Abstract
Increased glutamatergic neurotransmission appears to mediate the reinforcing properties of drugs of abuse, including ethanol (EtOH). We have shown that administration of ceftriaxone (CEF), a β-lactam antibiotic, reduced EtOH intake and increased glutamate transporter 1 (GLT-1) expression in mesocorticolimbic regions of male and female alcohol-preferring (P) rats. In the present study, we tested whether CEF administration would reduce nicotine (NIC) and/or EtOH intake by adult female P rats. P rats were randomly assigned to 4 groups: (a) 5% sucrose (SUC) and 10% SUC [SUC], (b) 5% SUC + 0.07 mg/ml NIC and 10% SUC + 0.14 mg/ml NIC [NIC-SUC], 15% EtOH and 30% EtOH [EtOH] and (d) 15% EtOH + 0.07 mg/ml NIC and 30% EtOH + 0.14 mg/ml NIC [NIC-EtOH]. After achieving stable intakes (4 weeks), the rats were administered 7 concurrent, daily i.p. injections of either saline or 100 mg/kg CEF. The effects of CEF on intake were significant but differed across the reinforcers; such that ml/kg/day SUC was reduced by ~30%, mg/kg/day NIC was reduced by ~70% in the NIC-SUC group and ~40% in the EtOH-NIC group, whereas g/kg/day EtOH was reduced by ~40% in both the EtOH and EtOH-NIC group. The effects of CEF on GLT-1 expression were also studied. We found that CEF significantly increased GLT-1 expression in the prefrontal cortex and the nucleus accumbens of the NIC and NIC-EtOH rats as compared to NIC- and NIC-EtOH saline-treated rats. These findings provide further support for GLT-1-associated mechanisms in EtOH and/or NIC abuse. The present results along with previous reports of CEF’s efficacy in reducing cocaine self-administration in rats suggest that modulation of GLT-1 expression and/or activity is an important pharmacological target for treating polysubstance abuse and dependence.
Keywords: Addiction, Alcohol, Ceftriaxone, Drinking, EAAT2, Polysubstance
Introduction
Changes in glutamatergic neurotransmission affect many aspects of neuroplasticity associated with alcohol and drug dependence (Szumlinski et al., 2008, Kalivas and Volkow, 2011, Uys and Reissner, 2011, Lenoir and Kiyatkin, 2013, Halbout et al., 2014, Wakabayashi and Kiyatkin, 2014). For example, neuroadaptations in the glutamatergic system appear to mediate ethanol tolerance, dependence, and withdrawal in animals and humans (Krystal et al., 2003, Albrecht and Buerger, 2009, Hermann et al., 2012). Additionally, the effects of ethanol withdrawal are linked to increases in extracellular glutamate concentrations in rats (Rossetti and Carboni, 1995). Ethanol-induced neuroadaptations of the glutamatergic system include alterations in N-methyl-D-aspartate (NMDA) and alpha-amino-3-hydroxyl-5-methyl-4-isoxale-propionate (AMPA) receptor activity (Grant et al., 1990, Sanna et al., 1993, Snell et al., 1996, Chen et al., 1997, Ary et al., 2012). For instance, rapid ethanol withdrawal results in phosphorylation and re-localization of synaptic NMDA receptors (Clapp et al., 2010). Studies from our laboratories and others have consistently demonstrated that ethanol exposure or self-administration leads to increases in extracellular glutamate concentration within the mesocorticolimbic system (Ding et al., 2012, Ding et al., 2013, Das et al., 2015). In addition, ethanol-induced increases in extracellular glutamate concentrations are associated with enhanced sensitivity of nucleus accumbens (Acb) NMDA-receptor stimulation (Siggins et al., 2003).
Importantly, experimenter- or self-administered ethanol decreases glutamate uptake in the Acb (Melendez et al., 2005) and cerebral cortex of rats (Schreiber and Freund, 2000). Glutamatergic activity in the posterior ventral tegmental area (pVTA) is also implicated; such that a low dose of ethanol (0.5 g/kg, i.p.) increases glutamate, a middle dose (1.0 g/kg) has no effect, whereas a high dose (2.0 g/kg) decreases glutamate levels in this region (Ding et al., 2012). In work with alcohol-preferring (P) rats, an animal model of alcoholism (c.f., (Bell et al., 2012, Bell et al., 2014)), chronic ethanol drinking increases basal extracellular glutamate and decreases glutamate clearance in both the pVTA and the Acb (Ding et al., 2013). Moreover, two weeks of ethanol deprivation restored both glutamate re-uptake and extracellular glutamate levels (Ding et al., 2013). Extracellular glutamate levels are regulated by several glutamate transporters located in neurons and glia (Gegelashvili and Schousboe, 1997, Seal and Amara, 1999, Anderson and Swanson, 2000). Glutamate transporter 1 [(GLT-1), or its human homolog, the excitatory amino acid transporter 2 (EAAT2)], is the primary transporter regulating removal of extracellular glutamate in the central nervous system (CNS) (Ginsberg et al., 1995, Danbolt, 2001, Mitani and Tanaka, 2003).
Second only to ethanol, tobacco/nicotine is the most abused substance in the United States and around the world. In the United States, approximately two thirds of individuals use ethanol and more than one quarter use tobacco (Falk et al., 2006). Their co-use is common as well, with more than 46 million co-users in the U.S., with co-use rates highest among adolescents and young adults (Falk et al., 2006). Individuals dependent upon one are also at least 3 times more likely, than the general population, to be dependent upon the other (Grant et al., 2004). Thus, individuals who smoke tobacco are more likely to drink alcohol than those who do not smoke tobacco; and, individuals who drink alcohol are more likely to smoke tobacco than those who do not drink alcohol (Bobo and Husten, 2000, Falk et al., 2006). Importantly, nicotine exposure also increases extracellular glutamate concentrations in the mesocorticolimbic reward circuit (Reid et al., 2000). Substantial evidence suggests that increases in extracellular glutamate concentrations are probably associated with downregulation of GLT-1, and possibly xCT, expression in the mesocorticolimbic reward system (Knackstedt et al., 2009, Knackstedt et al., 2010, Alhaddad et al., 2014a, Alhaddad et al., 2014b). Hence, the interaction between GLT-1 expression in mesocorticolimbic regions and nicotine-seeking behavior requires further investigation.
The role of GLT-1 in chemical dependency has been studied in drug abuse models, including studies investigating excessive ethanol intake. Functional activation of GLT-1 appears to reduce the rewarding effects of cocaine, morphine and methamphetamine (Nakagawa et al., 2005). In addition, upregulation of GLT-1 activity by ceftriaxone, a beta-lactam antibiotic, attenuated cue-induced cocaine-seeking behavior (Sari et al., 2009, Knackstedt et al., 2010). Importantly, our laboratory has reported that upregulation of GLT-1 expression/activity by ceftriaxone results in a dose-dependent, long-lasting reduction in ethanol intake by adult male P rats (Sari et al., 2011). In the present study, we investigated the effects of up-regulating GLT-1 expression, by ceftriaxone, on the maintenance of ethanol and/or nicotine intake by adult female P rats. Since ceftriaxone-induced reductions in ethanol intake by male P rats is associated with upregulation of GLT-1 expression in the prefrontal cortex (PFC) and Acb, the present study also assessed whether ceftriaxone-induced reductions in ethanol and/or nicotine intake by female P rats were associated with upregulation of GLT-1 expression in these two brain reward regions as well.
Materials and Methods
Animals
Adult female P rats (~75 days of age at the start of the experiment) were used in this study. No more than 2 rats from a litter were included in any condition or interaction between conditions. This was done to avoid litter effects and increase the generalizability of the findings (Holson and Pearce, 1992). Rats were housed in a temperature- (21°C) and humidity- (50%) controlled vivarium which was maintained on a 12h reverse-light/dark cycle (lights on at 2200h). All experimental procedures were approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine and are in accordance with guidelines set by the Institutional Animal Care and Use Committee of the National Institutes of Health, and the Guide for the Care and Use of Laboratory Animals.
Drinking protocol and treatment
Subjects were group-housed until ~75 days old. At ~75 days old, the animals were single-housed in hanging stainless steel cages. After adaptation to the caging, which included a Plexiglas® plate in each cage to reduce isolation and wire-mesh associated stress, the animals were randomly assigned to one of four conditions. In the first condition, the animals were given continuous (24h/day) free-choice, concurrent access to 5% and 10% sucrose [SUC] for 5 weeks to serve as an appetitive control. The concentrations of sucrose were chosen for their high palatability. In the second condition, the animals were given continuous (24h/day) free-choice, concurrent access to 15% and 30% ethanol [EtOH] for 5 weeks. Multiple concentrations of ethanol yield higher levels of intake compared to access to a single concentration of ethanol [e.g., (Bell et al., 2003)], moreover, this effect is independent of rat line/strain or even rodent species [c.f., (Bell et al., 2014)]. In the third condition, the animals were given continuous (24h/day) free-choice, concurrent access to 5% sucrose and 0.07 mg/ml nicotine along with 10% sucrose and 0.14 mg/ml nicotine [NIC-SUC] for 5 weeks. In the fourth condition, the animals were given continuous (24h/day) free-choice, concurrent access to 15% ethanol and 0.07 mg/ml nicotine along with 30% ethanol and 0.14 mg/ml nicotine [EtOH-NIC] for 5 weeks. The nicotine concentrations were chosen based on a previous study from our laboratory, which used multiple concentrations of nicotine as well as ethanol (Hauser et al., 2012).
Despite the reluctance to orally consume nicotine, our laboratory has found that when the nicotine solution is adulterated with either ethanol or sucrose P rats will readily ingest sufficient amounts of nicotine to attain blood nicotine and/or continine levels seen in tobacco-dependent individuals (Hauser et al., 2012). Although, the bitter taste of nicotine still modestly, albeit significantly, reduces ingestion levels for these reinforcers (ethanol and sucrose).
At the end of the 4th week of solution access, each condition was split into 2 dose groups for a ceftriaxone (0 vs. 200 mg/kg, injected intraperitoneally) treatment phase. Saline served as the vehicle. In animal models studying drug-seeking behavior and drug dependence performed in our laboratory and that of others has tested ceftriaxone at a dose of 200 mg/kg for the up-regulation of GLT-1. Hence, this treatment dose was selected for this study (Sari et al., 2009, Knackstedt et al., 2010, Sari et al., 2011, Ward et al., 2011, Fischer et al., 2013, Barr et al., 2015). Treatments were administered once-a-day for 7 consecutive days at the beginning of the dark-cycle, across the 5th week of solution access. The animals had ad libitum access to food and water throughout the experiment.
Brain harvesting
Twenty-four hours after the final injection brains were harvested from the animals for subsequent Western Blot analyses. This time-point coincided with lights out. We have found repeatedly that significantly less ethanol is consumed during the reduced activity period (lights on) for P rats. Thus, in general, negligible concentrations of ethanol are present in the blood and brain. The brains were removed flash-frozen and stored at −70°C. The PFC and Acb were subsequently micropunched using a cryostat maintained at −20°C, in order to keep the tissue frozen. After the brain regions were micropunched, they were returned to the −70°C freezer until GLT-1 protein levels were assayed with the Western blot procedure. Brains from a random subset of animals from each drinking-solution by ceftriaxone-treatment condition were used for the Western Blot analyses.
Western Blot analyses
Western blot procedures to examine the level of GLT-1 in the PFC and Acb were performed as described in previous studies (Sari et al., 2009, Sari et al., 2010, Sari et al., 2011). Briefly, proteins were loaded in 10-20% glycine gel (Invitrogen) and separated in a mini-cell apparatus. Proteins then were electrophoretically transferred onto a nitrocellulose membrane. The membrane was incubated in blocking buffer (3% milk in TBST, 50 mM Tris HCl; 150 mM NaCl, pH 7.4; 0.1% Tween20); and then incubated with primary antibody guinea pig anti-GLT-1 (Millipore) diluted 1:5000 in 3% milk in TBST overnight at 4°C. Membranes then were incubated with horseradish peroxidase (HRP)-labeled anti-guinea pig secondary antibody. The same membranes also were assessed for β-tubulin immunoblotting as a loading control. SuperSignal West Pico Chemiluminescence substrate (Pierce) was used to reveal HRP activity. Membranes then were exposed to Kodak BioMax MR film and developed using an SRX-101A developer. Immunoblots were analyzed using the MCID system. The data are presented as percentage ratios of GLT-1/β-tubulin, relative to saline-control levels.
Statistical Analyses
Drinking-solution data
Daily measures of sucrose (g/kg/day), ethanol (g/kg/day), nicotine (mg/kg/day) and water (ml/kg) intake as well as body weights (g) were recorded during the 7-day ceftriaxone-treatment phase. Because the experimental design was not balanced across solutions (i.e., a sucrose-nicotine condition was used instead of a nicotine-alone condition), three separate 2 × 2 × 7 (Test Solution by Dose by Test Day) mixed ANOVAs were conducted as omnibus analyses. Test Solution and Dose were the between-subject factors and Test Day was the within-subjects factor. Water intake and body weight were also analyzed for each respective drinking-solution by ceftriaxone-treatment group. Appropriate simple effect analyses, using Protected Fisher’s LSD tests, followed significant higher-order effects. In order to control for possible inflations in alpha-error due to separate omnibus mixed ANOVAs, alpha was set at p ≤ 0.025 for all analyses.
Western blot data
Western blot data from the saline-treated group was converted to 100% and the changes in expression of proteins, as a ratio to the loading control β-tubulin. The percentage ratios of GLT-1/β-tubulin in PFC and Acb were analyzed using independent one-sample t-test analyses versus 100% to determine changes in protein expression. The level of significance was set at p<0.05.
Results
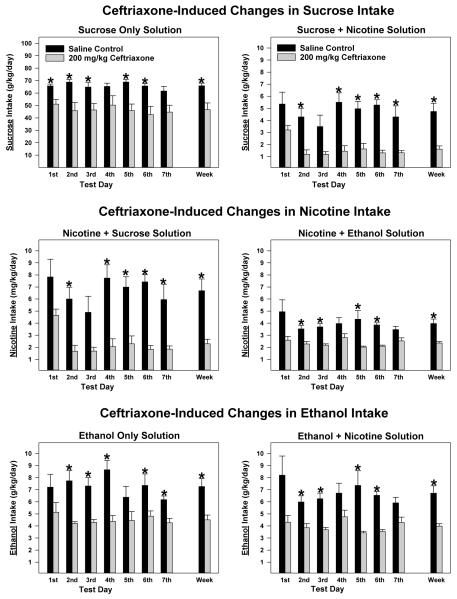
The daily sucrose intakes were measured in sucrose only group (termed “Sucrose Only Solution”) and nicotine + sucrose group [termed “Sucrose + Nicotine Solution”]. From the nicotine + sucrose group, daily nicotine intake was also measured (termed Nicotine + Sucrose Solution”) as reported in Figure 1. A similar nomenclature has been employed for the ethanol + nicotine concurrent-consuming group of P rats, when depicting levels of nicotine versus ethanol consumed/kg body weight/day by this group.
Figure 1.
The effects of 7 consecutive daily ceftriaxone (200 mg/kg, n = 5/treatment by test solution group) and saline (n = 5/treatment by test solution group) injections on average (± S.E.M.) intake of multiple test solutions by adult female alcohol-preferring P rats. Sucrose (g/kg/day) intakes by animals receiving the sucrose only test solution (5% and 10% available concurrently) are displayed in the left top panel; and sucrose intakes by animals receiving the sucrose + nicotine test solution (5% sucrose + 0.07 mg/ml nicotine and 10% sucrose + 0.14 mg/ml nicotine available concurrently) are displayed in the right top panel. Nicotine (mg/kg/day) intakes by animals receiving the nicotine + sucrose test solution (5% sucrose + 0.07 mg/ml nicotine and 10% sucrose + 0.14 mg/ml nicotine available concurrently) are displayed in the left middle panel; and nicotine intakes by animals receiving the nicotine + ethanol test solution (15% ethanol + 0.07 mg/ml nicotine and 30% ethanol + 0.14 mg/ml nicotine available concurrently) are displayed in the right middle panel. Ethanol (g/kg/day) intakes by animals receiving the ethanol only test solution (15% and 30% available concurrently) are displayed in the left bottom panel; and ethanol intakes by animals receiving the ethanol + nicotine test solution (15% ethanol + 0.07 mg/ml nicotine and 30% ethanol + 0.14 mg/ml nicotine available concurrently) are displayed in the right bottom panel. Within these panels test solution intake for each treatment day, the average across the 7 days of treatment and the 7 day average converted to percent relative to the saline group are displayed. *, indicates a significant (p < 0.025) difference between the ceftriaxone- and saline-treated animals. Values are expressed as mean ± SEM.
Sucrose Intake
The omnibus 2 × 2 × 7 (Test Solution by Dose by Test Day) mixed ANOVA on the sucrose consumption data revealed a significant Test Solution by Dose interaction [F(1,16) = 7.435, p = 0.015] as well as the main effects for Test Solution [F(1,16) = 325.346, p < 0.001] and Dose [F(1,16) = 14.344, p = 0.002]. As seen in Figure 1 (Top panels), the addition of nicotine significantly and precipitously (practically 10-fold) decreased sucrose intake and there were differential effects of ceftriaxone, such that the effect of ceftriaxone appears to be greater with the dual-reinforcer (sucrose + nicotine) solution compared with the single-reinforcer sucrose solution, despite the possibility for a floor effect with the dual-reinforcer solution (Figure 1, top panels). When examining the sucrose only data, the 2 × 7 (Dose by Test Day) mixed ANOVA revealed a significant main effect for Dose [F(1,8) = 10.779, p = 0.011] but no interaction indicating the relatively modest, but significant, reduction in sucrose consumption by ceftriaxone did not change significantly across days (Figure 1, Top left panel). When examining the sucrose intake data of animals consuming the sucrose + nicotine solution, the 2 × 7 (Dose by Test Day) mixed ANOVA revealed significant main effects for Dose [F(1,8) = 17.734, p = 0.003] and Test Day [F(1,6) = 6.855, p < 0.001] but no interaction indicating that ceftriaxone significantly reduced sucrose intake (Figure 1, Top right panel).
Nicotine Intake
The omnibus 2 × 2 × 7 (Test Solution by Dose by Test Day) mixed ANOVA on the nicotine consumption data revealed significant interactions for Test Solution by Dose [F(1,16) = 6.221, p = 0.024] and Test Solution by Test Day [F(6,96) = 2.693, p = 0.018] as well as significant main effects for Dose [F(1,16) = 14.344, p = 0.002] and Test Day [F(6,96) = 9.195, p < 0.001]. The 3-way interaction and Test Solution main effects approached significance (p = 0.039 and 0.030, respectively). As seen in Figure 1 (Middle panels), nicotine intake was higher when mixed with sucrose compared to ethanol; but, except for the first Test Day, ceftriaxone reduced nicotine intake to similar daily levels regardless of the adulterant (sucrose vs ethanol). When examining the nicotine intake data in the nicotine + sucrose solution, the 2 × 7 (Dose by Test Day) mixed ANOVA revealed significant main effects for Dose [F(1,8) = 17.654, p = 0.003] and Test Day [F(6,48) = 7.808, p < 0.001]. Figure 1 (Middle left panel) indicates that ceftriaxone significantly reduced nicotine intake in the presence of sucrose. When examining the nicotine intake data of animals consuming the nicotine + ethanol solution, the 2 × 7 (Dose by Test Day) mixed ANOVA only revealed a significant main effect for Dose [F(1,8) = 17.199, p = 0.003] indicating that (a) ceftriaxone significantly reduced nicotine intake and (b) there was no change in ceftriaxone’s effect across Test Days (Figure 1, Middle right panel).
Ethanol Intake
The omnibus 2 × 2 × 7 (Test Solution by Dose by Test Day) mixed ANOVA on the ethanol consumption data revealed a marginally significant 3-way Test Solution by Dose by Test Day interaction [F(6,96) = 2.507, p = 0.027] as well as significant main effects for Dose [F(1,16) = 29.805, p < 0.001] and Test Day [F(6,96) = 2.543, p = 0.025]. As with the sucrose + nicotine data, the addition of nicotine decreased concurrent ethanol intake, but not nearly to the extent seen with the sucrose solution (Figure 1, Top and Bottom panels). Also, as with the sucrose + nicotine data, ceftriaxone appears to be slightly more effective under the dual-reinforcer condition (Figure 1, Bottom right panel). When examining the ethanol only data, the 2 × 7 (Dose by Test Day) mixed ANOVA revealed a significant main effect for Dose [F(1,8) = 13.039, p = 0.007]. As seen in Figure 1 (Bottom left panel), in general ceftriaxone was equally effective in reducing ethanol intake across Test Days. When examining the ethanol intake data of animals consuming the ethanol + nicotine solution, the 2 × 7 (Dose by Test Day) mixed ANOVA revealed a significant main effect for Dose [F(1,8) = 17.445, p = 0.003], indicating that (a) ceftriaxone significantly reduced ethanol intake and (b) there was essentially no change in ceftriaxone’s efficacy across Test Days (Figure 1, Bottom right panel).
Effects of ceftriaxone on body weight (g), 2 hour and 24 hour water intake
When examining the rats that received sucrose as their test solution. Analyses of body weight, 2 hour and 24 hour water intake across test solution groups revealed no significant differences between ceftriaxone and saline during the treatment week (data not shown). When examining the rats that received sucrose-nicotine as their test solution. Analyses of body weight, 2 hour and 24 hour water intake across test solution groups revealed no significant difference in body weight; but, there were significant differences (p-values < 0.025) in water consumption between treatment groups, such that the ceftriaxone-treated animals consumed substantially more water relative to their saline-treated counterparts (2 hour: 20.4 ± 1.8 vs. 8.7 ± 1.0; 24 hour: 122.5 ± 9.5 vs. 67.1 ± 8.3 ml/day averaged across the week of treatment, respectively).
When examining the rats that received ethanol as their test solution. Analyses of body weight, 2 hour and 24 hour water intake across test solution groups revealed no significant difference in body weight; but, there were significant differences (p-values < 0.025) in water intake between treatment groups, such that the ceftriaxone-treated animals consumed more water relative to their saline-treated counterparts (2 hour: 27.6 ± 2.7 vs. 16.3 ± 1.7; 24 hour: 117.7 ± 7.5 vs. 81.3 ± 4.6 ml/day averaged across the week of treatment, respectively).
When examining the rats that received ethanol-nicotine as their test solution. Analyses of body weight, 2 hour and 24 hour water intake across test solution groups revealed no significant difference in body weight and 2 hour water intake; but, there was a significant difference (p < 0.025) between treatment groups for 24 hour water intake, such that the ceftriaxone-treated animals consumed more than their saline-treated counterparts (118.4 ± 11.1 vs. 91.0 ± 4.0 ml/day averaged across the week of treatment, respectively).
Effects of ceftriaxone on GLT-1 expression in Acb and PFC across test solutions
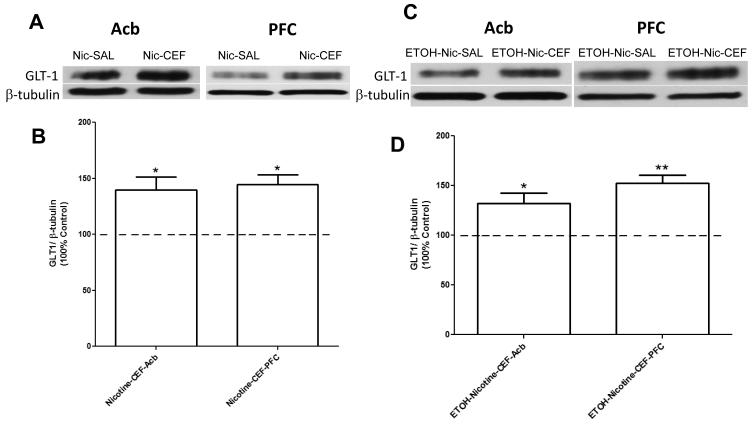
We further determined the effects of ceftriaxone on GLT-1 expression in Acb and PFC of sucrose-nicotine consuming P rats. Independent one-sample t-test analyses versus 100% showed significantly increased expression of GLT-1 in Acb [t(4) = 3.498, p= 0.0249; Figure 2A,B] and PFC [t(3) = 5.289, p= 0.0132; Figure 2A,B] of the nicotine-ceftriaxone group as compared to the nicotine-saline group. We also determined the effects of ceftriaxone on GLT-1 expression in Acb and PFC of ethanol-nicotine consuming P rats. Similar to the sucrose-nicotine results, independent one-sample t-test analyses versus 100% showed increased expression of GLT-1 in Acb [t(4) = 3.170, p= 0.0339; Figure 2C,D] and PFC [t(4) = 6.139, p= 0.0036; Figure 2C,D] of the ethanol-nicotine-ceftriaxone group as compared to the ethanol-nicotine-saline group. Note that there were no significant differences in β-tubulin expression in PFC and Acb between all groups (p>0.5).
Figure 2.
A) Effects of ceftriaxone on GLT-1 expression [GLT-1/β-tubulin (% of Control Group)] in nucleus accumbens (Acb) and prefrontal cortex (PFC) of nicotine consuming adult female P rats are depicted. B) As summarized in the bar graph, statistical analyses indicated significant differences between ceftriaxone-treated and saline-treated control animals (100%) in Acb (* p= 0.025) and PFC (* p= 0.013). C) Effects of ceftriaxone on GLT-1 expression [GLT-1/β-tubulin (% of Control Group)] in Acb and PFC of nicotine and ethanol consuming adult female P rats are depicted. D) As summarized in the bar graph, statistical analyses indicated significant differences between ceftriaxone-treated and saline-treated animals (100%) in Acb (* p= 0.034) and PFC (** p= 0.004). Values are expressed as mean ± SEM, (n = 5 for each group except Nicotine-CEF-PFC, with n = 4).
DISCUSSION
We report here that ceftriaxone treatment dramatically reduced the consumption of nicotine-sucrose by adult female P rats. Ceftriaxone treatment also reduced the consumption of ethanol as well as the ethanol/nicotine solution by adult female P rats. In addition, the reductions in consumption of nicotine and ethanol were negatively associated with upregulation of GLT-1 in Acb and PFC of these rats. Also, as it has been observed repeatedly in our past studies, ceftriaxone has opposite effects on a reinforcer vs water, when the two are presented together, such that reinforcer intake decreases and water intake increases (Sari et al., 2011, Rao and Sari, 2014, Rao et al., 2015).
Studies from our laboratory have revealed that ethanol consumption for five weeks leads to an increase in extracellular glutamate concentrations within Acb (Das et al., 2015). We and others have reported that ceftriaxone, known to upregulate GLT-1 expression and function, attenuated cue-induced cocaine relapse (Sari et al., 2009, Knackstedt et al., 2010). This effect was associated with an increase in GLT-1 expression in PFC and Acb and was blocked by drugs that block GLT-1 (Fischer et al., 2013, Das et al., 2015). Importantly, we recently reported that male P rats treated with ceftriaxone showed a significant reduction in ethanol intake and attenuated relapse-like behavior, as well as upregulation of GLT-1 and another glial glutamate transporter, cystine/glutamate exchange transporter (xCT), expression in Acb and PFC (Sari et al., 2011, Rao and Sari, 2012, Qrunfleh et al., 2013, Sari et al., 2013, Rao and Sari, 2014). We also have shown that upregulation of GLT-1 with GPI-1046 significantly reduces ethanol intake by P rats (Sari and Sreemantula, 2012). GLT-1 expression was found to be downregulated in Acb, but not PFC, of male P rats after chronic consumption of ethanol compared to ethanolnaïve animals (Sari and Sreemantula, 2012, Sari et al., 2013). While in another study, xCT expression was downregulated in both PFC and Acb of ethanol consuming P rats compared to ethanol-naïve male P rats (Alhaddad et al., 2014a). In addition, we recently reported that (R)-(−)-5-methyl-1-nicotinoyl-2-pyrazoline (MS-153) reduced ethanol intake and upregulated GLT-1 protein expression in Acb of P rats (Alhaddad et al., 2014b). Although, it is unclear by what mechanism these compounds up-regulate the expression of GLT-1, we and others have reported that ceftriaxone, as well as MS-153, activate NF-κB via IκBα degradation and p65 translocation to the nucleus, which leads to increases in GLT-1 expression (Lee et al., 2008, Alhaddad et al., 2014b, Rao et al., 2015).
Regarding nicotine, administration of a non-selective GLT-1 blocker, mecamylamine and L-trans-pyrolidine-2,4 dicarboxylic acid, exacerbated the increases in extracellular glutamate concentrations induced by nicotine (Reid et al., 2000). It is important to note that this study found that the effect of nicotine on glutamate release in Acb might not be directly associated with dopaminergic activity in the mesocorticolimbic circuit, which suggests the prominent role of glutamate transport during in vivo exposure to drugs of abuse. Pertinent to this discussion, the role of GLT-1 has been investigated in a nicotine-self-administration paradigm, which showed that GLT-1 expression was downregulated in Acb following chronic nicotine self-administration (Knackstedt et al., 2009). Similarly, increases in extracellular glutamate concentrations and downregulation of GLT-1 expression were found after rats experienced a reinstatement of nicotine-seeking paradigm (Gipson et al., 2013). We and others have demonstrated the effectiveness of ceftriaxone in upregulating GLT-1 expression, and putatively its function, which leads to a significant reduction in ethanol intake and cocaine seeking behavior (Sari et al., 2009, Knackstedt et al., 2010, Sari et al., 2011, Rao and Sari, 2014, Rao et al., 2015). Importantly, ceftriaxone also reduced reinstatement of a conditioned place preference induced by nicotine exposure (Alajaji et al., 2013). On a tangential note, a study demonstrated that ceftriaxone treatment facilitates the anti-nociceptive effects of nicotine and tolerance does not develop to this effect (Schroeder et al., 2011).
A few caveats need to be included on possible shortcomings of such study. First, the present study was conducted in female P rats and there is always the possibility for alteration of ethanol drinking behavior that might be associated with estrus cycle phase. In fact studies investigated the effects of estrous cycle phase in responses to female rats self-administering (Roberts et al., 1998). This study demonstrated that there were decrease of ethanol self-administration in estrus and proestrus than diestrus in rats with synchronized cycles, however, there were no effects of estrous phase when the rats were allowed to cycle freely (Roberts et al., 1998). In addition, our laboratory has shown previously that the effects of ceftriaxone on ethanol drinking are similar between female and male P rats (Sari et al., 2011; 2013). However, the role of sex differences, in nicotine intake, needs to be explored further. Regarding power of the statistical analyses, we must concede that the sample sizes (n=5/condition, with a total of 40 animals used) are small and may have prevented our detecting more robust effects. We should knowledge that the small sample size could have caused some level of inaccuracy in the estimates of the mean and standard deviation. However, it is important to note that ceftriaxone was found to upregulate GLT-1 expression in PFC and Acb, and consequently reduced ethanol intake in adolescent and adult female P rats (Sari et al., 2013). Further research, with larger sample sizes is needed to replicate our beta lactam-induced increases in GLT1 expression following nicotine-induced down-regulation of GLT1 expression.
Overall, it appears that elevated extracellular glutamate concentrations in the mesocorticolimbic system are associated with oral, and putatively intravenous, intake of nicotine and alterations (decreases) in GLT-1 expression and/or function mediates this effect. Nevertheless, other neuronal glutamate transporters may also be involved. For example, a recent study revealed that nicotine exposure reduced EAAT3, excitatory amino acid transporter-3, activity (Yoon et al., 2014). These authors reported that this effect was dependent on multiple intracellular kinase signaling pathways. Given these findings and our previous work examining changes in xCT expression following ethanol drinking and ceftriaxone treatment, future research is needed to determine the role of other neuronal- and glial-associated glutamate transporters in the development of nicotine/tobacco dependence. Nevertheless, the present study provides novel insights, and supports the existing literature, regarding the effectiveness of ceftriaxone treatment in attenuating drug-seeking behavior, presumably via upregulation of GLT-1 expression, in P rats which have a genetic predisposition for excessive ethanol intake.
Highlights.
-
➢
Ceftriaxone reduced nicotine intake by 70% in nicotine sucrose group.
-
➢
Ceftriaxone reduced nicotine intake by 40% in ethanol-nicotine group.
-
➢
CEF increased GLT-1 in PFC and Acb of nicotine and nicotine ethanol groups.
-
➢
GLT-1 as a target for the treatment of polysubstance abuse and dependence.
Acknowledgements
The presented research was supported in part by AA019458 (Y. Sari) as well as AA07611 (D.W. Crabb) and AA13522 (R.L. Bell) from the National Institute on Alcohol Abuse and Alcoholism.
Footnotes
Conflicts of Interest
None of the authors have real or perceived conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alajaji M, Bowers MS, Knackstedt L, Damaj MI. Effects of the beta-lactam antibiotic ceftriaxone on nicotine withdrawal and nicotine-induced reinstatement of preference in mice. Psychopharmacology. 2013;228:419–426. doi: 10.1007/s00213-013-3047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht S, Buerger E. Potential neuroprotection mechanisms in PD: focus on dopamine agonist pramipexole. Curr Med Res Opin. 2009;25:2977–2987. doi: 10.1185/03007990903364954. [DOI] [PubMed] [Google Scholar]
- Alhaddad H, Das SC, Sari Y. Effects of ceftriaxone on ethanol intake: a possible role for xCT and GLT-1 isoforms modulation of glutamate levels in P rats. Psychopharmacology. 2014a;231:4049–4057. doi: 10.1007/s00213-014-3545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaddad H, Kim NT, Aal-Aaboda M, Althobaiti YS, Leighton J, Boddu SHS, Wei YJ, Sari Y. Effects of MS-153 on chronic ethanol consumption and GLT1 modulation of glutamate levels in male alcohol-preferring rats. Front Behav Neurosci. 2014b;8 doi: 10.3389/fnbeh.2014.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- Ary AW, Cozzoli DK, Finn DA, Crabbe JC, Dehoff MH, Worley PF, Szumlinski KK. Ethanol up-regulates nucleus accumbens neuronal activity dependent pentraxin (Narp): implications for alcohol-induced behavioral plasticity. Alcohol. 2012;46:377–387. doi: 10.1016/j.alcohol.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr JL, Rasmussen BA, Tallarida CS, Scholl JL, Forster GL, Unterwald EM, Rawls SM. Ceftriaxone attenuates acute cocaine-evoked dopaminergic neurotransmission in the nucleus accumbens of the rat. British journal of pharmacology. 2015;172:5414–5424. doi: 10.1111/bph.13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd-Henricks ZA, Kuc KA, Lumeng L, Li TK, Murphy JM, McBride WJ. Effects of concurrent access to a single concentration or multiple concentrations of ethanol on the intake of ethanol by male and female periadolescent alcohol-preferring (P) rats. Alcohol. 2003;29:137–148. doi: 10.1016/s0741-8329(03)00022-3. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Engleman EA, Toalston JE, McBride WJ. Scheduled access alcohol drinking by alcohol-preferring (P) and high-alcohol-drinking (HAD) rats: modeling adolescent and adult binge-like drinking. Alcohol. 2014;48:225–234. doi: 10.1016/j.alcohol.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Sable HJ, Colombo G, Hyytia P, Rodd ZA, Lumeng L. Animal models for medications development targeting alcohol abuse using selectively bred rat lines: neurobiological and pharmacological validity. Pharmacology, biochemistry, and behavior. 2012;103:119–155. doi: 10.1016/j.pbb.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobo JK, Husten C. Sociocultural influences on smoking and drinking. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism. 2000;24:225–232. [PMC free article] [PubMed] [Google Scholar]
- Chen X, Michaelis ML, Michaelis EK. Effects of chronic ethanol treatment on the expression of calcium transport carriers and NMDA/glutamate receptor proteins in brain synaptic membranes. Journal of neurochemistry. 1997;69:1559–1569. doi: 10.1046/j.1471-4159.1997.69041559.x. [DOI] [PubMed] [Google Scholar]
- Clapp P, Gibson ES, Dell'acqua ML, Hoffman PL. Phosphorylation regulates removal of synaptic N-methyl-D-aspartate receptors after withdrawal from chronic ethanol exposure. The Journal of pharmacology and experimental therapeutics. 2010;332:720–729. doi: 10.1124/jpet.109.158741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Progress in neurobiology. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Das SC, Yamamoto BK, Hristov AM, Sari Y. Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology. 2015;97:67–74. doi: 10.1016/j.neuropharm.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Engleman EA, Rodd ZA, McBride WJ. Ethanol increases glutamate neurotransmission in the posterior ventral tegmental area of female wistar rats. Alcoholism, clinical and experimental research. 2012;36:633–640. doi: 10.1111/j.1530-0277.2011.01665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, Bailey JA, Lahiri DK, McBride WJ. Alcohol drinking and deprivation alter basal extracellular glutamate concentrations and clearance in the mesolimbic system of alcohol-preferring (P) rats. Addict Biol. 2013;18:297–306. doi: 10.1111/adb.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk DE, Yi HY, Hiller-Sturmhofel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism. 2006;29:162–171. [PMC free article] [PubMed] [Google Scholar]
- Fischer KD, Houston AC, Rebec GV. Role of the major glutamate transporter GLT1 in nucleus accumbens core versus shell in cue-induced cocaine-seeking behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:9319–9327. doi: 10.1523/JNEUROSCI.3278-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegelashvili G, Schousboe A. High affinity glutamate transporters: regulation of expression and activity. Molecular pharmacology. 1997;52:6–15. doi: 10.1124/mol.52.1.6. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Martin LJ, Rothstein JD. Regional deafferentation down-regulates subtypes of glutamate transporter proteins. Journal of neurochemistry. 1995;65:2800–2803. doi: 10.1046/j.1471-4159.1995.65062800.x. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, Kalivas PW. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9124–9129. doi: 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Grant KA, Valverius P, Hudspith M, Tabakoff B. Ethanol withdrawal seizures and the NMDA receptor complex. European journal of pharmacology. 1990;176:289–296. doi: 10.1016/0014-2999(90)90022-x. [DOI] [PubMed] [Google Scholar]
- Halbout B, Bernardi RE, Hansson AC, Spanagel R. Incubation of cocaine seeking following brief cocaine experience in mice is enhanced by mGluR1 blockade. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:1781–1790. doi: 10.1523/JNEUROSCI.1076-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Katner SN, Deehan GA, Jr., Ding ZM, Toalston JE, Scott BJ, Bell RL, McBride WJ, Rodd ZA. Development of an oral operant nicotine/ethanol co-use model in alcohol-preferring (p) rats. Alcoholism, clinical and experimental research. 2012;36:1963–1972. doi: 10.1111/j.1530-0277.2012.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann D, Weber-Fahr W, Sartorius A, Hoerst M, Frischknecht U, Tunc-Skarka N, Perreau-Lenz S, Hansson AC, Krumm B, Kiefer F, Spanagel R, Mann K, Ende G, Sommer WH. Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biological psychiatry. 2012;71:1015–1021. doi: 10.1016/j.biopsych.2011.07.034. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Molecular psychiatry. 2011;16:974–986. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas PW. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biological psychiatry. 2009;65:841–845. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biological psychiatry. 2010;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Mason G, Trevisan L, D'Souza DC. N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacol Ther. 2003;99:79–94. doi: 10.1016/s0163-7258(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Lee SG, Su ZZ, Emdad L, Gupta P, Sarkar D, Borjabad A, Volsky DJ, Fisher PB. Mechanism of ceftriaxone induction of excitatory amino Acid transporter-2 expression and glutamate uptake in primary human astrocytes. The Journal of biological chemistry. 2008;283:13116–13123. doi: 10.1074/jbc.M707697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Kiyatkin EA. Intravenous nicotine injection induces rapid, experience-dependent sensitization of glutamate release in the ventral tegmental area and nucleus accumbens. Journal of neurochemistry. 2013;127:541–551. doi: 10.1111/jnc.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas PW. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcoholism, clinical and experimental research. 2005;29:326–333. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- Mitani A, Tanaka K. Functional changes of glial glutamate transporter GLT-1 during ischemia: an in vivo study in the hippocampal CA1 of normal mice and mutant mice lacking GLT-1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:7176–7182. doi: 10.1523/JNEUROSCI.23-18-07176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Fujio M, Ozawa T, Minami M, Satoh M. Effect of MS-153, a glutamate transporter activator, on the conditioned rewarding effects of morphine, methamphetamine and cocaine in mice. Behavioural brain research. 2005;156:233–239. doi: 10.1016/j.bbr.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Qrunfleh AM, Alazizi A, Sari Y. Ceftriaxone, a beta-lactam antibiotic, attenuates relapse-like ethanol-drinking behavior in alcohol-preferring rats. Journal of psychopharmacology. 2013;27:541–549. doi: 10.1177/0269881113482529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PS, Sari Y. Glutamate transporter 1: target for the treatment of alcohol dependence. Current medicinal chemistry. 2012;19:5148–5156. doi: 10.2174/092986712803530511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PS, Sari Y. Effects of ceftriaxone on chronic ethanol consumption: a potential role for xCT and GLT1 modulation of glutamate levels in male P rats. Journal of molecular neuroscience : MN. 2014;54:71–77. doi: 10.1007/s12031-014-0251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PS, Saternos H, Goodwani S, Sari Y. Effects of ceftriaxone on GLT1 isoforms, xCT and associated signaling pathways in P rats exposed to ethanol. Psychopharmacology. 2015;232:2333–2342. doi: 10.1007/s00213-015-3868-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Fox L, Ho LB, Berger SP. Nicotine stimulation of extracellular glutamate levels in the nucleus accumbens: neuropharmacological characterization. Synapse. 2000;35:129–136. doi: 10.1002/(SICI)1098-2396(200002)35:2<129::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Smith AD, Weiss F, Rivier C, Koob GF. Estrous cycle effects on operant responding for ethanol in female rats. Alcoholism, clinical and experimental research. 1998;22:1564–1569. [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S. Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. European journal of pharmacology. 1995;283:177–183. doi: 10.1016/0014-2999(95)00344-k. [DOI] [PubMed] [Google Scholar]
- Sanna E, Serra M, Cossu A, Colombo G, Follesa P, Cuccheddu T, Concas A, Biggio G. Chronic ethanol intoxication induces differential effects on GABAA and NMDA receptor function in the rat brain. Alcoholism, clinical and experimental research. 1993;17:115–123. doi: 10.1111/j.1530-0277.1993.tb00735.x. [DOI] [PubMed] [Google Scholar]
- Sari Y, Franklin KM, Alazizi A, Rao PS, Bell RL. Effects of ceftriaxone on the acquisition and maintenance of ethanol drinking in peri-adolescent and adult female alcohol-preferring (P) rats. Neuroscience. 2013;241:229–238. doi: 10.1016/j.neuroscience.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Prieto AL, Barton SJ, Miller BR, Rebec GV. Ceftriaxone-induced up-regulation of cortical and striatal GLT1 in the R6/2 model of Huntington's disease. J Biomed Sci. 2010;17:62. doi: 10.1186/1423-0127-17-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sakai M, Weedman JM, Rebec GV, Bell RL. Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol Alcohol. 2011;46:239–246. doi: 10.1093/alcalc/agr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:9239–9243. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sreemantula SN. Neuroimmunophilin GPI-1046 reduces ethanol consumption in part through activation of GLT1 in alcohol-preferring rats. Neuroscience. 2012;227:327–335. doi: 10.1016/j.neuroscience.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R, Freund WD. Glutamate transport is downregulated in the cerebral cortex of alcohol-preferring rats. Med Sci Monit. 2000;6:649–652. [PubMed] [Google Scholar]
- Schroeder JA, Quick KF, Landry PM, Rawls SM. Glutamate transporter activation enhances nicotine antinociception and attenuates nicotine analgesic tolerance. Neuroreport. 2011;22:970–973. doi: 10.1097/WNR.0b013e32834d87eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Amara SG. Excitatory amino acid transporters: a family in flux. Annu Rev Pharmacol Toxicol. 1999;39:431–456. doi: 10.1146/annurev.pharmtox.39.1.431. [DOI] [PubMed] [Google Scholar]
- Siggins GR, Martin G, Roberto M, Nie Z, Madamba S, De Lecea L. Glutamatergic transmission in opiate and alcohol dependence. Annals of the New York Academy of Sciences. 2003;1003:196–211. doi: 10.1196/annals.1300.012. [DOI] [PubMed] [Google Scholar]
- Snell LD, Nunley KR, Lickteig RL, Browning MD, Tabakoff B, Hoffman PL. Regional and subunit specific changes in NMDA receptor mRNA and immunoreactivity in mouse brain following chronic ethanol ingestion. Brain Res Mol Brain Res. 1996;40:71–78. doi: 10.1016/0169-328x(96)00038-1. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD. Homers regulate drug-induced neuroplasticity: implications for addiction. Biochemical pharmacology. 2008;75:112–133. doi: 10.1016/j.bcp.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uys JD, Reissner KJ. Glutamatergic neuroplasticity in cocaine addiction. Progress in molecular biology and translational science. 2011;98:367–400. doi: 10.1016/B978-0-12-385506-0.00009-0. [DOI] [PubMed] [Google Scholar]
- Wakabayashi KT, Kiyatkin EA. Critical role of peripheral drug actions in experience-dependent changes in nucleus accumbens glutamate release induced by intravenous cocaine. Journal of neurochemistry. 2014;128:672–685. doi: 10.1111/jnc.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SJ, Rasmussen BA, Corley G, Henry C, Kim JK, Walker EA, Rawls SM. Beta-lactam antibiotic decreases acquisition of and motivation to respond for cocaine, but not sweet food, in C57Bl/6 mice. Behav Pharmacol. 2011;22:370–373. doi: 10.1097/FBP.0b013e3283473c10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HJ, Lim YJ, Zuo Z, Hur W, Do SH. Nicotine decreases the activity of glutamate transporter type 3. Toxicology letters. 2014;225:147–152. doi: 10.1016/j.toxlet.2013.12.002. [DOI] [PubMed] [Google Scholar]