb Test |
sham (n=8), 800 cGy (n=8) |
Hgb Test & Body Weight #2 |
Hgb Test & Body Weight #3 |
Harvest blood & tissues |
|||||
Four trials were used in two phases to develop this radiation-induced fatigue mouse model. The first phase consisted of finding the lowest radiation dose that produced fatigue behavior without signs of illness or mortality; while the second phase consisted of validating the selected radiation dose and characterizing the fatigue behavior observed. Voluntary wheel running activity (VWRA), measured by running distance and speed, in addition to body weight, saccharin preference, and hemoglobin (Hgb) concentration were assessed at baseline (pre-irradiation) and post-irradiation/sham irradiation. In validation trial 1, saccharin preference testing was repeated three times, each with a 48-hour period of ad libitum access to water and saccharin solution (0.1% w/v) in non-running wheel equipped home cages; hence, this trial lasted until day (D) 36.
2.5 Irradiation
On day 13 of the experiment, mice were moved from running wheel cages to their individual polyethylene cages. Irradiation or sham irradiation was given incrementally on days 13–15. Anesthetic solution (10mg/ml ketamine and 0.5mg/ml xylazine in 0.9% sterile saline) was administered at a dose of 0.1ml/10g body weight via intraperitoneal injection prior to irradiation. For irradiation/sham irradiation, animals were placed individually into a cylindrical lead shield with separable, symmetric sides within a plexiglass housing to prevent movement and ensure accurate exposure of the body area to be targeted for irradiation (Supplemental Figure 1). Once secured inside the shielding, mice were transported and placed into a Gammacell® 40 Exactor (MDS Nordion, Canada) with a 137Cesium source delivering approximately 100 cGy/minute. Sham controls were also anesthetized and transported inside the lead shielding in plexiglass housing to an adjoined shielded room next to the irradiator. All procedures described in this study occurred during the light cycle with mice treated in the same order each day on which they were performed. Immediately after returning from irradiation/sham irradiation, each mouse was placed back into its individual cage with an activated heating pad (SpaceDrapes, Inc., Manchester, MD) to aid in recovery from anesthesia.
2.6 Assessment of Radiation-Induced Fatigue
VWRA is an accepted and sensitive tool to objectively measure the motivational and neuromuscular components of fatigue [9, 11]. Prior studies of CRF in mice have measured VWRA between 2–3 weeks following the experimental intervention (e.g., tumor inoculation, drug infusion) [17, 18]. This study recorded VWRA for 15 days following irradiation. Mice were re-introduced to individual cages with running wheels on day 16, after completing a 3-day irradiation or sham treatment, and wheel activity data were recorded until the end of the study. Daily and cumulative distances measured by VWRA were compared between sham and irradiated groups.
2.7 Physiological & Behavioral Assessments
2.7.1 Active Cycle VWRA Measurements
To further describe changes in VWRA due to peripheral irradiation and characterize the general suppression of VWRA during the active cycle in this model of radiation-induced fatigue, average running distances and speeds were calculated for each 4 hour increment during the dark phase (6pm–10pm, 10pm–2am, and 2am–6am). Average incremental distance and speed were compared between groups for the baseline period (days 8–13) as well as for the first and second weeks after irradiation (days 16–23 and days 24–31, respectively). These parameters where not calculated for the light cycle because mice are nocturnal and rarely engage in any meaningful amount of VWRA during the light cycle [25, 28].
2.7.2 Hemoglobin and Body Weight Measurement
To determine if anemia accounted for changes in VWRA, hemoglobin levels were measured from tail vein blood drawn at baseline (day 8), and during both weeks post-irradiation (days 18 and 25) using a STAT-Site® MHgb Meter (Stanbio Laboratory, Boerne, TX). Body weights assessed one and two weeks after irradiation were compared to pre-irradiation (baseline) weight on day 10 to examine any relationship of body weight and VWRA.
2.7.3 Assessment of Anhedonia
CRF is often associated with depression [29]. To assess anhedonia, a measure of depressive behavior, mice were given ad libitum access to chow and water, as well as 0.1% w/v saccharin solution for three 48 hour periods. The first test (baseline) was on days 6–8, after handling was completed, but before the mice were introduced to the running wheels. The second and third saccharin preference tests occurred after irradiation during days 24–26 and days 34–36 of the study, weeks 1 and 2 post-irradiation, respectively. Because increased physical activity has been shown to influence depressive behavior in mice, specifically by lowering the threshold needed for reward (hyperhedonia) [30], the saccharine preference tests were conducted in cages without running wheels. Saccharine preference testing in mice is a measure of anhedonia, a depressive behavior [31]. Preference was calculated at each test by dividing the volume of saccharine solution consumed during each 48-hour test by the volume of total fluid (saccharin solution plus water) consumed.
2.8 Calcitonin Gene-Related Peptide (CGRP) ELISA
Blood was collected via cardiac puncture and placed in micro Z-gel tubes (Sarstedt, Numbrecht, Germany) at the termination of validation trial 2. Serum CGRP was measured using Mouse CGRP ELISA kit (MyBioSource, San Diego, CA). CGRP is a marker of gastrointestinal integrity [32]. Briefly, 100 µl of standard or undiluted serum was added in duplicate to each well. Following 2 hours incubation at 37 °C, plates were incubated for 1.5 hours with 100 µl of biotin antibody added to each well. Subsequently, plates were incubated in the presence of 100 µl of HRP-avidin solution per well for 1 hour at 37 °C. The plates were washed 5 times and incubated with 90 µl of TMB substrate solution added to each well. Absorbance was measured at 450 nm and CGRP concentrations were calculated based on a standard curve.
2.9 Tissue Harvest
Immediately following euthanasia of the validation trial 2 mice, skeletal muscle and visceral organs including lumbar bone marrow samples were harvested to examine histological changes that can indicate bystander irradiation damage. Tissues were stained with Haematoxylin and Eosin (H&E) and examined for evidence of pathology using a scale from 0 (normal) to 3 (highly damaged). Dissections, microscopic examination, and scoring were completed by an experienced pathologist blinded by the group assignments of the samples.
2.10 Statistics
The change patterns of cumulative VWRA distance, body weight, saccharin preference, and hemoglobin levels were analyzed using mixed design ANOVA. Pearson correlations between relevant variables were calculated to identify potential covariates of the fatigue behavior. Student t-tests were utilized to compare dark cycle activities (VWRA distance and speed), as well as serum CGRP levels of irradiated and control mice. Results were considered significant at p<0.05. All data are expressed as group mean ± standard error of the mean.
3. Results
3.1 Radiation Dose & VWRA
In determining the minimum dose to develop the radiation-induced fatigue mouse model, the initial pilot trial in phase I of the study did not show any change pattern difference in cumulative distance (in kilometers) between the sham irradiated (n=3) or irradiated mice before and post irradiation using 100, 200, 300, or 400 cGy/day for 3 successive days (n=4 mice/dose) radiation doses (F4,14=2.40, p=0.10). Mice displayed no signs of illness, so in the second pilot trial radiation dosages were increased to either 600 or 800 cGy per day × 3 days.
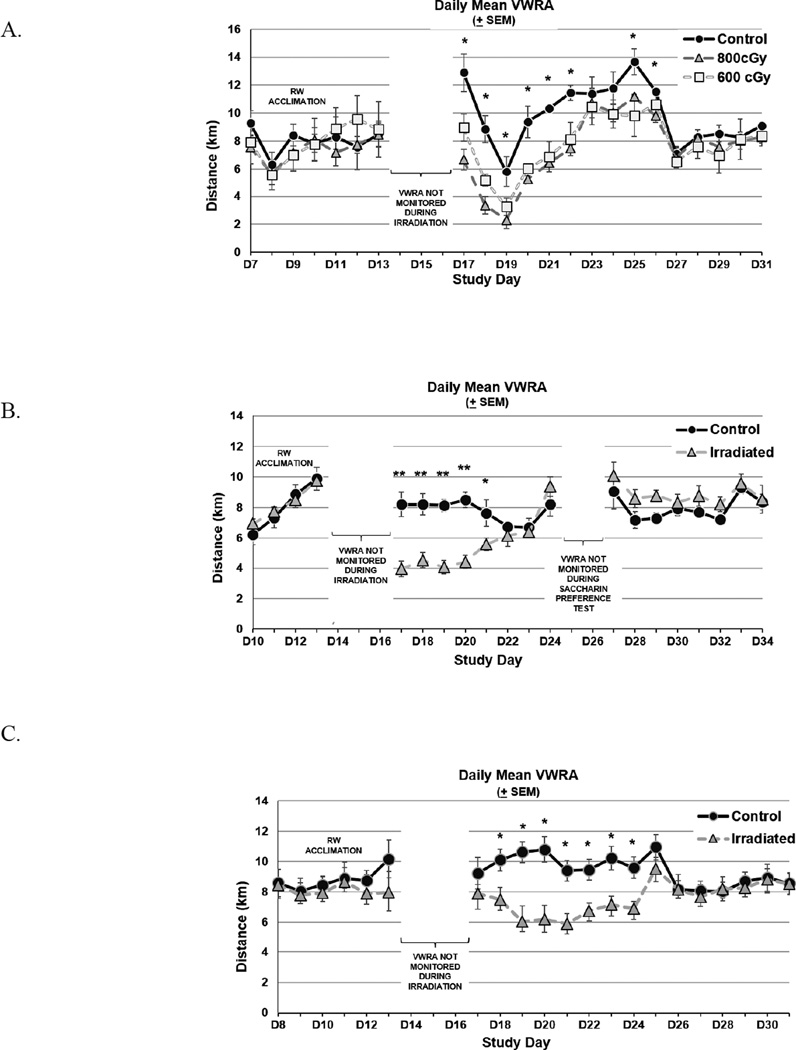
The second pilot trial showed a significant change of cumulative VWRA distance from pre- to post-irradiation between control and irradiated mice (F2,8=38.45, p<0.001). Post-irradiation cumulative VWRA distance in kilometers was less in mice irradiated with 800 cGy (n=4, 111.9 ± 10.3) compared to those irradiated with 600 cGy (n=4, 116.8 ± 17.9) and to non-irradiated mice (n=3, 148.2 ± 68.0) (Figure 1A). There was considerable variability in VWRA distance in the second pilot trial, which may be related to the small number of mice, as well as to unseen environmental and genetic factors [33–35]. All mice were observed to be in good health throughout this trial. These collective observations led to our selection of 800 cGy daily (2400 cGy total) dose regimen in the development of this radiation-induced fatigue mouse model.
Figure 1. Daily Voluntary Wheel Running Activity (VWRA).
A. 800 cGy daily radiation dose × 3 produced more pronounced fatigue behavior compared to 600 cGy daily radiation dose × 3, and sham controls in pilot trial 2. B. Validation trial 1 revealed significant decrease in distance (kilometers) for mice irradiated with 800 cGy daily radiation dose × 3. C. Validation trial 2 further confirms the development of fatigue behavior following irradiation with 800 cGy daily radiation dose × 3. The daily mean VWRA distance prior to irradiation days (days 13–15) reflect the VWRA distance covered during running wheel (RW) acclimation. Mice were allowed to rest in home cages on days 13–15 and during saccharin preference testing (days 24–26) in validation trial 1. Error bars show standard error of the mean. *p<0.05, **p<0.001.
Differences in cumulative VWRA distance by kilometers 7 days post-irradiation were confirmed in both validation trial 1 (800 cGy=115.3 ± 12.3; controls=126.3 ± 57.0, p<0.001) and validation trial 2 (800 cGy=113.6 ± 21.8; controls=141.0 ± 25.4, p=0.02). The daily mean VWRA for pilot trial 2 in phase 1 and the two validation trials in phase 2 are shown in Figure 1. No deaths or indications of declining health were observed related to the study procedures at any point during the experiment.
3.2 Active Cycle VWRA Measurements
3.2.1 Distance
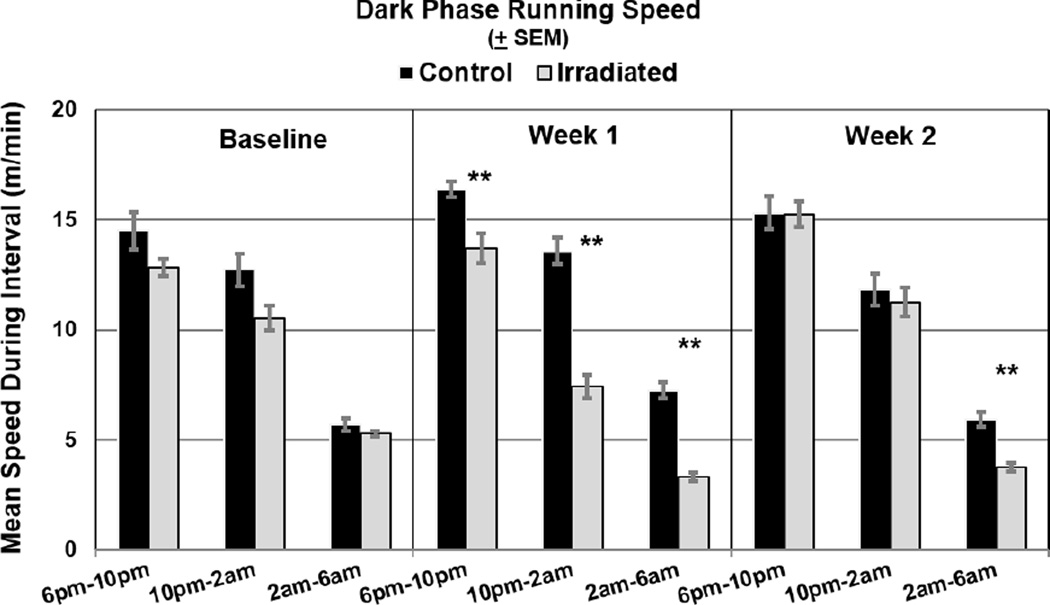
The 12-hour active phase (dark cycle) in validation trial 2 was divided into 4-hour increments to compare average VWRA distances of sham controls and irradiated mice at baseline (days 8–13) and the first (days 16–23) and second (days 24–31) weeks post-irradiation. After applying the Bonferroni correction, average VWRA distance, in kilometers from 6 pm to 10 pm (control=4.5 ± 0.01, irradiated=3.8 ± 0.12, p=3×10−4), from 10 pm to 2 am (control=3.3 ± 0.15, irradiated=1.8 ± 0.13, p=6.63×10−6), and from 2 am to 6 am (control=1.7 ± 0.1, irradiated=0.8 ± 0.05, p=5.79×10−7) one week post-irradiation were significantly different between sham control and irradiated mice. In the second week post-irradiation, average VWRA distance ran between 2am to 6am (control=1.4 ± 0.1, irradiated=0.9 ± 0.04, p=5.0×10−5) continued to be significantly different between the groups (Figure 2A).
Figure 2. Mean Dark Cycle Activity.
Mean distances (kilometers) (A) and running speeds (meters/minute) (B) were significantly lower (**p<0.001) for irradiated mice (n=8) than controls (n=8) during each 4 hour-increments of the dark (active) cycle during the first week post-irradiation and during the last 4 hour-increment (2am–6am) in the second week post-irradiation after applying Bonferroni correction.
3.2.2 Running Speed
Mean running speeds (meters/minute) during each 4-hour increment of the active phase in validation trial 2 were also compared between irradiated and control mice. Irradiated mice ran significantly slower up to two weeks post-irradiation during the last increment (2am–6am) of the dark cycle compared to controls (week 1: control=7.24 ± 0.35, irradiated=3.31 ± 0.21, p=5.8×10−7; week 2: control=5.91 ± 0.33, irradiated=3.74 ± 0.18, p=5.0×10−5) (Figure 2B).
3.3 Body Weight
There were no significant differences in body weight changes pre- and post-irradiation in control (sham irradiated) and irradiated mice, except in validation trial 1 (F1,11=5.68, p=0.04). The mice in the sham irradiation (control) group gained some weight (in grams) over time (n=5, mean=20.86 ± 0.67 on day 10, mean=21.72 ± 0.56 on day 22), while the irradiated mice had no change in weight post irradiation (n=8, mean=21.24 ± 0.30 on day 10, mean=21.11 ± 0.29 on day 22); however, a final mixed ANOVA model showed that body weight changes had no effect on cumulative VWRA distance post irradiation, as a significant change in VWRA cumulative distance post-irradiation was continually observed in all trials (pilot trial 2: F2,7=24.66, p=0.001; validation trial 2: F1,13=6.88, p=0.021). There were significant correlations between changes in body weight and dosage of radiation in pilot trial 2 (r= −0.62, p=0.04) and first validation trial (r=−0.58, p=0.04), but this relationship was not observed in the second validation trial (p=0.11).
3.4 Hemoglobin
Repeated measures ANOVA showed no significant change in hemoglobin concentration pre and post irradiation in validation trial 2 (F2,28=2.8, p=0.14). Additionally, there was no correlation between changes in hemoglobin levels and cumulative VWRA distance (p=0.08).
3.5 Anhedonia
There were no significant differences in the assessment of anhedonia tested by saccharin preference (percent of total 0.1% saccharin solution consumed) between irradiated (n=8) and sham irradiated (n=5) mice during baseline days 6–8 (sham=85±2%, irradiated=60±12%, p=0.10), days 24–26 post-irradiation (sham=73±6%, irradiated=74±9%, p=0.59), and days 34–36 post-irradiation (sham=68±14%, irradiated=81±6%, p=0.71).
3.6 Pathology Assessment
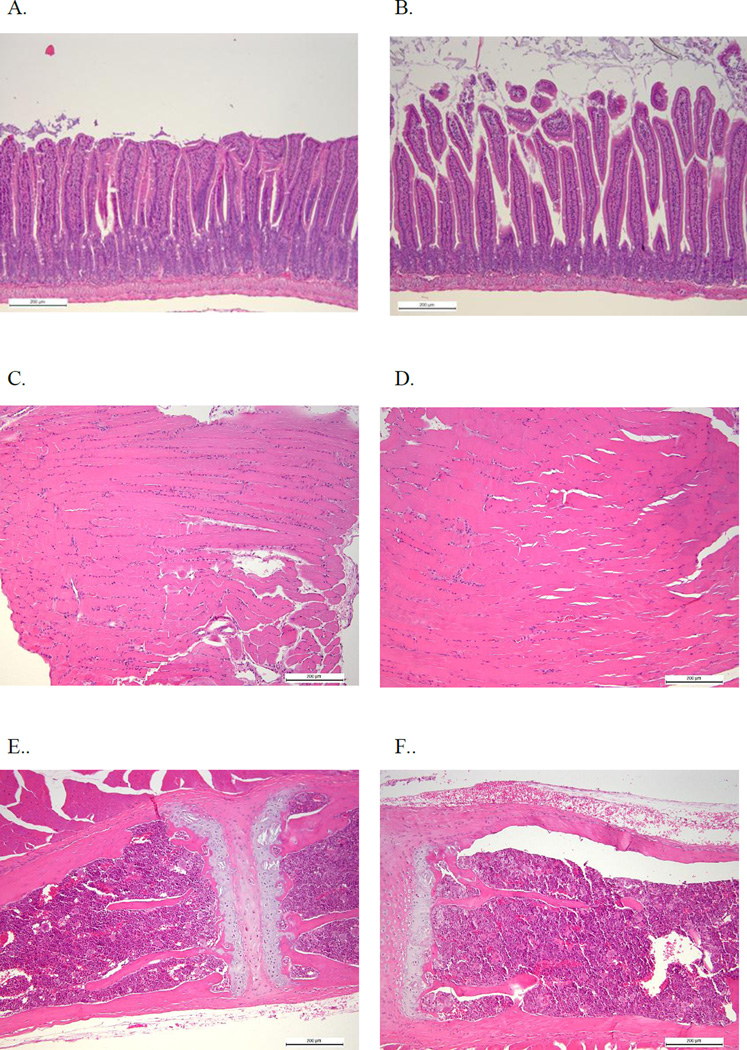
No differences were detected in serum CGRP concentrations, a biomarker indicative of gastrointestinal integrity, by ELISA between irradiated (n=8, 5.02±0.68 ng/ml) and sham irradiated (n=8, 5.56±0.52 ng/ml, p=0.68) mice. Additionally, histological evaluation of H&E stained tissues from all animals in these groups, which were dissected and scored by a pathologist blinded to the group assignments, showed no gross abnormalities or differences in pathology by way of average scores (0=normal, 1=mild damage, 2=moderate damage, 3=severe damage) of samples from irradiated and sham irradiated mice taken from skeletal muscle (control score=0.69±0.31, irradiated score=0.44±0.18, p=0.50), bone marrow (control=0.28±0.18, irradiated=0.28±0.20) and visceral organs (control=0.94±0.27, irradiated=0.99±0.21, p=0.86). Representative images from the small intestines, skeletal muscle, and bone marrow of control (A, C, and E, respectively) and irradiated (B, D, and F, respectively) animals are shown in Figure 3.
Figure 3. Representative Histological Images of the Small Intestine.
A. 100× H&E stain of the small intestine of a control mouse (pathology score=0.5). B. 100× H&E stain of the small intestine of an irradiated mouse (pathology score=0.5). C. 100× H&E stain of skeletal muscle from a control mouse (pathology score=0.5). D. 100× H&E stain of skeletal muscle from an irradiated mouse (pathology score=0). E. 100× H&E stain of bone marrow from a control mouse (pathology score=0). F. 100× H&E stain of bone marrow from an irradiated mouse (pathology score=0). Histological findings were scored from 0=normal, 1=mild damage, 2=moderate damage, 3=severe damage.
4. Discussion
In this study we describe development and validation of a novel model of radiation-induced fatigue in mice. Mice were given a simulated radiotherapy regimen in which irradiation was targeted to the pelvis and lower abdomen at a dose of 800 cGy per day × 3 days. Fatigue, modeled by decreased voluntary wheel running distance and speed, was observed for up to two weeks following irradiation. We also observed that decreases in voluntary wheel running distance and speed were most pronounced during the late hours of the active phase, which is consistent with observations in mouse models of chemotherapy-induced fatigue [10, 18]. Moreover, this aspect of our model displays similarity to the clinical trajectory of fatigue following localized radiation therapy for non-metastatic cancer, where patients complain of worsening fatigue in the afternoon or evening hours [36, 37], strengthening its validity.
Along with the intensification of fatigue behavior in late waking hours, the course of daily VWRA in this experiment offers a valuable insight for future investigations. Fatigue behavior of mice in our trials followed a consistent trajectory after irradiation, decreasing until reaching the lowest amount of VWRA between 3 and 4 days post-irradiation with mice recovering gradually over the next several days. These consistent changes in activity are an important finding to further phenotype fatigue behavior objectively and understand its biologic underpinnings. In future experiments these indicate times when measurement of biomarkers or the impact of interventions would be useful to evaluate.
Another important aspect of this model is that fatigue behavior was not related to anemia, anhedonia as assessed by saccharine preference testing, change in weight, or disruption of gastrointestinal integrity and damage to muscle or bone marrow. It demonstrates that our shielding mechanism was effective in targeting radiation to the desired peripheral region and validates our selection of the minimum radiation dose needed to generate a fatigue behavior, with no overt co-morbidities. The authors acknowledge the possibility that the fatigue behavior generated may be a result of unobserved radiation toxicity (e.g., neurotoxicity).
In future studies, we plan to incorporate additional phenotyping methods to assess the relationship of VWRA, our measure of fatigue, with skeletal muscle weakness and depression, both of which are often associated with CRF [11, 38]. Skeletal muscle weakness, assessed by grip strength testing of mice [39], together with measurements of skeletal muscle area, damage, and/or fiber composition would allow us to examine their contribution to any observed changes in strength [40]. Although anhedonia was not observed in this current study, the authors plan to incorporate additional measures of depressive behaviors in the future. We observed an initial correlation between body weight change and cumulative VWRA distance in the first validation trial, but did not see this relationship in the second validation trial. Further investigation is warranted to confirm this relationship, perhaps extending the duration of the experiment to observe long-term correlations between body weight change and VWRA distance.
Our current study successfully attained a model of radiation-induced fatigue in young C57BL/6 mice. Younger mice may have different fatigue behavior than older ones [41, 42], but this model can be applied to older mice in the future to translate to an older clinical population receiving localized radiation therapy by modifying the shielding design to accommodate the increase body size of aging C57BL/6 mice. Future studies that include addition of tumor prior to irradiation, or the addition of concomitant cancer therapies, such as chemotherapy or hormone therapy, will be greatly informative to understand the biologic mechanisms of CRF development.
This mouse model is advantageous for its potential to understand specific mechanisms causing fatigue. Radiation is a well-known trigger of inflammation [48, 49]. Prior studies have shown that localized, peripheral irradiation triggers differential expression of genes and proteins associated with inflammation [43–45], but no clear consensus exists as to which of these biomarkers are important for fatigue development. Peripheral inflammation also triggers alterations in neurotransmitter metabolism and changes in the function of the hypothalamic-pituitary-adrenal (HPA) axis, both of which influence fatigue behavior [46, 47]. This novel model system will enable further investigations to understand how peripheral inflammation influences neurotransmitter levels and activity, as well as to understand the behavioral responses from stress-inducing stimuli in an already fatigued mouse. Further, testing of experimental therapeutics that target pharmacodynamic markers that can modulate levels of neurotransmitters or HPA response would be important proof-of-concept activities to identify biomarkers of radiation-induced fatigue.
The importance of developing effective treatments for fatigue and understanding its etiology cannot be overstated. Continued development of preclinical models, such as the mouse model described here, are needed. This model offers objective, rapid assessment of interventions that may be translated to clinical setting. In addition, this animal model provides opportunities to separately investigate variables or symptoms that cluster with fatigue, and to determine how inflammation plays a role in the clustering of these symptoms. This information will provide researchers and clinicians with specific knowledge to offer more informed treatment options. The clinical implications in attenuating this debilitating symptom are significant and include improving patients’ quality of life and the outcomes of cancer treatment.
5. Conclusion
A novel mouse model of radiation-induced fatigue that is not associated with anemia, weight changes, or anhedonia is introduced in this study. This model provides an opportunity to advance mechanistic investigations into the etiology of fatigue related to radiation therapy. Quantifying the impact of manipulations on fatigue and associated factors via behavioral assays and biochemical analysis require the continued development and use of preclinical models such as the mouse model we present. However, models such as this one allow for convenient preclinical testing and objective assessment of the impact of potential treatments on fatigue, presenting a feasible approach to understanding this important symptom.
Supplementary Material
Highlights.
A novel radiation-induced fatigue mouse model that is not influenced by anemia, anhedonia, intestinal integrity disruption or weight changes.
Fatigue behavior is most prominent in the last four hours of the dark (active) cycle of the mouse model.
Voluntary wheel running wheel distance and speed are sensitive measures of fatigue behavior.
Acknowledgments
Financial Disclosures:
The authors would like to thank Michele Allen of the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH) for generously sharing her expertise in murine phenotyping methods and for her ongoing technical assistance, as well as for Timothy Hunt of NHLBI for helping us develop the shielding device. This study is supported by the Division of Intramural Research of the National Institute of Nursing Research of the NIH, and part of the validation trial is supported by a grant from the Oncology Nursing Society Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jean-Pierre P, Figueroa-Moseley CD, Kohli S, Fiscella K, Palesh OG, Morrow GR. Assessment of Cancer-Related Fatigue: Implications for Clinical Diagnosis and Treatment. The Oncologist. 2007;12(suppl 1):11–21. doi: 10.1634/theoncologist.12-S1-11. [DOI] [PubMed] [Google Scholar]
- 2.Ahlberg K, Ekmanb T, Gaston-Johansson F, Mock V. Assessment and management of cancer-related fatigue in adults. The Lancet. 2003;362(9384):640–650. doi: 10.1016/S0140-6736(03)14186-4. [DOI] [PubMed] [Google Scholar]
- 3.Minton O, Berger A, Barsevick A, Cramp F, Goedendorp M, Mitchell SA, Stone PC. Cancer-related fatigue and its impact on functioning. Cancer. 2013;119(Suppl 11):2124–2130. doi: 10.1002/cncr.28058. [DOI] [PubMed] [Google Scholar]
- 4.Barsevick AM, Irwin MR, Hinds P, Miller A, Berger A, Jacobsen P, Ancoli-Israel S, Reeve BB, Mustian K, O’Mara A, et al. Recommendations for High-Priority Research on Cancer-Related Fatigue in Children and Adults. J Natl Cancer Inst. 2013;105(19):1432–1440. doi: 10.1093/jnci/djt242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nature Reviews Clinical Oncology. 2014;11:597–609. doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vardy J, Dhillon HM, Pond GR, Rourke SB, Xu W, Dodd A, Renton C, Park A, Bekele T, Ringash J, et al. Cognitive function and fatigue after diagnosis of colorectal cancer. Ann Oncol. 2014 doi: 10.1093/annonc/mdu448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miaskowski C, Paul SM, Cooper BA, Lee K, Dodd M, West C, Aouizerat BE, Swift PS, Wara W. Trajectories of fatigue in men with prostate cancer before, during, and after radiation therapy. Journal of pain and symptom management. 2008;35(6):632–643. doi: 10.1016/j.jpainsymman.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhruva A, Dodd M, Paul SM, Cooper BA, Lee K, West C, Aouizerat BE, Swift PS, Wara W, Miaskowski C. Trajectories of fatigue in patients with breast cancer before, during, and after radiation therapy. Cancer nursing. 2010;33(3):201–212. doi: 10.1097/NCC.0b013e3181c75f2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ray M, Rogers LQ, Trammell RA, Toth LA. Fatigue and Sleep during Cancer and Chemotherapy: Translational Rodent Models. Comp Med. 2008;58(3):234–245. [PMC free article] [PubMed] [Google Scholar]
- 10.Ray MA, Trammell RA, Verhulst S, Ran S, Toth LA. Development of a Mouse Model for Assessing Fatigue during Chemotherapy. Comp Med. 2011;61(2):119–130. [PMC free article] [PubMed] [Google Scholar]
- 11.Dantzer R, Meagher MW, Cleeland CS. Translational approaches to treatment-induced symptoms in cancer patients. Nature reviews Clinical oncology. 2012;9(7):414–426. doi: 10.1038/nrclinonc.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. Drug therapy for the management of cancer-related fatigue. The Cochrane database of systematic reviews. 2010;(7):Cd006704. doi: 10.1002/14651858.CD006704.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saligan LN, Olson K, Filler K, Larkin D, Cramp F, Sriram Y, Escalante CP, del Giglio A, Kober KM, Kamath J, et al. The biology of cancer-related fatigue: a review of the literature. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2015;23(8):2461–2478. doi: 10.1007/s00520-015-2763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saligan LN, Olson K, Filler K, Larkin D, Cramp F, Yennurajalingam S, Escalante CP, del Giglio A, Kober KM, Kamath J, et al. Erratum to: The biology of cancer-related fatigue: a review of the literature. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2015;23(9):2853. doi: 10.1007/s00520-015-2815-5. [DOI] [PubMed] [Google Scholar]
- 15.Schrepf A, Lutgendorf SK, Pyter LM. Pre-treatment effects of peripheral tumors on brain and behavior: Neuroinflammatory mechanisms in humans and rodents. Brain, behavior, and immunity. 2015;49:1–17. doi: 10.1016/j.bbi.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saligan LN, Fernández-Martínez JL, deAndrés-Galiana EJ, Sonis S. Supervised Classification by Filter Methods and Recursive Feature Elimination Predicts Risk of Radiotherapy-Related Fatigue in Patients with Prostate Cancer. Cancer Inform. 2014;13:141–152. doi: 10.4137/CIN.S19745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norden DM, Bicer S, Clark Y, Jing R, Henry CJ, Wold LE, Reiser PJ, Godbout JP, McCarthy DO. Tumor growth increases neuroinflammation, fatigue and depressive-like behavior prior to alterations in muscle function. Brain, behavior, and immunity. 2015;43:76–85. doi: 10.1016/j.bbi.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zombeck JA, Fey EG, Lyng GD, Sonis ST. A clinically translatable mouse model for chemotherapy-related fatigue. Comp Med. 2013;63(6):491–497. [PMC free article] [PubMed] [Google Scholar]
- 19.Zaidi A, Jelveh S, Mahmood J, Hill RP. Effects of Lipopolysaccharide on the response of C57BL/6J mice to whole thorax irradiation. Radiother Oncol. 2012;105(3):341–349. doi: 10.1016/j.radonc.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y, Zhang Y, Pan F. The effects of EGb761 on lipopolysaccharide-induced depressive-like behaviour in C57BL/6J mice. Cent Eur J Immunol. 2015;40(1):11–17. doi: 10.5114/ceji.2015.49427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greene-Schloesser D, Moore E, Robbins ME. Molecular Pathways: Radiation-induced Cognitive Impairment. Clin Cancer Res. 2013;19(9):2294–2300. doi: 10.1158/1078-0432.CCR-11-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van der Meeren A, Lebaron-Jacobs L. Behavioural consequences of an 8 Gy total body irradiation in mice: regulation by interleukin-4. Canadian journal of physiology and pharmacology. 2001;79(2):140–143. [PubMed] [Google Scholar]
- 23.Yang M, Kim J, Kim JS, Kim SH, Kim JC, Kang MJ, Jung U, Shin T, Wang H, Moon C. Hippocampal dysfunctions in tumor-bearing mice. Brain, behavior, and immunity. 2014;36:147–155. doi: 10.1016/j.bbi.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 24.Novak CM, Burghardt PR, Levine JA. The use of a running wheel to measure activity in rodents: Relationship to energy balance, general activity, and reward. Neurosci Biobehav Rev. 2012;36(3):1001–1014. doi: 10.1016/j.neubiorev.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verwey M, Robinson B, Amir S. Recording and analysis of circadian rhythms in running-wheel activity in rodents. J Vis Exp. 2013;(71) doi: 10.3791/50186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Animals NRCUCftUotGftCaUoL. Guide for the Care and Use of Laboratory Animals. National Academies Press (US); 2011. [PubMed] [Google Scholar]
- 27.Innominato PF, Giacchetti S, Bjarnason GA, Focan C, Garufi C, Coudert B, Iacobelli S, Tampellini M, Durando X, Mormont MC, et al. Prediction of overall survival through circadian rest-activity monitoring during chemotherapy for metastatic colorectal cancer. International journal of cancer Journal international du cancer. 2012;131(11):2684–2692. doi: 10.1002/ijc.27574. [DOI] [PubMed] [Google Scholar]
- 28.Smythe GM, White JD. Voluntary wheel running in dystrophin-deficient (mdx) mice: Relationships between exercise parameters and exacerbation of the dystrophic phenotype. PLoS Curr. 2011;3:RRN1295. doi: 10.1371/currents.RRN1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reuter K, Classen CC, Roscoe JA, Morrow GR, Kirshner JJ, Rosenbluth R, Flynn PJ, Shedlock K, Spiegel D. Association of coping style, pain, age and depression with fatigue in women with primary breast cancer. Psycho-oncology. 2006;15(9):772–779. doi: 10.1002/pon.1012. [DOI] [PubMed] [Google Scholar]
- 30.Morris MJ, Na ES, Johnson AK. Voluntary running-wheel exercise decreases the threshold for rewarding intracranial self-stimulation. Behavioral neuroscience. 2012;126(4):582–587. doi: 10.1037/a0029149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Branchi I, Santarelli S, Capoccia S, Poggini S, D'Andrea I, Cirulli F, Alleva E. Antidepressant treatment outcome depends on the quality of the living environment: a pre-clinical investigation in mice. PloS one. 2013;8(4):e62226. doi: 10.1371/journal.pone.0062226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esfandyari T, Macnaughton WK, Quirion R, St Pierre S, Junien JL, Sharkey KA. A novel receptor for calcitonin gene-related peptide (CGRP) mediates secretion in the rat colon: implications for secretory function in colitis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2000;14(10):1439–1446. doi: 10.1096/fj.14.10.1439. [DOI] [PubMed] [Google Scholar]
- 33.Loos M, Koopmans B, Aarts E, Maroteaux G, van der Sluis S, Verhage M, Smit AB. Within-strain variation in behavior differs consistently between common inbred strains of mice. Mammalian genome : official journal of the International Mammalian Genome Society. 2015;26(7–8):348–354. doi: 10.1007/s00335-015-9578-7. [DOI] [PubMed] [Google Scholar]
- 34.Wahlsten D, Metten P, Phillips TJ, Boehm SL, 2nd, Burkhart-Kasch S, Dorow J, Doerksen S, Downing C, Fogarty J, Rodd-Henricks K, et al. Different data from different labs: lessons from studies of gene-environment interaction. Journal of neurobiology. 2003;54(1):283–311. doi: 10.1002/neu.10173. [DOI] [PubMed] [Google Scholar]
- 35.Leamy LJ, Pomp D, Lightfoot JT. Epistatic interactions of genes influence within-individual variation of physical activity traits in mice. Genetica. 2011;139(6):813–821. doi: 10.1007/s10709-011-9586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhruva A, Aouizerat BE, Cooper B, Paul SM, Dodd M, West C, Wara W, Lee K, Dunn LB, Langford DJ, et al. Differences in morning and evening fatigue in oncology patients and their family caregivers. European journal of oncology nursing : the official journal of European Oncology Nursing Society. 2013;17(6):841–848. doi: 10.1016/j.ejon.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miaskowski C, Aouizerat BE. BIOMARKERS: SYMPTOMS, SURVIVORSHIP, AND QUALITY OF LIFE. Semin Oncol Nurs. 2012;28(2):129–138. doi: 10.1016/j.soncn.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang XS, Zhao F, Fisch MJ, O’Mara AM, Cella D, Mendoza TR, Cleeland CS. Prevalence and characteristics of moderate-to-severe fatigue: a multicenter study in cancer patients and survivors. Cancer. 2014;120(3):425–432. doi: 10.1002/cncr.28434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark YY, Wold LE, Szalacha LA, McCarthy DO. Ubiquinol reduces muscle wasting but not fatigue in tumor-bearing mice. Biological research for nursing. 2015;17(3):321–329. doi: 10.1177/1099800414543822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hardee JP, Puppa MJ, Fix DK, Gao S, Hetzler KL, Bateman TA, Carson JA. The effect of radiation dose on mouse skeletal muscle remodeling. Radiology and oncology. 2014;48(3):247–256. doi: 10.2478/raon-2014-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung AP, Curtis TS, Turner MJ, Lightfoot JT. Influence of age of exposure to a running wheel on activity in inbred mice. Medicine and science in sports and exercise. 2006;38(1):51–56. doi: 10.1249/01.mss.0000181157.87366.f6. [DOI] [PubMed] [Google Scholar]
- 42.Turner MJ, Kleeberger SR, Lightfoot JT. Influence of genetic background on daily running-wheel activity differs with aging. Physiological genomics. 2005;22(1):76–85. doi: 10.1152/physiolgenomics.00243.2004. [DOI] [PubMed] [Google Scholar]
- 43.Hsiao CP, Araneta M, Wang XM, Saligan LN. The Association of IFI27 Expression and Fatigue Intensification during Localized Radiation Therapy: Implication of a Para-Inflammatory Bystander Response. Int J Mol Sci. 2013;14(8):16943–16957. doi: 10.3390/ijms140816943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Formenti SC, Demaria S. Systemic effects of local radiotherapy. The Lancet Oncology. 2009;10(7):718–726. doi: 10.1016/S1470-2045(09)70082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lukkahatai N PSGMHCPSLN. Proteomic Serum Profile of Fatigued Men Receiving Localized External Beam Radiation Therapy for Non-Metastatic Prostate Cancer. 2014;47(4):748–756. e744. doi: 10.1016/j.jpainsymman.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dantzer R. Cytokine, sickness behavior, and depression. Immunology and allergy clinics of North America. 2009;29(2):247–264. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Felger JC, Miller AH. Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Frontiers in neuroendocrinology. 2012;33(3):315–327. doi: 10.1016/j.yfrne.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.