Abstract
Objective
Following its recommendation for one-time hepatitis C virus (HCV) testing of people born between 1945 and 1965, CDC implemented the Hepatitis Testing and Linkage to Care (HepTLC) initiative to conduct birth-cohort hepatitis testing in U.S. health-care settings. We describe demographic characteristics, HCV infection prevalence, and HCV-related risk factors among people born between 1945 and 1965 who were tested as part of the program, which ran from 2012 to 2014.
Methods
As part of the HepTLC initiative, 14 grantees supporting 104 health-care sites in 21 U.S. municipalities tested participants born between 1945 and 1965 for HCV antibody (anti-HCV). Demographic characteristics and HCV risk factors were reported for people tested for anti-HCV and who were anti-HCV or HCV RNA positive. We evaluated outcomes along the HCV testing-to-care continuum using the following indicators: anti-HCV positive, HCV RNA test offered, HCV RNA positive, referred to care, and attended first medical appointment.
Results
Among 24,966 people tested for HCV infection, 2,900 (11.6%) were anti-HCV positive. Anti-HCV positivity was highest among those who self-identified as non-Hispanic black (n=1,701 of 12,202, 13.9%), men (n=2,073 of 12,130, 17.1%), and people born between 1951 and 1955 (n=795 of 5,768, 13.8%). Of the 2,900 people testing anti-HCV positive, 2,108 (72.7%) received an HCV RNA test, 1,497 (51.6%) were HCV RNA positive, 1,201 (41.4%) were referred to care, and 938 (32.3%) attended their first appointment.
Conclusion
Testing for HCV infection among those born between 1945 and 1965 without soliciting HCV risk factors was successful. Providers implementing birth-cohort testing should develop and evaluate strategies to improve outcomes along the testing-to-care continuum.
Approximately 2.7 million noninstitutionalized people, and an additional 375,000 to 750,000 homeless and incarcerated people, are currently infected with the hepatitis C virus (HCV) in the United States.1,2 Infections are often asymptomatic, and more than 50% of people are estimated to be unaware of their chronic HCV infection.3–6 Chronic HCV infections have contributed to an increasing incidence of chronic liver disease, hepatocellular carcinoma, and premature mortality.7–12 Absent interventions to increase awareness of chronic HCV infection and link people to care, HCV-associated morbidity and mortality will continue to rise.13
Since 1998, the Centers for Disease Control and Prevention (CDC) has recommended routine HCV antibody (anti-HCV) testing of people with exposures associated with HCV infection, including past or present injection drug use (IDU) and people with human immunodeficiency virus (HIV) infection.14 However, the high proportion of people who were unaware of their HCV infection status, coupled with rising HCV-associated morbidity and mortality, prompted CDC to expand these recommendations in 2012 to include a one-time HCV test for all people born between 1945 and 1965.3 The U.S. Preventive Services Task Force issued a similar recommendation in 2013.3,15
It is critical to assess how recommendations translate into practice to ensure guidelines are followed and to identify gaps to inform future policy development. For example, evaluations of HIV testing recommendations have identified gaps along the HIV care continuum to improve the proportion of HIV-infected individuals who are diagnosed, accessing and retained in medical care, obtaining antiretroviral therapy, and achieving viral suppression.16–18 Evaluating birth-cohort testing recommendations over time is also expected to identify gaps along the HCV testing-to-care continuum. Addressing these challenges may help to improve identification of HCV-infected people and link those infected to highly effective and tolerable HCV antiviral treatments to avert HCV-associated morbidity and mortality.
CDC's Hepatitis Testing and Linkage to Care (HepTLC) initiative provided an opportunity to assess the implementation of birth-cohort testing recommendations. This initiative explored methods for identifying people infected with HCV, including birth-cohort testing, and ways to link chronically infected people to care. For this article, we report HCV birth-cohort testing and linkage-to-care results, including demographic characteristics, risk-factor prevalence, and anti-HCV and HCV ribonucleic acid (RNA) positivity. We also discuss the proportion of people who completed each step of the HCV testing-to-care continuum, including the proportion of anti-HCV-positive people who were tested for HCV RNA and the proportion of chronically infected people who were linked to care. Finally, we examine how the use of HCV RNA same-day testing and assisted linkage-to-care strategies affects the testing-to-care continuum.
METHODS
Study population
CDC funded 14 grantees supporting 104 testing sites in 21 U.S. municipalities to implement birth-cohort testing for HepTLC. Grantees were asked to recruit previously undiagnosed people born between 1945 and 1965 for anti-HCV testing in different clinical settings, including emergency departments, federally qualified health centers, community health clinics, sexually transmitted disease (STD) clinics, and state health departments. Sites were located in San Diego, California; Denver, Colorado; Atlanta, Georgia; Bronx, Ellenville, Rochester, and Queens, New York; Durham, North Carolina; Philadelphia, Pennsylvania; Aguas Buenas, Caguas, San Juan, and Yabucoa, Puerto Rico; Bamberg, Cheraw, Fairfax, Florence, Mullens, and Orangeburg, South Carolina; San Antonio, Texas; and Washington, D.C. Each grantee targeted people in the birth cohort but also followed CDC recommendations to test people who reported HCV risk factors, such as IDU.
For the current study, we included all people born between 1945 and 1965 who were tested for anti-HCV or HCV RNA at each testing site. Grantees de-duplicated the data to exclude people with multiple testing sessions at different sites within the same grantee project.
Data sources
HCV testing occurred from October 1, 2012, to June 28, 2014. Data were extracted from the data collection and management system, EvaluationWeb®,19 on September 26, 2014, to allow 90 days of follow-up data for participants who received results, were referred to care, or started treatment after June 28, 2014. Participants self-reported birth year, sex, race/ethnicity, health insurance status and type, and risk-factor information, and site staff members entered these data into EvaluationWeb. Age was calculated based on reported birth year and date of HCV testing and grouped into four categories: 1945–1950 (aged 64–69 years), 1951–1955 (aged 59–63 years), 1956–1960 (aged 54–58 years), and 1961–1965 (aged 49–53 years). Race/ethnicity was categorized as non-Hispanic black, non-Hispanic white, Hispanic or Latino, and “other” (i.e., non-Hispanic Asian, Native Hawaiian or other Pacific Islander, and American Indian or Alaska Native). Health insurance was categorized as public, private, or no insurance. Risk factors included lifetime IDU (i.e., ever injected drugs), IDU in the past 12 months, and HIV infection. Project staff members at each testing site obtained anti-HCV test date and results, HCV RNA test date and results, referral to care, and attendance of first medical appointment, and entered these data into EvaluationWeb. Those with a positive HCV RNA test result were considered to have chronic HCV infection.
Analysis
We summed counts and calculated percentages for three groups (i.e., those who were anti-HCV positive, those who were tested for HCV RNA, and those who were HCV RNA positive) by demographic characteristic and risk factor. Percentages of those testing anti-HCV positive, tested for HCV RNA, and testing HCV RNA positive were calculated by dividing the total sums by the number of study participants, the number that tested anti-HCV positive, and the number that were tested for HCV RNA, respectively, for each demographic or risk-factor group.
We constructed a testing-to-care continuum to operationalize the steps required to confirm chronic HCV infection and link infected people to care. We defined steps along the testing-to-care continuum as anti-HCV-positive test result, HCV RNA test, HCV RNA-positive test result, referred to care, and attended first appointment. People were required to have completed each step in the continuum to be counted in successive steps. Discrepancies between the overall HCV RNA test and HCV RNA-positive counts and the counts reported along the testing-to-care continuum resulted from this inclusion requirement for the testing-to-care continuum and because some sites tested for HCV RNA first as part of their testing algorithm.
We examined the testing-to-care continuum using two different approaches. In the first approach, we calculated the proportion of anti-HCV-positive people who completed each step of the testing-to-care continuum; we divided the total number of people who completed each step of the continuum (numerator) by the initial number of those who tested anti-HCV positive (denominator). In the second approach, we calculated the proportion of people who completed each successive step in the testing-to-care continuum; we divided the total number of people who completed each step in the continuum (numerator) by the total number of people who completed the previous step (denominator).
Additionally, we evaluated the success of improving confirmatory testing (i.e., HCV RNA testing) by comparing the proportion of people who tested anti-HCV positive who received same-day HCV RNA testing with the proportion of people who tested anti-HCV positive and received an HCV RNA test one or more days after the positive anti-HCV test.
We also compared assisted-linkage strategies with passive-linkage strategies to ensure HCV-infected people were linked to medical care. We categorized scheduling a date for a follow-up medical appointment with a specialist or primary care physician as an assisted-linkage method, and we categorized only providing a referral to a specialist, primary care physician, or a medical facility as a passive-linkage method. We evaluated passive- and assisted-linkage methods by comparing the proportion of chronically infected people (i.e., HCV RNA positive) who attended a first medical appointment. We conducted analyses using SAS® version 9.3.20
RESULTS
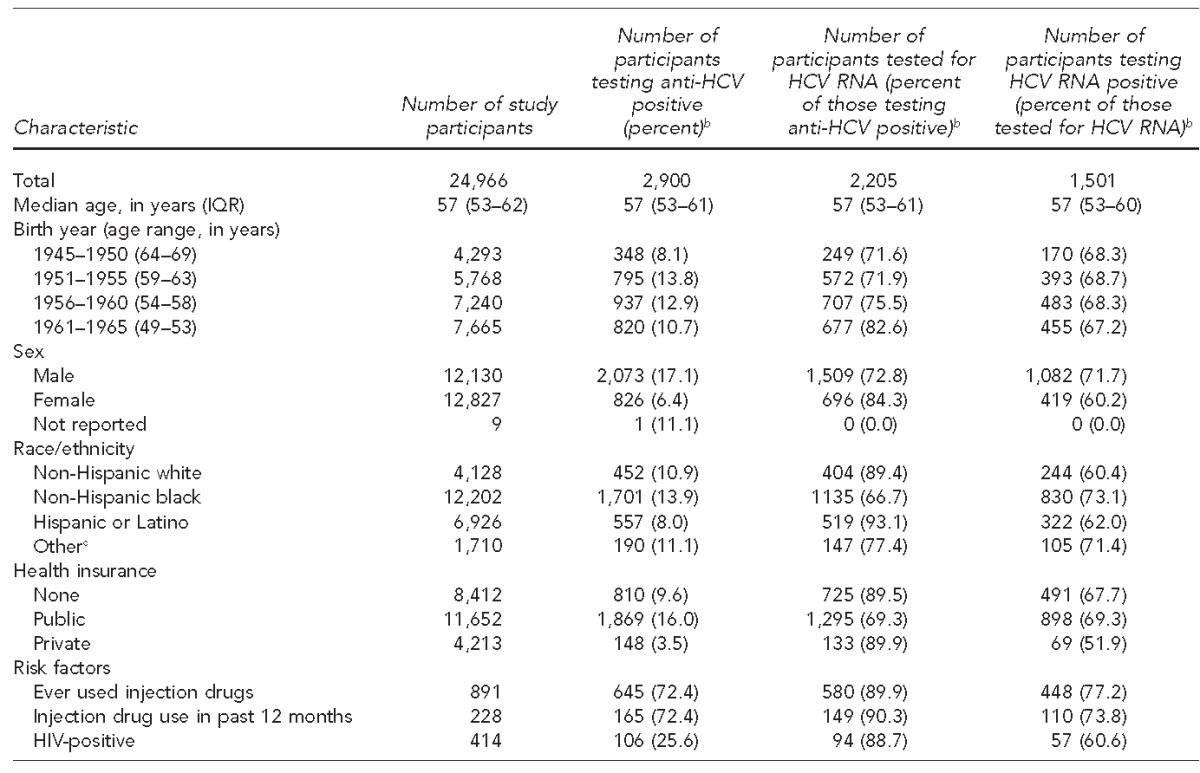
A total of 24,966 people born between 1945 and 1965 were tested for anti-HCV during the HepTLC project. Of the 24,966 people who were tested, testing frequency was highest among people born between 1961 and 1965 (n=7,665, 30.7%), those who self-identified as non-Hispanic black (n=12,202, 48.9%), and women (n=12,827, 51.4%). Furthermore, 11,652 (46.7%) patients were publicly insured and 891 (3.6%) reported ever injecting drugs (Table).
Table.
Characteristics and HCV test results of people born between 1945 and 1965 in the Hepatitis Testing and Linkage to Care (HepTLC) initiative, 21 U.S. municipalities, 2012–2014a
The HepTLC initiative promoted viral hepatitis B and hepatitis C screening, posttest counseling, and linkage to care at 34 U.S. sites. Hepatitis C testing sites were located in San Diego, California; Denver, Colorado; Atlanta, Georgia; Bronx, Ellenville, Rochester, and Queens, New York; Durham, North Carolina; Philadelphia, Pennsylvania; Aguas Buenas, Caguas, San Juan, and Yabucoa, Puerto Rico; Bamberg, Cheraw, Fairfax, Florence, Mullens, and Orangeburg, South Carolina; San Antonio, Texas; and Washington, D.C.
bPercentages are row percentages.
cIncludes non-Hispanic Asian, Native Hawaiian or Pacific Islander, and American Indian or Alaska Native
HCV = hepatitis C virus
anti-HCV = hepatitis C virus antibody
RNA = ribonucleic acid
IQR = interquartile range
HIV = human immunodeficiency virus
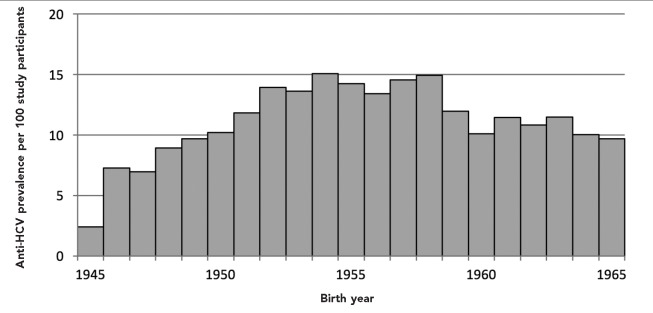
Of the 24,966 people who received an anti-HCV test, 2,900 (11.6%) were anti-HCV positive. Anti-HCV positivity was highest among those who self-identified as non-Hispanic black (n=1,701 of 12,202, 13.9%), men (n=2,073 of 12,130, 17.1%), and people born between 1951 and 1955 (n=795 of 5,768, 13.8%). Anti-HCV prevalence was lowest among people born in 1945 (2.4%), peaked among people born between 1954 and 1958 (range 13.4%–15.1%), and ranged from 9.7% to 11.9% for people born after 1958 (Figure 1). Demographic and risk characteristics among the 1,501 people who tested HCV RNA positive were similar to those who were anti-HCV positive.
Figure 1.
Anti-HCV prevalence per 100 study participants, by birth year, among people born between 1945 and 1965 who were tested for HCVa in the Hepatitis Testing and Linkage to Care (HepTLC) initiative, 21 U.S. municipalities, 2012–2014b
aOf 24,966 participants who were tested, 2,900 were anti-HCV positive.
bThe HepTLC initiative promoted viral hepatitis B and hepatitis C screening, posttest counseling, and linkage to care at 34 U.S. sites. Hepatitis C testing sites were located in San Diego, California; Denver, Colorado; Atlanta, Georgia; Bronx, Ellenville, Rochester, and Queens, New York; Durham, North Carolina; Philadelphia, Pennsylvania; Aguas Buenas, Caguas, San Juan, and Yabucoa, Puerto Rico; Bamberg, Cheraw, Fairfax, Florence, Mullens, and Orangeburg, South Carolina; San Antonio, Texas; and Washington, D.C.
anti-HCV = hepatitis C virus antibody
HCV = hepatitis C virus
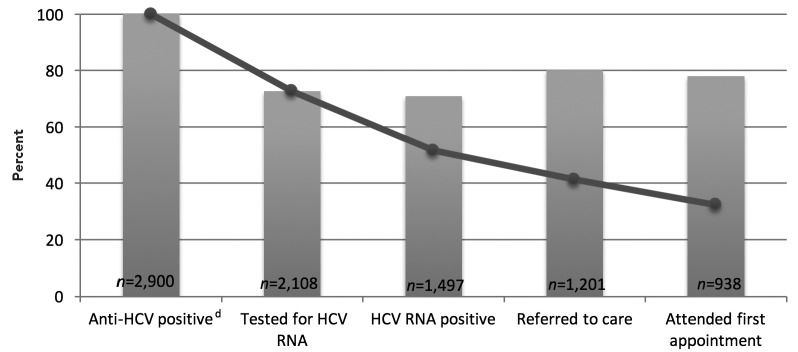
Of the 2,900 people who tested anti-HCV positive, 1,497 (51.6%) were confirmed with chronic infection by a positive HCV RNA test, and 938 (32.3%) attended a first medical appointment (Figure 2). Of the 2,900 people who tested anti-HCV positive, 2,108 (72.7%) received an HCV RNA test. Of the 1,497 people with chronic infection, 1,201 (80.2%) were referred to care.
Figure 2.
Percent of people born between 1945 and 1965 testing anti-HCV positive (n=2,900) who completed steps in the testing-to-care continuum, Hepatitis Testing and Linkage to Care (HepTLC) initiative, 21 U.S. municipalities, 2012–2014a–c
aThe HepTLC initiative promoted viral hepatitis B and hepatitis C screening, posttest counseling, and linkage to care at 34 U.S. sites. Hepatitis C testing sites were located in San Diego, California; Denver, Colorado; Atlanta, Georgia; Bronx, Ellenville, Rochester, and Queens, New York; Durham, North Carolina; Philadelphia, Pennsylvania; Aguas Buenas, Caguas, and San Juan, Yabucoa, Puerto Rico; Bamberg, Cheraw, Fairfax, Florence, Mullens, Orangeburg, South Carolina; San Antonio, Texas; and Washington, D.C.
bLine graph represents the percentage of all anti-HCV-positive people who completed each step of the testing-to-care continuum.
cBar graph represents the percentage of people who completed each successive step of the testing-to-care continuum.
dApproximately 20%–30% of HCV-infected individuals will clear the virus spontaneously.
anti-HCV = hepatitis C virus antibody
HCV = hepatitis C virus
RNA = ribonucleic acid
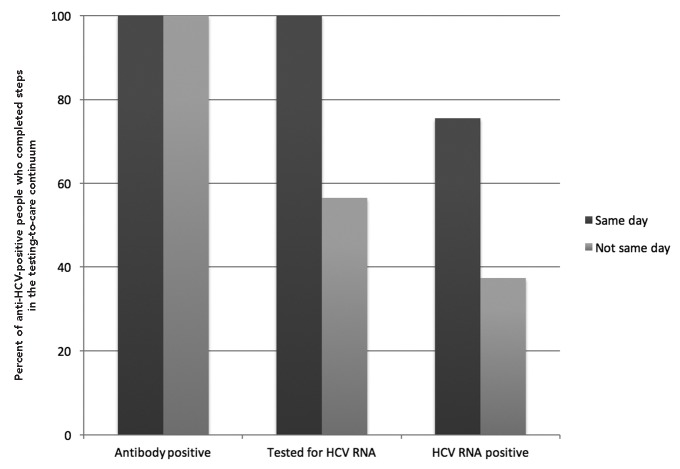
All 1,088 anti-HCV-positive people who received same-day HCV RNA testing were tested for HCV RNA, and 822 (75.6%) were identified as chronically infected (Figure 3). Of the 1,805 anti-HCV-positive people who did not receive same-day testing, 1,020 (56.5%) received an HCV RNA test and 675 (37.4%) were identified as chronically infected. Among the 818 chronically infected people who received assisted linkage to care, 696 (85.1%) successfully attended their first appointment. Among the 383 chronically infected people who were passively linked to care, 235 (61.4%) successfully attended their first medical appointment.
Figure 3.
Proportion of anti-HCV-positive people who were tested for HCV RNA and tested HCV RNA positive, by type of HCV RNA testing (n=2,893), in the Hepatitis Testing and Linkage to Care (HepTLC) initiative, 21 U.S. municipalities, 2012–2014a–d
aThe HepTLC initiative promoted viral hepatitis B and hepatitis C screening, posttest counseling, and linkage to care at 34 U.S. sites. Hepatitis C testing sites were located in San Diego, California; Denver, Colorado; Atlanta, Georgia; Bronx, Ellenville, Rochester, and Queens, New York; Durham, North Carolina; Philadelphia, Pennsylvania; Aguas Buenas, Caguas, and San Juan, Yabucoa, Puerto Rico; Bamberg, Cheraw, Fairfax, Florence, Mullens, Orangeburg, South Carolina; San Antonio, Texas; and Washington, D.C.
bSeven people without a valid antibody test date were excluded.
cAntibody positive for same-day testing included 1,088 participants.
dAntibody positive for non-same-day testing included 1,805 participants.
anti-HCV = hepatitis C virus antibody
HCV = hepatitis C virus
RNA = ribonucleic acid
DISCUSSION
HCV testing and follow-up
This project demonstrated that birth-cohort testing can be successfully implemented in a variety of health-care settings. CDC's 1998 HCV testing recommendations have been less successful in identifying people living with HCV infection; in a qualitative assessment of these recommendations, providers reported that the complexity of multiple risk factors and discomfort in asking questions about socially undesirable behaviors were barriers to effective risk-based screening implementation.14,21 An important strength of birth-cohort testing is identifying infected people who are reluctant to disclose a history of IDU or other risky behaviors, or providers who are uncomfortable discussing these behaviors with their patients.
Those who self-identified as non-Hispanic black, men, and people reporting past or present IDU were more likely to test anti-HCV positive—findings similar to previous HCV birth-cohort studies.1,22,23 However, anti-HCV prevalence was higher in the current analysis than in national birth-cohort studies,22,23 likely because of the higher level of risk for HCV infection among the populations served by the testing sites that participated in this project (i.e., federally qualified health centers, STD clinics, community health centers, and health department clinics).5,24–26 Providers seeking to implement birth-cohort testing should consider the clinic population being served and ensure that resources will be available to provide care to newly diagnosed patients.
Although anti-HCV testing was successfully implemented in health-care settings, grantees had difficulty obtaining follow-up HCV RNA tests for anti-HCV--positive patients. These results are consistent with previous research reporting that people born between 1945 and 1965 are less likely than other birth cohorts to have a follow-up HCV RNA test within six months of a positive anti-HCV test, and an estimated 63%–77% of people who test anti-HCV positive receive some follow-up viral hepatitis care, such as HCV RNA -testing.27,28 On the other hand, same-day HCV RNA testing compared with testing anti-HCV-positive patients on a different day following the antibody test enabled the identification of a greater proportion of people with chronic HCV infection. Providers implementing birth-cohort testing in the clinical setting should strive to provide all anti-HCV-positive people with an HCV RNA test in a single medical visit through same-day HCV RNA -testing or HCV RNA reflex testing. Reflex testing enables providers to use the same blood sample that was used for HCV-antibody testing for HCV RNA testing, without the need for patients to return to the testing site for another blood draw.29
Linkage to care
About three-quarters of all HCV RNA-positive people who were referred to care attended an initial medical appointment. However, assisted-linkage strategies were more effective than passive-linkage strategies in enabling people with chronic HCV infection to attend a first medical appointment (85.1% vs. 61.4%). Providers implementing birth-cohort testing should consider using assisted-linkage strategies or other effective linkage-to-care models to ensure that all HCV-infected people are successfully linked to care.
Factors that were beyond the scope of this project for which we did not collect data, but which may have negatively affected the testing-to-care continuum, included being underinsured, the perception that treatments are long and have undesirable side effects, geographic barriers to care, or shortage of skilled HCV clinical providers.3,30 Higher coverage of quality health insurance, the promise of shorter duration and highly effective all-oral treatment regimens, same-day testing, enhanced provider workforce, and utilization of assisted-linkage strategies may help to increase the number of people diagnosed, entered, and retained in care.
Limitations
This study was subject to several limitations. First, people with a positive anti-HCV result may have received HCV RNA testing and follow-up outside of the HepTLC grantee network. As such, the 71% of anti-HCV-positive people who received a follow-up HCV RNA test should be interpreted as the minimum percentage who received a follow-up HCV RNA test. Second, all demographic and risk-factor data from patients were self-reported. Disclosure of past or current IDU, a major risk factor for HCV infection, is often underreported. Third, although grantees were instructed to target undiagnosed people in the birth cohort for anti-HCV testing, data on previous HCV testing and diagnosis were not collected. It is possible that some participants may have known their anti-HCV status prior to this project. However, people with a previous diagnosis represent a small minority of the study population and most anti-HCV testing results represent newly diagnosed people. Fourth, we were unable to include analyses by clinical setting because of multiple limitations, such as lack of standardization or data collected on participant recruitment, testing procedures, and linkage-to-care interventions across clinical settings. These limitations may bias results by clinical setting or produce results that cannot be explained.
CONCLUSION
This study highlights successful implementation of birth-cohort HCV testing in a variety of health-care settings across 21 U.S. municipalities. Adoption of CDC recommendations for one-time HCV testing of people born between 1945 and 1965 should be supported, and efforts should prioritize reaching the populations at greatest risk for HCV infection. However, challenges remain to improve outcomes along the testing-to-care continuum. Adherence to CDC guidelines, use of same-day HCV RNA testing, ensuring availability of trained providers, and assisted-linkage strategies may help to improve the testing-to-care continuum. Continued birth-cohort HCV testing and identification of people with chronic HCV infection, and improved linkage to care in combination with new, effective all-oral HCV treatments, will help to decrease HCV-associated morbidity and mortality.
Footnotes
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). This study reported on a CDC-funded programmatic activity for which institutional review board approval was waived.
REFERENCES
- 1.Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chak E, Talal AH, Sherman KE, Schiff ER, Saab S. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090–101. doi: 10.1111/j.1478-3231.2011.02494.x. [DOI] [PubMed] [Google Scholar]
- 3.Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Teo CG, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012;61(RR-4):1–32. [PubMed] [Google Scholar]
- 4.Roblin DW, Smith BD, Weinbaum CM, Sabin ME. HCV screening practices and prevalence in an MCO, 2000–2007. Am J Manag Care. 2011;17:548–55. [PubMed] [Google Scholar]
- 5.Southern WN, Drainoni ML, Smith BD, Christiansen CL, McKee D, Gifford AL, et al. Hepatitis C testing practices and prevalence in a high-risk urban ambulatory care setting. J Viral Hepat. 2011;18:474–81. doi: 10.1111/j.1365-2893.2010.01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spradling PR, Rupp L, Moorman AC, Lu M, Teshale EH, Gordon SC, et al. Hepatitis B and C virus infection among 1.2 million persons with access to care: factors associated with testing and infection prevalence. Clin Infect Dis. 2012;55:1047–55. doi: 10.1093/cid/cis616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Kamary SS, Jhaveri R, Shardell MD. All-cause, liver-related, and non–liver-related mortality among HCV-infected individuals in the general US population. Clin Infect Dis. 2011;53:150–7. doi: 10.1093/cid/cir306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Serag HB. Epidemiology of hepatocellular carcinoma in USA. Hepatol Res. 2007;37(Suppl 2):S88–94. doi: 10.1111/j.1872-034X.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 9.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513–21. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 10.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156:271–8. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 11.Ly KN, Xing J, Klevens RM, Jiles RB, Holmberg SD. Causes of death and characteristics of decedents with viral hepatitis, United States, 2010. Clin Infect Dis. 2014;58:40–9. doi: 10.1093/cid/cit642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahajan R, Xing J, Liu SJ, Ly KN, Moorman AC, Rupp L, et al. Mortality among persons in care with hepatitis C virus infection: the Chronic Hepatitis Cohort Study (CHeCS), 2006–2010 [published erratum appears in Clin Infect Dis 2014;58:1792] Clin Infect Dis. 2014;58:1055–61. doi: 10.1093/cid/ciu077. [DOI] [PubMed] [Google Scholar]
- 13.Rein DB, Wittenborn JS, Weinbaum CM, Sabin M, Smith BD, Lesesne SB. Forecasting the morbidity and mortality associated with prevalent cases of pre-cirrhotic chronic hepatitis C in the United States. Dig Liver Dis. 2011;43:66–72. doi: 10.1016/j.dld.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Alter MJ, Margolis HS, Bell BP, Bice SD, Buffington J, Chamberland M, et al. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR-19):1–39. [PubMed] [Google Scholar]
- 15.Moyer VA U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:349–57. doi: 10.7326/0003-4819-159-5-201309030-00672. [DOI] [PubMed] [Google Scholar]
- 16.Skarbinski J, Rosenberg E, Paz-Bailey G, Hall HI, Rose CE, Viall AH, et al. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern Med. 2015;175:588–96. doi: 10.1001/jamainternmed.2014.8180. [DOI] [PubMed] [Google Scholar]
- 17.Ulett KB, Willig JH, Lin HY, Routman JS, Abroms S, Allison J, et al. The therapeutic implications of timely linkage and early retention in HIV care. AIDS Patient Care STDS. 2009;23:41–9. doi: 10.1089/apc.2008.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNairy ML, El-Sadr WM. The HIV care continuum: no partial credit given. AIDS. 2012;26:1735–8. doi: 10.1097/QAD.0b013e328355d67b. [DOI] [PubMed] [Google Scholar]
- 19.Luther Consulting, LLC. Carmel (IN): Luther Consulting, LLC; 2013. EvaluationWeb®: Version 5. [Google Scholar]
- 20.SAS Institute, Inc. Cary (NC): SAS Institute, Inc.; 2011. SAS®: Version 9.3. [Google Scholar]
- 21.Jewett A, Garg A, Meyer K, Wagner LD, Krauskopf K, Brown KA, et al. Hepatitis C virus testing perspectives among primary care physicians in four large primary care settings. Health Promot Pract. 2015;16:256–63. doi: 10.1177/1524839914532291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–14. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 23.Smith BD, Beckett GA, Yartel A, Holtzman D, Patel N, Ward JW. Previous exposure to HCV among persons born during 1945–1965: prevalence and predictors, United States, 1999–2008. Am J Public Health. 2014;104:474–81. doi: 10.2105/AJPH.2013.301549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galbraith JW, Franco RA, Donnelly JP, Rodgers JB, Morgan JM, Viles AF, et al. Unrecognized chronic hepatitis C virus infection among baby boomers in the emergency department. Hepatology. 2015;61:776–82. doi: 10.1002/hep.27410. [DOI] [PubMed] [Google Scholar]
- 25.Kelen GD, Green GB, Purcell RH, Chan DW, Qagish BF, Sivertson KT, et al. Hepatitis B and hepatitis C in emergency department patients. N Engl J Med. 1992;326:1399–404. doi: 10.1056/NEJM199205213262105. [DOI] [PubMed] [Google Scholar]
- 26.Litwin AH, Smith BD, Drainoni ML, McKee D, Gifford AL, Koppelman E, et al. Primary care-based interventions are associated with increases in hepatitis C virus testing for patients at risk. Dig Liver Dis. 2012;44:497–503. doi: 10.1016/j.dld.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med. 2013;368:1859–61. doi: 10.1056/NEJMp1302973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spradling PR, Tong X, Rupp LB, Moorman AC, Lu M, Teshale EH, et al. Trends in HCV RNA testing among HCV antibody-positive persons in care, 2003–2010. Clin Infect Dis. 2014;59:976–81. doi: 10.1093/cid/ciu509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Getchell JP, Wroblewski KE, DeMaria A, Jr, Bean CL, Parker MM, Pandori M, et al. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep. 2013;62(18):362–5. [PMC free article] [PubMed] [Google Scholar]
- 30.Yehia BR, Schranz AJ, Umscheid CA, Lo Re V., III The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS One. 2014;9:e101554. doi: 10.1371/journal.pone.0101554. [DOI] [PMC free article] [PubMed] [Google Scholar]