As many as 5.7 million people in the United States are living with hepatitis B virus (HBV) or hepatitis C virus (HCV) infection.1,2 Both infections are leading causes of liver cancer and infectious disease-related mortality.3 Therapies are available to suppress replication of HBV and eliminate or cure HCV infection, thereby dramatically decreasing the risk for liver cancer and death associated with these infections. However, at least half of the people living with HCV infection are unaware of their infection.4 Because most infected people develop few or no symptoms or signs of infection until their liver disease is far advanced, testing people for HBV and HCV is the critical first step toward identifying infected people and linking them to recommended care and treatment. As such, preventing HBV- and HCV-associated morbidity and mortality hinges on identifying effective testing strategies.
In 2011, the U.S. Department of Health and Human Services (HHS) released an action plan with goals for increasing the number of people living with HBV or HCV infection who are aware of their infection.5 Guided by the HHS action plan, the U.S. Centers for Disease Control and Prevention (CDC) developed the Hepatitis Testing and Linkage to Care (HepTLC) initiative to implement the agency's recommendations for HBV and HCV testing.4,6 In 2012, CDC received an appropriation from the Prevention and Public Health Fund (PPHF) for improvements in HBV and HCV testing. The PPHF is the nation's first mandatory funding stream dedicated to improving the U.S. public health system.7 With these funds, the U.S. Senate directed the CDC Division of Viral Hepatitis to increase the number of HBV- and HCV-infected people who are aware of their infection and receiving care.8 In 2013, CDC received resources from HHS to extend the PPHF-supported projects for another year.
In response to the Congressional directive, the HepTLC initiative included two demonstration projects: one targeting testing for HBV and the other targeting testing for HCV. The HBV demonstration project focused on improving HBV testing and linkage to care among U.S. communities of people born in countries with ≥2% prevalence of hepatitis B surface antigen (HBsAg), a marker of current HBV infection. Grantees were tasked with employing community-based approaches to provide services for populations that differed by English proficiency, familiarity with the U.S. health-care system, and other socioeconomic -factors. CDC supported nine programs that implemented HBV testing in seven states: California, Florida, Illinois, Minnesota, New York, Ohio, and Oregon.
The HCV demonstration project focused on improving HCV testing and linkage to care in clinical settings providing care for people born between 1945 and 1965 (i.e., baby boomers), who have the highest rate of chronic HCV infection and HCV-associated morbidity and mortality, and delivering care services for people who inject drugs, the population at greatest risk of transmitting and acquiring HCV infection. CDC supported 24 HCV testing and linkage-to-care programs located in Arizona, California, Colorado, Illinois, Maine, New York, North Carolina, Pennsylvania, Puerto Rico, South Carolina, Texas, Virginia, Washington, Washington (DC), and Wisconsin. For both HBV and HCV demonstration projects, CDC selected the targets for the measured activities deemed by agency subject experts as both ambitious and feasible for new local testing and linkage-to-care programs of short duration.
The articles in this supplemental issue of Public Health Reports (PHR) demonstrate the effectiveness of diverse HBV and HCV testing strategies employed by demonstration projects. During the project period, grantees executed project plans that approached or exceeded program goals regarding the number of people tested for HBV or HCV, the number of HBV- and HCV-positive people who received posttest counseling, and the number of people referred to recommended care and treatment services (Tables 1 and 2). A total of 23,144 participants received HBsAg testing and 64,716 participants received HCV antibody (anti-HCV) or HCV ribonucleic acid (RNA) testing. Compared with the prevalence of HBV and HCV in the general population,1,2 the high proportion of people testing positive for HBV infection (6%, 1,317/23,144) or with evidence of HCV infection (13%, 7,580/57,570) indicates that these demonstration projects appropriately targeted at-risk populations. To test foreign-born people for HBV infection, grantees employed community-based approaches and culturally and linguistically competent strategies in diverse settings, including refugee clinics, health fairs, and other community-based events targeting participants from multiple countries in Asia and Africa.
Table 1.
Benchmarks for hepatitis B virus testing and linkage-to-care programs during the Hepatitis Testing and Linkage to Care (HepTLC) initiative, nine U.S. programs, October 2012–September 2014
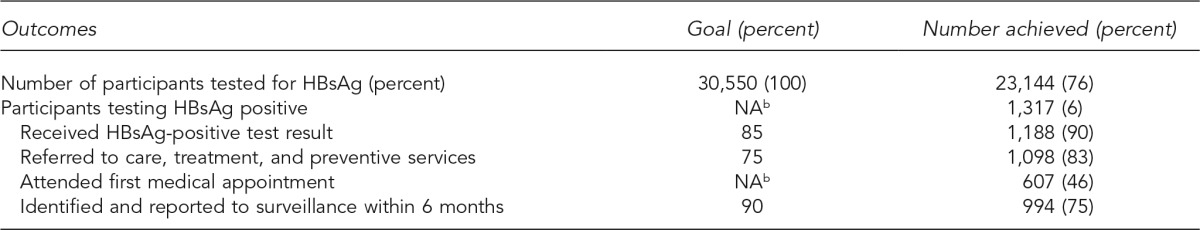
aThe HepTLC initiative promoted viral hepatitis B and hepatitis C screening, posttest counseling, and linkage to care at 34 U.S. sites, including nine programs for hepatitis B testing in California, Florida, Illinois, Minnesota, New York, Ohio, and Oregon.
bThere was no goal for this measure.
HBsAg = hepatitis B surface antigen
NA = not applicable
Table 2.
Benchmarks for hepatitis C virus testing and linkage to care during the Hepatitis Testing and Linkage to Care (HepTLC) initiative, 25 U.S. programs, October 2012–September 2014a
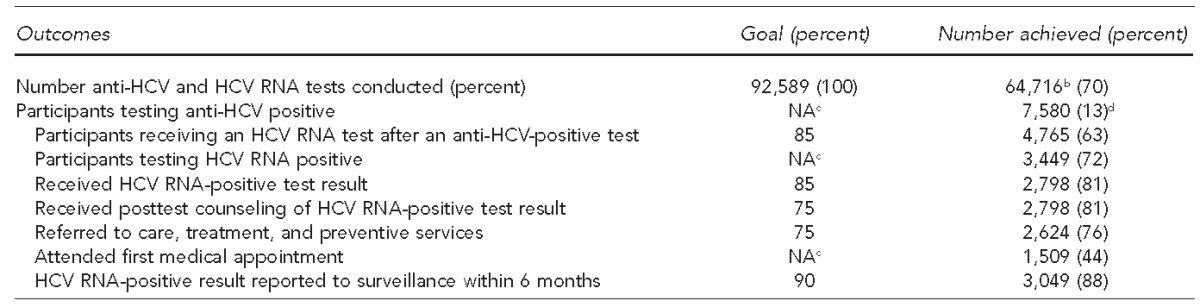
The HepTLC initiative promoted viral hepatitis B and hepatitis C screening, posttest counseling, and linkage to care at 34 U.S. sites, including 25 programs for hepatitis C testing in Arizona, California, Colorado, Illinois, Maine, New York, North Carolina, Pennsylvania, Puerto Rico, South Carolina, Texas, Virginia, Washington, Washington (DC), and Wisconsin.
bIncludes 57,570 participants who received an anti-HCV test and 7,146 participants who received an HCV RNA test without first obtaining an anti-HCV test.
cThere was no goal for this measure.
dBased on the 57,570 participants who received an anti-HCV test
anti-HCV = HCV antibody
HCV = hepatitis C virus
RNA = ribonucleic acid
NA = not applicable
For HBV and HCV programs, 1,188 of 1,317 (90%) HBsAg-positive participants and 2,798 of 3,449 (81%) HCV RNA-positive participants received their results, which exceeded the program goal of 75% for both indicators. Additionally, 1,098 of 1,317 (83%) HBsAg-positive participants and 2,624 of 3,449 (76%) HCV RNA-positive participants were referred to care, exceeding the program goal of 75% for both indicators. Data from these sites indicate that community-based organizations, public health agencies, and other stakeholders can collaborate with clinical care providers to implement HBV and HCV testing strategies, which successfully provide counseling and care referrals to people with chronic HBV and HCV infection.
Participating sites anecdotally highlighted factors associated with high rates of testing and linkage to care. These factors included establishing and maintaining strong relationships with local communities and clinical care providers, conducting linguistically appropriate outreach activities, and integrating services with existing community and clinic-based programs (e.g., refugee health clinics, community health centers) to reach target populations.
Data reported from these demonstration sites also revealed challenges. For example, HCV RNA testing, which is used to identify current HCV infection, was provided to 4,765 of 7,580 (63%) anti-HCV-positive participants, which was below the program goal of 85% completion of HCV testing to detect current HCV infection. Ensuring that HBV- and HCV-infected participants referred to care attended their first medical appointment also proved challenging. Only 607 of 1,098 (55%) participants referred for HBV care and 1,509 of 2,624 (58%) participants referred for HCV care attended their first medical appointment. For those infected with HBV, barriers to initial receipt of care included transportation, language, and lack of familiarity with the U.S. health-care system. The qualitative data provided by these sites suggest that patient navigators can help resolve some of these issues. For people infected with HCV, reflex testing (i.e., testing a single specimen for both anti-HCV and, for those with positive anti-HCV tests, HCV RNA) can increase the proportion of people diagnosed with current HCV infection. As HCV therapies become easier to administer, colocation of HCV testing and treatment in a primary care setting can increase the number and proportion of HCV-infected people who receive care and antiviral therapy.
With PPHF and HHS funding available, CDC guided the development of programs that increased testing for HBV and HCV infection, improved linkage to care, and made possible additional studies examining the implementation of CDC recommendations for HBV and HCV testing in emergency departments and other settings.6,7 The U.S. Preventive Services Task Force (USPSTF) has incorporated many of CDC's testing recommendations into its guidance for HCV (2013) and HBV (2014) testing.8,9
The data captured by the 34 programs highlighted in this supplemental issue of PHR can be used to guide implementation of CDC and USPSTF recommendations for viral hepatitis screening. With broad, equitable access to HBV and HCV testing, care, and treatment, the morbidity and mortality associated with HBV and HCV infection can be reduced, helping to eliminate hepatitis B and hepatitis C as public health threats in the United States.
REFERENCES
- 1.Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology. 2015;62:1353–63. doi: 10.1002/hep.27978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts H, Kruszon-Moran D, Ly KN, Hughes E, Iqbal K, Jiles RB, et al. Prevalence of chronic hepatitis B virus (HBV) infection in U.S. households: National Health and Nutrition Examination Survey (NHANES), 1988–2012. Hepatology. 2016;63:388–97. doi: 10.1002/hep.28109. [DOI] [PubMed] [Google Scholar]
- 3.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156:271–8. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 4.Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Ward JW. Hepatitis C virus testing of persons born during 1945–1965: recommendations from the Centers for Disease Control and Prevention. Ann Intern Med. 2012;157:817–22. doi: 10.7326/0003-4819-157-9-201211060-00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Department of Health and Human Services (US) Washington: HHS; 2011. Combating the silent epidemic of viral hepatitis: action plan for the prevention, care, and treatment of viral hepatitis. [Google Scholar]
- 6.Weinbaum CM, Williams I, Mast EE, Wang SA, Finelli L, Wasley A, et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57(RR-8):1–20. [PubMed] [Google Scholar]
- 7.Department of Health and Human Services (US) Prevention and Public Health Fund [cited 2016 Mar 18] Available from: http://www.hhs.gov/open/prevention/index.html.
- 8.Departments of Labor, Health and Human Services, and Education, and related agencies appropriation bill, 2012. The Committee on Appropriations. 112th Congress, 1st session; report 112-84 [cited 2016 Mar 18] Available from: https://www.congress.gov/112/crpt/srpt84/CRPT-112srpt84.pdf.
- 9.Galbraith JW, Franco RA, Donnelly JP, Rodgers JB, Morgan JM, Viles AF, et al. Unrecognized chronic hepatitis C virus infection among baby boomers in the emergency department. Hepatology. 2015;61:776–82. doi: 10.1002/hep.27410. [DOI] [PubMed] [Google Scholar]
- 10.Falade-Nwulia O, Mehta SH, Lasola J, Latkin C, Niculescu A, O'Connor C, et al. Public health clinic-based hepatitis C testing and linkage to care in Baltimore. J Viral Hepat. 2016 doi: 10.1111/jvh.12507. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moyer VA U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:349–57. doi: 10.7326/0003-4819-159-5-201309030-00672. [DOI] [PubMed] [Google Scholar]
- 12.LeFevre ML U.S. Preventive Services Task Force. Screening for hepatitis B virus infection in nonpregnant adolescents and adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:58–66. doi: 10.7326/M14-1018. [DOI] [PubMed] [Google Scholar]


