Abstract
Existing vaccines against human papillomavirus (HPV) require continuous cold-chain storage. Previously, we developed a bacteriophage virus-like particle (VLP) based vaccine for Human Papillomavirus (HPV) infection, which elicits broadly neutralizing antibodies against diverse HPV types. Here, we formulated these VLPs into a thermostable dry powder using a multi-component excipient system and by optimizing the spray drying parameters using a half-factorial design approach. Dry powder VLPs were stable after spray drying and after long-term storage at elevated temperatures. Immunization of mice with a single dose of reconstituted dry powder VLPs that were stored at 37°C for more than a year elicited high anti-L2 IgG antibody titers. Spray dried thermostable, broadly protective L2 bacteriophage VLPs vaccine could be accessible to remote regions of the world (where ~84% of cervical cancer patients reside) by eliminating the cold-chain requirement during transportation and storage.
Keywords: Virus-like particles, spray drying, thermostable vaccine, dry powder, cold-chain, Design of experiment
INTRODUCTION
Most vaccines must be transported and stored within a narrow temperature range, meaning that maintenance of the cold-chain is a critical factor in preserving vaccine potency. Cold-chain requirements, mostly associated with liquid vaccine formulations, are costly and often impractical in developing countries. Thus, techniques for enhancing vaccine stability over a wide range of environmental temperatures could improve vaccine access to remote regions.
Biologically active macromolecules are more stable in the dry state due to reduced mobility and least likelihood of hydrolysis, oxidation and deamidation; phenomena commonly observed for biopharmaceuticals in the liquid state 1. Spray drying is a one-step, scalable and continuous process that generates dry powders with precise particle size engineering. Spray drying has recently been used to formulate stable dry powder vaccines suitable for delivery via the gastrointestinal 2, intranasal 3, pulmonary 4, 5 and dermal 6 routes. Furthermore, these dry powder vaccines can also be administered by the parenteral route after reconstitution with water just prior to immunization 4.
Human papillomavirus (HPV) infection is responsible for almost all cervical cancers and a significant percentage of other anogenital and oral carcinomas 7, 8. Over 100 HPV types have been identified, but HPV-associated cancers are linked with one of 14–20 “high-risk” carcinogenic HPV types. Two HPV types (HPV16 and 18) are responsible for about 70% of cervical cancer cases. Three HPV virus-like particle (VLP)-based prophylactic vaccines that provide protection against subset of HPV types are currently available; Gardasil-4 is a quadrivalent vaccine that protects against HPV types 16 and 18 (70% of cervical cancers). It also protects against HPV types 6 & 11 (both associated with anogenital warts) while Cervarix is a bivalent vaccine targeting HPV16 & 18. Gardasil-9 (a nonavalent vaccine) is a recently approved second-generation vaccine that protects against the four HPV types, that Gardasil-4 protects against, in addition to five other HPV types (HPV31, 33, 45, 52 and 58) 9. However, all three of these vaccines require refrigeration, and temperature and geography can conspire to interfere with effective distribution of these vaccines to the developing world where the vast majority of cervical cancer cases occur 10.
As an alternative, we have developed a bacteriophage MS2 VLP-based candidate vaccine (MS2-16L2 VLP) that targets a highly conserved, broadly neutralizing epitope from the HPV minor capsid protein, L2. Using a well-established pre-clinical animal model, we showed that the L2-VLP vaccine protects mice from genital infection by diverse HPV types. Furthermore, a single dose of L2-VLPs elicits high-titer and long-lasting antibody responses 11–13.
Previously, we have shown that L2-VLPs are compatible with spray drying 11. In this study, we explored the effect of excipient ratio and spray drying parameters (inlet temperature, gas flow, and liquid feed rate) in order to optimize the formulation of the L2-VLP vaccine. We show that L2-VLPs dry powder vaccine has improved long-term thermostability at room temperature (RT) and 37°C for one year. We also evaluated the immunogenicity of L2-VLPs dry powder by the oral and parenteral route in vivo. Spray dried VLPs, that were stored for more than a year (14 months) at various temperatures, were highly immunogenic in a mouse model.
MATERIALS AND METHODS
Materials
L-leucine and D-mannitol were bought from Sigma-Aldrich, St. Louis, MO; D-(+)-trehalose dehydrate and dextran (MW 60,000–90,000) were from MP Biomedicals, Solon, OH; Eudragit® L30 D55 polymer was bought from Evonik Industries, Parsipanny, NJ.
Methods
Production of VLPs
MS2-16L2 VLPs were expressed and purified using previously published protocols 12–14. VLPs were then dialyzed against a solution that was based on the excipients used for spray drying the VLPs into a dry powder. The excipients (w/w) consisted of: 76.842% mannitol (M); 5.263% trehalose (T); 2.105% dextran (D) and 15.789% L-leucine; all these excipients are approved by Food Drug & Administration for pharmaceutical applications 15. The total solids concentration of this aqueous solution (MTDL) was 3% (w/v).
Design of Experiments (DoE)
Excipient optimization
A combination of three sugars and amino acid was selected as mentioned above to obtain a thermostable VLP vaccine formulation. A series of experiments were designed to define the most suitable dry powder formulation with regards to four response parameters: spray drying yield (response 1), moisture content (response 2), particle size (response 3) and particle size distribution (response 4) as shown in Table 1. Five factors (formulation and process variables) were chosen to design the experiments including excipient ratio (factors 1 & 2); inlet temperature (factor 3); gas flow rate (factor 4); and liquid feed rate (factor 5). Feed solutions of the four excipients: mannitol, L-leucine, trehalose and dextran (MLTD) were prepared in different ratios varying each excipient at two levels, high and low. Mannitol had two levels at 76.8 and 73.7%, leucine was varied at 15.8 and 10.5%, trehalose at 5.3 and 10.5% and lastly, dextran at 2.1 and 5.3%. A half-factorial design was used to select the best combination of excipients for spray drying VLPs (Table 1). The factorial design was constructed using the statistical software Design-Expert® (v.8.0.1, Stat-Ease Inc., Minneapolis, MN, USA).
Spray drying optimization
The dry powders were generated by using a Buchi Mini Spray Dryer B-290 with a standard two-fluid nozzle (0.7 mm diameter, Buchi Corporation, Flawil, Switzerland). Compressed nitrogen was used as the drying and atomizing gas. Amongst the spray drying parameters, inlet temperature, nitrogen gas flow rate and liquid feed rate were varied at the high and low levels. The inlet temperature was set at 135/155 °C resulting in an outlet temperature of 45/55 °C, respectively. The gas flow rate was varied at 450/750 L/h and the liquid feed rate at 2.4/3.6 ml/min. The aspirator rate and the concentration of feed solution was kept constant at 35 m3/h (100%) and 3% w/v, respectively, for all the runs. Subsequently, VLPs were spray dried with the optimized formulation (excipient ratio) and process variables (spray drying parameters). The VLPs concentration in the liquid feed solution was optimized at 2.5% w/w. The spray dryer was housed in a BioPROtect® III Jr. BSC (Baker Co., Sanford, ME) to provide protection to the dry powder VLPs (from contamination) and also prevent exposure to the operator.
Yield, moisture content and particle size
Process yield is defined as the weight fraction of the amount of dry powder recovered after spray drying, in comparison to the total solid content of the liquid material sprayed. Moisture content in the spray dried powders were analyzed after drying the powders at elevated temperatures. Briefly, 100 mg of freshly formulated powders were dried in aluminum weighing pans with perforated lids (44mm VWR International, Radnor, PA) in forced-air oven (VWR Scientific products, West Chester, PA) at 110 °C for 24 hours at atmospheric pressure; the samples were weighed to the nearest decimal (0.01 mg) after cooling. Additional drying steps of 1 h were performed to verify that constant mass was achieved i.e., the difference between two readings were less than 0.1% of the test sample. The particle size and its distribution were determined in duplicate by laser diffraction using the Malvern Mastersizer 3000 (Malvern instruments, UK) and the Malvern dry dispersion unit (Aero S cell). Data were expressed as volume median diameter and span ((D90-D10)/D50). Approximately 10 mg of the powder was loaded into the hopper at a liquid feed rate of 60%, and a dispersive pressure of 2 bar. The dispersing unit was cleaned after each analysis to avoid cross contamination between different formulations.
VLPs loading and Integrity
VLP loading in the dry powder is defined as the percent weight fraction of VLPs recovered after spray drying, in comparison to the VLPs in the initial feed solution. The loading was quantitatively determined by SDS-PAGE analysis. Briefly, ~8.0 mg of VLPs dry powder was mixed with 50 μL of phosphate buffer saline (PBS) and vortex-mixed. One, 2, 5 and 10μL of this mixture was loaded into a SDS-PAGE gel (NuPage 10% Bis-Tris; Invitrogen). In addition, 2μL of liquid VLPs suspension (not spray dried) was loaded into the same gel as a positive control. VLPs were visualized by Coomassie Blue staining. VLP loading was determined with the help of densitometric analysis using Image J (NIH USA, public domain). A volume of 10 μL of VLPs dry powder dissolved in PBS was studied chromatographically on agarose gel and visualized with Ethidium Bromide (EtBr) as well as Coomassie Blue staining to assess VLPs integrity. The reconstituted VLPs were also analyzed immediately after spray drying using transmission electron microscopy (TEM). Briefly, 20 ng/μl of the VLP suspension were adsorbed on to carbon-coated glow-discharged copper grids for 2 min and then stained with 2% uranyl acetate. VLPs were visualized using a Hitachi H7500 TEM at a magnification of 70,000x. Furthermore, the integrity of spray dried VLPs was confirmed using dynamic light scattering (DLS; Zetasizer Nano ZS, Malvern instruments, Worcestershire, UK) by analyzing equivalent concentrations (5μg/ml) of liquid VLPs and reconstituted dry powder VLPs. Size distribution data were expressed as percent number.
Stability Studies
The stability studies were conducted for twelve months (one year) at elevated storage conditions after spray drying with optimized formulation and process variables. MS2-16L2 VLPs dry powder were stored in glass vials with tightly sealed caps (caps wrapped with parafilm) in desiccators at different temperature conditions: 4 °C, room temperature (RT; 21–23 °C) and 37 °C. To assess the stability of VLPs powder, an aliquot was withdrawn at regular time intervals and resuspended in PBS. These samples were analyzed on agarose gel and TEM as described above.
In vivo studies
Two animal models and two different routes of immunization were used to evaluate the efficacy of spray dried VLPs. A rat model was used to evaluate the immunogenicity and the efficacy of VLPs when administered by the oral route. In addition, the VLPs dry powder was reconstituted and administered by the intramuscular route in mice. All animal studies were done in accordance with the National Institutes of Health and the University of New Mexico Institutional Animal Care and Use Committee (UNM IACUC) guidelines and was approved by the UNM IACUC (protocol 12- 100827-HSC).
Immunizations in rats
Prior to oral delivery the dry powder VLPs (containing 400 μg of VLPs) were manually filled in hard gelatin capsules for rats (size 9, Torpac Inc. Fairfield, NJ). After filling the capsules with VLPs powders (equivalent to 400 μg of VLPs per capsule), they were enteric coated by multiple dip coatings in Eudragit® L30 D55 polymer (Evonik Industries, Parsipanny, NJ) to provide protection to the capsules/VLPs from the harsh gastric environment as performed before 16, 17, 18. The capsules were allowed to air dry overnight after the final coating. Groups of five 6–8 week-old Sprague Dawley rats were immunized with VLP powders in enteric-coated capsules by oral gavage using a dosing syringe (Torpac Inc. Fairfield, NJ, USA) for rats following manufacturer’s instructions. Another group of rats was dosed with VLPs dry powder in uncoated capsules (no enteric coating). Groups of rats were administered three doses (one prime and two booster dose) at three-week intervals. As controls, two groups of rats were immunized intramuscularly with 10 μg of MS2-16L2 VLPs or just MS2 VLPs (on the same schedule). Two weeks after the final immunization, blood was withdrawn from the saphenous vein and vaginal washes were collected using 20 μl of phosphate buffered saline (PBS). Anti-L2 IgG antibody responses in sera were determined by end-point dilution ELISA (except for orally immunized mice 1:40 sera dilution was used) as described previously 12–14; mouse anti-rat IgG antibody was used as secondary antibody at 1:2000 (for sera from oral immunizations) and 1:5000 (for sera from intramuscular immunizations). To assess the induction of IgA antibodies, vaginal washes were diluted into 1:10 (in PBS with 0.5% non-fat milk) and used as primary antibody. Goat anti-Rat IgA at 1:500 dilution was used as secondary antibody.
Immunizations in mice
The spray dried VLPs (stored for fourteen months at RT and 37 °C) were reconstituted in sterile PBS immediately prior to IM administration. Four-six week-old Balb/c mice were immunized intramuscularly with a single dose of 5 μg reconstituted L2-VLPs or freshly made L2-VLPs. Sera was collected one, two, and three months after vaccination and then tested for anti-L2 antibodies by ELISA as described previously 12–14.
RESULTS
Design of Experiments- Half-factorial design
A two level, half-factorial design (25–1) was developed for the optimization of excipients ratio and spray drying parameters as shown in Table 1 using the Design-Expert® software. This design allowed us to decipher the appropriate formulation and process variables required to formulate a thermostable VLPs formulation in half the number of spray drying runs (16) as compared to a full factorial design (32). The 25–1 half-factorial design is a resolution V design in which main effects (e.g. A, B, C) or second-order interaction effects (e.g. AB, AD, BE) are not confounded with each other. However, in a resolution V design the main effects are confounded with three factor or higher order interactions. Sixteen design points and 3 midpoints were performed to check the reproducibility in terms of four response variables i.e., yield (%), moisture content (%), particle size (μm) and span (particle size distribution). To reduce the total number of runs, the four excipients were combined in two pairs based on their antagonistic effects, i.e., leucine vs. trehalose and mannitol vs. dextran; their ratios were also varied in pairs for the half-factorial design. Leucine:trehalose were varied at two different ratios 15.79:5.26 (3) and 10.53:10.53 (1). Similarly, mannitol:dextran were varied at 76.84:2.11 (37) and 73.68:5.26 (14). The spray drying process variables having the maximum effect on the response variables were identified as inlet temperature, gas flow rate and liquid feed rate 19. The optimization criteria for response variables were as follows: a) to maximize the yield (cost of the final product), b) to minimize moisture content (superior long-term storage stability), c) to achieve least span (narrow particle size distribution; uniform VLPs loading) and d) to achieve larger particle size (high VLP loading capacity).
Optimization of spray drying parameters
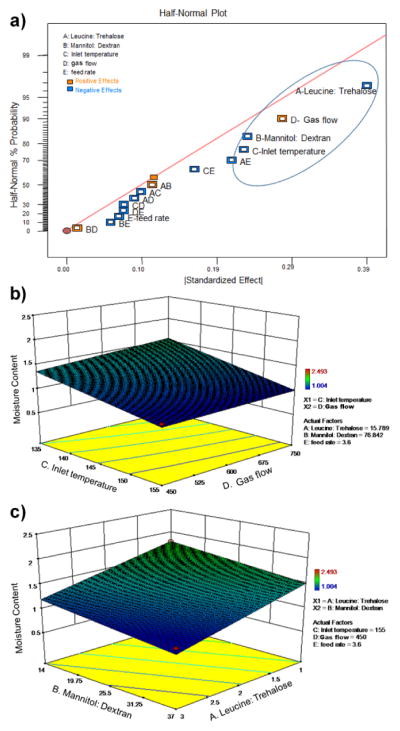
Among the four response variables, moisture content and yield were used to optimize the formulation and process variables (excipient ratio, inlet temperature, gas flow rate and liquid feed rate). Process variables were first optimized with respect to residual moisture content, since this is an important factor for maintaining the long-term storage stability of VLPs dry powder 20. The half-normal plot in Fig. 1a suggests that leucine:trehalose (A), mannitol:dextran (B), inlet temperature (C) and gas flow (D) are main contributors to the residual moisture content and contributed to 72% of all the overall effect (Supplemental table, highlighted values in column 3). In addition, excipient ratio and spray drying parameters were analyzed separately. As shown in Fig. 1b, residual moisture content in the powders decreased when the gas flow was decreased and the inlet temperature was increased. Furthermore, residual moisture content decreased when leucine:trehalose and mannitol:dextran ratio was increased, as shown in Fig. 1c.
Figure 1.
Optimization of spray drying parameters and multi-component excipients with respect to residual moisture content. a) half-normal plot of the spray dried powders; b) response surface plot in powders as a function of N2 gas flow and inlet temperature; c) response surface plot as a function of excipients ratio (A- leucine:trehalose and B- mannitol:dextran).
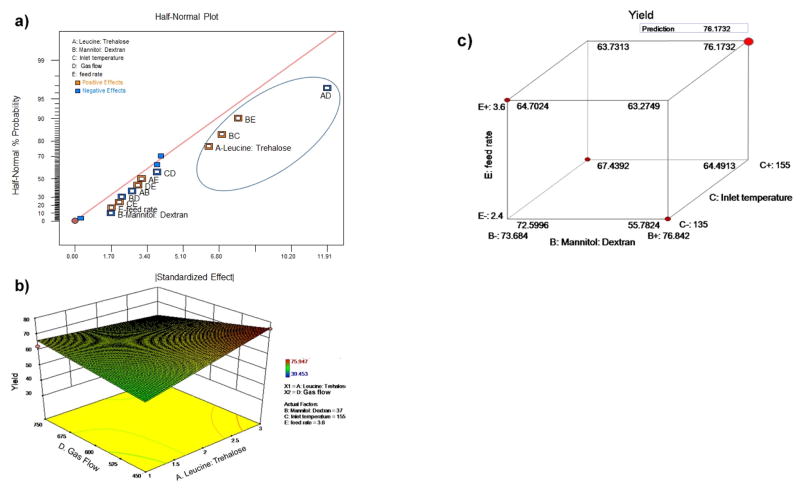
Formulation and process variables were next analyzed with respect to the final product yield after spray drying. Fig. 2a and the corresponding statistics (data not shown) suggests that the interaction between leucine:trehalose ratio (A) and gas flow (AD) contributed approximately 50% to the overall effect on the product yield. Further analysis of Fig. 2a and its corresponding statistics reveals that the interaction of mannitol:dextran ratio with inlet temperature (BC) and liquid feed rate (BE), accounts for only 28% of the overall effect on yield (Supplemental table, highlighted values in column 1). As shown in Fig. 2b, the maximum yield is achieved when gas flow is decreased and leucine:trehalose ratio (A) is increased. We also analyzed the three variables (mannitol:dextran ratio, inlet temperature and liquid feed rate) simultaneously as shown in the cube plot in Fig. 2c, where each corner is labelled with the high and low values set for each variable. Based on the combined analysis of the three parameters, increased mannitol:dextran ratio, liquid feed rate and inlet temperature all contributed towards a high product yield (marked by the bigger red dot).
Figure 2.
Optimization of spray drying parameters and multi-component excipients with respect to product yield. a) half-normal plot of the spray dried powders; b) response surface plot in powders as a function of N2 gas flow and leucine:trehalose ratio; c) cube plot showing the simultaneous optimization of mannitol:dextran ratio, liquid feed rate and inlet temperature towards achieving the highest product yield (shown by the large red dot).
The particle size (volume median diameter) of the dry powder, obtained by laser diffraction, was included as a parameter in the half-factorial design to achieve high VLPs loading since any change in particle size will affect the loading efficiency 21. The particle size was negatively affected by the gas flow which accounted for 94% of the overall effect (data and statistics not shown), indicating that the particle size increased with a decrease in gas flow. Therefore, as per the above analysis, the desirable parameters, i.e. low moisture content, high powder yield and larger particle size all required low gas flow rate. Hence, we used the low gas flow rate for spray drying VLPs. Although the span (particle size distribution) listed in Table I was not used for optimization, particles prepared by the optimized spray drying conditions showed a unimodal and narrow particle size distribution (Dv50- 4.60μm, span- 1.68).
Table I.
Half-factorial design (25–1) for the optimization of the excipients ratio (formulation) and the spray drying (process) parameters
| RUN | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Response 1 | Response 2 | Response 3 | Response 4 |
|---|---|---|---|---|---|---|---|---|---|
| leucine:trehalose | mannitol:dextran | Inlet temp. °C | Gas flow | Liquid feed rate ml/min | Yield | Moisture Content % | Particle size | Span | |
| w:w (ratio) | w:w (ratio) | L/h | % | μm | |||||
| 1 | 15.789: 5.263 (3) | 73.684: 5.263 (14) | 155 | 750 | 3.6 | 49.026 | 1.115 | 3.17 | 1.211 |
| 2 | 10.526:10.526 (1) | 76.842: 2.105 (37) | 155 | 750 | 3.6 | 62.48 | 1.735 | 2.94 | 1.151 |
| 3 | 13.158: 7.895 | 75.263: 3.684 (20) | 145 | 500 | 3 | 49.067 | 1.634 | 3.09 | 1.165 |
| 4 | 10.526:10.526 (1) | 76.842: 2.105 (37) | 135 | 450 | 3.6 | 48.44 | 1.614 | 4.60 | 1.603 |
| 5 | 15.789: 5.263 (3) | 73.684: 5.263 (14) | 135 | 450 | 3.6 | 68.8 | 1.613 | 5.03 | 1.705 |
| 6 | 10.526:10.526 (1) | 73.684: 5.263 (14) | 155 | 750 | 2.4 | 53.1067 | 2.045 | 2.83 | 1.095 |
| 7 | 15.789: 5.263 (3) | 73.684: 5.263 (14) | 155 | 450 | 2.4 | 67.213 | 1.400 | 4.36 | 1.494 |
| 8 | 10.526:10.526 (1) | 73.684: 5.263 (14) | 135 | 450 | 2.4 | 58.65 | 1.700 | 4.86 | 1.450 |
| 9 | 10.526:10.526 (1) | 76.842: 2.105 (37) | 155 | 450 | 2.4 | 52.16 | 1.505 | 4.34 | 1.369 |
| 10 | 13.158: 7.895 | 75.263: 3.684 (20) | 145 | 500 | 3 | 47.787 | 1.615 | 3.53 | 1.397 |
| 11 | 15.789: 5.263 (3) | 76.842: 2.105 (37) | 135 | 450 | 2.4 | 59.88 | 1.406 | 4.90 | 1.661 |
| 12 | 15.789: 5.263 (3) | 76.842: 2.105 (37) | 155 | 450 | 3.6 | 75.947 | 1.004 | 4.60 | 1.680 |
| 13 | 15.789: 5.263 (3) | 76.842: 2.105 (37) | 135 | 750 | 3.6 | 56.227 | 1.512 | 3.06 | 1.305 |
| 14 | 10.526:10.526 (1) | 73.684: 5.263 (14) | 135 | 750 | 3.6 | 61.533 | 2.493 | 3.16 | 1.243 |
| 15 | 10.526:10.526 (1) | 73.684: 5.263 (14) | 155 | 450 | 3.6 | 39.707 | 1.804 | 4.35 | 1.561 |
| 16 | 15.789: 5.263 (3) | 76.842: 2.105 (37) | 155 | 750 | 2.4 | 39.453 | 1.637 | 2.69 | 1.002 |
| 17 | 13.158: 7.895 | 75.263: 3.684 (20) | 145 | 500 | 3 | 39.87 | 1.503 | 3.51 | 1.321 |
| 18 | 10.526:10.526 (1) | 76.842: 2.105 (37) | 135 | 750 | 2.4 | 53.973 | 1.813 | 2.92 | 1.112 |
| 19 | 15.789: 5.263 (3) | 73.684: 5.263 (14) | 135 | 750 | 2.4 | 64.027 | 1.923 | 2.89 | 1.148 |
Labeled in bold are three experimental runs to check reproducibility
After the analysis of the half-factorial design, the optimized spray drying conditions for encapsulating VLPs were as follows: inlet temperature (155 °C), gas flow rate (450 L/hr), liquid feed rate (3.6 ml/min). Furthermore, the optimized formulation parameters for leucine:trehalose and mannitol:dextran was 15.789:5.263 (3) and 76.842:2.105 (37), respectively.
VLPs loading and integrity
VLPs (freshly prepared and immediately after spray drying) were co-electrophoresed in SDS-polyacrylamide gels with known concentrations of hen egg lysozyme as standards. Coomassie Blue-stained VLP bands were scanned and quantitated by densitometric analysis, using Image J software and a quantification method similar to the website http://lukemiller.org/index.php/2010/11/analyzing-gels-and-western-blots-with-image-j/.
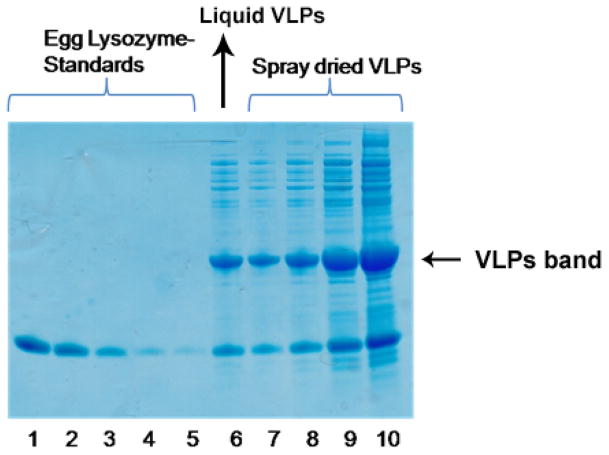
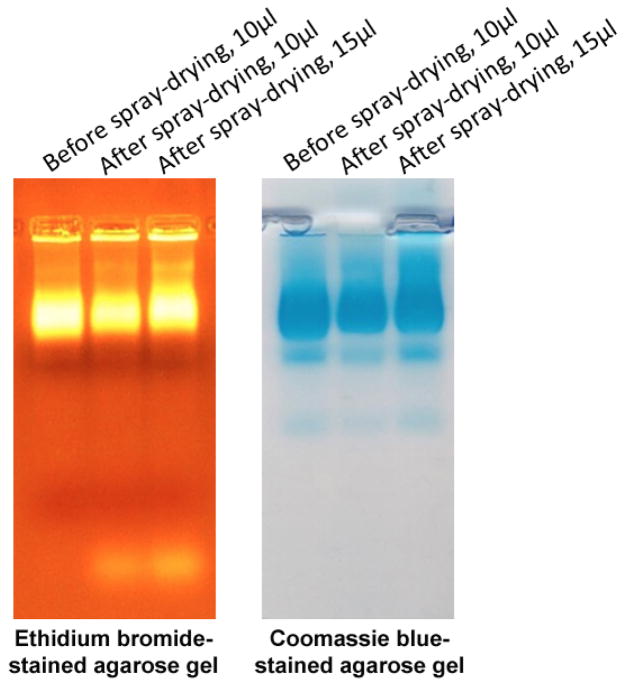
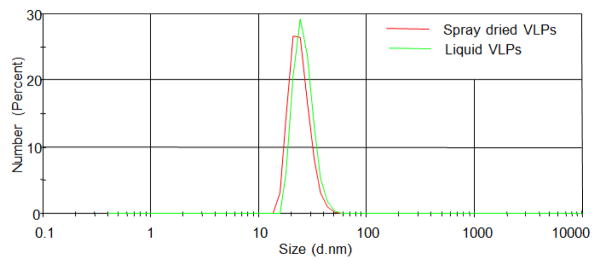
The concentration of VLPs in the suspension (pre-spray drying) and in spray dried powders were 2.5 mg/ml and 1.6 mg/ml, respectively (Fig 3). Therefore, the loading efficiency of VLPs in spray dried powder was 64%. The integrity of the VLPs after spray drying was assessed by agarose gel electrophoresis. As shown in Fig 4, the co-migration of encapsidated RNA (stained with ethidium bromide, EtBr) and protein (stained with Coomassie Blue) indicates the integrity of VLPs after spray drying. VLPs migrate through agarose gel due to their overall electrophoretic charge and can be visualized using EtBr by virtue of the RNA that is encapsidated by the particles and by Coomassie blue staining to detect protein. Thus, this assay was used to determine whether particles are present and whether they contain RNA. No additional protein bands were seen on the gel, indicating that the VLPs were not degraded. Moreover, DLS data (Fig 5) further showed the integrity of VLPs after spray drying as is evident by the overlapping size distribution of both liquid and dry powder VLPs (after reconstitution).
Figure 3.
Quantitation of VLPs loading in spray dried powder. Spray dried VLPs (Lane 7–10 containing 1, 2, 5 and 10 μl representing varying concentrations) and liquid VLPs (Lane 6- 2 μl) were co-electrophoresed in SDS-polyacrylamide gels with a range of hen egg lysozyme samples of defined concentrations (Lane 1–5). Gels were stained with Coomassie blue. VLPs protein bands were scanned and quantitated by densitometric analysis in comparison with known concentrations of egg lysozyme protein bands, using ImageJ software.
Figure 4.
Integrity of VLPs after spray drying. Reconstituted VLPs from dry powder were analyzed on agarose gel stained with ethidium bromide (left panel) and Coomassie blue (right panel) showing the VLP proteins at the same position as of encapsidated RNAs.
Figure 5.
Integrity of VLPs after spray drying. DLS data showing similar size distribution for liquid and dry powder VLPs
Long-term stability
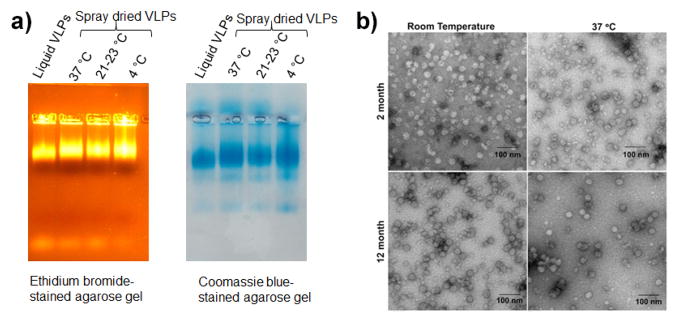
The stability of MS2-16L2 VLP dry powder at three different temperature conditions (RT and 37 °C) were studied for one year (twelve months). The integrity of VLPs was monitored by agarose gel electrophoresis and TEM. As shown in Fig 6a, the spray dried VLPs stored at elevated temperatures for 2 months and 12 months (data not shown) exhibit the same EtBr and Coomassie Blue staining patterns as freshly prepared VLPs, indicating that no degradation occurred during storage. Furthermore, as shown in Fig 6b, the morphology of spray dried VLPs remained the same as seen under TEM (two months and 12 months of storage of VLPs dry powder at RT and 37 °C). Immunization studies
Figure 6.
Stability of spray dried VLPs. Reconstituted VLPs from dry powder were analyzed a) Agarose gel stained with ethidium bromide (left panel) or Coomassie blue (right panel) at two months after storage and b) transmission electron microscopy images at two and twelve months after storage
Oral immunization in rats
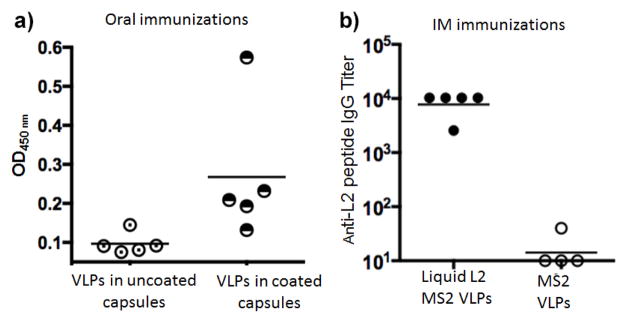
We were interested if oral immunization of spray dried VLPs will be as immunogenic as VLPs administered by the IM route. Rats were immunized orally with VLPs dry powder encapsulated in enteric-coated capsules. Rats were chosen as the animal model for oral immunization due to the availability of size 9 gelatin capsules for rats (Torpac Inc. Fairfield, NJ). For the in vivo studies, one group of rats (N=5) received enteric-coated capsules, another received uncoated capsules containing VLPs dry powder. As a positive control, a group of rats received intramuscular immunizations with VLPs. After three immunizations (each administered at three week intervals), rats were bled and anti-L2 antibody responses in sera were determined by peptide ELISA. As shown in Fig 7, oral immunization elicited weak antibody responses in a subset of the immunized rats; titers were much lower compared to rats immunized intramuscularly (Fig 7b). Rats immunized with enteric-coated capsules had detectable anti-L2 antibody responses. However, only one rat in the group immunized with uncoated capsules had detectable antibody levels above background (Fig 7a). No IgA antibody was detected in the vaginal washes from any of the immunized animal groups, except in one rat that was immunized intramuscularly with the L2 VLPs (data not shown).
Figure 7.
Immunogenicity of L2-VLPs in rats. Rats were immunized thrice orally with 400 μg of VLPs in a) coated or uncoated capsules or b) immunized intramuscularly with 10 μg of VLPs. Two weeks after the last immunization, sera were collected. In a), 1:40 serum dilution was used in an ELISA and b) antibodies titers were determined by end-point dilution ELISA.
IM immunization in mice
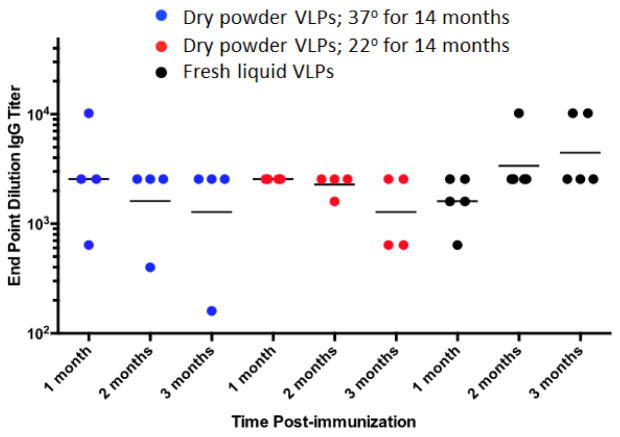
The immunogenicity of spray dried VLPs (stored for fourteen months at RT or 37°C) were evaluated by immunizing mice by the intramuscular route. Mice were given a single dose of spray dried (and reconstituted) or freshly prepared L2-VLPs and antibody titers were measured against the L2 peptide one, two, and three months post immunization. As shown in Fig 8, a single dose of spray dried VLPs induced high-titer anti-L2 IgG responses that were similar to mice immunized with freshly prepared (non-spray dried) L2-VLPs. Antibody titers were stable for three months after immunization, although the titer levels in a few mice immunized with spray dried L2-VLPs declined over this period.
Figure 8.
Immunogenicity of stored spray dried L2-VLPs. VLPs were prepared by spray drying and stored at 37°C or RT (21–23 °C) for 14 months. After this period, VLPs were reconstituted in PBS and used to immunize mice. Mice were given a single dose of 5 μg VLPs. A control group was immunized with freshly made L2-VLPs. Sera was collected 1, 2, and 3 months after immunization and anti-L2 IgG titers were determined by end-point dilution ELISA. Each dot refers to an individual immunized mice, lines denote geometric mean IgG titers.
DISCUSSION
Cervical cancer is the second most common and the fifth deadliest cancer in women, with over 500,000 new cases and 275,000 deaths worldwide with majority of the cases occurring in the developing world 10. Previously, we developed a broadly protective HPV vaccine in which a highly conserved neutralizing epitope from the HPV L2 protein was displayed multivalently on bacteriophage VLPs. The L2 bacteriophage VLPs induced a strong, long-lasting immune responses as well as broad protection against diverse HPV types 12, 14. L2-VLP vaccines elicit robust anti-L2 IgG responses while providing broad protection from genital and cutaneous HPV pseudovirus challenge in a mouse model of infection 11, 13, 14, 22.
In this study, we explored the effect of excipient ratios (formulation variables), inlet temperature, gas flow rate and liquid feed rate (spray drying variables) on MS2-16L2 VLP formulation. Liquid VLPs were formulated into dry powders by spray drying to provide long-term stability at elevated temperatures. The currently marketed vaccines against cervical cancer, Gardasil® (quadrivalent and nonavalent) and Cervarix®, like other liquid vaccines, require continuous cold-chain during storage and transportation. Spray drying has previously been used to formulate dry powder vaccines including peptides 4, 5, 23, live bacteria 24, viruses 25 and bacteriophages 26.
A half-factorial (25–1) DoE was used to achieve the best formulation and to optimize the spray drying conditions with the least experimental runs in order to save time and in reducing material costs 19, 27, 28. The DoE helped us to better understand the interaction between the spray drying processes and the formulation effects on dry powder characteristics, such as product yield, particle size, moisture content etc. Although, a half-factorial resolution V design has main effects and two-factor interactions unconfounded from themselves and each other, such a design may be confounding with three-factor or higher-order interactions. Spray drying usually involves exposure to process stress such as heat, shear and desiccation that can damage the proteins/peptides and therefore often require one or more excipients for stabilization 29. We optimized the adjustable spray drying parameters including the inlet temperature, liquid feed rate and the gas flow rate to achieve a thermostable VLPs dry powder.
A multi-component excipient system was used for spray drying, with each excipient providing certain properties towards achieving a stable dry powder. Incorporation of leucine in our formulation helped in protection against moisture uptake. In addition, leucine prevents the crystallization of mannitol possibly due to its ability to prevent moisture uptake by the dry powders 30. Furthermore, mannitol is a non-hygroscopic and non-reducing sugar that is effective in stabilizing spray dried proteins 31. Trehalose is also known to stabilize proteins after spray drying, possibly due to its high glass transition temperature. However, trehalose concentration was low in our dry powders since it is highly hygroscopic and could lead to powder agglomeration during long-term storage. A small percentage of dextran was included in our formulation as it imparts particle formation properties during spray drying. Dextran further provides stabilizing properties to the incorporated VLPs by preventing crystallization of the multi-component excipient system 1.
Long-term stability (twelve months) for spray dried VLPs was monitored at different temperatures (RT and 37°C) with the ultimate goal of eliminating cold-chain storage requirements for the VLPs vaccines. Cold-chain handling during transportation and storage of liquid vaccines is usually breached in remote regions of the world due to lack of well-developed and continuous refrigeration system 32. The risk of vaccine damage due to exposure to higher temperatures would lead to vaccine wastage, or more frightening causing a disease against which a child was immunized33. The significance of vaccine thermostability was first shown by the eradication of smallpox globally in the 1960’s immediately after a “heat-stable” vaccine was available; this allowed the vaccine’s widespread distribution without a cold-chain requirement to the remote regions of the world 34. More recently, Meningococcal A conjugate vaccine (MenAfrivac®) was approved in 2012 for its use in a controlled temperature chain (CTC) that allowed exposure to temperatures up to 40°C for four days. This vaccine not only reduced meningitis cases in sub-Saharan Africa significantly, but achieving short-term thermostability also reduced the cost of the vaccine by 50% 35. VLPs dry powder vaccine with enhanced thermostability is appropriate for deployment in resource-poor settings since majority of cervical cancers occur in the developing world 36.
VLP-based vaccines have been previously administered by various routes in preclinical and clinical studies, including sublingual 37, 38, oral 39, 40, skin 41, 42, vaginal 43, 44, pulmonary 45 and intranasal 3, 46 routes. We initially administered VLPs dry powder in rats by the oral route because of the many advantages offered by this route of immunization. The VLPs dry powder were delivered in enteric coated capsules to prevent its degradation in the harsh gastric environment and to retain its antigenicity. However, VLPs administered orally in enteric coated capsules largely elicited low antibody titers. VLPs delivered in capsules without enteric protection did not elicit an immune response in rats. The response elicited by VLPs in enteric coated capsules were lower compared to the response generated with IM administered VLPs. We speculate this variability due to non-uniform uptake of VLPs from the small intestine once released from the capsules 47. Future studies should incorporate permeation enhancers such as surfactants or fatty acids, or a mucosal adjuvant, along with enteric protection to the orally delivered VLPs vaccines 48, 49.
We also administered VLPs dry powder, stored at elevated temperatures for over one year, by the intramuscular route in mice. The sugar matrix of the dry powder protected the VLPs from the deleterious effects during spray drying, long-term storage as well as powder reconstitution. We have recently shown that spray dried VLPs dry powder are immunogenic 11. However, in the current study we showed that spray dried VLPs are immunogenic even after storing at 37 °C for fourteen months, thus demonstrating the enhanced thermostability of dry-powder VLPs.
CONCLUSIONS
We optimized the VLP dry powder formulations and spray drying parameters such that the vaccine withstood elevated temperatures during storage without losing its antigenicity. The optimized formulation consisted of three sugars (mannitol, trehalose and dextran) and an amino acid, leucine. Long-term stability testing at elevated temperatures can provide useful insights on the required storage conditions for these dry powder VLPs. This could have an enormous impact on the cost, efficiency and success of global immunization programs especially in the regions of the world where it is required the most. Consequently, this is a potentially significant achievement that could result in major economic benefits and allow broader vaccine coverage against HPV in order to be extended in low-resource countries.
Supplementary Material
Acknowledgments
This work was partially supported by a cooperative agreement from the US National Institutes of Health (National Institute of Allergy and Infectious Diseases) establishing the Epidemiology and Prevention Interdisciplinary Center for Sexually Transmitted Diseases (U19 AI113187). SS received stipend support from College of Pharmacy start-up funds. We would like to thank Dr. Stephen Jett for help with transmission electron microscopy and Ms. Monique Nysus with helping with oral dosing of size 9 capsules in rats.
Abbreviations
- VLP
Virus-like particle
- HPV
Human papillomavirus
- DoE
Design of Experiment
Footnotes
Additional data for the optimization of the spray drying parameters using the DoE- Half-factorial design is provided in the form of various factors and their interactions that contribute to the residual moisture content, particle size and the final powder yield.
References
- 1.Chen D, Kristensen D. Opportunities and challenges of developing thermostable vaccines. Expert review of vaccines. 2009 doi: 10.1586/erv.09.20. [DOI] [PubMed] [Google Scholar]
- 2.Esquisabel A, Pastor M, Talavera A, Cedré B, Fernández S, Sifontes S, Aranguren Y, Falero G, García L, Solís RL. A new oral vaccine candidate based on the microencapsulation by spray-drying of inactivated Vibrio cholerae. Vaccine. 2011;29(34):5758–5764. doi: 10.1016/j.vaccine.2011.05.098. [DOI] [PubMed] [Google Scholar]
- 3.Velasquez LS, Shira S, Berta AN, Kilbourne J, Medi BM, Tizard I, Ni Y, Arntzen CJ, Herbst-Kralovetz MM. Intranasal delivery of Norwalk virus-like particles formulated in an in situ gelling, dry powder vaccine. Vaccine. 2011;29(32):5221–31. doi: 10.1016/j.vaccine.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muttil P, Prego C, Garcia-Contreras L, Pulliam B, Fallon JK, Wang C, Hickey AJ, Edwards D. Immunization of guinea pigs with novel hepatitis B antigen as nanoparticle aggregate powders administered by the pulmonary route. The AAPS journal. 2010;12(3):330–7. doi: 10.1208/s12248-010-9192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muttil P, Pulliam B, Garcia-Contreras L, Fallon JK, Wang C, Hickey AJ, Edwards DA. Pulmonary immunization of guinea pigs with diphtheria CRM-197 antigen as nanoparticle aggregate dry powders enhance local and systemic immune responses. The AAPS journal. 2010;12(4):699–707. doi: 10.1208/s12248-010-9229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Padamwar MN, Patole MS, Pokharkar VB. Chitosan-reduced gold nanoparticles: a novel carrier for the preparation of spray-dried liposomes for topical delivery. Journal of liposome research. 2011;21(4):324–332. doi: 10.3109/08982104.2011.575380. [DOI] [PubMed] [Google Scholar]
- 7.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug-Saber M, Cozen W. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. Journal of Clinical Oncology. 2011;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLaughlin-Drubin ME, Meyers J, Munger K. Cancer associated human papillomaviruses. Current opinion in virology. 2012;2(4):459–466. doi: 10.1016/j.coviro.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fruscalzo A, Londero A, Bertozzi S, Lellé R. Second-generation prophylactic HPV vaccines: current options and future strategies for vaccines development. Minerva medica. 2015 [PubMed] [Google Scholar]
- 10.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin D, Forman D, Bray F. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] Vol. 2013. Lyon, France: International Agency for Research on Cancer; 2012. [Accessed 2015; 30]. GLOBOCAN. v1.0. http.globocan.iarc.fr. [Google Scholar]
- 11.Tumban E, Muttil P, Escobar CA, Peabody J, Wafula D, Peabody DS, Chackerian B. Preclinical refinements of a broadly protective VLP-based HPV vaccine targeting the minor capsid protein, L2. Vaccine. 2015;33(29):3346–53. doi: 10.1016/j.vaccine.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tumban E, Peabody J, Peabody DS, Chackerian B. A universal virus-like particle-based vaccine for human papillomavirus: longevity of protection and role of endogenous and exogenous adjuvants. Vaccine. 2013;31(41):4647–4654. doi: 10.1016/j.vaccine.2013.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tumban E, Peabody J, Tyler M, Peabody DS, Chackerian B. VLPs displaying a single L2 epitope induce broadly cross-neutralizing antibodies against human papillomavirus. PloS one. 2012;7(11):e49751. doi: 10.1371/journal.pone.0049751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tumban E, Peabody J, Peabody DS, Chackerian B. A pan-HPV vaccine based on bacteriophage PP7 VLPs displaying broadly cross-neutralizing epitopes from the HPV minor capsid protein, L2. PloS one. 2011;6(8):e23310. doi: 10.1371/journal.pone.0023310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Food and Drug Administration. [Accessed 13 March 2016];Inactive ingredient search for approved drug products. http://www.accessdata.fda.gov/scripts/cder/iig/index.Cfm.
- 16.Sonaje K, Chen YJ, Chen HL, Wey SP, Juang JH, Nguyen HN, Hsu CW, Lin KJ, Sung HW. Enteric-coated capsules filled with freeze-dried chitosan/poly(gamma-glutamic acid) nanoparticles for oral insulin delivery. Biomaterials. 2010;31(12):3384–94. doi: 10.1016/j.biomaterials.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen HN, Wey SP, Juang JH, Sonaje K, Ho YC, Chuang EY, Hsu CW, Yen TC, Lin KJ, Sung HW. The glucose-lowering potential of exendin-4 orally delivered via a pH-sensitive nanoparticle vehicle and effects on subsequent insulin secretion in vivo. Biomaterials. 2011;32(10):2673–82. doi: 10.1016/j.biomaterials.2010.12.044. [DOI] [PubMed] [Google Scholar]
- 18.Li P, Tan A, Prestidge CA, Nielsen HM, Mullertz A. Self-nanoemulsifying drug delivery systems for oral insulin delivery: in vitro and in vivo evaluations of enteric coating and drug loading. International journal of pharmaceutics. 2014;477(1–2):390–8. doi: 10.1016/j.ijpharm.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 19.Shi S, Hickey AJ. PLGA microparticles in respirable sizes enhance an in vitro T cell response to recombinant Mycobacterium tuberculosis antigen TB10.4-Ag85B. Pharmaceutical research. 2010;27(2):350–60. doi: 10.1007/s11095-009-0028-7. [DOI] [PubMed] [Google Scholar]
- 20.Paudel A, Worku ZA, Meeus J, Guns S, Van den Mooter G. Manufacturing of solid dispersions of poorly water soluble drugs by spray drying: formulation and process considerations. International journal of pharmaceutics. 2013;453(1):253–84. doi: 10.1016/j.ijpharm.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Maa Y, Hsu C. Effect of primary emulsions on microsphere size and protein-loading in the double emulsion process. Journal of microencapsulation. 1997;14(2):225–241. doi: 10.3109/02652049709015335. [DOI] [PubMed] [Google Scholar]
- 22.Caldeira JdC, Medford A, Kines RC, Lino CA, Schiller JT, Chackerian B, Peabody DS. Immunogenic display of diverse peptides, including a broadly cross-type neutralizing human papillomavirus L2 epitope, on virus-like particles of the RNA bacteriophage PP7. Vaccine. 2010;28(27):4384–4393. doi: 10.1016/j.vaccine.2010.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunda NK, Alfagih IM, Dennison SR, Tawfeek HM, Somavarapu S, Hutcheon GA, Saleem IY. Bovine Serum Albumin Adsorbed PGA-co-PDL Nanocarriers for Vaccine Delivery via Dry Powder Inhalation. Pharmaceutical research. 2014:1–13. doi: 10.1007/s11095-014-1538-5. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Contreras L, Wong YL, Muttil P, Padilla D, Sadoff J, Derousse J, Germishuizen WA, Goonesekera S, Elbert K, Bloom BR, Miller R, Fourie PB, Hickey A, Edwards D. Immunization by a bacterial aerosol. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(12):4656–60. doi: 10.1073/pnas.0800043105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amorij J, Huckriede A, Wilschut J, Frijlink H, Hinrichs W. Development of stable influenza vaccine powder formulations: challenges and possibilities. Pharmaceutical research. 2008;25(6):1256–1273. doi: 10.1007/s11095-008-9559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matinkhoo S, Lynch KH, Dennis JJ, Finlay WH, Vehring R. Spray-dried respirable powders containing bacteriophages for the treatment of pulmonary infections. Journal of pharmaceutical sciences. 2011;100(12):5197–5205. doi: 10.1002/jps.22715. [DOI] [PubMed] [Google Scholar]
- 27.Amaro MI, Tajber L, Corrigan OI, Healy AM. Optimisation of spray drying process conditions for sugar nanoporous microparticles (NPMPs) intended for inhalation. International journal of pharmaceutics. 2011;421(1):99–109. doi: 10.1016/j.ijpharm.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 28.Belotti S, Rossi A, Colombo P, Bettini R, Rekkas D, Politis S, Colombo G, Balducci AG, Buttini F. Spray dried amikacin powder for inhalation in cystic fibrosis patients: a quality by design approach for product construction. International journal of pharmaceutics. 2014;471(1–2):507–15. doi: 10.1016/j.ijpharm.2014.05.055. [DOI] [PubMed] [Google Scholar]
- 29.Jin TH, Tsao E, Goudsmit J, Dheenadhayalan V, Sadoff J. Stabilizing formulations for inhalable powders of an adenovirus 35-vectored tuberculosis (TB) vaccine (AERAS-402) Vaccine. 2010;28(27):4369–75. doi: 10.1016/j.vaccine.2010.04.059. [DOI] [PubMed] [Google Scholar]
- 30.Sou T, Kaminskas LM, Nguyen TH, Carlberg R, McIntosh MP, Morton DA. The effect of amino acid excipients on morphology and solid-state properties of multi-component spray-dried formulations for pulmonary delivery of biomacromolecules. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2012 doi: 10.1016/j.ejpb.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 31.Costantino HR, Andya JD, Nguyen PA, Dasovich N, Sweeney TD, Shire SJ, Hsu CC, Maa YF. Effect of mannitol crystallization on the stability and aerosol performance of a spray-dried pharmaceutical protein, recombinant humanized anti-IgE monoclonal antibody. Journal of pharmaceutical sciences. 1998;87(11):1406–1411. doi: 10.1021/js9800679. [DOI] [PubMed] [Google Scholar]
- 32.Kaufmann JR, Miller R, Cheyne J. Vaccine supply chains need to be better funded and strengthened, or lives will be at risk. Health Affairs. 2011;30(6):1113–1121. doi: 10.1377/hlthaff.2011.0368. [DOI] [PubMed] [Google Scholar]
- 33.Shrivastava A, Gupta N, Upadhyay P, Puliyel J. Caution needed in using oral polio vaccine beyond the cold chain: Vaccine vial monitors may be unreliable at high temperatures. The Indian journal of medical research. 2012;135(4):520. [PMC free article] [PubMed] [Google Scholar]
- 34.Cross R, Kaplan C, McClean D. The heat resistance of dried smallpox vaccine. The Lancet. 1957;269(6966):446–448. doi: 10.1016/s0140-6736(57)90521-4. [DOI] [PubMed] [Google Scholar]
- 35.Lydon P, Zipursky S, Tevi-Benissan C, Djingarey MH, Gbedonou P, Youssouf BO, Zaffran M. Economic benefits of keeping vaccines at ambient temperature during mass vaccination: the case of meningitis A vaccine in Chad. Bulletin of the World Health Organization. 2014;92(2):86–92. doi: 10.2471/BLT.13.123471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kane MA. Human papillomaviruses (HPV) vaccines: implementation and communication issues. Journal of Family Planning and Reproductive Health Care. 2015;34(1):3–4. doi: 10.1783/147118908783332113. [DOI] [PubMed] [Google Scholar]
- 37.Cuburu N, Kweon MN, Hervouet C, Cha HR, Pang YY, Holmgren J, Stadler K, Schiller JT, Anjuere F, Czerkinsky C. Sublingual immunization with nonreplicating antigens induces antibody-forming cells and cytotoxic T cells in the female genital tract mucosa and protects against genital papillomavirus infection. Journal of immunology. 2009;183(12):7851–9. doi: 10.4049/jimmunol.0803740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huo Z, Bissett SL, Giemza R, Beddows S, Oeser C, Lewis DJ. Systemic and mucosal immune responses to sublingual or intramuscular human papilloma virus antigens in healthy female volunteers. PloS one. 2012;7(3):e33736. doi: 10.1371/journal.pone.0033736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ball JM, Hardy ME, Atmar RL, Conner ME, Estes MK. Oral immunization with recombinant Norwalk virus-like particles induces a systemic and mucosal immune response in mice. Journal of virology. 1998;72(2):1345–53. doi: 10.1128/jvi.72.2.1345-1353.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ball JM, Graham DY, Opekun AR, Gilger MA, Guerrero RA, Estes MK. Recombinant Norwalk virus-like particles given orally to volunteers: phase I study. Gastroenterology. 1999;117(1):40–8. doi: 10.1016/s0016-5085(99)70548-2. [DOI] [PubMed] [Google Scholar]
- 41.Chen X, Fernando GJ, Crichton ML, Flaim C, Yukiko SR, Fairmaid EJ, Corbett HJ, Primiero CA, Ansaldo AB, Frazer IH, Brown LE, Kendall MA. Improving the reach of vaccines to low-resource regions, with a needle-free vaccine delivery device and long-term thermostabilization. Journal of controlled release : official journal of the Controlled Release Society. 2011;152(3):349–55. doi: 10.1016/j.jconrel.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 42.Corbett HJ, Fernando GJ, Chen X, Frazer IH, Kendall MA. Skin vaccination against cervical cancer associated human papillomavirus with a novel micro-projection array in a mouse model. PloS one. 2010;5(10):e13460. doi: 10.1371/journal.pone.0013460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hunter Z, Tumban E, Dziduszko A, Chackerian B. Aerosol delivery of virus-like particles to the genital tract induces local and systemic antibody responses. Vaccine. 2011;29(28):4584–92. doi: 10.1016/j.vaccine.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Rompay KK, Hunter Z, Jayashankar K, Peabody J, Montefiori D, LaBranche CC, Keele BF, Jensen K, Abel K, Chackerian B. A vaccine against CCR5 protects a subset of macaques upon intravaginal challenge with simian immunodeficiency virus SIVmac251. Journal of virology. 2014;88(4):2011–24. doi: 10.1128/JVI.02447-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hunter Z, Smyth HD, Durfee P, Chackerian B. Induction of mucosal and systemic antibody responses against the HIV coreceptor CCR5 upon intramuscular immunization and aerosol delivery of a virus-like particle based vaccine. Vaccine. 2009;28(2):403–14. doi: 10.1016/j.vaccine.2009.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balmelli C, Roden R, Potts A, Schiller J, De Grandi P, Nardelli-Haefliger D. Nasal immunization of mice with human papillomavirus type 16 virus-like particles elicits neutralizing antibodies in mucosal secretions. Journal of virology. 1998;72(10):8220–8229. doi: 10.1128/jvi.72.10.8220-8229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nature medicine. 2005;11:S45–S53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 48.Fasinu P, Pillay V, Ndesendo VM, du Toit LC, Choonara YE. Diverse approaches for the enhancement of oral drug bioavailability. Biopharmaceutics & drug disposition. 2011;32(4):185–209. doi: 10.1002/bdd.750. [DOI] [PubMed] [Google Scholar]
- 49.Hjelm BE, Kilbourne J, Herbst-Kralovetz MM. TLR7 and 9 agonists are highly effective mucosal adjuvants for norovirus virus-like particle vaccines. Hum Vaccin Immunother. 2014;10(2):410–6. doi: 10.4161/hv.27147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.