Abstract
The relationship of the stress response to the pathogenesis of alopecia areata (AA) was investigated by subjecting normal and skin graft-induced, AA-affected C3H/HeJ mice to light ether anesthesia or restraint stress. Plasma corticosterone (CORT), adrenocorticotropic hormone (ACTH), and estradiol (E2) levels were determined by RIA, whereas gene expression in brains, lymphoid organs, and skin was measured by quantitative RT-PCR for corticotropin-releasing hormone (Crh), arginine vasopressin (Avp), proopiomelanocortin (Pomc), glucocorticoid receptor (Nr3c1), mineralo corticoid receptor (Nr3c2), corticotropin-releasing hormone receptor types 1 and 2 (Crhr1, Crhr2), interleukin-12 (Il12), tumor necrosis factor-α (Tnfα), and estrogen receptors type-1 (Esr1) and type-2 (Esr2). AA mice had a marked increase in hypothalamic–pituitary–adrenal (HPA) tone and activity centrally, and peripherally in the skin and lymph nodes. There was also altered interaction between the adrenal and gonadal axes compared with that in normal mice. Stress further exacerbated changes in AA mouse HPA activity both centrally and peripherally. AA mice had significantly blunted CORT and ACTH responses to acute ether stress (physiological stressor) and a deficit in habituation to repeated restraint stress (psychological stressor). The positive correlation of HPA hormone levels with skin Th1 cytokines suggests that altered HPA activity may occur as a consequence of the immune response associated with AA.
INTRODUCTION
Alopecia areata (AA) is a non-scarring inflammatory hair loss disease with a lifetime risk of 1.7% (Safavi et al., 1995). It is typically characterized as a rapidly developing, patchy hair loss. In severe cases, the hair loss can affect the entire scalp (alopecia totalis) and body (alopecia universalis). AA is thought to involve an autoimmune mechanism, although the target autoantigen has not yet been identified (McElwee et al., 1999; Hordinsky and Ericson, 2004). Although AA has been associated with the presence of certain gene alleles, it is generally accepted that environmental factors can also contribute to disease development and severity (McElwee et al., 2001).
Anecdotally, stressful life events have been suggested as a trigger for onset of AA (Barber, 1921; Brauner and Goodheart, 1988). However, the results of controlled clinical studies on the association of stress with AA have been inconclusive (Greenberg, 1955; Perini et al., 1984; Gupta et al., 1997). Some studies did not find a significant correlation of hair loss onset with stressful life events (van der Steen et al., 1992; Brajac et al., 2003; Picardi et al., 2003; Gulec et al., 2004) whereas others report finding stressful life events occurring to AA patients before the onset of disease (Muller and Winkelmann, 1963; Chrousos, 1995; Garcia-Hernandez et al., 1999; Gulec et al., 2004; Kakourou et al., 2007).
In contrast, several studies have shown that individuals with AA are more likely to exhibit aberrant psychosocial traits, such as increased anxiety, depression, and aggression (Gupta et al., 1997; Liakopoulou et al., 1997; Brajac et al., 2003; Picardi et al., 2003). Psychosocial issues in AA patients are commonly assumed to be an emotional response to the sudden loss of hair and its negative image perception in our highly image-orientated society. However, studies with stress models show that activation of the immune system can modulate the hypothalamic–pituitary–adrenal (HPA) axis and vice versa (Jessop et al., 1987; Chrousos, 1995; Shanks et al., 1998; Elenkov and Chrousos, 1999, 2002; Harbuz et al., 2006). As such, aberrant stress responses in AA patients might be due to a biochemical link between the chronic inflammatory action of AA and the HPA axis. It has even been suggested that treatment of psychosomatic problems in patients might have a positive impact on AA lesions (Garcia-Hernandez et al., 1999).
The term “stress” describes a state of threatened homeostasis. Stressors activate neurobiological circuits in the brain, which in turn initiate the release of neurotransmitters and hormones (Chrousos, 1998; Dhabhar, 2002, 2003). The main components of the stress system are the locus coeruleus, sympathetic-adrenal medullary system or autonomic nervous system, and the HPA axis (Zhang et al., 2005). Stressors act through central neural pathways to the paraventricular nuclei of the hypothalamus, where corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) are synthesized. AVP is a weak secretagogoue on its own, but acts to potentiate the effects of CRH, particularly under conditions of prolonged or chronic stress. CRH is released into the portal circulation and regulates the expression of pro-opiomelanocortin (POMC), stimulating the release of POMC-derived peptides (adrenocorticotropin (ACTH) and β-endorphin) from the anterior pituitary (Chrousos, 1998).
ACTH travels through the systemic circulation and acts at the adrenal cortex to stimulate the synthesis and release of glucocorticoid hormones which in turn inhibit the HPA axis at the pituitary, hypothalamus and higher brain areas (Chrousos, 1998). Evidence suggests that hypothalamic peptidergic drive and the efficacy of corticosterone (CORT)-mediated feedback largely determine the characteristics of basal and stress-related HPA activity (Park et al., 2005). Glucocorticoids also have a profound effect on body metabolism and act to reestablish homeostasis among body systems including the immune system (Chrousos, 1998).
Nearly every component of the HPA axis has been identified in the skin and hair follicles suggesting a strong potential for interaction between the skin and the central HPA axis (Zouboulis, 2000; Ito et al., 2005; Slominski, 2005; Arck et al., 2006). Neurogenic and neuroendocrine factors have been proposed to be involved in the pathogenesis and recurrence of AA. Neuropeptides such as substance P (SP) and SP-degrading enzymes have been found at an elevated level in AA lesions (Rossi et al., 1997; Siebenhaar et al., 2007). A recent study of stress hormones in AA lesions identified increased ACTH and α-melanocyte-stimulating hormone, suggesting the presence of an active neurogenic system and local HPA axis activity in AA lesions (Kim et al., 2006).
In investigating the relationship between stress and AA, two issues seem most prominent: (1) how might stress impact the development of AA, and (2) how might the presence of AA modify the HPA axis and subsequent stress responses? We examined the latter in this study using the skin graft-induced C3H/HeJ mouse model for AA (Carroll et al., 2002; McElwee et al., 2002, 2005; Zoller et al., 2002). The interactions within and between the HPA axis, stress responses, and the immune system are well explored in other mouse models (Kariagina et al., 2004; Sheridan et al., 2004). In this study, we investigated the functional status of both the central and peripheral HPA axis under basal (unstressed) conditions as well as following stress, in AA-affected mice in comparison to non-AA-affected “normal” mice. We hypothesized that the presence of AA would significantly modify the HPA axis and stress responses. The goal was to understand the nature of stress and the stress response, and the relationships between central, systemic, and peripheral HPA activities in the C3H/HeJ mouse induced AA model.
RESULTS
CORT and ACTH expression
Analysis of CORT levels following an acute physiological stressor (ether inhalation) in experiment 1 revealed a significant main effect of treatment (F(1,20)=15.30, P<0.001) and a significant group×treatment interaction (F(1,20)=6.69, P<0.05). Post hoc tests indicated that normal and AA mice did not differ significantly under basal conditions. At 30 minutes post-stress, however, normal mice exhibited a marked CORT elevation compared with the basal condition (P<0.01), whereas AA-affected mice showed no significant change in CORT levels (Table 1). This suggests that AA mice have a blunted response to an acute physiological stressor. Similar to CORT, analysis of ACTH levels revealed a main effect of treatment (F(1,19)=8.23, P<0.01)). Normal and AA mice did not differ under basal conditions. However, normal mice showed a marked ACTH elevation at 30 minutes post-stress (P<0.05), whereas AA mice showed no significant change in ACTH levels following stress (Table 1).
Table 1.
Plasma CORT and ACTH levels in AA and normal mice, experiment 1
| Normal basal1 | Normal stressed1 | AA basal1 | AA stressed1 | |
|---|---|---|---|---|
| CORT (ng ml−1) | 32.6575±8.70 | 188.99±37.80 | 86.65±18.12 | 118.51±21.93 |
| ACTH (pg ml−1) | 98.05±6.69 | 150.21±13.30 | 129.03±9.45 | 152.21±7.93 |
AA, alopecia areata; ACTH, adrenocorticotropic hormone; CORT, corticosterone.
Mean value for each group±SEM using three analyses per mouse.
Similar to experiment 1, exposure to a psychological stressor (restraint) in experiment 2 unmasked significant differences between normal and AA mice. Analysis revealed significant main effects of both group (F(1,28)=4.54, P<0.05) and treatment (F(2,28)=55.11, P<0.001). CORT levels were similar in normal and AA mice under basal conditions, and both normal and AA mice showed significant CORT increases in response to both acute and repeated stress (P<0.001). Interestingly, however, although normal and AA mice showed similar CORT elevations in response to acute restraint stress, AA mice showed greater CORT elevations than normal mice following five exposures to repeated restraint stress (P<0.05, Figure 1a), suggesting a deficit in habituation to psychological stress in AA mice.
Figure 1. Plasma levels of ACTH and CORT.

Plasma ACTH and CORT levels were measured by RIA. Unfilled bars represent data from normal mice (n = 18) and black bars represent data from AA mice (n = 16). *P<0.05 and ***P<0.001.
Analysis of plasma ACTH levels revealed only a main effect of treatment (F(2,28)=11.56, P<0.001). Overall, plasma ACTH levels were elevated following both acute and repeated restraint stress compared with basal levels (P<0.001), but there was no significant difference between normal and AA mice under any condition (Figure 1).
Plasma estradiol (E2) concentration and correlation between plasma CORT and E2
Normal and AA mice had similar basal E2 levels and showed similar E2 responses to repeated restraint stress (Figure 2). Although it appeared that normal mice also showed a greater E2 response to acute stress than AA mice, differences between the groups failed to reach statistical significance. Notably, however, we found a positive correlation between plasma CORT and E2 levels in normal mice (r=0.6238, P<0.05), but not in AA mice (r=0.0496, P=0.87) (Figure 3).
Figure 2. Plasma levels of E2.
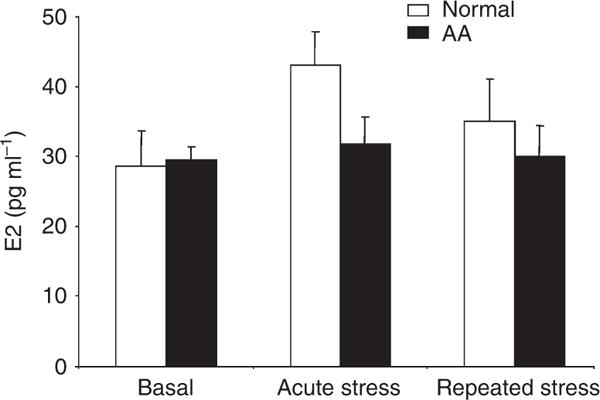
Plasma E2 levels were measured by RIA. Unfilled bars represent data from normal mice (n = 18) and black bars represent data from AA mice (n = 16).
Figure 3. Correlation between plasma levels of CORT and E2.
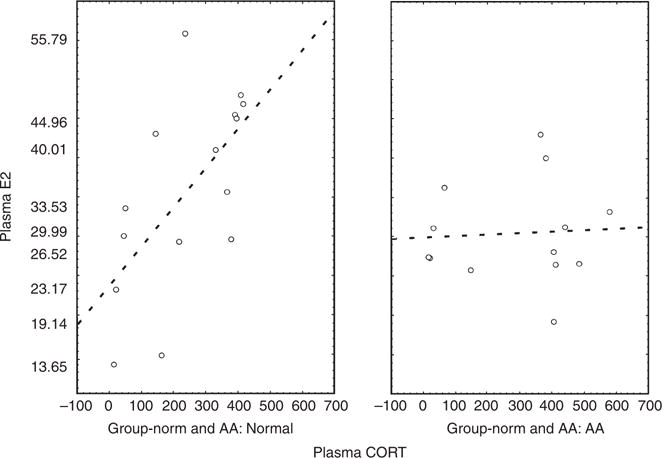
Correlation between plasma levels of CORT and E2 in normal mice (left, n = 18) (r = 0.6238, P<0.05) and AA mice (right, n = 16) (r = 0.0496, P = 0.87). y axis represents plasma level of E2 and x axis represents plasma levels of CORT.
Central changes in gene expression
HPA-related genes: Crh, Avp, Pomc, Crhr1, Crhr2, Nr3c1, and Nr3c2
There were no significant changes in hypothalamic Crh or pituitary Crhr1 or Crhr2 mRNA levels under any treatment condition. Compared with normal mice, AA mice had 2.89- and 1.6-fold increases in hypothalamic Avp mRNA levels under acute and repeated stress conditions, respectively. Pituitary Pomc mRNA levels were also increased in AA mice by 2.61- and 2.76-fold under basal and repeated stress conditions, respectively. This finding was supported by the statistical analysis. A significant main effect of treatment (F(2, 26)=4.83, P<0.05), indicated that Pomc mRNA expression was significantly increased in AA mice compared with normal mice under both basal and repeated stress conditions (P<0.05). Levels of pituitary Crh, Crhr1, and Crhr2 were approximately equal to, or less than, 1.5-fold different from those of normal mice (Table 2). Finally, in AA mice, hippocampal Nr3c1 levels were 1.96- and 1.53-fold higher than those in normal mice under basal and acute stress conditions, respectively. In contrast, hippocampal Nr3c2 levels were 1.60-fold lower than those in normal mice following acute stress (Table 2).
Table 2.
Gene expression levels in brains of AA and normal mice
| Basal | Acute stress | Repeated stress | |
|---|---|---|---|
| Hypothalamus | |||
| Crh | 1.33 | 1.36 | 1.48 |
| Avp | 1.01 | 2.89 | 1.60 |
| Esr1 | −1.15 | 1.40 | 1.13 |
| Esr2 | −1.51 | 2.21 | −1.06 |
|
| |||
| Hippocampus | |||
| Esr1 | −1.54 | −1.16 | 1.00 |
| Esr2 | −1.47 | −2.70 | −1.27 |
| Nr3c1 | 1.96 | 1.53 | 1.00 |
| Nr3c2 | −1.23 | −1.60 | −1.27 |
|
| |||
| Pituitary | |||
| Pomc* | 2.61 | −1.01 | 2.76 |
| Crhr1 | 1.15 | 1.46 | 1.30 |
| Crhr2 | −1.21 | −1.17 | 1.42 |
AA, alopecia areata; Avp, arginine vasopressin; Crh, corticotropin-releasing hormone; Esr, estrogen receptor; Crhr, corticotropin-releasing hormone receptor; Nr3c, glucocorticoid receptor; Pomc, pro-opiomelanocortin.
Gene expression level of normal mice is set at ±1 fold. Significant differences are indicated in bold. All values are based on mean values from experiments conducted in triplicate for each mouse sample.
Pomc in the pituitary: acute stress<basal=chronic stress, P<0.05; AA: acute<chronic<basal, P<0.05.
HPG-related genes: Esr1 and Esr2
When compared with normal mice, AA mice had 1.51-fold lower expression of hypothalamic Esr2 under basal conditions, but 2.21-fold greater expression following acute stress. There were no significant differences in expression levels of Esr1, under any treatment condition (Table 2). In contrast, in the hippocampus Esr1 levels were 1.54-fold lower in AA compared with normal mice under basal conditions, and Esr2 mRNA levels were 2.7-fold lower in AA compared with normal mice under acute stress conditions (Table 2).
Statistical analysis of gene co-expression correlations
Correlations in expression between genes in the CNS of both normal and AA mice were examined based on the analysis of results for each individual mouse. A positive correlation was found between Crh and Avp in the hypothalamus (r=0.6991, P<0.01) in normal mice, at both basal and acute stress conditions (r=0.8773, P<0.05; r=0.8533, P<0.05, respectively), but the correlation was not present in AA mice.
Peripheral changes in gene expression
Gene expression levels in lymph nodes
Messenger RNA levels for HPA hormones, hormone receptors and cytokines were tested in skin draining lymph nodes. In AA compared with normal mice, several HPA hormones and their receptors, including Crh, Avp, Pomc, Crhr1, and Esr2 were observed to be synchronously lower under both basal (−1.58- to −1.94-fold) and acute stress (−1.61- to −5.88-fold) conditions, but significantly higher (3.40- to 5.91-fold) under repeated stress conditions (Table 3). In addition, Esr1 Acthr and Nr3c1(GR) gene expression levels were lower (−1.61- to −2.28-fold) in AA compared with normal mice following acute stress, whereas Crhr2 mRNA levels were lower (−2.09-fold) in AA compared with normal mice under basal conditions. In contrast, higher gene expression levels in AA compared with normal mice were seen for Nr3c2(MR) under both basal (1.89-fold) and repeated stress (1.82-fold) conditions, and for Acthr (1.81-fold) following repeated stress. Il12, an indicator of proinflammatory activity, was increased in AA compared with normal mice under all conditions (1.55- to 4.56-fold). Statistical analysis supported this latter finding, with a main effect of group (F(1,28)=7.71, P<0.01) reflecting higher Il12 mRNA expression in AA compared with normal mice (P<0.05, post hoc analysis).
Table 3.
Gene expression levels in lymph nodes of AA and normal mice
| Target gene | Basal | Acute stress | Repeated stress |
|---|---|---|---|
| Crh | −1.68 | −2.67 | 3.40 |
| Avp | −1.40 | −1.61 | 3.64 |
| Pomc | −1.58 | −2.33 | 5.91 |
| Crhr1 | −1.90 | −5.88 | 5.30 |
| Crhr2 | −2.09 | −1.43 | 1.43 |
| Esr1 | −1.37 | −2.28 | −1.18 |
| Esr2 | −1.94 | −3.71 | 3.28 |
| Nr3r1 | −1.25 | −1.69 | −1.33 |
| Nr3r2 | 1.89 | 1.17 | 1.82 |
| Acthr | 1.27 | −1.61 | 1.81 |
| Il12* | 4.56 | 1.55 | 2.46 |
AA, alopecia areata; Avp, arginine vasopressin; Crh, corticotropin-releasing hormone; Esr, estrogen receptor; Crhr, corticotropin-releasing hormone receptor; Nr3c, glucocorticoid receptor; Pomc, pro-opiomelanocortin.
Gene expression level of normal mice is set at ±1 fold. Significant differences are indicated in bold. All values are based on mean values from experiments conducted in triplicate for each mouse sample.
Il12 expression: AA>normal, P<0.01.
Statistical analysis of gene co-expression correlations
Correlations in expression between genes in lymph nodes of both normal and AA mice were examined based on the analysis of results for each individual mouse. In both normal and AA mice, levels of three hormones of the HPA axis, Crh, Avp, and Pomc, were positively correlated to each other (r was between 0.72 and 0.97, P<0.01). Also, they were all positively correlated to Crhr1, Acthr, Nr3c2, and Esr2, in both normal and AA mice, and to Nr3c1 and Esr1 in AA mice only (r was between 0.60 and 0.94, P<0.01). Interestingly, Crhr2 expression levels were not correlated to any other gene in normal mice but were positively correlated with Crhr1 and Nr3c2 in AA mice (r was between 0.6782 and 0.8999, P<0.01). No correlation was found between local HPA hormones and Il12 production in lymph nodes.
Gene expression levels in skin
Locally in the skin, AA mice showed enhanced HPA activity. Avp mRNA expression was elevated in the skin in AA compared with normal mice under all conditions, by 1.93- to 9.57-fold. Pomc expression was also significantly elevated under basal (7.74-fold) and repeated stress (16.28-fold) conditions. Statistical analysis supported this finding; a main effect of group for Pomc expression (F(2,28)=7.175, P<0.05) indicated significantly higher Pomc mRNA levels in AA compared with normal mice (P<0.05) (Figure 4; Table 4).
Figure 4. mRNA levels of HPA hormones and their receptors in the skin of AA mice in comparison to normal haired mice.

Gene expression levels of non-AA affected normal mice (n = 18) were set at ±1-fold, and gene expression changes in AA mice (n = 16) were expressed as the normalized “fold” changes in mRNA expression compared with those of normal mice. Statistical analysis for significant differences in expression between AA-affected mice and normal mice was performed on the data using ANOVA. *P<0.05 and **P<0.01.
Table 4.
Gene expression levels in skin of AA and normal mice
| Basal condition | Acute stress | Repeated stress | |
|---|---|---|---|
| Avp | 1.930458 | 5.023554 | 9.574854 |
| Pomc* | 7.741041 | 1.434298 | 16.27736 |
| Crhr1* | −6.69844 | −4.89092 | −29.0653 |
| Crhr2* | 3.101853 | 4.612316 | 50.68268 |
| Esr1* | −20.3236 | 2.084355 | −3.47242 |
| Nr3r1* | −3.33694 | −2.03101 | −1.8185 |
| Nr3r12 | −2.4507 | 1.653299 | 1.693722 |
| Acthr | 2.325701 | 1.349734 | 1.928571 |
| Tnfα* | 2.58766 | 3.98049 | 3.966478 |
AA, alopecia areata; Avp, arginine vasopressin; Crh, corticotropin-releasing hormone; Esr, estrogen receptor; Crhr, corticotropin-releasing hormone receptor; Nr3c, glucocorticoid receptor; Pomc, pro-opiomelanocortin; Tnfα, tumor necrosis factor-α.
Gene expression level of normal mice is set at ±1 fold. All values are based on mean values from experiments conducted in triplicate for each mouse sample.
AA4 or <normal, P<0.05.
With the exception of Crhr2 and Acthr, receptors for stress hormones were mainly decreased in AA compared with normal mice. Crhr1 and Nr3c1 were lower in all treatment conditions, whereas Nr3c2 levels were reduced under basal conditions and Esr1 levels were lower under both basal and repeated stress conditions. Statistical analyses supported these findings. Main effects of group for Crhr1 (F(1,28)=45.34, P<0.001), Nr3c1 (F(2,27)=12.751, P<0.01), and Esr1 (F(2,27=6.4797, P<0.05), reflected significantly lower levels of expression in AA compared with normal mice. Significant main effects of treatment were also found for Crhr1 (F(2,28)=8.017, P<0.01) and Esr1 (F(2,27)=4.6098, P<0.05). Crhr1 mRNA levels were significantly lower in AA under basal and repeated stress conditions (P<0.05). Similarly, Esr1 expression was lower in AA under basal conditions compared with both acute and repeated stress conditions and compared with all treatment conditions in normal mice (Ps<0.05–0.001).
In contrast, Crhr2 levels were markedly upregulated in AA mouse skin compared with normal mouse skin, as indicated by significant main effects for group (F(1,28)=22.61, P<0.001) and treatment (F(2,28)=5.02, P<0.05), and a group×treatment interaction (F(2,28)=3.68, P<0.05). Post hoc analyses revealed a significant increase in Crhr2 levels in AA compared with normal mice following repeated stress (P<0.001).
Tumor necrosis factor-α (Tnfα) mRNA levels were also increased in AA mice under both basal and stress conditions, as indicated by a significant main effect of group (F(1,25)=9.537, P<0.01) (Figure 4; Table 4).
Protein expression in skin
CRHR2 protein expression was mainly found in hair follicles, with no significant expression observed in other skin structures in either normal- and AA-affected mice. Histologically, CRHR2 expression in normal mice was found mainly in the upper two-thirds of the hair follicle, toward the epidermis. In contrast, in AA mice, CRHR2 was found to extend deeper into the follicular bulb toward the dermal papillae, predominantly located in the inner root sheath (Figure 5).
Figure 5. CRH-R2 protein expression in the skin.

Immunohistochemical examination of CRH-R2 in the skin of mice under repeated stress conditions indicated limited expression intensity in anagen stage hair follicles of normal haired mice (a), whereas AA mice exhibited expression primarily in the inner root sheath of dystrophic anagen hair follicles (arrowheads, b). Bar = 30 μm.
Central and peripheral HPA measures
Links between systemic hormones and cutaneous hormone receptors were found in both normal and AA mice. In normal mice, plasma CORT levels were negatively correlated with skin Nr3c1 (r=−0.8347, P<0.05), but positively correlated with skin Nr3c2 (r=0.6520, P<0.01) expression under the repeated stress condition. In AA mice, on the other hand, there was a strong negative correlation between plasma CORT levels and skin Nr3c2 expression under the repeated stress condition (r=−0.9600, P<0.05).
HPA hormones and cytokine production
In AA mice, both plasma ACTH levels and cutaneous Acthr expression levels were positively correlated with Tnfα levels in skin (r=0.8816 and 0.5776, P<0.05) under the repeated stress condition.
DISCUSSION
Stress elicits significant defects in the HPA response in rodents with compromised stress response systems (Weinberg et al., 1996). In this study, a number of findings demonstrated that AA and normal mice exhibit significantly different peripheral and central HPA responses to stress. We found that systemically, AA mice had blunted CORT and ACTH responses to an acute physiological stressor (ether inhalation). In contrast, they did not have a blunted response to an acute psychological stressor (restraint), but they did show a deficit in habituation to repeated psychological stress. It is known that rodents exhibit different responses to physical and psychological stressors, suggesting physical and psychological stress may be regulated by different pathways (Endo and Shiraki, 2000; Oishi et al., 2003; Amano et al., 2007; Cui et al., 2007). The burden of immune system activity on the HPA axis may elicit differences in response to stress from different origins. Further research is required to investigate why AA mice exhibited differential stress responses to acute physiological and psychological challenges and failed to adapt to a repeated psychological stressor. Here we focused on responses in AA mice to psychological stress as the more relevant stressor experienced by humans with AA.
AA mice have an aberrant central HPA response to psychological stress
Central neuroendocrine changes support and extend the findings of altered HPA activity and regulation in AA mice in response to psychological stress. AA mice were similar to normal controls in Crh and Crhr expression. Importantly however, AA mice exhibited increased expression of hypothalamic Avp following stress exposure and increased pituitary Pomc under both basal and stress conditions. Expression levels of receptors in the HPA system at different levels of the axis usually reflect the functional status of the system, as well as the negative feedback mechanism. Hippocampal Nr3c1 mRNA levels were upregulated and Nr3c2 mRNA levels were downregulated in the hippocampus of AA mice. Taken together, these findings provide strong evidence for a marked increase in HPA tone, reflected in HPA hyperactivity at all levels of the axis.
In comparison to normal mice, AA mice had increased hypothalamic Avp mRNA levels under both acute and repeated stress conditions. Together with significantly increased Pomc mRNA expression under both basal and repeated stress conditions, this strongly suggests increased central HPA drive in AA mice. The finding of increased hypothalamic Avp instead of Crh expression in AA mice is consistent with findings in arthritic rodent models. AVP usually potentiates the effects of CRH, especially under conditions of prolonged or chronic stress. An increased AVP:CRH ratio increases hypothalamic drive and appears to be critical for maintaining pituitary responsiveness to repeated stress (Chowdrey et al., 1995).
HPA activity might play a role in modulating AA-associated inflammation
Interactions between the neuroendocrine and immune systems are multifaceted and bidirectional. Altered interaction between the neuroendocrine and immune systems can result in decreased immunosuppression and an exaggerated inflammatory response in some disease models (Harbuz et al., 2006). There is strong evidence for modulatory effects of stress on inflammatory autoimmune disease (Jessop et al., 2004). In turn, increased peripheral levels of cytokine production, including IL-1, IL-6 and TNFα, have been shown to activate the HPA axis (Dunn, 2000). In our study we found that both plasma ACTH levels and local cutaneous Acthr expression were positively correlated to Tnfa expression in AA mouse skin. This suggests that there may be interactions between systemic HPA hormones, expression levels of cutaneous HPA hormone receptors, and proinflammatory cytokine production in AA skin, although the decisive role of HPA hormones in the interaction remains to be determined.
AA mice have an aberrant HPG response to stress
The hypothalamic–pituitary–gonadal (HPG) axis activity was also investigated in our study. The HPA system usually inhibits the reproductive system to save energy (Mastorakos et al., 2006). In turn, E2 within the physiological range can enhance HPA activity, but may also inhibit the HPA stress response of females (Young et al., 2001). We found that there was a strong positive correlation between plasma CORT and E2 levels in normal mice not present in AA mice. The data suggest that E2 has the expected facilitatory effect on HPA activity in normal mice but that the relationship between the HPA and HPG axes is disrupted in AA mice.
Estrogens exert their physiological effects through two estrogen receptor (ER) subtypes, ER-α and ER-β (Muramatsu and Inoue, 2000). ER-α is of primary importance in relation to HPG function and reproduction, whereas ER-β in the brain has roles in HPA regulation, stress and spatial learning. The characterization of mice lacking ER-α, or ER-β, or both has revealed that both receptor subtypes have overlapping, but also unique roles in estrogen-dependent action in vivo (Matthews and Gustafsson, 2003; Imamov et al., 2005). Experiments have shown that ER-β may critically modulate the HPA axis response to stress and it is regulated by circulating CORT (Isgor et al., 2003). In our study, AA mice had decreased hypothalamic and hippocampal expression of Esr2 (ER-β) under basal and acute stress conditions, respectively, but an increased hypothalamic expression following acute stress. This may further contribute to the dysfunctional hyperactivity of the HPA axis centrally.
AA is associated with significant changes in the peripheral HPA system
There is very limited literature published about HPA hormone production and receptor expression in lymphoid organs. However, data from our study suggested that lymphoid organs may be able to produce components of the HPA axis and express their receptors. As such, the skin may not be the only peripheral unit of the HPA axis. In AA-affected mice, lymph node Crh, Avp, Pomc, Crhr1, and Esr2 mRNA levels were lower under basal and acute stress conditions but upregulated following repeated stress. In addition, strong positive correlations were found in our study among several HPA hormones and their receptors in lymph nodes, such as Crh, Avp, Pomc, Crhr1, Acthr, and Nr3c2.
The fact that expression of HPA hormone genes and their receptors were detected in an organ in which the majority of cells are lymphocytes might indicate that lymphocytes produce HPA hormones in an autocrine fashion. Decreased Nr3c1 levels in lymph nodes found in our study could explain that, despite higher HPA activity, an immunosuppressive effect of steroids on the local pathogenic lymphocytes was not functionally significant owing to decreased receptor levels on the cells.
Also of note, Crhr2 expression levels were positively correlated with Crhr1 and Nr3c2 in AA mice. Others have also observed a high degree of colocalization of Crhr1 and Crhr2 receptors on circulating and resident immune cells from rats with adjuvant arthritis (Mousa et al., 2003). The synchronized expression of Crhr1 and Crhr2 receptors in immune cells may be involved in conveying CRH signals to release β-endorphin from immune cells and produce antinociceptive effects by activating opioid receptors on peripheral sensory nerve endings (Mousa et al., 2003).
AA is associated with significant changes to HPA components expressed in skin
Studies have shown that the human hair follicle is capable of producing HPA hormones and may form a functional loop which may be reflected by changes to the central HPA axis (Slominski et al., 1998, 2000, 2007; Ito et al., 2005). In our study, we also found highly active local HPA activity in the skin of AA mice, shown by significantly increased Avp and Pomc mRNA levels and a strong positive correlation between them. Consistent with our data demonstrating cutaneous HPA activity, a recent study in human AA lesions also found elevated CRH and POMC production at the protein level (Kim et al., 2006). We were unable to detect cutaneous Crh at the mRNA level in our study, which is consistent with the findings that, unlike human skin, de novo production of Crh mRNA is not found in mouse skin (Paus et al., 2006).
In skin, differential expression levels were also seen among the receptors for HPA hormones. We found increased levels Crhr2 and Acthr, but decreased mRNA levels of Crhr1, Nr3c1, and Nr3c2 in the skin of AA mice. In human skin, CRH-R1 is the major receptor expressed in epidermal and dermal compartments and CRH-R2 is predominantly found in dermal structures (Slominski et al., 2006). Increased levels of CRH-R2 in human AA lesions was also found by others (Katsarou-Katsari et al., 2001). Therefore, increased CRH-R2 expression at both mRNA and protein levels found in our study may well be a component of an overreactive local skin HPA axis. Decreased Nr3c1 and Nr3c2 mRNA levels may be a negative feedback mechanism in AA mice to adjust the local highly active HPA functional status.
Estrogen receptors have been identified in hair follicles (Conrad and Paus, 2004; Kwon et al., 2004; Koehler et al., 2005) and estrogens can modulate hair growth, although whether estrogens promote or inhibit hair loss is still a matter of debate (Moverare et al., 2002; Thornton, 2002, 2005; Ohnemus et al., 2004, 2006). In our study, we could not find Esr2 in either normal or AA mouse skin. AA mice had decreased Esr1 expression at basal and repeated stress conditions, but increased expression under acute stress conditions. Therefore, at least in this mouse model, Esr1 may be the predominant receptor for estrogen in skin. The functional significance of changes in receptor expression with stress exposure remains to be determined. Changes in regulation seen under basal conditions reflect a basic defect of the HPA axis in response to chronic inflammatory stress.
The central HPA and peripheral HPA may interact in a feedback loop with the inflammatory response in AA
There is significant evidence that the skin and hair follicles have their own local equivalent of the HPA axis, termed the brain-hair follicle axis (BHA) (Arck et al., 2001, 2003). Neuropeptides, particularly SP, have been shown to be expressed in the skin and hair follicles and may regulate the hair growth cycle (Maurer et al., 1997; Zhou et al., 2006). SP promotes catagen and also induces a loss of immune privilege markers in hair follicles (Arck et al., 2001; Peters et al., 2007). These events may be mediated in part via SP signaling to mast cells and promotion of mast cell degranulation leading to hair follicle regression. Exposure to stress can curtail hair growth in mice with associated increased expression of SP (Arck et al., 2001, 2003; Katayama et al., 2007). In contrast, mice deficient in mast cells, or mast cell-expressed SP receptor NK1R, do not exhibit curtailment of their hair growth cycle in response stress (Arck et al., 2005). SP is highly expressed in the skin of AA-affected humans (Toyoda et al., 2001) and in the C3H/HeJ mouse model (Siebenhaar et al., 2007), suggesting the potential for a role in the development of dystrophic AA-affected hair follicles. It has been hypothesized that AA onset may require stress-induced SP expression in the skin and other BHA activity (Peters et al., 2006, 2007).
The local skin HPA axis is highly active in human and mouse AA as shown here and elsewhere (Toyoda et al., 2001; Siebenhaar et al., 2007). However, the data presented here indicate there may also be a feedback loop, from the AA disease focus and the local BHA, to the central HPA axis. In essence, the central HPA axis may impact the local BHA and the AA disease mechanism in the skin. In turn, the local inflammation and BHA may impact the central HPA axis. Inflammatory cells release cytokines that can act systemically and, in other stress models, have been shown to significantly alter the HPA axis (Jessop et al., 1987; Chrousos, 1995; Shanks et al., 1998; Elenkov and Chrousos, 1999, 2002; Harbuz et al., 2006). In addition, local products produced by the BHA might be released systemically and may feedback to the HPA axis. For example, ACTH, CRH, CORT, and POMC are all produced in the skin (Slominski et al., 2007). These factors have significant modulatory action on hair follicle cycling (Maurer et al., 1997; Slominski et al., 1998, 1999), but it is possible that their release from the skin may also impact the central HPA axis. It is also feasible that the release of ACTH, CRH, and CORT by the central HPA axis to the periphery directly impacts the hair growth cycle bypassing the local BHA. Published knowledge, and the data from this study, suggest the potential for a close, and complex, interrelationship between AA, and central, and peripheral stress responses (Figure 6).
Figure 6. Hypothetical interactions between the HPA and the immune system in AA.

A diagrammatic representation of the potential mode of interaction between components of the central HPA axis, skin BHA axis, and the immune system associated with AA development. ACTH, adrenocorticotropic hormone; AVP, arginine vasopressin; BHA, brain-hair follicle axis; CRF, corticotropin-releasing factor; and HPA, hypothalamic–pituitary–adrenal axis.
In conclusion, the data from our study show that AA mice have a significantly blunted systemic HPA response to acute physiological stress and a defective adaptation to repeated psychological stress. AA mice also have highly active central and peripheral HPA activity, and a defective interaction between the HPA and HPG axes, as compared with normal mice. The immune response in AA may underlie the altered HPA activity observed in AA-affected mice. However, we also observed a feedback loop whereby increased HPA activity correlated with increased inflammatory activity. Stress further exacerbated many of the differences in the HPA axis observed between AA and normal haired mice systemically, centrally and peripherally in the skin and the lymphoid organs. The data revealed a positive HPA hormone level correlation with skin proinflammatory cytokine levels. As HPA hormones increased in AA mice in response to chronic stress exposure, so did cytokine levels in the skin. This suggests that altered HPA activity may be involved in modulating the severity or course of AA. From this study, however, we cannot answer the question of whether the HPA axis participates in the onset of the AA. Overall, the mouse model data suggest that individuals with AA might also have increased HPA tone or activity and an inability to adapt to stressors. As modified HPA activity may be a characteristic of AA disease development, stress management may be of potential adjunctive benefit in the clinical handling of the alopecia patient.
MATERIALS AND METHODS
Mice and surgical procedure
All research studies were conducted with the approval of the University of British Columbia Animal Care Committee. Subjects were all female C3H/HeJ mice supplied from colonies at The Jackson Laboratory (Bar Harbor, ME) specific pathogen-free production facility. Mice had access to food and water ad libitum, and were housed throughout all phases of the study in a facility isolated from other mice and optimized for low noise and low interference levels.
Previously, we have shown that grafting skin from spontaneous AA-affected mice to histocompatible mice transfers the disease to the graft host (McElwee et al., 1998). This allows for a controlled model of AA in matched groups. Briefly, mice spontaneously affected with AA were euthanized and dorsal and ventral full-thickness skin grafts, approximately 1–1.5 cm in diameter, were aseptically removed. Recipient, normal haired C3H/HeJ mice were anesthetized and engrafted with AA-affected skin graft. Grafted mice were allowed to age and developed AA with first hair loss beginning 7–9 weeks post grafting.
Experimental design and stress paradigms
Three months after surgery, female C3H/HeJ mice with induced AA lesions, and age-matched (all aged 10 months) normal female C3H/HeJ mice were assigned to stress or no stress conditions. Mice were housed in polycarbonate cages with stainless steel lids for a 1–2 week adaptation period in an animal room isolated from other animal facilities and directly adjacent to the processing laboratory. During this time, they were given access to water and standard mouse laboratory chow ad libitum. The colony room was controlled to ~21 °C and lights were on from 0600 to 1800 hours.
In experiment 1, induced AA mice (n = 12) and normal controls (n = 12) were subjected to a physiological stressor consisting of light ether anesthesia, that is, exposure to ether vapors for approximately 15–20 seconds, without loss of consciousness, at a consistent time of day (1100 hr). Half the mice in each group (n = 6) were decapitated immediately (within 30 seconds) upon removal from the home cage and trunk blood collected for measurement of basal hormone levels (basal condition). We have shown that this time frame is fast enough to obtain a measure of true basal CORT and ACTH levels without any effects of exposure to ether itself (Hofmann et al., 1999). The remaining mice (n = 6) were returned to their home cages for 30 minutes before decapitation to obtain a measure of stress levels of CORT and ACTH.
In experiment 2, restraint stress was used as a psychological stressor (Pare and Glavin, 1986; Glavin et al., 1994). Mice were confined in small containers (5×8 cm) with ventilated caps (Giberson and Weinberg, 1995). Induced AA mice (n = 16) and normal controls (n = 18) were assigned to one of three conditions: (1) acute stress: mice exposed once only to 30 minutes restraint stress at a consistent time of day (1100 hr), and terminated immediately following stress (n = 5–6); (2) repeated restraint: mice exposed to multiple stress events consisting of exposure to 30 minutes restraint stress daily, for 5 consecutive days (n = 6) at a consistent time of day (1100 hr); and (3) basal or no stress: mice were left undisturbed in their home cages and terminated immediately on removal from the home crate (n = 5–6) at an equivalent time. Care was taken to create a quiet work condition in the laboratory to ensure the true basal condition of the mice.
Sampling
All mice were terminated by decapitation. The procedure was carried out on all mice at approximately 1130–1200 hr. Therefore, all the animals were killed within a short time period to ensure they were all at a similar point in their circadian rhythm. Trunk blood was collected for measurement of plasma levels of ACTH, CORT, and E2. Hypothalami and hippocampi were dissected and pituitary glands were collected (Lan et al., 2006). Skin-draining lymph nodes (cervical, axillary, brachial, and inguinal) were harvested. Skin samples containing only anagen stage (normal mice) or dystrophic anagen stage (AA mice) hair follicles, as determined by visual inspection of pigment distribution in the dorsal mouse skin, were removed (McElwee et al., 2004). Telogen stage skin was discarded. Skin samples from each mouse were subdivided and were either embedded in OCT compound (Sakura Tissue-Tek, Torrance, CA) or immersed in RNA later (Qiagen, Mississauga, ON, Canada) and frozen at −80 °C.
Hormone measurement by radioimmunoassay
Plasma ACTH was assayed using a modification of the DiaSorin ACTH 125I RIA Kit (DiaSorin Inc., Stillwater, MN) (Lan et al., 2006). Antiserum cross-reactivity was 100% for ACTH and less than 0.1% for all other peptides assessed (including α-melanocyte-stimulating hormone, β-endorphin and β-lipotropin). All reagent volumes were halved and 50 μl plasma was used per tube. The minimum detectable concentration for ACTH was 20 pg ml−1, and intra- and interassay coefficients of variation were 3.9 and 6.5%, respectively. Total plasma CORT (bound plus free) was measured by standard radioimmunoassay (RIA). Antiserum was obtained from MP Biomedicals (Orangeberg, NY) (Lan et al., 2006). The minimum detectable CORT concentration was 25 ng ml−1. The intra- and interassay coefficients of variation were 4.4 and 7.1%, respectively. Plasma E2 was measured with the ADVIA Centaur Estradiol-6 III Assay RIA kit (Diagnostic Products Corporation, Siemens Medical Solutions Diagnostics, Los Angeles, CA). The minimum detectable E2 concentration was 16 pg ml−1. The intra- and interassay coefficients of variation were 2.9 and 4.9%, respectively.
Quantitative PCR (qPCR)
Total RNA was extracted from RNA later-preserved tissue samples using RNAeasy Extraction kits (Qiagen). Fibrous kits were used for RNA extraction from lymph nodes and skin; lipid kits for were used for RNA extraction from hypothalamus, hippocampus, and pituitary. Gene-specific primers for the templates of interest were designed from published cDNA sequences from GeneBank (Table S1). The target genes evaluated were Crh, Avp, Pomc1, Crhr1, Crhr2, Acthr, Esr1 (equivalent to human ERα), Esr2 (equivalent to human ERβ), Nr3c1 (equivalent to human GR), Nr3c2 (equivalent to human MR), Tnfa, and Il12 as indicators of proinflammatory activity (McElwee et al., 2002). Primer pairs for target genes were designed using Primer 3 software (v.0.3.0) available online (http://frodo.wi.mit.edu/) and then obtained from commercial sources (Invitrogen, Burlington, ON, Canada). Total RNA was extracted from tissues harvested from AA and normal mice, quantified and reverse transcribed into cDNA using the Superscript First-Strand Synthesis System for RT-PCR (Invitrogen). Samples of cDNA templates were mixed with gene-specific primers and Master mix (Qiagen) containing PCR buffer, DEPC-treated water, MgCl2, dNTPs, Taq, and SYBR green. β-Actin was used as an endogenous control. Test and control samples were transferred to wells of a 96-well plate, loaded into a sequence detection system (ABI 7000; MJ Research, Waltham, MA), and thermal cycling was initiated using standard protocols. Analysis was conducted using the supplied computer and software linked to the thermal cycler and detection system.
Immunohistochemistry
Protein expression levels of CRHR2 were explored in the skin tissues of both normal and AA mice using the indirect immunoperoxidase technique (Zhang et al., 2003). Briefly, rehydrated sections were blocked with 15 ml of Tris-buffered saline solution with 200 μl normal donkey serum and 0.015 g BSA, for 30 minutes at RT. After rinsing three times in buffered saline solution, the slides were incubated goat anti-CRHR2 (Santa Cruz Biotech, Santa Cruz, CA) for an hour at room temperature, followed by washing and 30 minute’s incubation with peroxidase-conjugated donkey anti-goat IgG (Jackson ImmunoResearch, West Grove, PA) secondary antibodies. After further washing, bound conjugate was detected by incubation in buffered saline solution containing 0.02% hydrogen peroxide and 5 mg ml−1 diaminobenzidine (Sigma, Oakville, ON, Canada). Sections were counterstained with Harris’ hematoxylin and mounted in Permount (Fisher Scientific, Ottawa, ON, Canada). We also attempted to determine GR expression by immunohistochemistry but were unsuccessful (not shown).
Statistical analyses
Data were analyzed by appropriate analyses of variance for the factors of experimental group (normal, AA) and treatment (no stress, stress for experiment 1; no stress, acute stress, repeated stress for experiment 2). Significant main effects and interactions were further analyzed by Newman–Keul’s post hoc tests. For q-PCR data, gene expression levels of normal mice were set at ± 1-fold, and gene expression changes in AA mice were expressed as the normalized “fold” change in mRNA expression compared with those of normal mice. Appropriate analyses of variance were also performed to confirm significant differences between groups. Correlations were also run to determine relationships between measurements within individuals using Pearson’s R simple linear correlations (Statistica version 6; Stat Soft. Tulsa, OK).
Supplementary Material
Table S1. Primers used in this study.
Acknowledgments
This research was supported by the National Alopecia Areata Foundation (NAAF), the Canadian Dermatology Foundation (CDF); and the Canadian Institutes of Health Research (CIHR No.: MOP82927) to KJM; and NAAF and the North American Hair Research Society (NAHRS) to XZ. Further financial support was provided by AA007789 from NIH/NIAAA, and grants from the BC Ministry of Children and Family Development (through the UBC Human Early Learning Partnership) and the Canadian Institute for Advanced Research to JW.
Abbreviations
- AA
alopecia areata
- ACTH
adrenocorticotropic hormone
- AVP
arginine vasopressin
- BHA
brain-hair follicle axis
- CNS
central nervous system
- CORT
corticosterone
- CRH
corticotropin-releasing hormone
- CRHR
corticotropin-releasing hormone receptor (1 and 2)
- E2
estradiol
- GR
glucocorticoid receptor
- HPA axis
hypothalamic–pituitary–adrenal axis
- HPG axis
hypothalamic-pituitary-gonadal axis
- MR
mineralocorticoid receptor
- POMC
pro-opiomelanocortin
- SP
substance P
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Amano M, Suemaru K, Cui R, Umeda Y, Li B, Gomita Y, et al. Effects of physical and psychological stress on 5-HT2A receptor-mediated wet-dog shake responses in streptozotocin-induced diabetic rats. Acta Med Okayama. 2007;61:205–12. doi: 10.18926/AMO/32870. [DOI] [PubMed] [Google Scholar]
- Arck PC, Handjiski B, Hagen E, Joachim R, Klapp BF, Paus R. Indications for a ‘brain-hair follicle axis (BHA)’: inhibition of keratinocyte proliferation and up-regulation of keratinocyte apoptosis in telogen hair follicles by stress and substance P. FASEB J. 2001;15:2536–8. doi: 10.1096/fj.00-0699fje. [DOI] [PubMed] [Google Scholar]
- Arck PC, Handjiski B, Kuhlmei A, Peters EM, Knackstedt M, Peter A, et al. Mast cell deficient and neurokinin-1 receptor knockout mice are protected from stress-induced hair growth inhibition. J Mol Med. 2005;83:386–96. doi: 10.1007/s00109-004-0627-z. [DOI] [PubMed] [Google Scholar]
- Arck PC, Handjiski B, Peters EM, Peter AS, Hagen E, Fischer A, et al. Stress inhibits hair growth in mice by induction of premature catagen development and deleterious perifollicular inflammatory events via neuropeptide substance P-dependent pathways. Am J Pathol. 2003;162:803–814. doi: 10.1016/S0002-9440(10)63877-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arck PC, Slominski A, Theoharides TC, Peters EM, Paus R. Neuroimmunology of stress: skin takes center stage. J Invest Dermatol. 2006;126:1697–704. doi: 10.1038/sj.jid.5700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber H. Alopecia areata. Br J Dermatol Syph. 1921;33:1–14. [Google Scholar]
- Brajac I, Tkalcic M, Dragojevic DM, Gruber F. Roles of stress, stress perception and trait-anxiety in the onset and course of alopecia areata. J Dermatol. 2003;30:871–8. doi: 10.1111/j.1346-8138.2003.tb00341.x. [DOI] [PubMed] [Google Scholar]
- Brauner GJ, Goodheart HP. Dermatologic care behind bars. J Am Acad Dermatol. 1988;18:1066–73. doi: 10.1016/s0190-9622(88)70107-3. [DOI] [PubMed] [Google Scholar]
- Carroll JM, McElwee KJ, E King L, Byrne MC, Sundberg JP. Gene array profiling and immunomodulation studies define a cell-mediated immune response underlying the pathogenesis of alopecia areata in a mouse model and humans. J Invest Dermatol. 2002;119:392–402. doi: 10.1046/j.1523-1747.2002.01811.x. [DOI] [PubMed] [Google Scholar]
- Chowdrey HS, Larsen PJ, Harbuz MS, Jessop DS, Aguilera G, Eckland DJ, et al. Evidence for arginine vasopressin as the primary activator of the HPA axis during adjuvant-induced arthritis. Br J Pharmacol. 1995;116:2417–24. doi: 10.1111/j.1476-5381.1995.tb15089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–62. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Stressors, stress, and neuroendocrine integration of the adaptive response. The 1997 Hans Selye Memorial Lecture. Ann N Y Acad Sci. 1998;851:311–35. doi: 10.1111/j.1749-6632.1998.tb09006.x. [DOI] [PubMed] [Google Scholar]
- Conrad F, Paus R. Estrogens and the hair follicle. J Dtsch Dermatol Ges. 2004;2:412–23. doi: 10.1046/j.1439-0353.2004.04037.x. [DOI] [PubMed] [Google Scholar]
- Cui R, Li B, Suemaru K, Araki H. Differential effects of psychological and physical stress on the sleep pattern in rats. Acta Med Okayama. 2007;61:319–27. doi: 10.18926/AMO/32876. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. Stress-induced augmentation of immune function—the role of stress hormones, leukocyte trafficking, and cytokines. Brain Behav Immun. 2002;16:785–98. doi: 10.1016/s0889-1591(02)00036-3. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. Stress, leukocyte trafficking, and the augmentation of skin immune function. Ann N Y Acad Sci. 2003;992:205–17. doi: 10.1111/j.1749-6632.2003.tb03151.x. [DOI] [PubMed] [Google Scholar]
- Dunn AJ. Cytokine activation of the HPA axis. Ann N Y Acad Sci. 2000;917:608–17. doi: 10.1111/j.1749-6632.2000.tb05426.x. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Chrousos GP. Stress hormones, Th1/Th2 patterns, pro/anti-inflammatory cytokines and susceptibility to disease. Trends Endocrinol Metab. 1999;10:359–68. doi: 10.1016/s1043-2760(99)00188-5. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Chrousos GP. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann N Y Acad Sci. 2002;966:290–303. doi: 10.1111/j.1749-6632.2002.tb04229.x. [DOI] [PubMed] [Google Scholar]
- Endo Y, Shiraki K. Behavior and body temperature in rats following chronic foot shock or psychological stress exposure. Physiol Behav. 2000;71:263–8. doi: 10.1016/s0031-9384(00)00339-5. [DOI] [PubMed] [Google Scholar]
- Garcia-Hernandez MJ, Ruiz-Doblado S, Rodriguez-Pichardo A, Camacho F. Alopecia areata, stress and psychiatric disorders: a review. J Dermatol. 1999;26:625–32. doi: 10.1111/j.1346-8138.1999.tb02063.x. [DOI] [PubMed] [Google Scholar]
- Giberson PK, Weinberg J. Effects of prenatal ethanol exposure and stress in adulthood on lymphocyte populations in rats. Alcohol Clin Exp Res. 1995;19:1286–94. doi: 10.1111/j.1530-0277.1995.tb01613.x. [DOI] [PubMed] [Google Scholar]
- Glavin GB, Pare WP, Sandbak T, Bakke HK, Murison R. Restraint stress in biomedical research: an update. Neurosci Biobehav Rev. 1994;18:223–49. doi: 10.1016/0149-7634(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Greenberg SI. Alopecia areata, a psychiatric survey. AMA Arch Derm. 1955;72:454–7. doi: 10.1001/archderm.1955.03730350056010. [DOI] [PubMed] [Google Scholar]
- Gulec AT, Tanriverdi N, Duru C, Saray Y, Akcali C. The role of psychological factors in alopecia areata and the impact of the disease on the quality of life. Int J Dermatol. 2004;43:352–6. doi: 10.1111/j.1365-4632.2004.02028.x. [DOI] [PubMed] [Google Scholar]
- Gupta MA, Gupta AK, Watteel GN. Stress and alopecia areata: a psychodermatologic study. Acta Derm Venereol. 1997;77:296–8. doi: 10.2340/0001555577296298. [DOI] [PubMed] [Google Scholar]
- Harbuz MS, Richards LJ, Chover-Gonzalez AJ, Marti-Sistac O, Jessop DS. Stress in autoimmune disease models. Ann NY Acad Sci. 2006;1069:51–61. doi: 10.1196/annals.1351.005. [DOI] [PubMed] [Google Scholar]
- Hofmann C, Glavas M, Yu W, Weinberg J. Glucocorticoid fast feedback is not altered in rats prenatally exposed to ethanol. Alcohol Clin Exp Res. 1999;23:891–900. [PubMed] [Google Scholar]
- Hordinsky M, Ericson M. Autoimmunity: alopecia areata. J Investig Dermatol Symp Proc. 2004;9:73–8. doi: 10.1111/j.1087-0024.2004.00835.x. [DOI] [PubMed] [Google Scholar]
- Imamov O, Shim GJ, Warner M, Gustafsson JA. Estrogen receptor beta in health and disease. Biol Reprod. 2005;73:866–71. doi: 10.1095/biolreprod.105.043497. [DOI] [PubMed] [Google Scholar]
- Isgor C, Cecchi M, Kabbaj M, Akil H, Watson SJ. Estrogen receptor beta in the paraventricular nucleus of hypothalamus regulates the neuroendocrine response to stress and is regulated by corticosterone. Neuroscience. 2003;121:837–45. doi: 10.1016/s0306-4522(03)00561-x. [DOI] [PubMed] [Google Scholar]
- Ito N, Ito T, Paus R. The human hair follicle has established a fully functional peripheral equivalent of the hypothamalic-pituitary-adrenal-axis (HPA) Exp Dermatol. 2005;14:158. [Google Scholar]
- Jessop DS, Richards LJ, Harbuz MS. Effects of stress on inflammatory autoimmune disease: destructive or protective? Stress. 2004;7:261–6. doi: 10.1080/10253890400025497. [DOI] [PubMed] [Google Scholar]
- Jessop JJ, Gale K, Bayer BM. Enhancement of rat lymphocyte proliferation after prolonged exposure to stress. J Neuroimmunol. 1987;16:261–71. doi: 10.1016/0165-5728(87)90080-4. [DOI] [PubMed] [Google Scholar]
- Kakourou T, Karachristou K, Chrousos G. A case series of alopecia areata in children: impact of personal and family history of stress and autoimmunity. J Eur Acad Dermatol Venereol. 2007;21:356–9. doi: 10.1111/j.1468-3083.2006.01931.x. [DOI] [PubMed] [Google Scholar]
- Kariagina A, Romanenko D, Ren SG, Chesnokova V. Hypothalamic-pituitary cytokine network. Endocrinology. 2004;145:104–12. doi: 10.1210/en.2003-0669. [DOI] [PubMed] [Google Scholar]
- Katayama M, Aoki E, Suzuki H, Kawana S. Foot shock stress prolongs the telogen stage of the spontaneous hair cycle in a non-depilated mouse model. Exp Dermatol. 2007;16:553–60. doi: 10.1111/j.1600-0625.2007.00558.x. [DOI] [PubMed] [Google Scholar]
- Katsarou-Katsari A, Singh LK, Theoharides TC. Alopecia areata and affected skin CRH receptor upregulation induced by acute emotional stress. Dermatology. 2001;203:157–61. doi: 10.1159/000051732. [DOI] [PubMed] [Google Scholar]
- Kim HS, Cho DH, Kim HJ, Lee JY, Cho BK, Park HJ. Immunoreactivity of corticotropin-releasing hormone, adrenocorticotropic hormone and alpha-melanocyte-stimulating hormone in alopecia areata. Exp Dermatol. 2006;15:515–22. doi: 10.1111/j.1600-0625.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- Koehler KF, Helguero LA, Haldosen LA, Warner M, Gustafsson JA. Reflections on the discovery and significance of estrogen receptor beta. Endocr Rev. 2005;26:465–78. doi: 10.1210/er.2004-0027. [DOI] [PubMed] [Google Scholar]
- Kwon OS, Han JH, Yoo HG, Lee SR, Kim KH, Eun HC, et al. Expression of androgen receptor, estrogen receptor alpha and beta in the dermal papilla of human hair follicles in vivo. J Dermatol Sci. 2004;36:176–9. doi: 10.1016/j.jdermsci.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Lan N, Yamashita F, Halpert AG, Ellis L, Yu WK, Viau V, et al. Prenatal ethanol exposure alters the effects of gonadectomy on hypothalamic-pituitary-adrenal activity in male rats. J Neuroendocrinol. 2006;18:672–84. doi: 10.1111/j.1365-2826.2006.01462.x. [DOI] [PubMed] [Google Scholar]
- Liakopoulou M, Alifieraki T, Katideniou A, Kakourou T, Tselalidou E, Tsiantis J, et al. Children with alopecia areata: psychiatric symptomatology and life events. J Am Acad Child Adolesc Psychiatry. 1997;36:678–84. doi: 10.1097/00004583-199705000-00019. [DOI] [PubMed] [Google Scholar]
- Mastorakos G, Pavlatou MG, Mizamtsidi M. The hypothalamic-pituitary-adrenal and the hypothalamic-pituitary-gonadal axes interplay. Pediatr Endocrinol Rev. 2006;3(Suppl 1):172–81. [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv. 2003;3:281–92. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- Maurer M, Fischer E, Handjiski B, von Stebut E, Algermissen B, Bavandi A, et al. Activated skin mast cells are involved in murine hair follicle regression (catagen) Lab Invest. 1997;77:319–32. [PubMed] [Google Scholar]
- McElwee K, Freyschmidt-Paul P, Ziegler A, Happle R, Hoffmann R. Genetic susceptibility and severity of alopecia areata in human and animal models. Eur J Dermatol. 2001;11:11–6. [PubMed] [Google Scholar]
- McElwee KJ, Boggess D, Burgett B, Bates R, Bedigan HG, Sundberg JP, et al. Murine cytomegalovirus is not associated with alopecia areata in C3H/HeJ mice. J Invest Dermatol. 1998;110:986–7. doi: 10.1046/j.1523-1747.1998.00207.x. [DOI] [PubMed] [Google Scholar]
- McElwee KJ, Hoffmann R, Freyschmidt-Paul P, Wenzel E, Kissling S, Sundberg JP, et al. Resistance to alopecia areata in C3H/HeJ mice is associated with increased expression of regulatory cytokines and a failure to recruit CD4+ and CD8+ cells. J Invest Dermatol. 2002;119:1426–33. doi: 10.1046/j.1523-1747.2002.19620.x. [DOI] [PubMed] [Google Scholar]
- McElwee KJ, Huth A, Kissling S, Hoffmann R. Macrophage-stimulating protein promotes hair growth ex vivo and induces anagen from telogen stage hair follicles in vivo. J Invest Dermatol. 2004;123:34–40. doi: 10.1111/j.0022-202X.2004.22712.x. [DOI] [PubMed] [Google Scholar]
- McElwee KJ, Tobin DJ, Bystryn JC, King LE, Jr, Sundberg JP. Alopecia areata: an autoimmune disease? Exp Dermatol. 1999;8:371–9. doi: 10.1111/j.1600-0625.1999.tb00385.x. [DOI] [PubMed] [Google Scholar]
- McElwee KJ, Yu M, Park SW, Ross EK, Finner A, Shapiro J. What can we learn from animal models of Alopecia areata? Dermatology. 2005;211:47–53. doi: 10.1159/000085580. [DOI] [PubMed] [Google Scholar]
- Mousa SA, Bopaiah CP, Stein C, Schafer M. Involvement of corticotropin-releasing hormone receptor subtypes 1 and 2 in peripheral opioid-mediated inhibition of inflammatory pain. Pain. 2003;106:297–307. doi: 10.1016/S0304-3959(03)00302-6. [DOI] [PubMed] [Google Scholar]
- Moverare S, Lindberg MK, Faergemann J, Gustafsson JA, Ohlsson C. Estrogen receptor alpha, but not estrogen receptor beta, is involved in the regulation of the hair follicle cycling as well as the thickness of epidermis in male mice. J Invest Dermatol. 2002;119:1053–8. doi: 10.1046/j.1523-1747.2002.00637.x. [DOI] [PubMed] [Google Scholar]
- Muller SA, Winkelmann RK. Alopecia areata. An evaluation of 736 patients. Arch Dermatol. 1963;88:290–7. doi: 10.1001/archderm.1963.01590210048007. [DOI] [PubMed] [Google Scholar]
- Muramatsu M, Inoue S. Estrogen receptors: how do they control reproductive and nonreproductive functions? Biochem Biophys Res Commun. 2000;270:1–10. doi: 10.1006/bbrc.2000.2214. [DOI] [PubMed] [Google Scholar]
- Ohnemus U, Uenalan M, Inzunza J, Gustafsson JA, Paus R. The hair follicle as an estrogen target and source. Endocr Rev. 2006;27:677–706. doi: 10.1210/er.2006-0020. [DOI] [PubMed] [Google Scholar]
- Ohnemus U, Unalan M, Handjiski B, Paus R. Topical estrogen accelerates hair regrowth in mice after chemotherapy-induced alopecia by favoring the dystrophic catagen response pathway to damage. J Invest Dermatol. 2004;122:7–13. doi: 10.1046/j.0022-202X.2003.22120.x. [DOI] [PubMed] [Google Scholar]
- Oishi K, Nishio N, Konishi K, Shimokawa M, Okuda T, Kuriyama T, et al. Differential effects of physical and psychological stressors on immune functions of rats. Stress. 2003;6:33–40. doi: 10.1080/1025389031000101330. [DOI] [PubMed] [Google Scholar]
- Pare WP, Glavin GB. Restraint stress in biomedical research: a review. Neurosci Biobehav Rev. 1986;10:339–70. doi: 10.1016/0149-7634(86)90017-5. [DOI] [PubMed] [Google Scholar]
- Park E, Chan O, Li Q, Kiraly M, Matthews SG, Vranic M, et al. Changes in basal hypothalamo-pituitary-adrenal activity during exercise training are centrally mediated. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1360–71. doi: 10.1152/ajpregu.00103.2005. [DOI] [PubMed] [Google Scholar]
- Paus R, Theoharides TC, Arck PC. Neuroimmunoendocrine circuitry of the ‘brain-skin connection’. Trends Immunol. 2006;27:32–9. doi: 10.1016/j.it.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Perini GI, Veller Fornasa C, Cipriani R, Bettin A, Zecchino F, Peserico A. Life events and alopecia areata. Psychother Psychosom. 1984;41:48–52. doi: 10.1159/000287786. [DOI] [PubMed] [Google Scholar]
- Peters EM, Arck PC, Paus R. Hair growth inhibition by psychoemotional stress: a mouse model for neural mechanisms in hair growth control. Exp Dermatol. 2006;15:1–13. doi: 10.1111/j.0906-6705.2005.00372.x. [DOI] [PubMed] [Google Scholar]
- Peters EM, Liotiri S, Bodo E, Hagen E, Biro T, Arck PC, et al. Probing the effects of stress mediators on the human hair follicle: substance P holds central position. Am J Pathol. 2007;171:1872–86. doi: 10.2353/ajpath.2007.061206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picardi A, Pasquini P, Cattaruzza MS, Gaetano P, Baliva G, Melchi CF, et al. Psychosomatic factors in first-onset alopecia areata. Psychosomatics. 2003;44:374–81. doi: 10.1176/appi.psy.44.5.374. [DOI] [PubMed] [Google Scholar]
- Rossi R, Del Bianco E, Isolani D, Baccari MC, Cappugi P. Possible involvement of neuropeptidergic sensory nerves in alopecia areata. Neuroreport. 1997;8:1135–8. doi: 10.1097/00001756-199703240-00015. [DOI] [PubMed] [Google Scholar]
- Safavi KH, Muller SA, Suman VJ, Moshell AN, Melton LJ., III Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628–33. doi: 10.4065/70.7.628. [DOI] [PubMed] [Google Scholar]
- Shanks N, Harbuz MS, Jessop DS, Perks P, Moore PM, Lightman SL. Inflammatory disease as chronic stress. Ann N Y Acad Sci. 1998;840:599–607. doi: 10.1111/j.1749-6632.1998.tb09599.x. [DOI] [PubMed] [Google Scholar]
- Sheridan JF, Padgett DA, Avitsur R, Marucha PT. Experimental models of stress and wound healing. World J Surg. 2004;28:327–30. doi: 10.1007/s00268-003-7404-y. [DOI] [PubMed] [Google Scholar]
- Siebenhaar F, Sharov AA, Peters EM, Sharova TY, Syska W, Mardaryev AN, et al. Substance P as an immunomodulatory neuropeptide in a mouse model for autoimmune hair loss (alopecia areata) J Invest Dermatol. 2007;127:1489–97. doi: 10.1038/sj.jid.5700704. [DOI] [PubMed] [Google Scholar]
- Slominski A. Neuroendocrine system of the skin. Dermatology. 2005;211:199–208. doi: 10.1159/000087012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Botchkareva NV, Botchkarev VA, Chakraborty A, Luger T, Uenalan M, et al. Hair cycle-dependent production of ACTH in mouse skin. Biochim Biophys Acta. 1998;1448:147–52. doi: 10.1016/s0167-4889(98)00124-4. [DOI] [PubMed] [Google Scholar]
- Slominski A, Botchkareva NV, Botchkarev VA, Chakraborty A, Luger T, Uenalan M, et al. ACTH production in C57BL/6 mouse skin. Ann N Y Acad Sci. 1999;885:448–50. doi: 10.1111/j.1749-6632.1999.tb08709.x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Tuckey RC, Paus R. Differential expression of HPA axis homolog in the skin. Mol Cell Endocrinol. 2007;265–6:143–9. doi: 10.1016/j.mce.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Zmijewski M, Slominski RM, Kauser S, Wortsman J, et al. Corticotropin releasing hormone and the skin. Front Biosci. 2006;11:2230–48. doi: 10.2741/1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton MJ. The biological actions of estrogens on skin. Exp Dermatol. 2002;11:487–502. doi: 10.1034/j.1600-0625.2002.110601.x. [DOI] [PubMed] [Google Scholar]
- Thornton MJ. Oestrogen functions in skin and skin appendages. Expert Opin Ther Targets. 2005;9:617–29. doi: 10.1517/14728222.9.3.617. [DOI] [PubMed] [Google Scholar]
- Toyoda M, Makino T, Kagoura M, Morohashi M. Expression of neuropeptide-degrading enzymes in alopecia areata: an immunohistochemical study. Br J Dermatol. 2001;144:46–54. doi: 10.1046/j.1365-2133.2001.03951.x. [DOI] [PubMed] [Google Scholar]
- van der Steen P, Boezeman J, Duller P, Happle R. Can alopecia areata be triggered by emotional stress? An uncontrolled evaluation of 178 patients with extensive hair loss. Acta Derm Venereol. 1992;72:279–80. [PubMed] [Google Scholar]
- Weinberg J, Taylor AN, Gianoulakis C. Fetal ethanol exposure: hypothalamic-pituitary-adrenal and beta-endorphin responses to repeated stress. Alcohol Clin Exp Res. 1996;20:122–31. doi: 10.1111/j.1530-0277.1996.tb01054.x. [DOI] [PubMed] [Google Scholar]
- Young EA, Altemus M, Parkison V, Shastry S. Effects of estrogen antagonists and agonists on the ACTH response to restraint stress in female rats. Neuropsychopharmacology. 2001;25:881–91. doi: 10.1016/S0893-133X(01)00301-3. [DOI] [PubMed] [Google Scholar]
- Zhang X, Fitzsimmons RL, Cleland LG, Ey PL, Zannettino AC, Farmer EA, et al. CD36/fatty acid translocase in rats: distribution, isolation from hepatocytes, and comparison with the scavenger receptor SR-B1. Lab Invest. 2003;83:317–32. doi: 10.1097/01.lab.0000059923.67198.ba. [DOI] [PubMed] [Google Scholar]
- Zhang X, Sliwowska JH, Weinberg J. Prenatal alcohol exposure and fetal programming: effects on neuroendocrine and immune function. Exp Biol Med (Maywood) 2005;230:376–88. doi: 10.1177/15353702-0323006-05. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Kawana S, Aoki E, Katayama M, Nagano M, Suzuki H. Dynamic changes in nerve growth factor and substance P in the murine hair cycle induced by depilation. J Dermatol. 2006;33:833–41. doi: 10.1111/j.1346-8138.2006.00191.x. [DOI] [PubMed] [Google Scholar]
- Zoller M, McElwee KJ, Engel P, Hoffmann R. Transient CD44 variant isoform expression and reduction in CD4(+)/CD25(+) regulatory T cells in C3H/HeJ mice with alopecia areata. J Invest Dermatol. 2002;118:983–92. doi: 10.1046/j.1523-1747.2002.01745.x. [DOI] [PubMed] [Google Scholar]
- Zouboulis CC. Human skin: an independent peripheral endocrine organ. Horm Res. 2000;54:230–42. doi: 10.1159/000053265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primers used in this study.


