Abstract
The REL gene, encoding the NF-κB subunit c-Rel, is frequently amplified in B-cell lymphoma and functions as a tumour-promoting transcription factor. Here we report the surprising result that c-rel–/– mice display significantly earlier lymphomagenesis in the c-Myc driven, Eμ-Myc model of B-cell lymphoma. c-Rel loss also led to earlier onset of disease in a separate TCL1-Tg-driven lymphoma model. Tumour reimplantation experiments indicated that this is an effect intrinsic to the Eμ-Myc lymphoma cells but, counterintuitively, c-rel–/– Eμ-Myc lymphoma cells were more sensitive to apoptotic stimuli. To learn more about why loss of c-Rel led to earlier onset of disease, microarray gene expression analysis was performed on B cells from 4-week-old, wild-type and c-rel–/– Eμ-Myc mice. Extensive changes in gene expression were not seen at this age, but among those transcripts significantly downregulated by the loss of c-Rel was the B-cell tumour suppressor BTB and CNC homology 2 (Bach2). Quantitative PCR and western blot analysis confirmed loss of Bach2 in c-Rel mutant Eμ-Myc tumours at both 4 weeks and the terminal stages of disease. Moreover, Bach2 expression was also downregulated in c-rel–/– TCL1-Tg mice and RelA Thr505Ala mutant Eμ-Myc mice. Analysis of wild-type Eμ-Myc mice demonstrated that the population expressing low levels of Bach2 exhibited the earlier onset of lymphoma seen in c-rel–/– mice. Confirming the relevance of these findings to human disease, analysis of chromatin immunoprecipitation sequencing data revealed that Bach2 is a c-Rel and NF-κB target gene in transformed human B cells, whereas treatment of Burkitt's lymphoma cells with inhibitors of the NF-κB/IκB kinase pathway or deletion of c-Rel or RelA resulted in loss of Bach2 expression. These data reveal a surprising tumour suppressor role for c-Rel in lymphoma development explained by regulation of Bach2 expression, underlining the context-dependent complexity of NF-κB signalling in cancer.
Introduction
The tumour-promoting role of the NF-κB pathway is well established and results from its ability to regulate the expression of genes involved in multiple aspects of cancer cell biology.1 This is also true in haematological malignancies2 and in several B-cell lymphoma types, such as activated B-cell-like-diffuse large B-cell lymphomas,3 primary mediastinal large B-cell lymphoma4, 5 and classical Hodgkin lymphoma6 NF-κB activity is required for survival and proliferation. However, the contribution of individual NF-κB subunits is generally not known. In particular, whereas NF-κB subunits have been reported to exhibit characteristics of tumour suppressors in vitro,1 it has not been investigated whether these properties have relevance to lymphoma development in vivo.
There are five NF-κB subunits in mammalian cells, RelA/p65, RelB, c-Rel, p50/p105 (NF-κB1) and p52/p100 (NF-κB2). RelA and c-Rel function as effector subunits for the IκB kinase β-dependent, canonical NF-κB pathway.7 Of these NF-κB subunits, c-Rel is most closely associated with lymphoma and was first identified as the cellular homologue of the avian Rev-T retroviral oncogene v-Rel.8, 9, 10 c-Rel is ubiquitously expressed in B cells regardless of developmental stage, although the highest levels are observed in mature B cells.11, 12, 13 c-rel knockout mice developed normally with no effects on B-cell maturation but do exhibit some immunological defects, including reduced B-cell proliferation and activation, abnormal germinal centres and reduced number of marginal zone B cells.14, 15, 16, 17
c-Rel is distinct from other NF-κB family members in its ability to transform chicken lymphoid cells in vitro.8, 18, 19, 20 Moreover, genomic and cytogenetic studies of human lymphomas have shown gains of chromosome 2p13, which encodes the REL gene. Amplifications and gains of REL have been detected in ~50% of HL21, 22, 23 and 10–25% or 50% in two studies of primary mediastinal large B-cell lymphoma.4, 24 REL has also been identified as a susceptibility locus for HL,25 whereas c-Rel nuclear localisation has been identified as a poor prognostic factor in both activated B-cell-like- and germinal centre B-cell-like-diffuse large B-cell lymphomas.26
Despite this, relatively little is known about the role of c-Rel or other NF-κB subunits in c-Myc-driven lymphomas. However, a recent study of Myc-driven B-cell lymphoma in mice revealed a tumour suppressor role for RelA.27 Here, short hairpin RNA silencing of RelA did not affect progression of established lymphomas, but after cyclophosphamide treatment its loss resulted in chemoresistance as a consequence of impaired induction of senescence.27 Similarly, NF-κB was required for both therapy-induced senescence and resistance to cell death in the Eμ-Myc mouse model of B-cell lymphoma upon expression of a degradation-resistant form of IκBα.28 c-Myc can also inhibit expression of NF-κB2, and loss of this NF-κB subunit in the Eμ-Myc mouse model resulted in moderately earlier onset of disease as a consequence of impaired apoptosis.29 By contrast, deletion of NF-κB1 displayed no effects on Eμ-Myc lymphoma development.30 These results imply a more complicated role for NF-κB in Myc-driven lymphoma, with both tumour-promoting and -suppressing functions being reported, although any role for c-Rel has not been established.
Here, we have investigated the role of c-Rel in mouse models of B-cell lymphomagenesis. We demonstrate that, opposite to the expected result, c-rel–/– Eμ-Myc and TCL1-Tg mice exhibit earlier onset of lymphoma and that this result can be explained by c-Rel-dependent regulation of the B-cell tumour suppressor BTB and CNC homology 2 (Bach2).
Results
NF-κB is active in Eμ-Myc-derived lymphoma
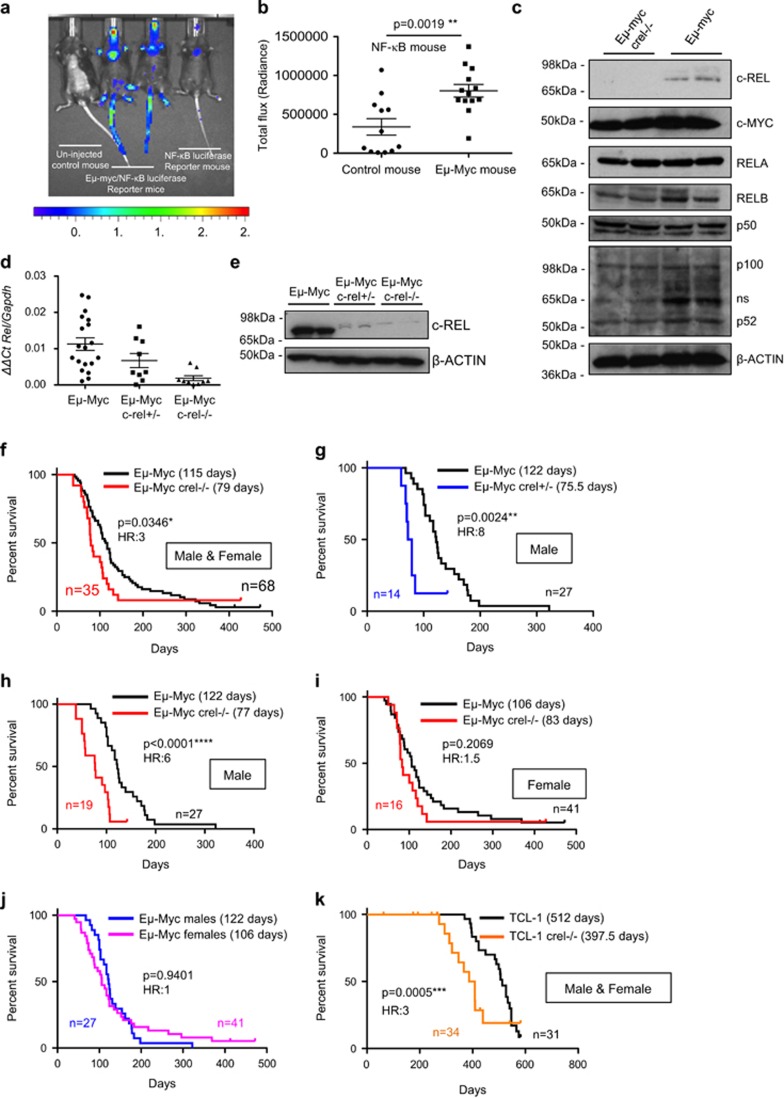
To determine if there are significant levels of NF-κB activity in Myc-driven B-cell lymphoma, with the potential to affect disease driven by this oncogene, we crossed 3 × κB-luc (NF-κB-Luc) reporter mice onto Eμ-Myc transgenic mice, allowing in vivo visualisation of NF-κB activity.31 The median onset of aggressive lymphoma in Eμ-Myc mice is between the ages of 3 and 6 months but they exhibit the hallmarks of Myc overexpression by 4 weeks.32 This analysis revealed significantly higher levels of NF-κB activity in Eμ-Myc mice at 8 weeks of age, in lymphoid organ sites, including mesenteric/inguinal lymph nodes and thymus (Figures 1a and b).
Figure 1.
c-Rel functions as a tumour suppressor in Eμ-Myc-driven B-cell lymphoma in mice. (a) Representative image of in vivo NF-κB bioluminescence (radiance) of age-matched littermates of NF-κB-Luc and Eμ-Myc/NF-κB-Luc mice. Eight-week-old mice underwent in vivo imaging using the IVIS Spectrum system (Perkin Elmer, Beaconsfield, UK) after being intraperitoneally administration with 150 mg/kg VivoGlo d-luciferin (Promega, Southampton, UK) dissolved in sterile phosphate-buffered saline. Ten-min post-d-luciferin-administration, mice were imaged using a photon emission over 5 min, under isoflurane anaesthesia. Luminescence was seen in the thymic area and also in the tails and other exposed regions of the Eμ-Myc/NF-κB-Luc mice, the latter likely due to a higher number of circulating lymphocytes with increased NF-κB activity. (b) Quantification of NF-κB bioluminescence (radiance) of thymic regions in NF-κB-Luc (n=12) and Eμ-Myc/NF-κB-Luc (n=13) mice. Bioluminescence was quantified using the Living Image software version 4.3.1 (Perkins Elmer) and region of interest tool. Data shown as mean±s.e.m., **P<0.01, unpaired Student's t-tests. For all tests, where appropriate, analyses were undertaken to test for normal distribution. (c) Western blot analysis of the NF-κB subunits, c-REL, RELA, RELB, p100/p52 and p50 together with c-MYC in extracts prepared from Eμ-Myc and Eμ-Myc/c-rel–/– mouse tumorigenic spleens. Whole-cell extracts were prepared from Eμ-Myc or Eμ-Myc/c-rel–/– tumour cell suspensions. Cell pellets were washed with ice-cold phosphate-buffered saline and lysed using PhosphoSafe Extraction Reagent (Merck Millipore, Watford, UK). Antibodies used were c-Rel (sc-71 Santa Cruz, Insight Biotechnology, Wembley, UK), c-Myc (sc-42 Santa Cruz), RelA (sc-372 Santa Cruz), RelB (4954 Cell Signaling, Hitchin, UK), p50 (06-886 Merck Millipore), p100/p52 (sc-848 Santa Cruz) and β-Actin (A5441 Sigma-Aldrich, Gillingham, UK). (d) Quantitative-PCR analysis showing relative Rel expression in end-stage tumorigenic spleens from Eμ-Myc (n=20), Eμ-Myc/c-rel+/– (n=12) and Eμ-Myc/c-rel–/– (n=11) mice. Data shown as mean±s.e.m., each point is an individual mouse. (e) Western blot analysis of c-REL levels in tumorigenic spleens from Eμ-Myc, Eμ-Myc/c-rel+/– and Eμ-Myc/c-rel–/– mice. (f–j) Reduced survival of Eμ-Myc/c-rel+/– and Eμ-Myc/c-rel–/– mice. Kaplan–Meier plots showing survival curves for Eμ-Myc and (f) Eμ-Myc/c-rel–/– mice, (g) Eμ-Myc/c-rel+/– male mice, (h) Eμ-Myc/c-rel–/– male mice, (i) Eμ-Myc/c-rel–/– female mice and relative survival of male versus female Eμ-Myc mice is shown in (j). P-values (Mantel–Cox test) and hazard ratios are shown. (k) Kaplan–Meier plot showing reduced survival of TCL1/c-rel–/– mice. Animal handling, husbandry and experimentation were undertaken in compliance with UK Home Office regulations under project licences and approved by the local ethical review committee. All mice used in these experiments were on C57BL/6 background and bred at the Comparative Biology Centre, Newcastle University. c-rel–/– mice were provided by Dr Fiona Oakley (Newcastle University). NF-κB-luc (NF-κB-Luc–/+) reporter mice were a gift from Professor Matthew Wright (Newcastle) and originated in the laboratory of Professor Harald Carlsen (Norwegian University of Life Sciences). Eμ-Myc and TCL1-Tg mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Eμ-Myc/c-rel+/– offspring were generated by mating c-rel–/– female mice with Eμ-Myc male mice. Eμ-Myc/c-rel–/– mice were then generated by crossing Eμ-Myc/c-rel+/– males with c-rel–/– female mice. In TCL1-Tg mice, a human TCL1 coding sequence is expressed from a B29 minimal promoter, coupled with the IgH intronic enhancer resulting in B- and T-cell expression. TCL1/c-rel–/– offspring were generated as for Eμ-Myc by mating c-rel–/– female mice with TCL1-Tg male mice. All mice were designated to an experimental group-dependent on their strain and no blinding was undertaken during analysis. For survival analysis, mice were monitored daily and were killed at predetermined end points, defined as the animal becoming moribund, losing bodyweight/condition and/or having palpable tumour burden at any lymphoid organ site, at which point animals underwent necropsy. Kaplan–Meier survival curves were drawn using GraphPad Prism (Version 5.0, GraphPad Software, La Joll, CA, USA).
Loss of c-Rel results in earlier onset of Eμ-Myc-driven lymphoma
To investigate the role of c-Rel in MYC-induced lymphomagenesis, Eμ-Myc/c-rel–/– mice were generated. Western blot analysis confirmed no significant effects on the other NF-κB subunits or c-Myc in splenic tumour B cells, although slightly lower levels of the non-canonical NF-κB subunits p52 and RelB were found in c-rel–/– cells (Figure 1c). Eμ-Myc/c-rel+/– mice, despite having intermediate levels of c-Rel mRNA (Figure 1d), had almost no detectable c-Rel protein in Eμ-Myc lymphoma cells (Figure 1e).
Given the known tumour-promoting role of c-Rel in B-cell lymphoma, we were surprised to find that Eμ-Myc/c-rel–/– mice had a significantly shorter overall survival (median survival 79 days) than Eμ-Myc mice (median survival 115 days; Figure 1f). Earlier onset of disease was also seen in heterozygote Eμ-Myc/c-rel+/– male mice (median onset 75.5 days; Figure 1g). Although survival times of male and female Eμ-Myc/c-rel–/– mice were similar (77 vs 83 days, respectively; Figures 1h and i), this effect appeared more pronounced in male c-Rel mutant mice due to gender differences in wild-type Eμ-Myc mice (122 days in males vs 106 days in females), although this difference was not statistically significant (Figure 1j).
To determine if earlier onset of disease could be seen in other lymphoma models, we generated c-rel–/– strains of pEμ-B29-TCL1 (TCL1-Tg) transgenic mice.33 These mice exhibit slower disease progression than in the Eμ-Myc model and in our experiments many mice developed tumours at non-lymphoid sites (not shown). Nonetheless, c-rel–/– mice again displayed significantly reduced survival relative to wild-type TCL1 mice, confirming that this effect is not restricted to the Eμ-Myc model (Figure 1k).
Reimplanted Eμ-Myc tumours grow equally well in wild-type and c-rel–/– mice
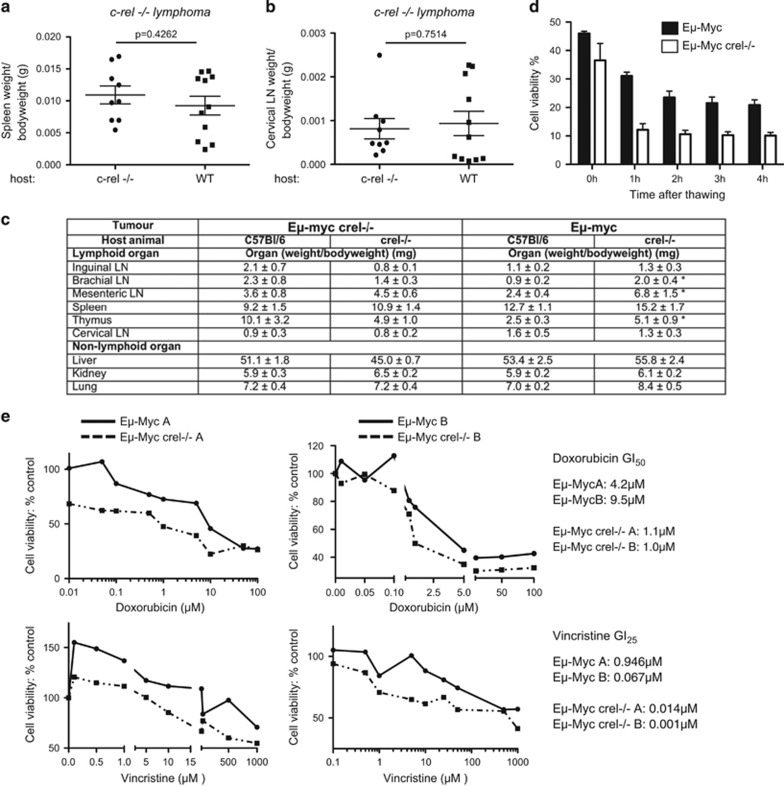
These results revealed an apparent tumour suppressor role for c-Rel, but it was unclear if this resulted from an effect intrinsic to the tumour cells or from other effects of the c-rel–/– mice. Therefore, to investigate whether non-tumour cells in the wild-type and c-rel–/– mice might contribute to earlier onset of disease in c-Rel null mice, we performed a series of reciprocal tumour reimplantation studies. Tumours derived from either wild-type or c-rel–/– male Eμ-Myc mice were transplanted into either C57Bl/6 or c-rel–/– male host mice. Importantly, whether the host mice were wild type or c-rel–/– did not affect the rate of c-rel–/– lymphoma growth (Figures 2a and c). A more mixed effect was seen with reimplanted wild-type Eμ-Myc cells, where increased lymphoma growth was seen at some sites but not others in the c-rel–/– host mice (Figure 2c). Reimplanted c-rel–/– lymphomas were also slower to develop than wild type (~4 weeks vs 2 weeks) but this may reflect the reduced viability of Eμ-Myc/c-rel–/– tumour cells after thawing frozen samples (Figure 2d). This analysis does not rule out a contribution from the non-tumour background in the development of Eμ-Myc lymphoma in these mice. However, given that we saw no effects of the host animal on the growth of reimplanted c-rel–/– cells, we investigated if there were intrinsic differences between wild-type and c-rel–/– lymphoma cells.
Figure 2.
Eμ-Myc/c-rel–/– tumours grow equally well in wild-type and c-rel–/– mice and are more sensitive to apoptotic stimuli. (a, b) Reimplanted Eμ-Myc/c-rel–/– tumours grow equally well in wild-type and c-rel–/– mice. Lymph node tumours derived from three different Eμ-Myc/c-rel–/– mice were reimplanted in parallel into either three wild-type (C57Bl/6) or three c-rel–/– host mice. Four weeks after implantation, the mice were killed and tumour sizes at different sites were assessed. Data shown here are from the spleen (a) and cervical lymph nodes (b). Data representing mean±s.e.m. and P-values were calculated using Student's unpaired t-tests. (c) Tumour burden in lymphoid organs (weight of organ/bodyweight of animal in gram) following reimplantation of either Eμ-Myc or Eμ-Myc c-rel–/– tumour cells into either C57Bl/6 or c-rel–/– mice. Data shown are the means of three independent tumours each implanted into three mice±s.e.m. *P<0.05 in an unpaired Student's t-test, but otherwise there were no significant differences between tumour burden in wild-type and c-rel knock-out animals at any of the sites assessed. (d) Cell viability of Eμ-Myc and Eμ-Myc/c-rel–/– tumour cells grown ex vivo. Cell viability was measured using the trypan blue exclusion assay over a 4-h period after freeze thawing. (e) Eμ-Myc/c-rel–/– tumour cells are more sensitive to apoptotic stimuli. Freshly isolated Eμ-Myc or Eμ-Myc/c-rel–/– lymph node tumour cells (5 × 105 per well) were seeded into 96-well plates. Increasing concentrations of the chemotherapeutic agents, doxorubicin (Sigma-Aldrich) or vincristine (Sigma-Aldrich) or solvent controls were added to three replicate wells. After 96 h, viability was quantified using the CellTiter96 AQueous One Solution Cell Proliferation Assay (MTS; Promega), according to the manufacturer's instructions. Single-cell suspensions were prepared from tumour-bearing organs of Eμ-Myc and Eμ-Myc/c-rel–/– mice upon necropsy. These were then used for downstream analyses or frozen in 90% fetal bovine serum/10% dimethyl sulfoxide for long-term storage and transplantation. For reciprocal microenvironment experiments, 2 × 106 Eμ-Myc/c-rel–/– lymph node tumour cells from male mice were transplanted intravenously via the lateral tail vein into 8-week-old male C57BL/6 or c-rel–/– recipients. Mice were necropsied when they became moribund and the tumour burden assessed. C57BL/6 mice used for reimplantation studies were purchased from Charles River (Margate, UK).
c-rel–/– B-cell lymphomas are more sensitive to apoptotic stimuli
c-Rel and the other NF-κB subunits can contribute towards tumorigenesis by inducing the expression of antiapoptotic genes34 and, consistent with this and the results in Figure 2d, we found that when cultured ex vivo, tumour cell isolates from Eμ-Myc/c-rel–/– mice showed increased sensitivity to the R-CHOP therapy components doxorubicin and vincristine (Figure 2e). Therefore, Eμ-Myc/c-rel−/− cells appear more prone to apoptosis when compared with their wild-type equivalents. These effects are consistent with the known antiapoptotic effects of c-Rel but did not explain the earlier onset of disease in c-Rel null mice.
The tumour suppressor Bach2 is a c-Rel target gene
The p53 and ARF pathways are frequently disrupted in Eμ-Myc lymphoma.35 However, we found that mRNA levels of p53 target genes, such as Mdm2 and Bax, as well as the CDKN2A gene that encodes the ARF protein were similar across end-stage Eμ-Myc and Eμ-Myc/c-rel–/– tumour cells (not shown), suggesting that c-Rel loss does not lead to further disruption of these pathways. Moreover, no significant differences in BCL2L1 mRNA, an NF-κB target gene that encodes the antiapoptotic protein Bcl-xL,34 were observed (not shown).
We therefore wanted to learn more about other changes in gene expression associated with the earlier onset of lymphoma in the Eμ-Myc/c-rel–/– mice. Consequently, we decided to perform microarray-based genome-wide mRNA expression analyses on B cells from 4-week-old Eμ-Myc, Eμ-Myc/c-rel+/– and Eμ-Myc/c-rel–/– mice.
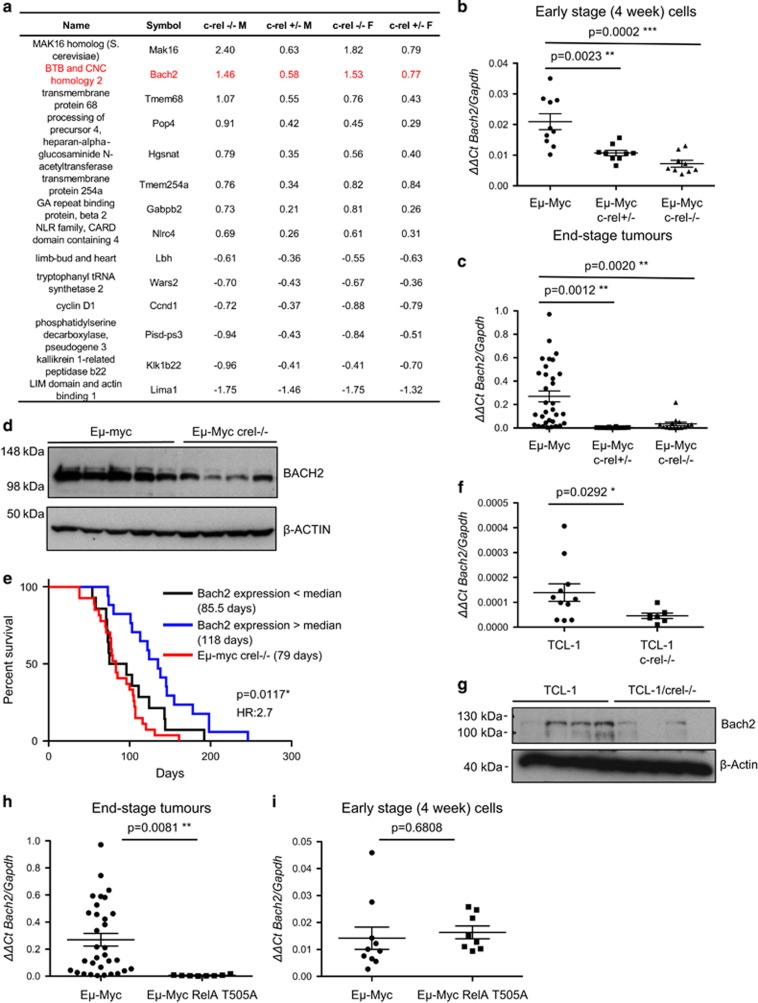
Analysis of these microarray data identified a number of genes misregulated in Eμ-Myc/c-rel–/– mice (Figure 3a). Of these, the loss of expression of Bach2 in c-Rel mutant mice was of particular interest. Bach2 is a lymphoid-specific transcription factor with a role in B-cell development36 and the response to oxidative stress.37, 38 Bach2 has also been identified as a tumour suppressor in acute lymphoblastic leukaemia.39 Importantly, quantitative PCR analysis confirmed that Bach2 mRNA expression is lost in B cells from 4-week-old Eμ-Myc/c-rel+/– and Eμ-Myc/c-rel–/– mice (Figure 3b), and also from the tumours taken from mice killed with end-stage disease (Figure 3c). Bach2 protein levels were also significantly reduced in the Eμ-Myc/c-rel−/− tumours (Figure 3d). Quantitative PCR also validated a number of other potential targets identified in the microarray, including Cyclin D1 and Lima1 (not shown). Although Bach2 levels were reduced in normal, untransformed B cells from c-rel–/– 4-week-old mice, this was not a statistically significant effect (not shown).
Figure 3.
Expression of the B-cell tumour suppressor Bach2 is dependent on c-Rel in Eμ-Myc lymphoma. (a) Table showing genes whose expression is regulated by c-Rel from microarray analysis of bone marrow-derived B cells from 4-week-old Eμ-Myc, Eμ-Myc/c-rel+/– and Eμ-Myc/c-rel–/– mice. Fold changes shown are compared with equivalent wild-type cells and are in log2 (a positive number indicates higher expression in wild-type cells). Bone marrow-derived B cells were purified from 4-week-old Eμ-Myc or Eμ-Myc/c-rel+/–, Eμ-Myc/c-rel+/– mice using CD19 microbeads (MACS Miltenyi Biotec, Surrey, UK). Total B-cell RNA, purified using the PeqGold total RNA extraction kit (Peqlab, VWR, Lutterworth, UK), was then used for microarray analysis at Cambridge Genomic Services (University of Cambridge, Cambridge, UK) using the Illumina mouse WG-6 Expression BeadChip system (San Diego, CA, USA). These data were background corrected in Illumina GenomeStudio and subsequent analysis proceeded using the lumi and limma packages in R (Bioconductor, Seattle, WA, USA).46, 47, 48 Variant stabilisation transform and robust spline normalisation were applied in lumi. Differential expression was detected using linear models and empirical Bayes statistics in limma. A list of genes for each comparison was generated using a Benjamini–Hochberg false discovery rate-corrected P-value of 0.05 as a cutoff. (b, c) Confirmation that Bach2 mRNA levels are c-Rel regulated. Quantitative-PCR (q-PCR) showing relative Bach2 expression in (b) bone marrow-derived B cells from Eμ-Myc (n=10), Eμ-Myc/c-rel+/– (n=9) and Eμ-Myc/c-rel–/– (n=9) mice and (c) end-stage tumorigenic spleens from Eμ-Myc (n=30), Eμ-Myc/c-rel+/– (n=12) and Eμ-Myc/c-rel–/– (n=11) mice. q-PCR was performed in triplicate on 20 ng cDNA (Reverse Transcriptase kit, Qiagen, Crawley, UK), using predesigned Bach2 Quanititect Primer assays (Qiagen). Samples were run and analysed on a Rotor-gene Q system (Qiagen), using murine Gapdh primers as an internal control. All cycle threshold values were normalised to Gapdh levels using the Pfaffl method.49 Data represent mean±s.e.m. **P<0.01, ***P<0.001 (unpaired Student's t-test). (d) Bach2 protein levels are reduced in Eμ-Myc/c-rel–/– mice. Whole-cell extracts were prepared from Eμ-Myc or Eμ-Myc/c-rel–/– tumourigenic spleens. Cell pellets were washed with ice-cold phosphate-buffered saline, and lysed using PhosphoSafe Extraction Reagent (Merck Millipore), according to the manufacturer's protocols. Western blot analysis was performed using antibodies to BACH2 (ab83364 Abcam, Cambridge, UK) or the loading control β-ACTIN (A5441 Sigma-Aldrich). (e) Low levels of Bach2 mRNA correlate with poor survival in wild-type Eμ-Myc mice. Kaplan–Meier analysis of the survival of mice with below and above the median levels of Bach2 mRNA (from data in c). Also shown for comparison is the survival data from Eμ-Myc/c-rel–/– mice shown in Figure 1f. (f) Bach2 mRNA levels are c-Rel regulated in TCL1-Tg mice. q-PCR showing relative Bach2 expression in end-stage tumorigenic spleens from TCL1-Tg (n=11) and TCL1-Tg/c-rel–/– (n=7) mice. Data represent mean±s.e.m. *P<0.05. (g) Bach2 protein levels are reduced in TCL1/c-rel–/– mice. Whole-cell extracts were prepared from TCL1-Tg or TCL1/c-rel–/– tumourigenic spleens and western blot analysis was performed as indcated. (h, i) Low Bach2 mRNA levels in RelA T505A mice. q-PCR showing relative Bach2 expression in (h) end-stage tumorigenic spleens from Eμ-Myc (n=30) and Eμ-Myc/relaT505A (n=8) mice and (i) bone marrow-derived B cells from Eμ-Myc (n=10) and Eμ-Myc/relaT505A (n=8) mice. Note, data from wild-type Eμ-Myc mice are the same as shown in c. Data represent mean±s.e.m. **P<0.01 (unpaired Student's t-test). RelA T505A knock-in mice were generated by Taconic Artemis (Cologne, Germany) using C57Bl/6 ES cells.
Although Bach2 mRNA levels are uniformly low in all Eμ-Myc/c-rel–/– and c-rel+/– lymphoma samples analysed, we observed a wide range of Bach2 mRNA expression in end-stage wild-type Eμ-Myc tumours (Figure 3c). We were therefore interested in whether this would correlate with survival of these wild-type Eμ-Myc mice. Significantly, we found that Eμ-Myc mice with below-the-median level of Bach2 mRNA displayed decreased survival, with a median survival of 85.5 versus 135 days for mice with high Bach2 levels (Figure 3e). Therefore, wild-type mice with reduced levels of Bach2 have a very similar pattern of lymphoma onset to that seen in the c-rel–/– mice, providing a potential mechanism that allows this NF-κB subunit to function as a tumour suppressor in this model of c-Myc-driven B-cell lymphoma (Figure 3e).
To determine the generality of these effects we also analysed Bach2 levels in the spleens of TCL1-Tg mice, where we observed a reduction in mRNA and protein levels (Figures 3f and g). Furthermore, in a separate NF-κB knock in mouse model, where the RelA subunit was engineered to contain a Thr505Ala mutation in its transactivation domain, a site previously shown to affect NF-κB function,40 loss of Bach2 expression was also seen in end-stage lymphoma cells (Figure 3h) but not in 4-week B cells from Eμ-Myc mice (Figure 3i). The RelA T505A mouse will be described in more detail elsewhere.
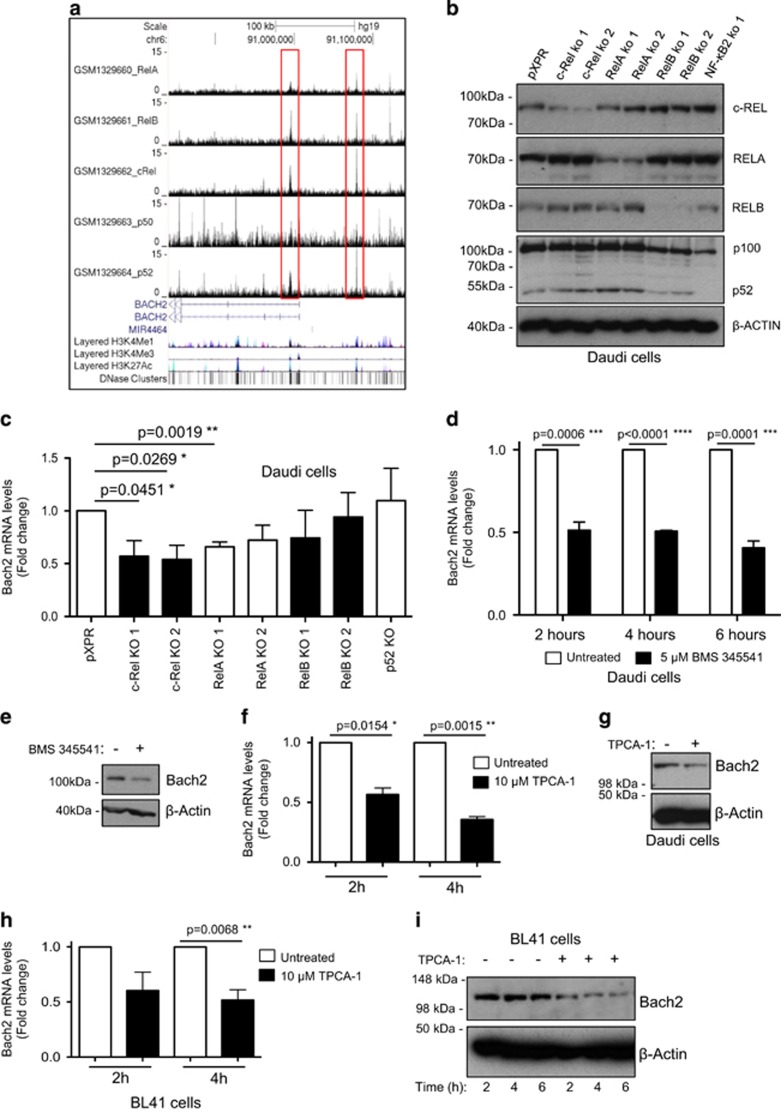
Although these data indicated that Bach2 expression is regulated by c-Rel, Bach2 has not been previously described as a direct NF-κB target gene. To address this, we analysed chromatin immunoprecipitation sequencing (ChIP-Seq) data from the Epstein–Barr-virus-transformed human lymphoblastoid B-cell line GM12878.41 This revealed that the Bach2 promoter is bound by c-Rel together with the other NF-κB subunits, RelA, RelB and p52 (Figure 4a). Moreover, further analysis of ChIP-Seq data obtained for the RelA NF-κB subunit by the Encode consortium confirmed that Bach2 is an NF-κB target gene in multiple B-cell lines (not shown). Consistent with these data, analysis of the human Burkitt lymphoma cell line Daudi, where NF-κB subunits had been depleted by CRISPR/Cas9 mutagenesis, revealed that loss of either c-Rel or RelA reduced Bach2 mRNA levels (Figures 4b and c). However, no effect on Bach2 protein level was seen (not shown) suggesting functional compensation between c-Rel and RelA in these cells, as has been reported previously for these subunits.42 Treatment of Daudi cells with the IκB kinase-β inhibitors BMS 345541 or TPCA-1, which inhibit the classical NF-κB pathway and so target both RelA and c-Rel, did result in loss of both Bach2 mRNA and protein (Figures 4d and g), and similar results were seen in the Burkitt cell line BL41 treated with TPCA-1 (Figures 4h and i).
Figure 4.
Bach2 is an NF-κB target gene on human B-cell malignancies. (a) ChIP-Seq data showing NF-κB subunit binding in the region of the human BACH2 gene in the Epstein–Barr-virus-transformed lymphoblastoid B-cell line (LCL) GM12878. ChIP-Seq data were extracted from a previously published analysis of the Epstein–Barr-virus-transformed LCL GM12878 using validated anti-RelA, RelB, c-Rel, p52 and p50 antibodies.41 Reads from all ChIP-Seq experiments were mapped to the hg19/GRCh37 build of the human genome using the UCSC genome browser. (b, c) c-Rel and RelA regulate Bach2 mRNA levels in Daudi cells. In b western blot analysis shows depletion of NF-κB subunits in the Daudi Burkitt's lymphoma cell line using CRISPR/Cas9 mutagenesis. In c q-PCR shows relative Bach2 expression in the Daudi cells with mutated NF-κB subunits. Data are obtained from separately derived pools of Daudi Cas9+ cells that express either a control single-guide RNA (sgRNA) against GFP (pXPR) or an sgRNA against the indicated NF-κB subunit. RNA or protein was extracted for either q-PCR (b) or western blot (c) analysis, as indicated. Daudi Cas9/CRISPR analysis: Daudi cells with stable Cas9 expression were derived as previously described.50 Briefly, Daudi cells with stable Streptococcus pyogenes Cas9 expression were established by infection with lentiviral transduction and blasticidin selection, using pLentiCas9-Blast (Addgene plasmid #52962). Cas9 activity was validated by transduction of the Daudi Cas9+ cells with a test lentivirus, which encodes a GFP and a sgRNA that targets GFP.51 The PXPR-011 plasmid (provided by John Doench, Broad Institute, Cambridge, MA, USA) was used to construct this test virus. Cas9 activity was evident in >85% of the selected Daudi cells by flow cytometry analysis (the residual 15% of cells that continue to express GFP may be cells where the non-homologous end-joining pathway correctly repaired the Cas9-induced DNA double-strand break).51 To obtain NF-κB subunit knockdown by CRISPR/Cas9 genome editing, the following sgRNAs were designed using CRISPRdirect (http://crispr.dbcls.jp/):52 RelA 5′-AGTCCTTTCCTACAAGCTCG-3′ and 5′-AGCTGATGTGCACCGACAAG-3′ RelB 5′-GGTCTGGCGACGCGGCGACT-3′ and 5′-AGCGGCCCTCGCACTCGTAG-3′ c-Rel 5′-AAATGTGAAGGGCGATCAGC-3′ and 5′-ATTGGGTTCGAGACAACAGG-3′ p52 5′-TAGGCTGTTCCACGATCACC-3′. Oligonucleotides were synthesized by Life Technologies (Paisley, UK), were individually cloned into the lentiGuide-Puro vector (Addgene plasmid #52963), according to the protocol from the Zhang laboratory (http://genome-engineering.org/),53 and were sequence verified. VSV-G pseudotyped lentiviruses encoding a sgRNA were produced in 293 T cells and used to transduce Daudi Cas9+ cells. Cells transduced with sgRNA-encoding lentivirus were selected by puromycin. (d–g) Treatment of the Daudi Burkitt's lymphoma cell line with the IκB kinase inhibitors BMS 345541 and TPCA-1 reduces BACH2 mRNA and protein levels. Daudi cells were treated with either 5 μM BMS 345541 (Calbiochem, San Diego, CA, USA) or 10 μM TPCA-1 (Calbiochem) for the times shown. RNA or protein was extracted for either q-PCR (d, f) or western blot (e, g) analysis using the Bach2 antibody, ABN171 (Merck Millipore). (h, i) Treatment of the BL41 Burkitt's lymphoma cell lines with the IκB kinase inhibitor TPCA-1 reduces BACH2 mRNA and protein levels. BL41 cells were treated with 10μM TPCA-1 for the times shown. RNA or protein was extracted for either q-PCR (h) or western blot (i) analysis. q-PCR data represent the mean of three independent experiments±s.e.m., *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 (unpaired Student's t-test). Daudi and BL41 cells were obtained from the American Type Culture Collection (Teddington, UK) and grown in RPMI-1640 medium (Lonza, Basel, Switzerland; supplemented with 10% (v/v) fetal bovine serum (Invitrogen, Paisley, UK) and 2 mM l-glutamine (Lonza)). Cell lines were sent to LGC Standards for authentication by short tandem repeat profiling.
The role of c-Rel in B-cell lymphoma
Given the large number of studies indicating tumour-promoting roles for c-Rel in lymphoma,2, 3, 4, 5, 6, 21, 22, 23, 24, 25, 26, 43 our results showing earlier onset of disease in c-Rel mutant mice were surprising. However, a number of in vitro studies have, in addition to their known tumour-promoting activities, revealed tumour suppressor functions for NF-κB subunits.1 Moreover, previous reports using mouse models of c-Myc-driven lymphoma have demonstrated that through induction of therapy-induced senescence, NF-κB can function as a tumour suppressor in this context.27, 28 Importantly, previous studies of the role of c-Rel in lymphoma have used either patient cells or established laboratory cell lines. In both cases, by analysing 'end-stage' cancer cells, these investigations will have focused on the antiapoptotic effects of NF-κB, which we also see, but will have missed any more complex roles that might occur during the process of lymphomagenesis itself. Our study has therefore allowed the description of a previously unknown role for c-Rel in the prevention of B-cell lymphoma development by regulating the expression of Bach2. However, NF-κB regulation of Bach2 is not restricted to c-Rel and our data also support a role for RelA. Interestingly, in Eμ-Myc mice RelA regulation of Bach2 was only seen in the 'end-stage' lymphomas (Figures 3h and i), suggesting that c-Rel is the primary driver of Bach2 expression. Nonetheless, this demonstrates the complex interplay between NF-κB subunits, as well as the potential for stage-specific regulation of gene expression during lymphomagenesis. It will be of interest to see if c-Rel can also contribute to the regulation of NF-κB-dependent senescence reported in Eμ-Myc lymphoma cells.27, 28
Bach2 is a transcription factor and known as B-cell tumour suppressor. Interestingly, a recent report illustrated that Bach2 is required for c-Myc-dependent induction of p53 in pre-B cells.39 Moreover, loss of Bach2 is associated with the development of pre-B acute lymphoblastic leukaemia.39 Bach2 promoter activity is also reduced upon BCR-ABL expression in chronic myeloid leukaemia, through regulation by the transcription factor, Pax5, suggesting that suppression of Bach2 may contribute to lymphoid blast crisis in chronic myeloid leukaemia.44 Although we cannot rule out contributions from the other c-Rel-regulated genes we identified, we propose that induction of Bach2 expression by c-Rel/NF-κB provides one mechanism that allows these factors to function as tumour suppressors in the early stages of B-cell lymphoma development. However, some reports have suggested that Bach2 may also contribute towards malignancy in some contexts.45 Since the tumour suppressor functions of Bach2 are associated with p53, it is possible that p53 loss or mutation is also the trigger for a change in Bach2 function. Therefore, the consequences of NF-κB regulation of Bach2 expression may vary depending on the stage of lymphoma development.
Accession numbers
NF-κB ChIP-Seq data sets have been published41 (gene expression omnibus, accession code GSE55105).
Microarray data have been submitted to ArrayExpress, accession code E-MTAB-2774.
Acknowledgments
We thank Fiona Oakley, Sonia Rocha, Derek Mann, Claire Richardson, Saimir Luli, Elaine Willmore and all members of the NDP laboratory for their helpful advice and assistance. We are very grateful to Michael J. Walsh for assistance with generation of CRISPR/Cas9 knock-out cell lines. JEH is funded by Leukemia Lymphoma Research grant 11022, JAB and HS are funded by the Wellcome Trust grant 094409, further funding from the NDP lab was obtained from Cancer Research UK grant C1443/A12750. The IVIS Spectrum was funded by Welcome Trust Equipment grant 087961. BZ and BEG are funded by US National Institutes of Health (grants K08 CA140780 and RO1 CA12850) and by a Burroughs Wellcome Medical Scientist career award.
Author contributions
JEH performed the majority of the experimental work and contributed to concept and design of experiments and manuscript writing. JAB and HS assisted with procedures involving Eμ-Myc mice. BZ performed ChIP-Seq analysis. KJC provided advice on working with Eμ-Myc mice and assisted with data analysis. HDT provided training and assisted with lymphoma reimplantation studies. CB provided advice on B-cell lymphoma and contributed to experimental concepts and design. SJC performed bioinformatics analysis of microarray data. BEG performed analysis of ChIP-Seq data and contributed to experimental concepts and design. NDP contributed to concept and design of experiments and manuscript writing.
The authors declare no conflict of interest.
References
- Perkins ND. The diverse and complex roles of NF-κB subunits in cancer. Nat Rev Cancer 2012; 12: 121–132. [DOI] [PubMed] [Google Scholar]
- Staudt LM. Oncogenic activation of NF-κB. Cold Spring Harb Perspect Biol 2010; 2: a000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RE, Brown KD, Siebenlist U, Staudt LM. Constitutive nuclear factor κB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med 2001; 194: 1861–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerhake F, Kutok JL, Monti S, Chen W, LaCasce AS, Cattoretti G et al. NFκB activity, function, and target-gene signatures in primary mediastinal large B-cell lymphoma and diffuse large B-cell lymphoma subtypes. Blood 2005; 106: 1392–1399. [DOI] [PubMed] [Google Scholar]
- Rosenwald A, Wright G, Leroy K, Yu X, Gaulard P, Gascoyne RD et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med 2003; 198: 851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppers R. The biology of Hodgkin's lymphoma. Nat Rev Cancer 2009; 9: 15–27. [DOI] [PubMed] [Google Scholar]
- Perkins ND. Integrating cell-signalling pathways with NF-κB and IKK function. Nat Rev Mol Cell Biol 2007; 8: 49–62. [DOI] [PubMed] [Google Scholar]
- Gilmore TD. Multiple mutations contribute to the oncogenicity of the retroviral oncoprotein v-Rel. Oncogene 1999; 18: 6925–6937. [DOI] [PubMed] [Google Scholar]
- Fullard N, Wilson CL, Oakley F. Roles of c-Rel signalling in inflammation and disease. Int J Biochem Cell Biol 2012; 44: 851–860. [DOI] [PubMed] [Google Scholar]
- Courtois G, Gilmore TD. Mutations in the NF-κB signaling pathway: implications for human disease. Oncogene 2006; 25: 6831–6843. [DOI] [PubMed] [Google Scholar]
- Grumont RJ, Gerondakis S. The subunit composition of NF-κB complexes changes during B-cell development. Cell Growth Differ 1994; 5: 1321–1331. [PubMed] [Google Scholar]
- Liou HC, Sha WC, Scott ML, Baltimore D. Sequential induction of NF-κB/Rel family proteins during B-cell terminal differentiation. Mol Cell Biol 1994; 14: 5349–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weih F, Carrasco D, Bravo R. Constitutive and inducible Rel/NF-κB activities in mouse thymus and spleen. Oncogene 1994; 9: 3289–3297. [PubMed] [Google Scholar]
- Kontgen F, Grumont RJ, Strasser A, Metcalf D, Li R, Tarlinton D et al. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev 1995; 9: 1965–1977. [DOI] [PubMed] [Google Scholar]
- Tumang JR, Hsia CY, Tian W, Bromberg JF, Liou HC. IL-6 rescues the hyporesponsiveness of c-Rel deficient B cells independent of Bcl-xL, Mcl-1, and Bcl-2. Cell Immunol 2002; 217: 47–57. [DOI] [PubMed] [Google Scholar]
- Tumang JR, Owyang A, Andjelic S, Jin Z, Hardy RR, Liou ML et al. c-Rel is essential for B lymphocyte survival and cell cycle progression. Eur J Immunol 1998; 28: 4299–4312. [DOI] [PubMed] [Google Scholar]
- Cariappa A, Liou HC, Horwitz BH, Pillai S. Nuclear Factor κB is required for the development of marginal zone B lymphocytes. J Exp Med 2000; 192: 1175–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starczynowski DT, Reynolds JG, Gilmore TD. Deletion of either C-terminal transactivation subdomain enhances the in vitro transforming activity of human transcription factor REL in chicken spleen cells. Oncogene 2003; 22: 6928–6936. [DOI] [PubMed] [Google Scholar]
- Chin M, Herscovitch M, Zhang N, Waxman DJ, Gilmore TD. Overexpression of an activated REL mutant enhances the transformed state of the human B-lymphoma BJAB cell line and alters its gene expression profile. Oncogene 2009; 28: 2100–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Rayet B, Gelinas C. Divergent C-terminal transactivation domains of Rel/NF-κB proteins are critical determinants of their oncogenic potential in lymphocytes. Oncogene 2004; 23: 1030–1042. [DOI] [PubMed] [Google Scholar]
- Barth TF, Martin-Subero JI, Joos S, Menz CK, Hasel C, Mechtersheimer G et al. Gains of 2p involving the REL locus correlate with nuclear c-Rel protein accumulation in neoplastic cells of classical Hodgkin lymphoma. Blood 2003; 101: 3681–3686. [DOI] [PubMed] [Google Scholar]
- Joos S, Menz CK, Wrobel G, Siebert R, Gesk S, Ohl S et al. Classical Hodgkin lymphoma is characterized by recurrent copy number gains of the short arm of chromosome 2. Blood 2002; 99: 1381–1387. [DOI] [PubMed] [Google Scholar]
- Martin-Subero JI, Gesk S, Harder L, Sonoki T, Tucker PW, Schlegelberger B et al. Recurrent involvement of the REL and BCL11A loci in classical Hodgkin lymphoma. Blood 2002; 99: 1474–1477. [DOI] [PubMed] [Google Scholar]
- Lenz G, Davis RE, Ngo VN, Lam L, George TC, Wright GW et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science 2008; 319: 1676–1679. [DOI] [PubMed] [Google Scholar]
- Enciso-Mora V, Broderick P, Ma Y, Jarrett RF, Hjalgrim H, Hemminki K et al. A genome-wide association study of Hodgkin's lymphoma identifies new susceptibility loci at 2p16.1 (REL), 8q24.21 and 10p14 (GATA3). Nat Genet 2010; 42: 1126–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry CV, Ewton AA, Olsen RJ, Logan BR, Preti HA, Liu YC et al. Prognostic impact of C-REL expression in diffuse large B-cell lymphoma. J Hematop 2009; 2: 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien Y, Scuoppo C, Wang X, Fang X, Balgley B, Bolden JE et al. Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes Dev 2011; 25: 2125–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing H, Kase J, Dorr JR, Milanovic M, Lenze D, Grau M et al. Opposing roles of NF-κB in anti-cancer treatment outcome unveiled by cross-species investigations. Genes Dev 2011; 25: 2137–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller U, Huber J, Nilsson JA, Fallahi M, Hall MA, Peschel C et al. Myc suppression of Nfkb2 accelerates lymphomagenesis. BMC cancer 2010; 10: 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller U, Nilsson JA, Maclean KH, Old JB, Cleveland JL. Nfkb 1 is dispensable for Myc-induced lymphomagenesis. Oncogene 2005; 24: 6231–6240. [DOI] [PubMed] [Google Scholar]
- Carlsen H, Moskaug JO, Fromm SH, Blomhoff R. In vivo imaging of NF-κB activity. J Immunol 2002; 168: 1441–1446. [DOI] [PubMed] [Google Scholar]
- Harris AW, Pinkert CA, Crawford M, Langdon WY, Brinster RL, Adams JM. The E mu-myc transgenic mouse. A model for high-incidence spontaneous lymphoma and leukemia of early B cells. J Exp Med 1988; 167: 353–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer KK, French SW, Turner DE, Nguyen MT, Renard M, Malone CS et al. Dysregulated TCL1 promotes multiple classes of mature B cell lymphoma. Proc Natl Acad Sci USA 2002; 99: 14392–14397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Dutta J, Gupta N, Fan G, Gelinas C. Regulation of programmed cell death by NF-κB and its role in tumorigenesis and therapy. Adv Exp Med Biol 2008; 615: 223–250. [DOI] [PubMed] [Google Scholar]
- Eischen CM, Weber JD, Roussel MF, Sherr CJ, Cleveland JL. Disruption of the ARF-Mdm2-p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev 1999; 13: 2658–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S, Ito E, Toki T, Maekawa T, Kanezaki R, Umenai T et al. Cloning and expression of human B cell-specific transcription factor BACH2 mapped to chromosome 6q15. Oncogene 2000; 19: 3739–3749. [DOI] [PubMed] [Google Scholar]
- Muto A, Tashiro S, Tsuchiya H, Kume A, Kanno M, Ito E et al. Activation of Maf/AP-1 repressor Bach2 by oxidative stress promotes apoptosis and its interaction with promyelocytic leukemia nuclear bodies. J Biol Chem 2002; 277: 20724–20733. [DOI] [PubMed] [Google Scholar]
- Hoshino H, Kobayashi A, Yoshida M, Kudo N, Oyake T, Motohashi H et al. Oxidative stress abolishes leptomycin B-sensitive nuclear export of transcription repressor Bach2 that counteracts activation of Maf recognition element. J Biol Chem 2000; 275: 15370–15376. [DOI] [PubMed] [Google Scholar]
- Swaminathan S, Huang C, Geng H, Chen Z, Harvey R, Kang H et al. BACH2 mediates negative selection and p53-dependent tumor suppression at the pre-B cell receptor checkpoint. Nat Med 2013; 19: 1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Msaki A, Sanchez AM, Koh LF, Barre B, Rocha S, Perkins ND et al. The role of RelA (p65) threonine 505 phosphorylation in the regulation of cell growth, survival, and migration. Mol Biol Cell 2011; 22: 3032–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Barrera LA, Ersing I, Willox B, Schmidt SC, Greenfeld H et al. The NF-κB genomic landscape in lymphoblastoid B cells. Cell Rep 2014; 8: 1595–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann M, Metcalf D, Merryfull J, Beg A, Baltimore D, Gerondakis S. The combined absence of the transcription factors Rel and RelA leads to multiple hemopoietic cell defects. Proc Natl Acad Sci USA 1999; 96: 11848–11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham LV, Tamayo AT, Yoshimura LC, Lin-Lee YC, Ford RJ. Constitutive NF-κB and NFAT activation in aggressive B-cell lymphomas synergistically activates the CD154 gene and maintains lymphoma cell survival. Blood 2005; 106: 3940–3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casolari DA, Makri M, Yoshida C, Muto A, Igarashi K, Melo JV. Transcriptional suppression of BACH2 by the Bcr-Abl oncoprotein is mediated by PAX5. Leukemia 2013; 27: 409–415. [DOI] [PubMed] [Google Scholar]
- Ichikawa S, Fukuhara N, Katsushima H, Takahashi T, Yamamoto J, Yokoyama H et al. Association between BACH2 expression and clinical prognosis in diffuse large B-cell lymphoma. Cancer Sci 2014; 105: 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du P, Kibbe WA, Lin SM. lumi: a pipeline for processing Illumina microarray. Bioinformatics 2008; 24: 1547–1548. [DOI] [PubMed] [Google Scholar]
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 2004; 5: R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth GK. limma: linear models for microarray data. In: Gentleman RC, Carey VJ, Dudoit S, Irizarry R, Huber W (eds). Bioinformatics and Computational Biology Solutions Using R and Bioconductor. Springer:, New York, 2005, pp 397–420. [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfeld H, Takasaki K, Walsh MJ, Ersing I, Bernhardt K, Ma Y et al. TRAF1 coordinates polyubiquitin signaling to enhance Epstein-Barr virus LMP1-mediated growth and survival pathway activation. PLoS Pathog 2015; 11: e1004890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doench JG, Hartenian E, Graham DB, Tothova Z, Hegde M, Smith I et al. Rational design of highly active sgRNAs for CRISPR-Cas9-mediated gene inactivation. Nat Biotechnol 2014; 32: 1262–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y, Hino K, Bono H, Ui-Tei K. CRISPRdirect: software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics 2015; 31: 1120–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods 2014; 11: 783–784. [DOI] [PMC free article] [PubMed] [Google Scholar]