Abstract
West Nile virus (WNV), a member of the Flaviviridae family, is the leading cause of viral encephalitis in the United States. Despite efforts to control the spread of WNV, there has been an increase in the number of outbreaks and clinical cases with neurological problems. There are no antiviral compounds currently in trials for WNV. NITD008 is an adenosine analogue inhibitor that interrupts the RNA-dependent RNA polymerase of flaviviruses. While NITD008 has shown promise as an antiviral for dengue virus, the ability of this drug to block WNV replication is only limited to Vero cells. Neuroinflammation is also a major cause of the WNV-associated pathology, therefore we evaluated the effect of NITD008 and a newly characterized anti-inflammatory drug vorinostat (SAHA), a histone deacetylase inhibitor, on WNV replication and disease progression in a mouse model. When administered at 10 and 25 mg/kg at days 1 to 6 after WNV infection in C57BL/6 mice, NITD008 conferred complete protection from clinical symptoms and death, which correlated with reduced viral load in the serum and restriction of virus-CNS entry. Delay of NITD008 treatment to days 3 to 6 and days 5 to 9 after infection, when WNV replication was high in the periphery and brain, resulted in the gradual loss of protection against WNV infection. However, co-treatment with SAHA and NITD008 during the CNS phase of disease improved disease outcome significantly by reducing inflammation and neuronal death. Our results support potential synergistic effect of combination therapy of NITD008 with SAHA for the treatment of WNV encephalitis.
1. Introduction
West Nile virus (WNV), a mosquito-borne neurotropic flavivirus, is closely related to Japanese encephalitis virus (JEV) and dengue virus (DENV) and has caused annual outbreaks in the North America since its arrival in 1999. The most recently reported outbreak of WNV in the United States 2012 reported 5,674 total cases including 2,873 neuroinvasive cases and 286 deaths (Beasley et al., 2013). In spite of continuing efforts, there is neither a vaccine for the prevention of WNV infection nor a specific therapy for the treatment of WNV encephalitis in humans.
The WNV RNA genome is transcribed into three structural proteins (core, membrane and envelope) and seven non-structural (NS) proteins. The NS proteins, specifically NS3 (helicase and protease) and NS5 (RNA-dependent RNA polymerase), are critical for virus replication (Lim and Shi, 2013). Robust induction of innate immune responses, such as type-1 interferon (IFN) and pro-inflammatory cytokines, facilitates clearance of WNV from the periphery and prevents neuroinvasion (Suthar et al., 2013). Following virus entry into the central nervous system (CNS), neurons are the primary target of WNV replication. However, uncontrolled inflammation caused by activated astrocytes and microglia and infiltrating peripheral leukocytes also contributes to neuronal injury (Suthar et al., 2013). Collective data from mouse studies indicate that timely clearance of WNV from the periphery and resolution of inflammation in the brain determines the disease outcome.
Multiple efforts so far have led to the discovery of several small molecule-based inhibitors targeting viral proteins critical for WNV replication (Aravapalli et al., 2012; Lim and Shi, 2013; Mueller et al., 2008; Stahla-Beek et al., 2012). However, data on their efficacy in vivo is limited and not promising. Recently, Yin and colleagues demonstrated that an adenosine analogue inhibitor (NITD008) exhibited potent antiviral activity against DENV in vivo (Yin et al., 2009). NITD008 competes with natural ATP substrates to incorporate into the growing RNA chain and terminates elongation. In the IFN α/β- and γ receptor-deficient AG129 mouse model of DENV infection, treatment with NITD008 suppressed peak viremia and completely protected mice from DENV infection and associated mortality in a dose-dependent manner. This study further demonstrated that NITD008 could block replication of other flaviviruses including WNV in Vero cells.
Another therapeutic strategy to improve neuroinflammatory disorders is to target the host inflammatory response in the CNS (Krishnan and Garcia-Blanco, 2014). Epigenetic control of histone proteins in the chromatin via histone-modifying enzymes, such as histone deacetylase (HDAC) and histone acetyltransferase (HAT), is a mechanism by which a cell regulates expression of genes including inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-1beta (IL-1β) (Leoni et al., 2002; Licciardi et al., 2012; van Essen et al., 2010). Alterations in the activity and expression of these enzymes, specifically HDACs, have been reported in several inflammatory diseases (Leoni et al., 2002; Wu et al., 2013) and have become the basis of proposing HDAC inhibitors as attractive candidates to attenuate inflammation. In the light of these studies, we first examined whether NITD008 can block WNV replication in vivo and improve disease outcome. We further tested the hypothesis that treatment with a novel anti-inflammatory compound, suberanilohydroxamic acid (SAHA or vorinostat), an HDAC inhibitor, during the CNS phase of infection will further improve WNV disease outcome by limiting neuroinflammation.
2. Materials and Methods
2.1 Animal experiments: infection with WNV and treatment with drugs
This study was approved by the University of Hawaii Institutional Animal Care and Use Committee and was conducted in strict accordance with guidelines established by the National Institutes of Health. All infections were conducted on 9 to 11-week old mice by inoculation of 1,000 PFU of WNV-NY99 isolated from crow brain and very similar to the currently circulating strains like SW/WN03 and NA/WN02 (Mann et al., 2013) in the footpad, as described previously (Kumar et al., 2013; Roe et al., 2012). NITD008 was dissolved to a stock solution of 1.25 mg/mL and was administered via oral gavage twice per day, as described previously (Yin et al., 2009). The SAHA was dissolved to 12.5 mg/mL in DMSO and sterile PBS and was administered via intraperitoneal injection once per day. Control animals were injected with PBS via IP. The animals were observed for clinical signs, morbidity and mortality for 17 days. At different time points after inoculation, blood was collected from the tail vein and serum was separated and stored at −80°C. In separate experiments, mice were anesthetized, perfused with 20 mL cold PBS and the tissues (brain, kidney, spleen) were harvested and flash frozen for future analysis.
2.2 Viral quantification in blood and tissues
WNV titers in serum and tissue homogenates were analyzed by either plaque assay using Vero cells (serum, spleen and kidney) or qRT-PCR (brain) as described previously (Verma et al., 2011b). The data were expressed as WNV PFU per μL serum or μg RNA.
2.3 Detection of host inflammatory genes using quantitative real-time RT-PCR
Total RNA was extracted from the brains and mRNA levels of inflammatory cytokines (IL-6 and TNF-α) were determined using SYBR Green supermix and specific cytokine primers. Fold change in the brains, as compared to relevant uninfected controls, was calculated after normalizing to the GAPDH gene, as previously described (Verma et al., 2010).
2.4 Immunohistochemistry
At day 9 after infection, mice were transcardially perfused with 20 mL PBS followed by 20 mL of 4% paraformaldehyde (PFA). Brains were harvested and were either cryoprotected in 30% sucrose and frozen in Optimal Cutting Temperature solution or processed for paraffin embedding. Brain sections of 10μm thickness were cut and terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining was performed using an in situ cell death detection kit (Roche), as previously described (Kumar et al., 2013). Tissue sections were also stained with haematoxylin and eosin (H&E) and examined for pathological changes.
2.5 Statistical analysis
For survival analyses, GraphPad Prism 5.0 was used to perform Kaplan-Meier log-rank test to compare curves. Comparison of mRNA and WNV titers data was carried out using unpaired student t test using Prism 5.0. Differences of P<0.05 were considered significant.
3. Results
3.1 NITD008 protects mice against WNV infection in a dose-dependent manner
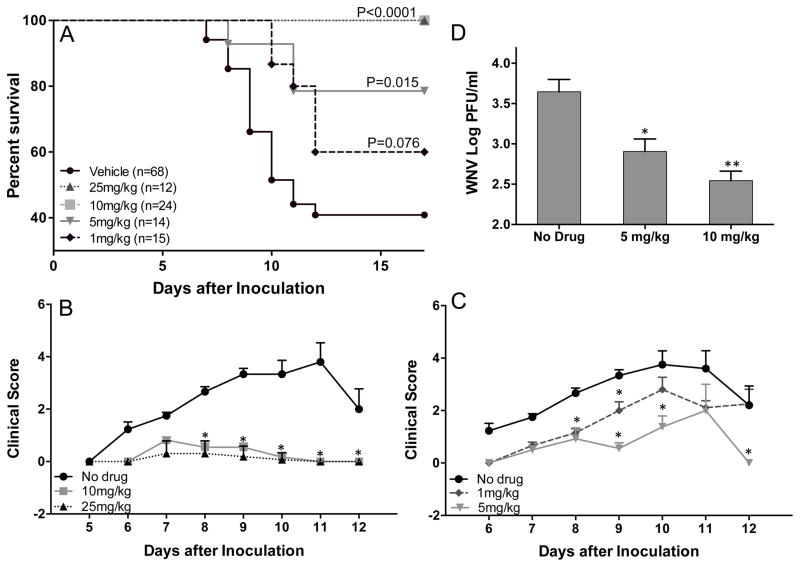
To assess the therapeutic effect of NITD008, mice were administered with 25, 10, 5 or 1 mg/kg of NITD008 via oral gavage on days 1 to 6 after WNV infection. As seen in Fig. 1, infected mice exhibited clinical signs typical of WNV disease, such as ruffled fur, ataxia, hunchback, etc. and resulted in 67% mortality. In contrast, mice treated with 25 and 10 mg/kg of NITD008 were completely protected and did not show any signs of clinical disease (Fig. 1A and 1B). At the lower dose of 5 mg/kg of NITD008, infected mice exhibited a reduced mortality rate (P<0.05) with very mild disease signs, as compared to untreated mice (Fig. 1C). However, treatment with 1 mg/kg NITD008 did not have any significant difference in the mortality or clinical score as compared to the control group. Accordingly, WNV titers in the serum decreased in a dose-dependent manner at day 3 after infection (Fig. 1D). Further, treatment with these doses of NITD008 for 6 days did not result in any signs of toxicity in control mice, such as weight loss or bloody stools (data not shown).
Figure 1.
NITD008 protects against lethal challenge with WNV. (A–C) C57BL/6 mice inoculated with 1000 PFU WNV (NY99) via footpad injection were administered with different doses of NITD008 via oral gavage twice per day from day 1 to 6 after inoculation and observed for clinical symptoms and mortality. Clinical score criteria: 1, ruffled fur; 2, ruffled fur with distinct hunched back; 3, paresis/difficulty walking; 4, paralysis; 5, moribund/euthanized; (D) WNV titers measured in the serum at day 3 after infection using plaque assay. Error bar represent ± SEM from at least 4 mice/group. **p< 0.005, *p<0.05
3.2 NITD008 loses efficacy when administered during late phase of WNV infection
The therapeutic window of efficacy of NITD008, specifically when the virus has entered the brain was evaluated by treating WNV-infected mice with 10 mg/kg of NITD008 during the peak viremia stage (days 3 to 6 after infection) or during the CNS invasion and replication phase (days 5 to 9 after infection). As seen in Fig. 2, delay of NITD008 treatment by two days (days 3 to 6), albeit not completely protective, was able to significantly reduce the mortality of WNV-infected mice. However, treatment on days 5 to 9 days after infection exhibited a marked decrease in the survival of infected mice, which correlated with no significant difference in the clinical score of these mice when compared to mice treated on days 1 to 6. These results suggest that NITD008 looses its protective effect when administered during the CNS stage of WNV disease.
Figure 2.
Protective effect is lost when NITD008 treatment is delayed. WNV-infected mice were administered with vehicle or 10 mg/kg NITD008 on either days 1 to 6, day 3 to 6 or days 5 to 9 and observed for (A) mortality and (B) clinical symptoms. Error bar represent ± SEM. **p< 0.005, *p<0.05
3.3 NITD008 blocks WNV replication at different stages of infection
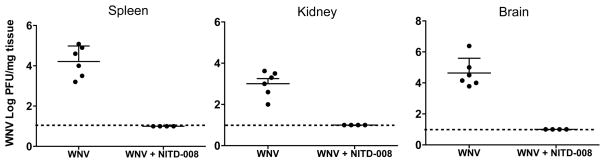
To understand the correlation of disease outcome in NITD008-treated mice with virus replication, we determined the levels of WNV in different groups of mice. As shown in our previous studies (Kumar et al., 2012; Kumar et al., 2013; Roe et al., 2012), WNV replication in the peripheral tissues (spleen and kidney) is detected between days 2 to 6 after infection and the virus is completely cleared from the periphery by day 7 after infection. On the other hand, WNV replication in the brain peaks at day 8 after infection. As depicted in Fig. 3, at day 5 after infection WNV was not detected in the spleen and kidney of NITD008-treated mice (10 mg/kg dose on days 1 to 6), which correlated well with significant reduction of virus replication in the serum (Fig. 1D). Further, no virus was detected in the brains of mice treated with NITD008 starting day 1 after infection, suggesting that reduced viremia completely restricts WNV neuroinvasion in this mouse model.
Figure 3.
NITD008 blocks WNV dissemination to peripheral tissues and brain. WNV-infected mice treated with vehicle or 10 mg/kg NITD008 on days 1–6 after inoculation were sacrificed at day 5 (spleen and kidney) and day 8 (brain) after infection and WNV titers were analyzed in the tissue homogenates using plaque assay. Dotted line- detection limit of plaque assay. Error bar represent ± SEM from at least 4 mice/group. *p<0.05
3.4 Combined treatment with SAHA increases the therapeutic window of NITD008
Having demonstrated that NITD008 loses its protective effect when WNV has established infection in the CNS, we next evaluated the impact of the HDAC inhibitor SAHA on the neuroinflammation and WNV disease outcome. Mice were treated with 100 mg/kg of SAHA either on days 3 to 6 or days 5 to 9 after infection, and as seen in Fig. 4A, SAHA treatment on days 3 to 6 after infection had no significant effect on the mortality rate of WNV-infected mice. However, when SAHA was administered during the CNS stage of infection, there was a trend of decreased mortality, but it was not significant. The virus titers in the serum at day 3 after infection before beginning the treatment with SAHA were comparable between all groups (data not shown).
Figure 4.
Co-treatment of SAHA with NITD008 improves outcome of lethal challenge with WNV: (A) SAHA was administered on days 3–6 or 5–9 after WNV inoculation, once per day and survival was monitored (B) Survival of mice treated with 100 mg/kg of SAHA or 10 mg/kg NITD008 alone was compared with mice treated with 100 mg/kg of SAHA and 10 mg/kg NITD008 on days 5–9 after inoculation (C) WNV titers in the brain at day 9 after infection from mice treated with SAHA (days 3–6 and 5–9) and SAHA plus NITD008 (days 5–9) using qRT-PCR. Error bars represent ± SEM from at least 4 mice/group.
Based on our observation that both NITD008 and SAHA independently had slight but not significant effect on the survival of infected mice when treated during the CNS phase of the disease, we further tested the efficacy of combined treatment of these two drugs in protecting mice from progressing to encephalitis. Mice receiving both 10 mg/kg NITD008 and 100 mg/kg SAHA (days 5 to 9) showed a significant reduction in the mortality rate (10%) when compared to groups treated with SAHA and NITD008 alone (Fig. 4B, P=0.002).
3.5 Co-treatment with SAHA and NITD008 attenuates the mRNA levels of inflammatory cytokines in the brain
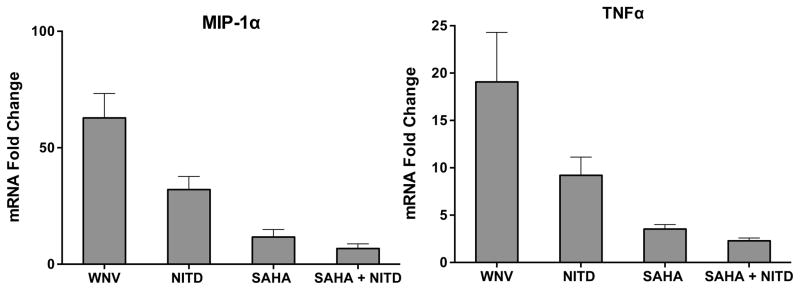
Since pan-HDAC inhibitors are shown to exert multiple neuroprotective properties, we examined the specific effect of SAHA treatment on WNV replication and neuroinflammation in the brain. WNV loads in the brains of different groups of treated mice demonstrated that SAHA treatment had no effect on virus replication in the brains when given alone or in combination with NITD008 (Fig. 4C), suggesting that SAHA does not exhibit anti-WNV property in mice. We then assessed the mRNA levels of key WNV-associated cytokines and chemokines. As seen in Fig. 5, WNV infection led to significant increase in the transcripts of TNF-α and macrophage inflammatory protein-1alpha (MIP-1α) in the mouse brains, which reduced slightly following NITD008 treatment at days 5 to 9 after infection. However, the group that received SAHA alone or SAHA plus NITD008 (days 5 to 9 after infection) exhibited significantly reduced levels of TNF-α and MIP-1α mRNA.
Figure 5.
Treatment with NITD008 and SAHA attenuates inflammatory mediators in infected mouse brains. Brains harvested from mice treated with NITD008 and SAHA were used to analyze the levels of mRNA of TNF-α and MIP-1α. The data from at least 4mice/group was normalized to GAPDH and is represented as fold change as compared to un-infected controls.
3.6 Treatment with NITD008 and SAHA reduce WNV-associated neuropathology
Neuronal death caused by direct virus infection and increased neuroinflammation is an important hallmark of WNV disease pathology (King et al., 2007). Since SAHA reduced inflammatory mediators, we examined the apoptotic neurons in the brains of different groups of mice. As seen in Fig. 6A and 6B, a significant increase in the number of dying neurons was observed in the brain at day 9 after WNV infection using H&E staining and TUNEL assay. Not surprisingly, mice that received NITD008 beginning 1 day after infection exhibited very few TUNEL-positive cells. However, when the NITD008 treatment began at 5 days after infection, apoptotic cells were clearly visible in the brain, which decreased significantly following combined treatment of both SAHA and NITD008 (Fig. 6B and 6C).
Figure 6.
Treatment with NITD008 and SAHA reduces markers of WNV-associated neuropathology in the brain: (A) H&E staining of PFA perfused brains depicts shrunken neuron body (white arrows) with light pink cytoplasmic staining representing degenerating neurons in the brain of WNV infected mice at day 9 after inoculation. NITD008 treatment demonstrated significant improvement in the neuron body (white arrow head). (B) TUNEL positive cells (red) were prominent in the brain of WNV-infected mice at day 9 after infection, which reduced significantly in the brains from mice treated with early NITD008 and combined treatment of NITD008 + SAHA. (C) Quantitative representation of TUNEL positive cells from at least 5 fields per coverslip from two independent experiments. *p<0.05 compared to WNV infected brains.
4. Discussion
The continuing outbreaks of WNV-associated neurological disease in the United States underscore the urgent need for effective antiviral strategies. Previously described adenosine analog NITD008 has been shown to potently inhibit DENV replication in vivo (Lim and Shi, 2013). Using a well-established mouse model of WNV infection, our data highlights that (i) the treatment of mice with NITD008 significantly attenuates viremia and prevents neuroinvasion, resulting in complete protection from WNV-associated mortality and morbidity; (ii) NITD008 loses its protective effect when administered during the CNS phase of the disease; and (iii) co-treatment with SAHA and NITD008 during the later stages of WNV disease improves disease outcome by reducing inflammation and neuronal death. Our findings further extend the WNV in vitro studies and show that NITD008 significantly reduced viral load and was protective against WNV-associated mortality in mice in a dose- dependent manner (Fig. 1). Further, the lowest protective dose of 10 mg/kg in WNV was similar to the in vivo efficacy of NITD008 observed in DENV-infected AG129 mice (Yin et al., 2009). Although NITD008 was found to be toxic in rats and dogs after two weeks of treatment (Yin et al., 2009), nucleoside analogue inhibitors remain the most promising class of antiviral compounds against viruses that are cleared within 7 to 14 days (Lim et al., 2013). Interestingly, in addition to flaviviruses, recent studies demonstrate that NITD008 has potent antiviral activity in vitro and in vivo against enterovirus 71 (Deng et al., 2014). Another nucleoside analog, 3′-azidothymidine, has been shown to efficiently block the HIV replication and has evolved into the main component of antiretroviral drug combinations now used in the treatment of HIV-infected patients (Cihlar and Ray, 2010).
The time of administration of any antiviral drug is a critical factor in determining the efficacy of novel compounds. Our data indicating no substantial difference in disease outcome when the treatment of NITD008 was delayed by 5 days is not surprising. A possible explanation is that by day 5, WNV has already entered the CNS and NITD008 is probably too late to completely restrict virus replication. Secondly, although leakiness in the blood-brain barrier (BBB) is well documented in the WNV-infected mouse model (Dai et al., 2008; Roe et al., 2012), the ability of NITD008 to cross the BBB is not yet characterized. Therefore, it is likely that the concentration of NITD008 in the CNS is not optimum to block robust WNV replication in the neurons. However, Yin and colleagues reported similar loss of protection following delayed start of treatment with NITD008 at 48 hrs after DENV infection in AG129 mice (Yin et al., 2009) once the virus infection was established in the periphery. Likewise, previous studies using other disease models support the fact that the time of treatment is critical for the disease outcome. For example, Oestereich and co-workers (2014) recently showed that antiviral favipiravir lost its efficacy against Ebola virus infection in mice when the treatment was delayed by two days.
Several in vitro and in vivo proof-of-principle studies using knock-out mouse models have identified host factors contributing to the inflammation and neuronal injury to improve disease outcome of WNV and other neurotropic pathogens (Wang et al., 2008). Our previous in vitro studies demonstrated improvement in BBB disruption and neuronal injury using inhibitors of matrix metalloproteinase-9 and cyclooxygenase-2 and neutralizing antibodies against cytokines (Verma et al., 2010; Verma et al., 2011a). In this study, as compared to other HDAC inhibitors, such as Trichostatin A and MS-275, we chose SAHA as an anti-inflammatory drug to attenuate neuroinflammation because this drug can cross the BBB and has shown promise in treating inflammatory encephalopathy (Fang et al., 2014; Ge et al., 2013; Singh et al., 2011; Wang et al., 2013). SAHA treatment was reported to reduce the levels of pro-inflammatory cytokines, inducible nitric oxide and activation of NF-κB in activated mouse primary astrocytes (Singh et al., 2011). Similarly, increased expression of HDAC6 was noted in mouse model of Alzheimer’s disease (AD), loss of which restored amyloid beta-associated cognitive deficits (Govindarajan et al., 2013). Although a more detailed study is warranted to carefully delineate the role of epigenetic mechanisms and specific HDACs associated with WNV-associated neuropathology, our results provide first evidence that epigenetic machinery is involved in the cytokine production in WNV-infected brain. This study did not characterize the cell types that might respond to SAHA treatment in the brain and future studies will investigate the response of SAHA treatment on other hallmarks of WNV-encephalitis such as BBB disruption.
A major limitation of targeting host machinery is the possibility of undesirable side effects in long-term treatments, because these factors also play important roles in cell survival. However, the approach of short-term treatment for controlling acute diseases, such as encephalitis associated with WNV and related neurotropic pathogens such as JEV, may not face similar shortcomings as compared to long-term blocking of inflammatory molecules in chronic diseases, such as AD or HIV infections (Zemek et al., 2014).
5. Conclusions
To our knowledge, our results for the first time provide conclusive evidence that NITD008 is an effective antiviral compound that protects mice from WNV encephalitis when administered during the early stage of disease. If the ongoing research to address the toxicity associated with long-term usage of NITD008 is successful, this drug has high chances of being developed as an effective prophylactic antiviral against WNV and other flaviviruses. Our study acknowledges the limitation of NITD008 to completely protect against acute WNV encephalitis as virus is almost cleared when the patient exhibits severe disease. Given that combined therapy of NITD008 with SAHA was protective in a mouse model of WNV infection, our findings provide a platform for future studies in mice and humans to develop novel therapeutic options to manage WNV and other neurotropic viruses.
NITD008 treatment of WNV-infected mice attenuates viremia, prevents neuroinvasion and completely prevents mortality
NITD008 loses its protective effect when administered during the CNS phase of the WNV disease
Co-treatment with histone deacetylase inhibitor and NITD008 improves mortality by reducing inflammation and neuronal death
Acknowledgments
This study was supported by the Centers of Biomedical Research Excellence (P20GM103516), National Institute of General Medical Sciences, and the Research Centers in Minority Institutions (U54MD008149), National Institute on Minority Health and Health Disparities, National Institutes of Health, and Institutional funds. We thank Histopathology Core of the Research Centers in Minority Institutions Program (G12MD007601) and the ABSL-3 Biocontainment Core staff for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aravapalli S, Lai H, Teramoto T, Alliston KR, Lushington GH, Ferguson EL, Padmanabhan R, Groutas WC. Inhibitors of Dengue virus and West Nile virus proteases based on the aminobenzamide scaffold. Bioorganic & medicinal chemistry. 2012;20:4140–4148. doi: 10.1016/j.bmc.2012.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley DW, Barrett AD, Tesh RB. Resurgence of West Nile neurologic disease in the United States in 2012: what happened? What needs to be done? Antiviral research. 2013;99:1–5. doi: 10.1016/j.antiviral.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Cihlar T, Ray AS. Nucleoside and nucleotide HIV reverse transcriptase inhibitors: 25 years after zidovudine. Antiviral research. 2010;85:39–58. doi: 10.1016/j.antiviral.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Dai J, Wang P, Bai F, Town T, Fikrig E. Icam-1 participates in the entry of west nile virus into the central nervous system. Journal of virology. 2008;82:4164–4168. doi: 10.1128/JVI.02621-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng CL, Yeo H, Ye HQ, Liu SQ, Shang BD, Gong P, Alonso S, Shi PY, Zhang B. Inhibition of enterovirus 71 by adenosine analog NITD008. Journal of virology. 2014;88:11915–11923. doi: 10.1128/JVI.01207-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Lian Y, Xie K, Cai S, Wen P. Epigenetic modulation of neuronal apoptosis and cognitive functions in sepsis-associated encephalopathy. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2014;35:283–288. doi: 10.1007/s10072-013-1508-4. [DOI] [PubMed] [Google Scholar]
- Ge Z, Da Y, Xue Z, Zhang K, Zhuang H, Peng M, Li Y, Li W, Simard A, Hao J, Yao Z, Zhang R. Vorinostat, a histone deacetylase inhibitor, suppresses dendritic cell function and ameliorates experimental autoimmune encephalomyelitis. Experimental neurology. 2013;241:56–66. doi: 10.1016/j.expneurol.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Govindarajan N, Rao P, Burkhardt S, Sananbenesi F, Schluter OM, Bradke F, Lu J, Fischer A. Reducing HDAC6 ameliorates cognitive deficits in a mouse model for Alzheimer’s disease. EMBO molecular medicine. 2013;5:52–63. doi: 10.1002/emmm.201201923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King NJ, Getts DR, Getts MT, Rana S, Shrestha B, Kesson AM. Immunopathology of flavivirus infections. Immunology and cell biology. 2007;85:33–42. doi: 10.1038/sj.icb.7100012. [DOI] [PubMed] [Google Scholar]
- Krishnan MN, Garcia-Blanco MA. Targeting host factors to treat West Nile and dengue viral infections. Viruses. 2014;6:683–708. doi: 10.3390/v6020683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Roe K, Nerurkar PV, Namekar M, Orillo B, Verma S, Nerurkar VR. Impaired virus clearance, compromised immune response and increased mortality in type 2 diabetic mice infected with West Nile virus. PLoS ONE. 2012;7:e44682. doi: 10.1371/journal.pone.0044682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Roe K, Orillo B, Muruve DA, Nerurkar VR, Gale M, Jr, Verma S. Inflammasome adaptor protein Apoptosis-associated speck-like protein containing CARD (ASC) is critical for the immune response and survival in west Nile virus encephalitis. Journal of virology. 2013;87:3655–3667. doi: 10.1128/JVI.02667-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoni F, Zaliani A, Bertolini G, Porro G, Pagani P, Pozzi P, Dona G, Fossati G, Sozzani S, Azam T, Bufler P, Fantuzzi G, Goncharov I, Kim SH, Pomerantz BJ, Reznikov LL, Siegmund B, Dinarello CA, Mascagni P. The antitumor histone deacetylase inhibitor suberoylanilide hydroxamic acid exhibits antiinflammatory properties via suppression of cytokines. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:2995–3000. doi: 10.1073/pnas.052702999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licciardi PV, Kwa FA, Ververis K, Di Costanzo N, Balcerczyk A, Tang ML, El-Osta A, Karagiannis TC. Influence of natural and synthetic histone deacetylase inhibitors on chromatin. Antioxidants & redox signaling. 2012;17:340–354. doi: 10.1089/ars.2011.4480. [DOI] [PubMed] [Google Scholar]
- Lim SP, Shi PY. West Nile virus drug discovery. Viruses. 2013;5:2977–3006. doi: 10.3390/v5122977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SP, Wang QY, Noble CG, Chen YL, Dong H, Zou B, Yokokawa F, Nilar S, Smith P, Beer D, Lescar J, Shi PY. Ten years of dengue drug discovery: progress and prospects. Antiviral research. 2013;100:500–519. doi: 10.1016/j.antiviral.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Mann BR, McMullen AR, Swetnam DM, Barrett AD. Molecular epidemiology and evolution of West Nile virus in North America. International journal of environmental research and public health. 2013;10:5111–5129. doi: 10.3390/ijerph10105111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller NH, Pattabiraman N, Ansarah-Sobrinho C, Viswanathan P, Pierson TC, Padmanabhan R. Identification and biochemical characterization of small-molecule inhibitors of west nile virus serine protease by a high-throughput screen. Antimicrobial agents and chemotherapy. 2008;52:3385–3393. doi: 10.1128/AAC.01508-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe K, Kumar M, Lum S, Orillo B, Nerurkar VR, Verma S. West Nile virus-induced disruption of the blood-brain barrier in mice is characterized by the degradation of the junctional complex proteins and increase in multiple matrix metalloproteinases. The Journal of general virology. 2012;93:1193–1203. doi: 10.1099/vir.0.040899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Khan M, Singh I. HDAC inhibitor SAHA normalizes the levels of VLCFAs in human skin fibroblasts from X-ALD patients and downregulates the expression of proinflammatory cytokines in Abcd1/2-silenced mouse astrocytes. Journal of lipid research. 2011;52:2056–2069. doi: 10.1194/jlr.M017491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahla-Beek HJ, April DG, Saeedi BJ, Hannah AM, Keenan SM, Geiss BJ. Identification of a novel antiviral inhibitor of the flavivirus guanylyltransferase enzyme. Journal of virology. 2012;86:8730–8739. doi: 10.1128/JVI.00384-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthar MS, Diamond MS, Gale M., Jr West Nile virus infection and immunity. Nature reviews Microbiology. 2013;11:115–128. doi: 10.1038/nrmicro2950. [DOI] [PubMed] [Google Scholar]
- van Essen D, Zhu Y, Saccani S. A feed-forward circuit controlling inducible NF-kappaB target gene activation by promoter histone demethylation. Mol Cell. 2010;39:750–760. doi: 10.1016/j.molcel.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Verma S, Kumar M, Gurjav U, Lum S, Nerurkar VR. Reversal of West Nile virus-induced blood-brain barrier disruption and tight junction proteins degradation by matrix metalloproteinases inhibitor. Virology. 2010;397:130–138. doi: 10.1016/j.virol.2009.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S, Kumar M, Nerurkar VR. Cyclooxygenase-2 inhibitor blocks the production of West Nile virus-induced neuroinflammatory markers in astrocytes. The Journal of general virology. 2011a;92:507–515. doi: 10.1099/vir.0.026716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S, Kumar M, Nerurkar VR. Cyclooxygenase-2 inhibitor blocks the production of West Nile virus-induced neuroinflammatory markers in astrocytes. Journal of General Virology. 2011b;92:507–515. doi: 10.1099/vir.0.026716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Zhao M, Chen G, Cheng X, Han X, Lin S, Zhang X, Yu X. The histone deacetylase inhibitor vorinostat prevents TNFalpha-induced necroptosis by regulating multiple signaling pathways. Apoptosis : an international journal on programmed cell death. 2013;18:1348–1362. doi: 10.1007/s10495-013-0866-y. [DOI] [PubMed] [Google Scholar]
- Wang P, Dai J, Bai F, Kong KF, Wong SJ, Montgomery RR, Madri JA, Fikrig E. Matrix metalloproteinase 9 facilitates west nile virus entry into the brain. Journal of virology. 2008;82:8978–8985. doi: 10.1128/JVI.00314-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Dong L, Zhang M, Jia M, Zhang G, Qiu L, Ji M, Yang J. Class I histone deacetylase inhibitor valproic acid reverses cognitive deficits in a mouse model of septic encephalopathy. Neurochemical research. 2013;38:2440–2449. doi: 10.1007/s11064-013-1159-0. [DOI] [PubMed] [Google Scholar]
- Yin Z, Chen YL, Schul W, Wang QY, Gu F, Duraiswamy J, Kondreddi RR, Niyomrattanakit P, Lakshminarayana SB, Goh A, Xu HY, Liu W, Liu B, Lim JY, Ng CY, Qing M, Lim CC, Yip A, Wang G, Chan WL, Tan HP, Lin K, Zhang B, Zou G, Bernard KA, Garrett C, Beltz K, Dong M, Weaver M, He H, Pichota A, Dartois V, Keller TH, Shi PY. An adenosine nucleoside inhibitor of dengue virus. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20435–20439. doi: 10.1073/pnas.0907010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemek F, Drtinova L, Nepovimova E, Sepsova V, Korabecny J, Klimes J, Kuca K. Outcomes of Alzheimer’s disease therapy with acetylcholinesterase inhibitors and memantine. Expert opinion on drug safety. 2014;13:759–774. doi: 10.1517/14740338.2014.914168. [DOI] [PubMed] [Google Scholar]