Abstract
Background
Autism spectrum disorder (ASD) encompasses a complex presentation of symptoms that include deficits in social interaction and repetitive or stereotyped interests/behaviors. In keeping with the increasing recognition of both the dimensional characteristics of ASD symptoms and the categorical nature of a diagnosis, we sought to delineate their neural mechanisms based on the functional connectivity of four known neural networks (i.e., the default-mode network, the dorsal attention network, the salience network, and the executive control network).
Methods
We leveraged an open data resource (ABIDE) providing rsfMRI datasets from 90 male children with ASD and 95 typically-developing male children. This dataset also included the Social Responsiveness Scale (SRS) as a dimensional measure of ASD traits. Seed-based functional connectivity was paired with linear regression to identify functional connectivity abnormalities associated with categorical effects of ASD diagnosis, dimensional effects of ASD-like behaviors, and their interaction.
Results
Our results revealed the existence of dimensional mechanisms of ASD uniquely affecting each network based on the presence of connectivity-behavioral relationships; these were independent of diagnostic category. However, we also found evidence of categorical differences (i.e., diagnostic group differences) in connectivity strength for each network, as well as categorical differences in connectivity-behavioral relationships (i.e., diagnosis-by-behavior interactions), supporting the coexistence of categorical mechanisms of ASD.
Conclusions
Overall, our findings support a hybrid model for ASD characterization that includes a combination of both categorical and dimensional brain mechanisms and provide a novel understanding of the neural underpinnings of ASD.
Keywords: Autism spectrum disorder, resting-state fMRI, functional connectivity, social cognition, dimensional measures, default-mode network
INTRODUCTION
Autism spectrum disorder (ASD) is characterized by poor social and reciprocal communication skills combined with repetitive or stereotyped interests/behaviors (1, 2). However, a range of symptom severity and functional impairment exists within and across these disorders, in agreement with the notion that ASD represents a spectrum. In fact, studies have revealed that multiple subtypes of ASD exist along a continuum of the same disorder (3–5). Furthermore, children without a diagnosis of ASD may also exhibit varying degrees of social impairment qualitatively similar to ASD without meeting diagnostic criteria, suggesting that the continuum of ASD symptoms may span beyond the categorical diagnosis of ASD (6, 7). Therefore, a dimensional characterization of ASD has become increasingly favored within the clinical and research community, prompting a revision to the Diagnostic and Statistical Manual for Mental Disorders (DSM5) to include severity ratings for ASD rather than categorical subgroups. In parallel with this clinical evidence, recent studies have identified dimensional brain-behavior relationships related to ASD (8, 9). However, it remains unknown whether behaviors observed in children with ASD are similarly represented in the brain as in typically-developing children (TDC). Moreover, diagnoses ultimately remain categorical in nature, yet the particular contributions of categorical brain mechanisms, especially after controlling for dimensional relationships, remain poorly defined. Therefore, studies that systematically examine both the categorical and dimensional mechanisms of ASD are highly desired to disentangle the complex neural correlates of ASD.
ASD has been increasingly recognized as a disorder of disrupted neural interactions (10). The largest resting-state functional magnetic resonance imaging (rsfMRI) investigation of ASD to date provides convincing support for this notion (11), as have many other studies (e.g. (12–17)). Therefore, an examination of functional connectivity measurements represents a promising direction for delineating the potential categorical and dimensional neural mechanisms of ASD. In fact, we (18) and others (19) have recently demonstrated the feasibility of such an endeavor in studies of attention-deficit hyperactivity disorder (ADHD). Specifically, we explored functional connectivity alterations associated with both categorical diagnosis and ADHD symptom severity in relation to four large-scale neural networks, namely, the dorsal attention network (DAN, (20)), the default-mode network (DMN, (21)), the salience network (SAL, (22)), and the executive control network (ECN, (23)). Findings demonstrated three distinct pattern of brain-behavioral relationships: 1) categorical differences in network-level functional connectivity strength between children with and without a diagnosis of ADHD, supporting the existence of categorically-represented neural mechanisms; 2) quantitative relationships between network-level functional connectivity and behavioral measures that were independent of categorical diagnosis, indicating dimensional mechanisms; and 3) diagnostic group differences in the quantitative relationships between network-level functional connectivity and behavioral measures, suggesting qualitatively different behavioral representations in the brain, reinforcing the categorical differences. The demonstration of the presence of three categories of neural mechanisms in ADHD provides a compelling model for studies of other categorically-defined disorders that are known to occur along a spectrum; ASD is the next natural candidate given the evidence that ASD symptoms exhibit both categorical and dimensional qualities (24). Moreover, the same four networks previously investigated in ADHD are also involved in processes that are disrupted in ASD, including social processing (i.e., DMN (25) and SAL (26)), restricted and repetitive behaviors (i.e., SAL, (27)), cognitive control (i.e., ECN and SAL (28)), and attention (i.e., DAN (29)). Thus, a parallel investigation of these networks in ASD to examine the categorical and/or dimensional nature of this disorder may ultimately aid ASD diagnosis and characterization.
In this study, rsfRMI data from 107 TDC and 109 children with ASD selected from a large data repository (i.e., Autism Brain Imaging Data Exchange (ABIDE) (11)) were analyzed. Functional connectivity measures, derived from four large-scale higher-order cognitive networks (i.e., DAN, DMN, SAL, and ECN) were tested to identify three types of effects: 1) categorical differences between TDC and ASD in the magnitude of functional connectivity; 2) congruent dimensional relationships between symptom severity and functional connectivity existing across both TDC and ASD; and 3) categorical differences between TDC and ASD in the relationship between symptom severity and functional connectivity. Our results demonstrate evidence of all three categories of neural mechanisms of ASD.
METHODS
Subjects
Data were selected from the Autism Brain Imaging Data repository of rsfMRI scans of children, adolescents, and adults both with and without ASD from multiple international sites (http://fcon_1000.projects.nitrc.org/indi/abide/). All sites provided ASD diagnostic status for each subject and several sites offered various continuous measures of autism-related symptoms. For the current study, sites were selected based on their inclusion of MRI data, categorical diagnosis, and Social Responsiveness Scale (SRS) scores (30) from both TDC and ASD children and adolescents (age range 6.5–18.7 years). This limited age range was selected to ensure a similar age distribution across sites and to minimize potential developmental effects of ASD-related neural alterations (31). Furthermore, as males are most often affected by this disorder, there were too few datasets available from females with ASD to draw meaningful estimates of sex effects (32), limiting our analyses to males. Datasets were further limited to those passing the quality assessment protocol performed prior to release of the preprocessed ABIDE datasets to the public. This selection process resulted in a total of 185 subjects, including 95 TDC and 90 ASD across four sites (Leuven 2, NYU, USM, Yale; see Table 1).
Table 1.
Demographic and symptom severity information of included subjects.
| All sites | Leuven | NYU | USM | Yale | |
|---|---|---|---|---|---|
| N | 185 | 25 | 101 | 25 | 34 |
| TDC | 95 | 14 | 51 | 11 | 19 |
| ASD | 90 | 11 | 50 | 14 | 15 |
| Autism/Aspergers/PDD-NOS | 85/17/14 | 11/0/0 | 38/8/4 | 13/0/1 | 3/4/8 |
| Age | 13.2 (3.2) | 14.1 (1.4) | 12.0 (3.0) | 16.3 (2.4) | 12.5 (3.0) |
| TDC | 13.2 (3.1) | 14.5 (1.6) | 12.5 (3.1) | 15.8 (2.6) | 12.3 (2.9) |
| ASD | 13.1 (3.3) | 13.6 (1.0) | 11.5 (2.9) | 16.7 (2.2) | 12.8 (3.4) |
| SRS | 56.4 (43.4) | 49.8 (45.0) | 57.5 (41.8) | 62.0 (46.6) | 52.0 (43.1) |
| TDC | 19.1 (14.7) | 17.6 (13.6) | 22.4 (13.6) | 14.6 (12.8) | 20.4 (21.3) |
| ASD | 93.0 (28.4) | 91.0 (36.0) | 91.9 (29.6) | 99.3 (22.2) | 95.7 (21.1) |
Mean and standard deviation (italics) are provided for each continuous measure. N, number; ASD, autism spectrum disorder; TDC, typically-developing children; SRS, Social Responsiveness Scale; PDD-NOS, pervasive developmental disorder - not otherwise specified; NYU, New York University; USM, Utah School of Medicine.
SRS total raw scores, indicating the severity of impairment related to ASD, provided our dimensional measure of ASD. The SRS is a 65-item quantitative assessment based on parent ratings of core deficits pertaining to autism. This assessment offers a continuous measure of ASD as an alternative to other categorically-oriented diagnostic tools (30), providing a single score of symptom severity. Thus, both children with a categorical diagnosis of ASD and children not meeting ASD diagnostic criteria (TDC) will fall somewhere along the continuum of behaviors measured by SRS.
Categorical diagnoses of ASD were determined by clinician evaluation at each site and were supported by additional ASD-related dimensional measures, which varied by site (see Supplemental Information). ASD subtypes described by the DSM-IV-TR were included as a single ASD group (see Table 1), consistent with emerging views that these subtypes represent different presentations of the same disorder (American Psychiatric 2, 4). Detailed inclusion and exclusion criteria for each site are described in the Supplemental Information.
fMRI Acquisition
RsfMRI scans and MPRAGE structural images were acquired on a Philips Intera (Leuven), Siemens Allegra (NYU) and Siemens Trio (USM, Yale) 3 Tesla MRI scanners. Image acquisition parameters for each site are detailed in Table S1.
Preprocessing
RsfMRI datasets were downloaded in their preprocessed form following the Configurable Pipeline for Analysis of Connectomes (C-PAC, http://fcp-indi.github.com, (33)). Preprocessing steps using Analysis of Functional Neuroimages (AFNI) software (34) and custom scripts included slice time correction, motion correction, global mean intensity normalization, nuisance signal regression including 24 motion parameters (6 directions head motion, motion from one time point prior, and their squares), the top five principal components from white matter and cerebrospinal fluid signals, and linear and quadratic trends, and band-pass filtering (0.01–0.1Hz). Registration to MNI standard space included linear registration to anatomical images using FSL’s FLIRT (35) and application of the non-linear anatomical-to-MNI transformation calculated with ANTS (36). Final voxel size was 3×3×3 mm3. To further minimize effects of motion on our analyses, only datasets with a frame-wise displacement across all volumes of no more than 0.2 mm were included. Finally, linear regression was performed and no significant relationships between mean frame-wise displacement and categorical diagnosis (t=1.57, p=0.12) or SRS scores (t=−0.57, p=0.57) were detected, ensuring that the results would not be secondary to motion parameters.
Functional Connectivity
Functional connectivity was calculated using a seed-based approach by applying 3dfim+ in AFNI software. Consistent with our previous study in ADHD (18), we examined ASD-related functional connectivity associated with four well-described neural networks: DAN (20), DMN (21), SAL (22), and ECN (23). Each network was defined by the voxel-wise Pearson correlation with a reference time series extracted as the simple average time series of all voxels within a 6 mm spherical seed at coordinates obtained from the literature (22, 23, 37). Specifically, the ECN was defined by a seed in the right dorsolateral prefrontal cortex (MNI: 44, 36, 20) and the SAL by a seed in the right anterior insula (MNI: 38, 26, −10), based on Seeley et al. (22). Seeds for DMN and DAN were placed in the posterior cingulate cortex (MNI: 1, −55, 17) and bilateral intraparietal sulcus (MNI: −27, −52, 57; 24, −56, 55), respectively (23, 37). Pearson correlation maps were normalized using a Fisher z-transform.
Statistical Models
To identify categorical effects of ASD diagnosis and dimensional effects of symptom severity on brain functional connectivity, hierarchical linear regression analyses were employed. This model was selected to account for the nested nature of our data since site-specific characteristics may influence categorical and/or dimensional effects of interest. Therefore, we have designed a linear mixed-effects model and added random intercepts and slopes (capturing potential site-specific categorical and dimensional effects) for each site to better account for the nested nature of the multi-site data. To further minimize the effects of motion and/or other systematic differences (e.g., scanner, scanning parameters/procedures, data quality, etc.) across sites on global connectivity, we employed mean connectivity regression, a technique in which the mean value of each subject’s functional connectivity map is entered as a covariate of no interest in the group analysis (38). The first model tested ASD diagnosis (1 or 0) and SRS score as predictors of network functional connectivity, covarying for age, mean connectivity, and site effects. This model was designed to identify those categorical effects associated with an ASD diagnosis that were not driven by differences in symptom severity scores, which we term “categorical effects” in functional connectivity magnitude. Furthermore, significant effects of symptoms measured by the SRS that were not due to effects of categorical diagnosis were also explored. These effects are subsequently referred to as “congruent dimensional effects,” since the dimensional relationships are congruent across the groups. A second analysis included the interaction of ASD diagnosis and SRS score as a predictor in the model in order to test whether there are categorical effects in the relationship of ASD behaviors to functional connectivity. Such categorical-by-dimensional interactions are subsequently described as “incongruent dimensional effects.” Results were cluster-level corrected for multiple comparisons using 3dClustSim in AFNI at p<0.05 with a minimum cluster size of 66 voxels providing a corrected false positive rate of 0.05. Finally, a composite map of regions showing dimensional relationships, categorical effects in magnitude, and categorical effects in brain-behavior relationships was calculated, identifying regions showing each of the three effects as well as the overlap of effects.
RESULTS
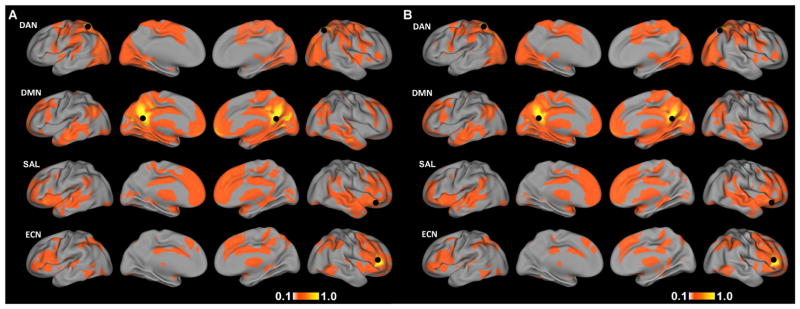
Demographic variables and clinical measures for the TDC and ASD groups are presented in Table 1. Mean functional connectivity maps for each of the four networks for TDC and ASD are presented in Figure 1A, B, respectively. Spatial maps of functional connectivity for each network in TDC largely resembled the networks reported in adult populations. For the DAN, functional connectivity was observed bilaterally in the frontal eye fields, intraparietal sulcus, and ventral visual association regions, including visual motion area MT+ (39). For the DMN, functional connectivity was observed in the posterior cingulate cortex, precuneus, medial prefrontal cortex, and bilateral angular gyrus (21, 40). The SAL consisted of the bilateral inferior frontal gyrus/anterior insula, anterior cingulate cortex, and bilateral middle temporal gyrus (22). ECN connectivity included the bilateral middle and inferior frontal gyrus, dorsomedial prefrontal cortex, and bilateral parietal cortex (22, 23).
Figure 1.
Mean functional connectivity maps for A) TDC and B) ASD for the dorsal attention network (DAN), default-mode network (DMN), salience network (SAL), and executive control network (ECN). Black circles mark the location of seed regions used to define each network. Images are displayed at a threshold of |r|>0.1.
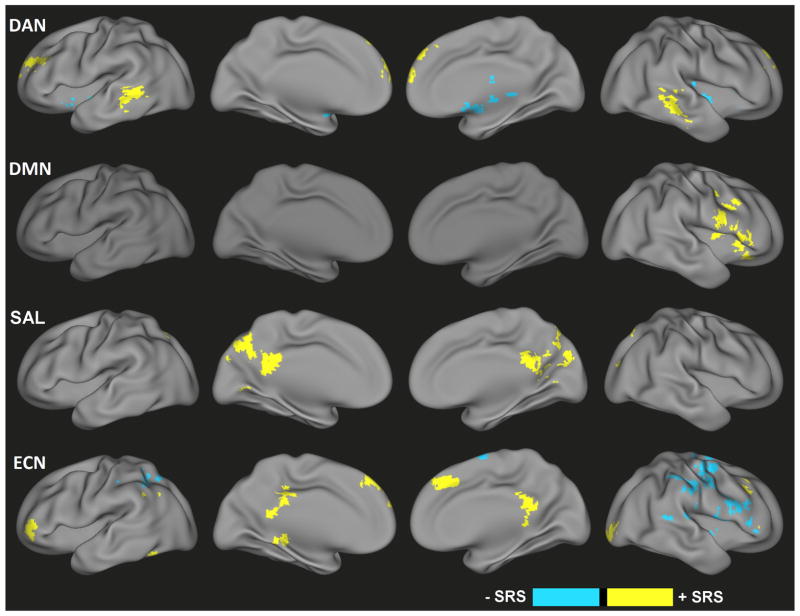
Congruent Dimensional Effects
Dimensional brain-behavior relationships that were consistent across both TDC and ASD groups were observed in each of the four networks (Figure 2, Table S2). For the DAN, higher scores on the SRS were associated with greater connectivity with the medial frontal gyrus and bilateral middle temporal gyrus. and bilateral putamen across both groups, whereas negative relationships were observed in the thalamus and bilateral putamen. For the DMN, consistent positive relationships between SRS scores and connectivity were observed in precentral gyrus, right insula, and right inferior frontal gyrus. Significant brain-behavior relationships for SAL connectivity were detected in right middle and superior frontal cortex (positive), as well as bilateral superior temporal gyrus, left superior temporal sulcus, and precentral gyrus (negative). For the ECN, significant positive dimensional relationships were present in the medial frontal gyrus, bilateral middle frontal gyrus, right lingual gyrus and posterior cingulate cortex. Negative relationships were primarily observed across the right precentral, postcentral and inferior frontal gyri. The exact coordinates and sizes of all detected regions showing congruent dimensional effects are listed in Table S2.
Figure 2.
Congruent dimensional effects of ASD symptoms (i.e., SRS score) across all subjects (i.e., independent of categorical diagnosis) for the dorsal attention network (DAN), default-mode network (DMN), salience network (SAL), and executive control network (ECN) functional connectivity. Yellow represents positive relationships with ASD symptoms; blue represents negative relationships with ASD symptoms.
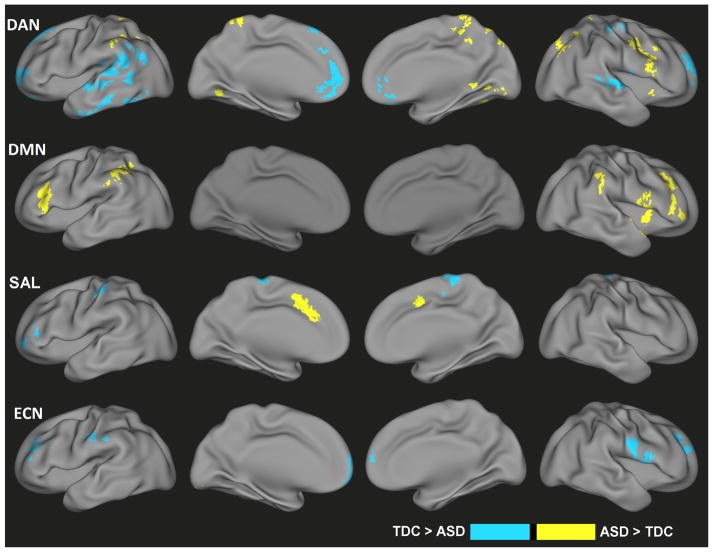
Categorical Effects in Magnitude
After controlling for dimensional effects, a number of brain regions demonstrated categorical differences in functional connectivity for each of the four networks (Figure 3, Table S3). In particular, the ASD group demonstrated enhanced DAN functional connectivity in regions including the precuneus, cerebellum, and right precentral gyrus but decreased connectivity in the medial frontal gyrus and lateral temporal cortices. For the DMN, the ASD group was associated with increased connectivity in the bilateral middle frontal gyrus, bilateral inferior parietal lobules and right insula. SAL connectivity increases associated with a categorical ASD diagnosis were found in the dorsal anterior cingulate cortex, whereas decreases were noted along the medial frontal gyrus, left middle frontal gyrus, and left postcentral gyrus. For the ECN, greater connectivity for ASD was detected in the left cerebellum. Categorical ECN connectivity decreases for the ASD group were detected in the medial prefrontal cortex, right superior frontal gyrus, right precentral gyrus, left middle frontal gyrus, left postcentral gyrus and medial frontal gyrus. Coordinates of all regions demonstrating categorical effects on functional connectivity magnitude are listed in Table S3.
Figure 3.
Categorical differences in functional connectivity values associated with an ASD diagnosis but not explained by ASD-related symptom severity (i.e., SRS score) for the dorsal attention network (DAN), default-mode network (DMN), salience network (SAL), and executive control network (ECN). Yellow indicates ASD > TDC; blue indicates TDC > ASD.
Incongruent Dimensional Effects
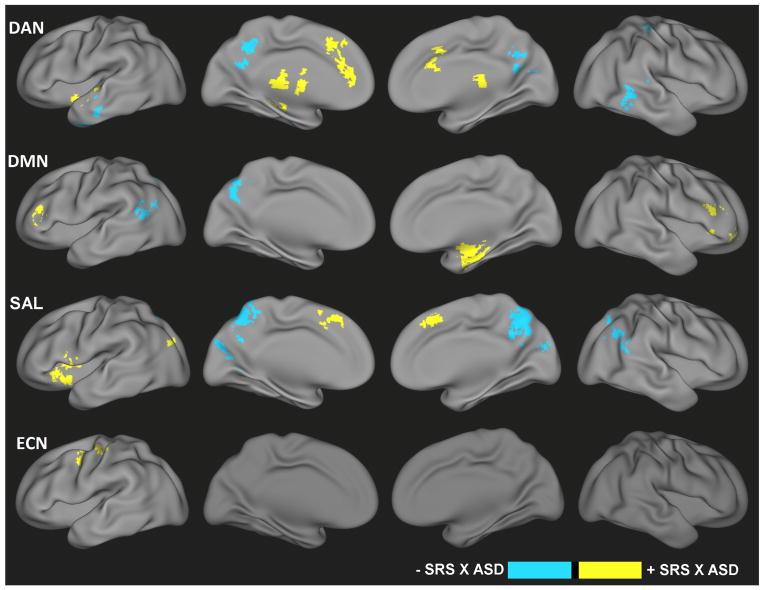
Tests of the interaction between categorical groups and dimensional relationships indicated differential brain-behavior relationships for subjects with an ASD diagnosis compared to TDC (Figure 4, Table S4). For example, for the DAN, ASD demonstrated increased slopes in the brain-behavior relationships between SRS and connectivity with the anterior cingulate cortex, thalamus, and left insula and decreased slopes in the brain-behavior relationships between SRS and connectivity within the posterior cingulate cortex and bilateral middle temporal gyrus. Categorical differences in brain-behavior correlations were observed for DMN in the right parahippocampal gyrus and bilateral middle frontal gyrus (ASD>TDC in slope), as well as precuneus and left superior temporal gyrus (TDC>ASD in slope). Increased slopes in brain-behavior relationships for the ASD group were also detected in left insula, bilateral superior temporal gyrus, and left middle occipital gyrus for SAL, in addition to decreased slopes in the brain-behavior relationships in the precuneus and right angular gyrus. Finally, increased slopes in the brain-behavior relationships for the ASD group were found in the cerebellum and left middle/precentral gyrus for ECN. Table S4 contains coordinates for all regions demonstrating incongruent dimensional effects.
Figure 4.
Significant categorical-by-dimensional interaction effects of ASD for the dorsal attention network (DAN), default-mode network (DMN), salience network (SAL), and executive control network (ECN). Yellow indicates those regions for which the relationship between functional connectivity and symptoms was increased in slope (i.e., either become more positive or change from negative to positive) for ASD versus TDC, whereas blue indicates regions with a decrease in slope (i.e., either become more negative or change from positive to negative) relationship between functional connectivity and symptoms for ASD versus TDC.
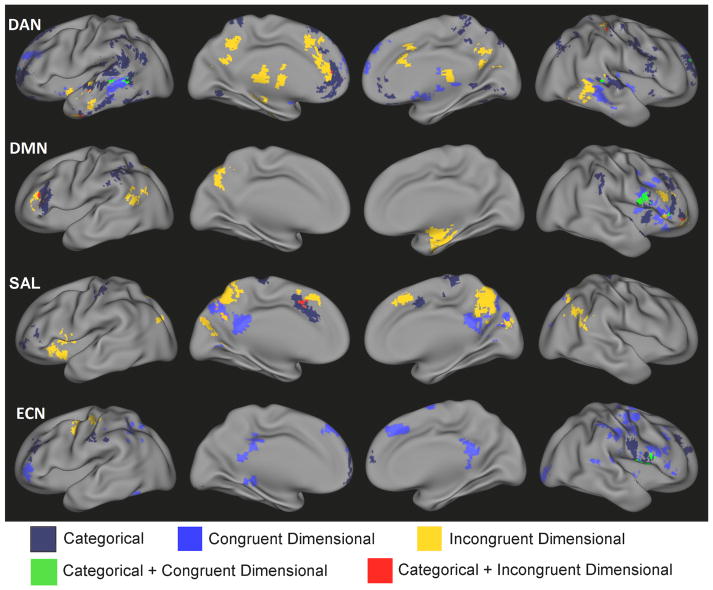
Overlap of Categorical and Dimensional Effects
Categorical and dimensional effects largely impacted distinct regions as demonstrated by Figure 5. However, several regions also demonstrated a convergence of effects (Figure 5). For example, a diagnosis of ASD was associated with greater connectivity between the DMN and right inferior frontal gyrus (categorical effect), whereas this same region was also positively associated with ASD symptoms as measured by the SRS (congruent dimensional effect). There was also a small degree of overlap of categorical and incongruent dimensional effects for SAL connectivity with the dorsomedial prefrontal cortex/supplementary motor area.
Figure 5.
Composite maps of the dorsal attention network (DAN), default-mode network (DMN), salience network (SAL), and executive control network (ECN) representing the regions demonstrating categorical effects of ASD on functional connecitivty (white), consistent dimensional relationships for both ASD and TDC (green), categorical differences in dimensional relationships between ASD and TDC (red), an overlap between categorical and congruent dimensional effects (blue) and an overlap between categorical and incongruent dimensional effects (yellow).
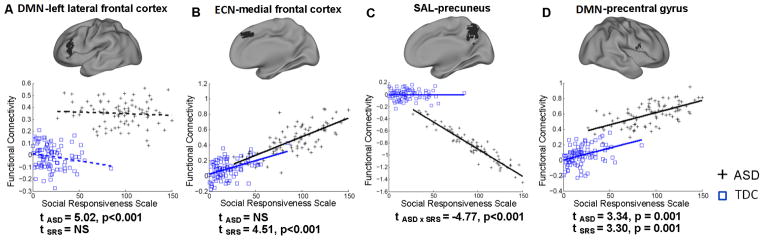
To further demonstrate the separability of categorical and dimensional effects of ASD, we produced scatter plots of the different types of relationships (Figure 6). An example of a categorical effect without a significant dimensional effect for DMN connectivity is displayed in Figure 6A, whereas a dimensional effect of ECN connectivity in the absence of a significant categorical effect is displayed in Figure 6B. A significant diagnosis-by-behavior interaction demonstrates an incongruent dimensional effect for SAL connectivity (Figure 6C). An example of overlapping categorical and congruent dimensional effects on DMN connectivity is presented in Figure 6D.
Figure 6.
Scatter plots depicting the relationship between SRS scores and functional connectivity for TDC and ASD groups for selected regions. Statistically significant linear relationships (solid lines) or non-significant linear relationships (dashed lines) are fit to data points for TDC and ASD. T-statistics for categorical and dimensional effects on regional connectivity are reported below each plot demonstrating A) categorical effects only, B) dimensional effects only, C) an interaction of categorical and dimensional effects, and D) both dimensional and categorical effects. Plotted functional connectivity values represent residuals after removing nuisance effects.
DAN, dorsal attention network; DMN, default mode network; SAL, salience network; ECN, executive control network.
DISCUSSION
Based on a large, multisite analysis of rsfMRI scans, we demonstrate functional connectivity abnormalities related to ASD that encompass both categorical and dimensional brain-behavior relationships. The effects of ASD group were not restricted to a particular brain region or network, but rather demonstrated extensive functional connectivity alterations across each of the four networks tested (i.e., DAN, DMN, SAL, and ECN). The functional connectivity variations associated with a continuous measure of ASD symptoms (i.e., SRS) consistently across both ASD and TDC group support the existence of dimensional brain mechanisms in ASD. On the other hand, we also found evidence to support categorical brain mechanisms. There were a number of regions that exhibited categorical differences in magnitude of functional connectivity, which could not be explained by quantitative relationships with ASD symptoms; another set of regions demonstrated categorical differences in their linear relationship with ASD symptoms. Therefore, consistent with previously-reported findings in ADHD children (18, 19), the characterization of functional connectivity alterations in this sample points to combined categorical and dimensional brain mechanisms underlying ASD-related deficits.
The detection of brain regions exhibiting a consistent association with ASD-like behaviors across both TDC and ASD groups (Figure 2, 6B, 6D) suggests that ASD impairments are represented in the brain – at least to some degree – as alterations in brain circuits supporting typical behaviors. In the current study, the SRS was used to characterize behavioral abnormalities associated with ASD. This instrument largely measures impairments in reciprocal social interactions (30), but some questions also tap into restricted, repetitive behaviors. Its scores capture the two major symptom categories required for a DSM-5 diagnosis of ASD (41) as a single measure of severity distributed continuously in the population (30, 42). Therefore, the regions in which functional connectivity was associated with SRS scores consistently across the two groups suggest that ASD symptoms partly stem from a single and continuously-distributed factor. For example, an interpretation of the relationship between higher SRS scores and heightened connectivity of the SAL seed with the posterior cingulate cortex (Figure 2) would be that a greater connectivity between these regions is related to a greater severity of social impairment, regardless of diagnosis. The posterior cingulate cortex has been linked to social cognition (25) and is a key brain region of the default mode network (21)(Figure 1). An inference of this finding is that greater connectivity between these regions at rest could signal impairment in normal interactions between the DMN and SAL in response to salient social stimuli, resulting in a poorer understanding of intentions and actions of others during social interactions. Overall, regions showing congruent brain-behavior relationships between ASD and TDC groups may underlie the normal expression of social behaviors that form a continuum, on which ASD children fall towards one end, and therefore provide support for the dimensional nature of ASD.
On the other hand, functional connectivity of a number of other regions that exhibited categorical differences – either diagnostic group differences in functional connectivity or differences in the relationship between behavioral scores and functional connectivity – suggest that ASD also represents a discrete syndrome. Although it is possible that categorical differences detected after controlling for symptoms may be due to the inability of SRS to fully explain the entirety of ASD behavioral deficits (e.g., intellectual deficits, language deficits, comorbidities), this explanation seems unlikely to account for the extensive categorical effects we observed (Figure 3). Rather, we suggest that there are factors that contribute to ASD that are either themselves categorical or affect the brain in a categorical manner (i.e., genetic polymorphisms, environmental insults). For example, the DMN demonstrated greater connectivity in ASD children compared to TDC in the bilateral middle frontal gyrus, bilateral inferior parietal lobules and right insula; this was not related to severity of social impairments (Figure 3). The impacted regions are closely related to the executive control network (Figure 1). Given the importance of the DMN for social cognition (25) and the role of ECN in attentional control (43, 44) and coordination of activity in other networks (37, 45), the abnormal connectivity between these sets of regions at rest suggests altered regulation of the DMNN activity by the ECN, which may promote, in a categorical way (i.e., only in the group of children with ASD), an increased bias towards internal cognitive processes and reduced reaction to external (i.e. social) stimuli (37, 45).
Differences between ASD and TDC in the relationship between functional connectivity and ASD symptom severity scores also support the existence of categorical brain mechanisms of ASD. Such findings imply that the brain representation of ASD symptom severity is qualitatively different from the brain representation of the normal spectrum of social behaviors in TDC (41, 46). Regions demonstrating this type of effect included functional connectivity between the middle occipital gyrus and SAL (Figure 4, 6C), for which there was a positive brain-behavior relationship for ASD but a negative relationship for TDC, suggesting a potential role of altered integration of visual processing with salience detection in the expression of social impairment within ASD. Another interesting discrepancy between ASD and TDC in brain-behavior relationships was detected for DAN connectivity with the posterior cingulate cortex, a key region of the DMN. The opposing functions of these two networks has been well-described (40) and seems to be important for behavior (47). Although lesser connectivity between these regions is associated with reduced social impairments in TDC, this relationship is altered in children with ASD, indicating that ASD-related impairments are associated with a categorical disruption in the intrinsic organization of these two opposing neural networks. Thus, these findings point to potential categorical mechanisms of ASD, and provide support for the existence of a dual categorical-dimensional characterization of ASD.
Although dimensional and categorical effects were each identified while covarying for the other, there were a couple of regions in which both type of effects demonstrated overlap (Figure 5). An example of such a region was found for right precentral gyrus functional connectivity with the DMN (Figure 6D). Functional connectivity of this region exhibited a consistent positive dimensional relationship for ASD and TDC groups; however, after controlling for differences in symptom severity, there remained a significant categorical effect in which children with ASD exhibited hyper-connectivity of these regions. Although it is conceivable that such effects are unrelated, the possibility that categorical and dimensional mechanisms can work in tandem deserves further exploration.
Limitations
Selecting an all-male sample may limit the extension of the study inferences to females. Future studies should consider sex differences in the brain representations of ASD to elucidate the neural mechanisms contributing to the strong male bias in the prevalence of this disorder. Additionally, we did not statistically control for medication use as information regarding psychoactive medication use was inconsistently available across sites, and sites further varied as to whether stimulants were withheld prior to scanning. As the heterogeneity of ASD is well documented, both in terms of etiology and behavioral expression, it remains unclear to what extent our findings are representative of these variations, particularly in behavioral domains not fully captured by the SRS. Moreover, previous work has demonstrated that SRS scores may be biased by non-ASD factors (48), including non-ASD behavioral problems, age, language skills and cognitive skills. As such, future studies should also include other complementary measures of ASD severity that were currently not available across both TDC and ASD groups. Finally, although we performed parallel examinations of ASD and ADHD, the lack of corresponding dimensional measures for these datasets precluded a formal statistical comparison of their functional connectivity alterations. Future studies that explore the potential overlapping and distinct neural mechanisms underlying these two neurodevelopmental disorders are highly desired (49–51).
Conclusions
Based on combined analyses of functional connectivity of four large-scale neural networks, this study demonstrated the presence of distinct categorical and dimensional brain abnormalities associated ASD. The detection of shared brain-behavior relationships across both children with ASD and TDC supports a dimensional characterization of ASD. On the other hand, functional connectivity deficits associated with a categorical ASD diagnosis or diagnosis-by-behavior interaction suggest that children with ASD are also categorically distinct from TDC. Taken together, these findings shed light on the neural bases of ASD and support the use of a categorical-dimensional hybrid model for researchers and clinicians to conceptualize this disorder.
Supplementary Material
Acknowledgments
We thank Dr. Joseph Piven at the University of North Carolina at Chapel Hill for helpful comments and discussions during the preparation of this manuscript. Funding was provided to contributing research institutions by a multitude of sources which are listed for each site at http://fcon_1000.projects.nitrc.org/indi/abide/ and included the Fund for Scientific Research-Flanders (FWO), Belgian Inter University Attraction Pole, KU Leuven Research Council, National Institutes of Health (NIH), Autism Speaks, Stavros Niarchos Foundation, Leon Levy Foundation, an endowment provided by Phyllis Green and Randolph Cōwen, University of Utah Multidisciplinary Research Seed Grant, Ben B. and Iris M. Margolis Foundation, Simons Foundation, John Merck Scholars Fund, Autism Science Foundation.
Footnotes
Financial Disclosures
ADM is a co-author of the Italian version of the SRS from which she is entitled to royalties. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Amanda Elton, Biomedical Research Imaging Center, University of North Carolina at Chapel Hill.
Adriana Di Martino, Phyllis Green and Randolph Cowen Institute for Pediatric Neuroscience at the NYU Child Study Center, New York University Langone Medical Center, New York, NY, USA.
Heather Cody Hazlett, Department of Psychiatry and Carolina Institute for Developmental Disabilities, University of North Carolina at Chapel Hill.
Wei Gao, Department of Radiology and Biomedical Research Imaging Center, University of North Carolina at Chapel Hill. Biomedical Imaging Research Institute, Department of Biomedical Sciences and Imaging, Cedars-Sinai Medical Center.
References
- 1.Ousley O, Cermak T. Autism Spectrum Disorder: Defining Dimensions and Subgroups. Current Developmental Disorders Reports. 2014;1:20–28. doi: 10.1007/s40474-013-0003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Association AP. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: American Psychiatric Publishing, Incorporated; 2013. [Google Scholar]
- 3.Wiggins L, Robins D, Adamson L, Bakeman R, Henrich C. Support for a Dimensional View of Autism Spectrum Disorders in Toddlers. Journal of autism and developmental disorders. 2012;42:191–200. doi: 10.1007/s10803-011-1230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamp-Becker I, Smidt J, Ghahreman M, Heinzel-Gutenbrunner M, Becker K, Remschmidt H. Categorical and dimensional structure of autism spectrum disorders: the nosologic validity of Asperger syndrome. Journal of autism and developmental disorders. 2010;40:921–929. doi: 10.1007/s10803-010-0939-5. [DOI] [PubMed] [Google Scholar]
- 5.Grzadzinski R, Huerta M, Lord C. DSM-5 and autism spectrum disorders (ASDs): an opportunity for identifying ASD subtypes. Molecular Autism. 2013;4:12–12. doi: 10.1186/2040-2392-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Constantino JN, Przybeck T, Friesen D, Todd RD. Reciprocal Social Behavior in Children With and Without Pervasive Developmental Disorders. Journal of Developmental & Behavioral Pediatrics. 2000;21:2–11. doi: 10.1097/00004703-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Grzadzinski R, Di Martino A, Brady E, Mairena MA, O’Neale M, Petkova E, et al. Examining autistic traits in children with ADHD: does the autism spectrum extend to ADHD? J Autism Dev Disord. 2011;41:1178–1191. doi: 10.1007/s10803-010-1135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Martino A, Shehzad Z, Kelly C, Roy A, Gee D, Uddin L, et al. Relationship between cingulo-insular functional connectivity and autistic traits in neurotypical adults. American Journal of Psychiatry. 2009;166:891–899. doi: 10.1176/appi.ajp.2009.08121894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von dem Hagen EA, Nummenmaa L, Yu R, Engell AD, Ewbank MP, Calder AJ. Autism spectrum traits in the typical population predict structure and function in the posterior superior temporal sulcus. Cerebral Cortex. 2011;21:493–500. doi: 10.1093/cercor/bhq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Misra V. The Social Brain Network and Autism. Annals of neurosciences. 2014;21:69. doi: 10.5214/ans.0972.7531.210208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Martino A, Yan C, Li Q, Denio E, Castellanos F, Alaerts K, et al. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Molecular psychiatry. 2014;19:659–667. doi: 10.1038/mp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. Neuro Report. 2006;17:1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- 13.Weng S-J, Wiggins JL, Peltier SJ, Carrasco M, Risi S, Lord C, et al. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Research. 2010;1313:202–214. doi: 10.1016/j.brainres.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paakki J-J, Rahko J, Long X, Moilanen I, Tervonen O, Nikkinen J, et al. Alterations in regional homogeneity of resting-state brain activity in autism spectrum disorders. Brain Research. 2010;1321:169–179. doi: 10.1016/j.brainres.2009.12.081. [DOI] [PubMed] [Google Scholar]
- 15.Padmanabhan A, Lynn A, Foran W, Luna B, O’Hearn K. Age related changes in striatal resting state functional connectivity in autism. Frontiers in Human Neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and Anatomical Cortical Underconnectivity in Autism: Evidence from an fMRI Study of an Executive Function Task and Corpus Callosum Morphometry. Cerebral Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minshew NJ, Williams DL. The new neurobiology of autism: Cortex, connectivity, and neuronal organization. Archives of Neurology. 2007;64:945–950. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elton A, Alcauter S, Gao W. Network connectivity abnormality profile supports a categorical-dimensional hybrid model of ADHD. Human Brain Mapping. 2014 doi: 10.1002/hbm.22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chabernaud C, Mennes M, Kelly C, Nooner K, Di Martino A, Castellanos FX, et al. Dimensional Brain-Behavior Relationships in Children with Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2012;71:434–442. doi: 10.1016/j.biopsych.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, et al. A Common Network of Functional Areas for Attention and Eye Movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- 21.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frazier TW, Youngstrom EA, Speer L, Embacher R, Law P, Constantino J, et al. Validation of Proposed DSM-5 Criteria for Autism Spectrum Disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2012;51:28–40.e23. doi: 10.1016/j.jaac.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mars RB, Neubert F-X, Noonan MP, Sallet J, Toni I, Rushworth MFS. On the relationship between the ‘default mode network’ and the ‘social brain’. Frontiers in Human Neuroscience. 2012;6 doi: 10.3389/fnhum.2012.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eger E, Moretti L, Dehaene S, Sirigu A. Decoding the representation of learned social roles in the human brain. Cortex. 2013;49:2484–2493. doi: 10.1016/j.cortex.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Uddin LQ, Supekar K, Lynch CJ, et al. Salience network–based classification and prediction of symptom severity in children with autism. JAMA Psychiatry. 2013;70:869–879. doi: 10.1001/jamapsychiatry.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solomon M, Ozonoff SJ, Ursu S, Ravizza S, Cummings N, Ly S, et al. The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia. 2009;47:2515–2526. doi: 10.1016/j.neuropsychologia.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Remington A, Swettenham J, Campbell R, Coleman M. Selective attention and perceptual load in autism spectrum disorder. Psychological Science. 2009;20:1388–1393. doi: 10.1111/j.1467-9280.2009.02454.x. [DOI] [PubMed] [Google Scholar]
- 30.Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. Journal of autism and developmental disorders. 2003;33:427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- 31.Dickstein DP, Pescosolido MF, Reidy BL, Galvan T, Kim KL, Seymour KE, et al. Developmental Meta-Analysis of the Functional Neural Correlates of Autism Spectrum Disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52:279–289.e216. doi: 10.1016/j.jaac.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Werling DM, Geschwind DH. Sex differences in autism spectrum disorders. Curr Opin Neurol. 2013;26:146–153. doi: 10.1097/WCO.0b013e32835ee548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sikka S, Cheung B, Khanuja R, Ghosh S, Yan C-g, Li Q, et al. Towards Automated Analysis of Connectomes: The Configurable Pipeline for the Analysis of Connectomes (C-PAC) 2014. [Google Scholar]
- 34.Cox RW. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 35.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SMFSL. NeuroImage. 62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 36.Tustison NJ, Cook PA, Klein A, Song G, Das SR, Duda JT, et al. Large-scale evaluation of ANTs and FreeSurfer cortical thickness measurements. Neuroimage. 2014 Oct 1;99:166–79. doi: 10.1016/j.neuroimage.2014.05.044. doi:101016/jneuroimage201405044. Epub 2014 May 29. [DOI] [PubMed] [Google Scholar]
- 37.Gao W, Lin W. Frontal parietal control network regulates the anti-correlated default and dorsal attention networks. Hum Brain Mapp. 2012;33:192–202. doi: 10.1002/hbm.21204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan C-G, Craddock RC, Zuo X-N, Zang Y-F, Milham MP. Standardizing the intrinsic brain: towards robust measurement of inter-individual variation in 1000 functional connectomes. Neuroimage. 2013;80:246–262. doi: 10.1016/j.neuroimage.2013.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 40.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frazier TW, Ratliff KR, Gruber C, Zhang Y, Law PA, Constantino JN. Confirmatory factor analytic structure and measurement invariance of quantitative autistic traits measured by the Social Responsiveness Scale-2. Autism. 2013 doi: 10.1177/1362361313500382. 1362361313500382. [DOI] [PubMed] [Google Scholar]
- 42.Constantino JN, Gruber CP, Davis S, Hayes S, Passanante N, Przybeck T. The factor structure of autistic traits. Journal of Child Psychology and Psychiatry. 2004;45:719–726. doi: 10.1111/j.1469-7610.2004.00266.x. [DOI] [PubMed] [Google Scholar]
- 43.Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, et al. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends in Cognitive Sciences. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elton A, Gao W. Divergent task-dependent functional connectivity of executive control and salience networks. Cortex. 2014;51:56–66. doi: 10.1016/j.cortex.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Frazier TW, Youngstrom EA, Sinclair L, Kubu CS, Law P, Rezai A, et al. Autism spectrum disorders as a qualitatively distinct category from typical behavior in a large, clinically ascertained sample. Assessment. 2010;17:308–320. doi: 10.1177/1073191109356534. [DOI] [PubMed] [Google Scholar]
- 47.Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 48.Hus V, Bishop S, Gotham K, Huerta M, Lord C. Factors influencing scores on the social responsiveness scale. Journal of child psychology and psychiatry, and allied disciplines. 2013;54:216–224. doi: 10.1111/j.1469-7610.2012.02589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christakou A, Murphy CM, Chantiluke K, Cubillo AI, Smith AB, Giampietro V, et al. Disorder-specific functional abnormalities during sustained attention in youth with Attention Deficit Hyperactivity Disorder (ADHD) and with Autism. Mol Psychiatry. 2013;18:236–244. doi: 10.1038/mp.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Di Martino A, Zuo X-N, Kelly C, Grzadzinski R, Mennes M, Schvarcz A, et al. Shared and Distinct Intrinsic Functional Network Centrality in Autism and Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2013;74:623–632. doi: 10.1016/j.biopsych.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ray S, Miller M, Karalunas S, Robertson C, Grayson DS, Cary RP, et al. Structural and functional connectivity of the human brain in autism spectrum disorders and attention-deficit/hyperactivity disorder: A rich club-organization study. Human Brain Mapping. 2014;35:6032–6048. doi: 10.1002/hbm.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.