Abstract
Objective
People who inject drugs (PWID) are at increased risk for hepatitis C virus (HCV) infection. We examined HCV testing outcomes among PWID through CDC's Hepatitis Testing and Linkage to Care initiative, which promoted viral hepatitis B and hepatitis C screening, posttest counseling, and linkage to care at 34 U.S. sites during 2012–2014. Ten grantees in nine geographically diverse cities conducted HCV testing among PWID.
Methods
Among those testing positive for HCV antibody (anti-HCV), we calculated the proportion who were offered a confirmatory HCV ribonucleic acid (RNA) test, positively diagnosed, and referred to a specialist for care. We stratified anti-HCV-positive people who completed each step by same-day testing (i.e., an HCV RNA test administered on the same date as an anti-HCV test) vs. person not receiving same-day testing to evaluate whether the need for follow-up testing affected diagnosis of chronic infection and linkage to care.
Results
A total of 15,274 people received an anti-HCV test at 84 testing sites targeting PWID. Of those, 11,159 (73%) reported having injected drugs in their lifetime, 7,789 (51%) reported injecting drugs in the past 12 months, and 3,495 (23%) tested anti-HCV positive. A total of 1,630 people received testing for HCV RNA, of whom 1,244 (76%) were HCV RNA positive. When not receiving both tests on the same day, 601 of 2,465 (24%) anti-HCV-positive people received an HCV RNA test.
Conclusion
Strategies to diagnose PWID for HCV infection are needed to reduce associated morbidity and mortality. Agencies can substantially increase the number of PWID who are diagnosed and informed of their HCV infection by administering both anti-HCV and HCV RNA tests during a single testing event.
Approximately three million people in the United States are currently infected with the hepatitis C virus (HCV).1 HCV infection substantially increases the risk of liver failure, cirrhosis, and hepatocellular carcinoma, and contributes to an estimated 17,000 deaths annually.2,3 Percutaneous exposure to contaminated blood via illicit drug injecting is the chief risk factor for HCV infection. Roughly 6.6 million people have reported injecting drugs in their lifetime, and more than 700,000 people are estimated to have injected in the past year.4 Among people who inject drugs (PWID), approximately 30%–70% are HCV antibody (anti-HCV) positive,5 and HCV incidence among PWID is high (<40 per 100 person-years), especially among PWID aged 18–29 years.6
An estimated 15%–25% of people infected with HCV will clear the virus within six months of initial exposure.7 Those who develop chronic infection can be asymptomatic for years while still remaining at risk for sequelae associated with disease progression.8 Without such early symptoms, many people infected with HCV are unaware of their infection.9 Yet, anti-HCV positivity alone (i.e., without a confirmatory HCV ribonucleic acid [RNA] test) is not a diagnosis for current infection, because it can also indicate a past HVC infection that has resolved or a biologic false positivity. An estimated 30% of those testing anti-HCV positive never receive an HCV RNA test to confirm current infection, leaving them undiagnosed for chronic infection and ineligible for follow-up care.10
In 2013, the Centers for Disease Control and Prevention (CDC) published updated guidelines for clinicians that recommended conducting two tests, anti-HCV followed by HCV RNA by polymerase chain reaction, to accurately identify current infection.11 Administering both tests on the same day during a single testing appointment has been shown to increase both the number of anti-HCV-positive people who receive a confirmatory HCV RNA test12 and the number of people diagnosed with current infection.13,14
To increase the number of people with viral hepatitis who are tested, diagnosed, and linked to care, CDC implemented the Hepatitis Testing and Linkage to Care (HepTLC) initiative. HepTLC was designed to support programs that could effectively target populations most affected by hepatitis B virus (HBV) and HCV infection (e.g., PWID) and link them to care.15 We present results from one aspect of the HepTLC initiative that targeted PWID to highlight persistent gaps in the testing-to-care continuum for PWID seeking diagnosis and treatment for HCV infection.
METHODS
Study population
Ten CDC grantees supported 84 sites in nine geographically diverse U.S. cities: Tucson, Arizona; Chicago, Illinois; Los Angeles and Oakland, California; Portland, Maine; New York City, New York; Seattle, Washington; Richmond, Virginia; and Milwaukee, Wisconsin. Grantees aimed to increase the number of PWID who were tested for HCV infection and linked to care. The study population included all individuals tested for anti-HCV at these sites. Testing sites comprised syringe services programs, Ryan White-funded clinics, sexually transmitted disease clinics, local and state health departments, and other community-care organizations. Sites used several methods to recruit PWID for testing, including peer-based recruitment and targeted outreach at community health events and clinics.
As part of the patient recruitment for this initiative, CDC recommended that HepTLC grantees follow all CDC guidelines for HCV testing and linkage to care.16 Grantees targeting PWID followed CDC recommendations for identifying HCV infection,11 which included testing people born between 1945 and 1965 for anti-HCV—otherwise known as birth-cohort testing—and testing those with reported behavioral risk (i.e., injection drug use).17 Because both groups are included in CDC's HCV testing recommendations, we were unable to determine if individuals were tested because of their birth year or because they injected drugs. Accordingly, we included in the analysis all people tested at grantee-supported sites targeting PWID. Furthermore, to allow maximum flexibility in testing capacity and workflow, testing sites were allowed to use existing anti-HCV testing infrastructure or provided recommendations on which anti-HCV test kits were most effective to identify anti-HCV-positive participants. Similar flexibility was allowed for confirmatory HCV RNA tests; sites collaborated with hospitals or other laboratories to determine whether qualitative or quantitative HCV RNA tests were applicable. Grantees de-duplicated data to exclude participants with multiple testing sessions.
Data collection
People visiting testing sites were asked to provide social and demographic information, including sex, birth year, race/ethnicity, and health insurance status. We calculated age based on reported birth year and date of testing and categorized race/ethnicity as non-Hispanic black, non-Hispanic white, and Hispanic. Because of small sample sizes, we categorized people who self-identified as Asian, American Indian/Alaska Native, and Native Hawaiian/Pacific Islander as “other.”
Participants reported any behavioral risks associated with their HCV infection, including injection drug use in the past 12 months or having ever injected drugs. Staff members collected data on HCV testing and linkage to care, including anti-HCV and HCV RNA test results, dates of testing, referrals, and attendance at first medical appointment. Participants with an indeterminate anti-HCV test result were given an additional anti-HCV or HCV RNA test. Anti-HCV-positive participants who agreed to an HCV RNA test received either a quantitative or qualitative test. For the quantitative HCV RNA test, ≥10 international units/milliliter was categorized as chronic HCV infection.
We included participants whose first testing visit occurred between October 1, 2012, and June 28, 2014, in our analysis. Staff members at testing sites entered all data into EvaluationWeb®, a centralized Internet-based data management system.18 Data were extracted from EvaluationWeb on September 26, 2014, to allow inclusion of 90 days of follow-up data for anti-HCV-positive participants receiving HCV RNA tests and results, referrals, and follow-up medical care.
Data analysis
We reported frequencies and proportions for demographic and risk-factor data for the entire study population, and then for anti-HCV-positive and HCV RNA-positive participants. Next, we used indicators to evaluate the HCV testing-to-care continuum in the following order: (1) anti-HCV-positive test, (2) HCV RNA test received, (3) HCV RNA-positive test, (4) referred to care, and (5) attended first medical appointment.
We evaluated the indicators in the testing-to-care continuum using two methods. First, we calculated the proportion of anti-HCV-positive participants who completed every step of the continuum; we divided the number of anti-HCV-positive participants who completed each step of the continuum by the total number of anti-HCV-positive participants (Figure 1). This calculation methodology is commonly used to determine completion rates for the HCV care continuum.14 Second, to more accurately assess the proportion of participants who completed each successive step of the care continuum, we adjusted the -denominators at each step to equal the number of participants who completed the previous step (-Figure 2). We adjusted the denominators at each step because only anti-HCV-positive participants testing positive for HCV RNA should be referred to care, and because 15%–25% of anti-HCV-positive people will spontaneously clear the virus.
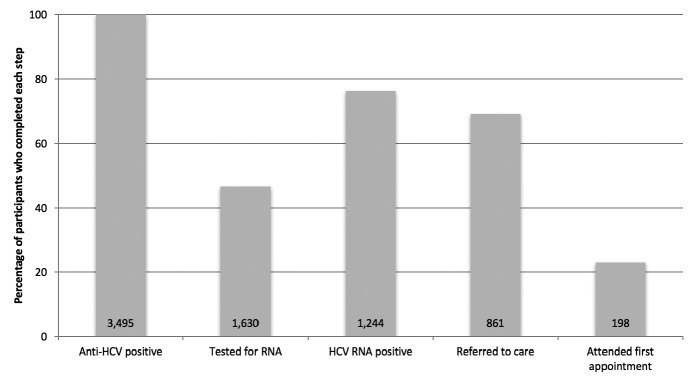
Figure 1.
Percentage of anti-HCV-positive people who inject drugs completing each step of the testing-to-care continuum during the Hepatitis Testing and Linkage to Care (HepTLC) initiative, nine U.S. cities, 2012–2014a,b
aThe HepTLC initiative promoted viral hepatitis B and hepatitis C screening, posttest counseling, and linkage to care at 34 U.S. sites. The sites that targeted people who inject drugs for HCV testing were located in Tucson, Arizona; Chicago, Illinois; Los Angeles and Oakland, California; Portland, Maine; New York City, New York; Seattle, Washington; Richmond, Virginia; and Milwaukee, Wisconsin.
bThe total number of people who completed each step of the continuum was divided by the initial count of anti-HCV-positive people.
anti-HCV = hepatitis C virus antibody
HCV = hepatitis C virus
RNA = ribonucleic acid
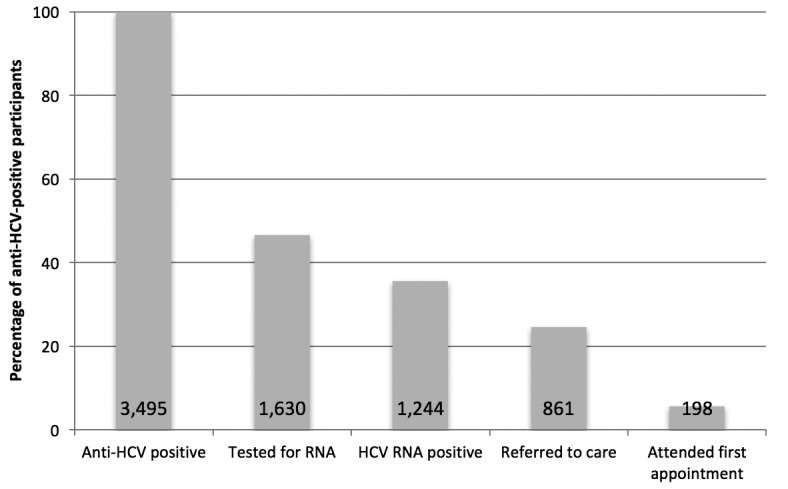
Figure 2.
Percentage of HCV RNA-positive people who inject drugs who completed each successive step of the testing-to-care continuum during the Hepatitis Testing and Linkage to Care (HepTLC) initiative, nine U.S. cities, 2012–2014a,b
aThe HepTLC initiative promoted viral hepatitis B and hepatitis C screening, posttest counseling, and linkage to care at 34 U.S. sites. The sites that targeted people who inject drugs for HCV testing were located in Tucson, Arizona; Chicago, Illinois; Los Angeles and Oakland, California; Portland, Maine; New York City, New York; Seattle, Washington; Richmond, Virginia; and Milwaukee, Wisconsin.
bThe total number of people who completed each step of the continuum was divided by the total number of people who completed the previous step.
HCV = hepatitis C virus
RNA = ribonucleic acid
anti-HCV = hepatitis C virus antibody
We then examined the proportion of anti-HCV-positive participants who completed each step of the testing-to-care continuum by receipt of same-day testing. We defined same-day testing as receiving either a quantitative or qualitative HCV RNA test on the same date as an anti-HCV test. We defined not same-day testing as receiving a quantitative or qualitative HCV RNA test more than one day after an anti-HCV test. We performed all analyses using SAS® version 9.3.19
RESULTS
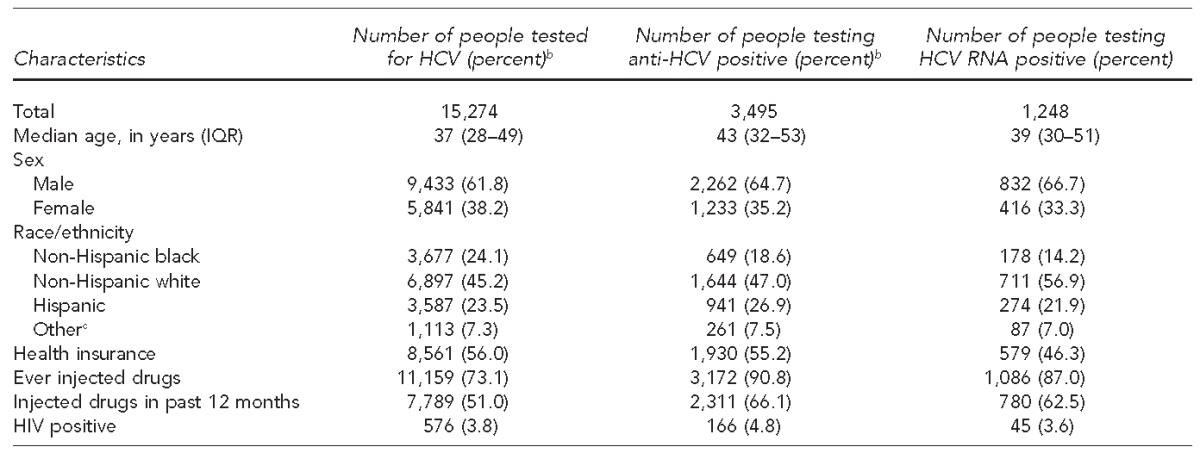
The 10 HepTLC grantees targeting PWID tested 15,274 participants for anti-HCV. Of the total population tested, the median age was 37 years (interquartile range [IQR] = 28–49), 8,561 (56.1%) participants had health insurance, 11,159 (73.1%) participants reported ever injecting drugs, and 7,789 (51.0%) participants reported injecting drugs in the past 12 months (Table).
Table.
Characteristics and HCV test results of people who inject drugs, Hepatitis Testing and Linkage to Care (HepTLC) initiative, nine U.S. cities, 2012–2014a
The HepTLC initiative promoted viral hepatitis B and hepatitis C screening, posttest counseling, and linkage to care at 34 U.S. sites. The sites that targeted people who inject drugs for HCV testing were located in Tucson, Arizona; Chicago, Illinois; Los Angeles and Oakland, California; Portland, Maine; New York City, New York; Seattle, Washington; Richmond, Virginia; and Milwaukee, Wisconsin.
bPercentages may not total to 100 because of rounding.
cIncludes non-Hispanic Asian, Native Hawaiian or Pacific Islander, and American Indian or Alaska Native
HCV = hepatitis C virus
anti-HCV = hepatitis C virus antibody
RNA = ribonucleic acid
IQR = interquartile range
HIV = human immunodeficiency virus
Among the 15,274 PWID participants, 3,495 (22.9%) tested anti-HCV positive. The median age of anti-HCV-positive participants (43 years, IQR=32–53) was higher than the median age of those who received an anti-HCV test. Among the 3,495 anti-HCV-positive participants, 1,930 (55.2%) had health insurance, 3,172 (90.8%) reported ever injecting drugs, and 2,311 (66.1%) reported injecting drugs in the past 12 months. Demographic and risk-factor characteristics among the 1,248 HCV RNA-positive participants were similar to the anti-HCV-positive population (Table).
Among the 3,495 anti-HCV-positive participants, 1,630 (46.6%) received an HCV RNA test (Figure 1). Among those tested for HCV RNA, 1,244 (76.3%) participants tested HCV RNA positive, of whom 861 (69.2%) were referred to care and 198 of 861(22.9%) attended their first medical appointment (Figure 2).
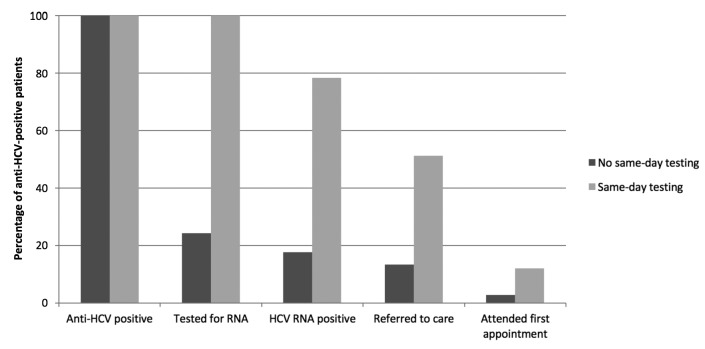
Among the 1,030 anti-HCV-positive participants who received same-day HCV RNA testing, 808 (78.4%) tested HCV RNA positive and 125 (12.1%) attended their first medical appointment (Figure 3). Among the 2,465 anti-HCV-positive participants who did not receive same-day testing, 601 (24.4%) received an HCV RNA test, 436 (17.7%) tested HCV RNA positive, and 73 (3.0%) attended their first medical appointment.
Figure 3.
Completion of the testing-to-care continuum among anti-HCV-positive people who inject drugs, by receipt of same-day or not same-day HCV RNA testing, during the Hepatitis Testing and Linkage to Care (HepTLC) initiative, nine U.S. cities, 2012–2014a
aThe HepTLC initiative promoted viral hepatitis B and hepatitis C screening, posttest counseling, and linkage to care at 34 U.S. sites. The sites that targeted people who inject drugs for HCV testing were located in Tucson, Arizona; Chicago, Illinois; Los Angeles and Oakland, California; Portland, Maine; New York City, New York; Seattle, Washington; Richmond, Virginia; and Milwaukee, Wisconsin.
anti-HCV = hepatitis C virus antibody
HCV = hepatitis C virus
RNA = ribonucleic acid
DISCUSSION
During the HepTLC initiative, grantees performed HCV testing on previously untested individuals with a history of injecting drugs. During the two-year project period, 15,274 anti-HCV tests were administered. Yet, given the successes in recruitment and the number of anti-HCV tests administered, fewer than half of those who received a positive anti-HCV test result (n=3,495) received a confirmatory HCV RNA test (n=1,630), and fewer than 10% of anti-HCV-positive participants (n=198) attended their first medical appointment. The considerable decrease in the number of people completing each step in the continuum of care—from receiving an anti-HCV-positive test result to attending a first medical appointment—is consistent with the hepatitis treatment literature.17,20 Taken together, these data highlight the persistent and recurring problem of PWID not receiving confirmatory HCV RNA testing following a positive anti-HCV test result, which translates to a substantial number of people who remain undiagnosed and uninformed of their HCV infection status and thus ineligible to receive medical care.21
Our findings demonstrated that performing a venous blood draw for an HCV RNA test on the same day and at the same appointment when an anti-HCV test is administered can improve diagnostic outcomes by eliminating the need for a follow-up appointment to diagnose current infection. The benefits of this testing algorithm are most notable in the number of anti-HCV-positive participants who did not receive same-day testing: 601 (24.4%) of 2,465 anti-HCV-positive participants who did not receive same-day testing received a confirmatory HCV RNA test compared with 100.0% of anti-HCV-positive participants who received a confirmatory HCV RNA test on the same day as their anti-HCV test.
Reducing the number of appointments needed to diagnose current infection may help increase the number of infected people linked to care given the many obstacles PWID face when trying to access health care.20–27 For example, economic barriers, such as unemployment, chronic poverty, and/or homelessness, can prevent PWID from returning to a site for an HCV RNA test,21 while being uninsured or underinsured can contribute to individuals not seeking care for HCV.22 Additionally, many health-care providers are hesitant to treat PWID because of personal concerns about adherence and reinfection.23 For those who do initiate care, institutional stigma and provider bias against treating people who continue to inject drugs may prevent HCV-infected PWID from accessing treatment.24,25
The benefits associated with same-day testing also support the delivery of reflex testing as a practical strategy to increase the number of anti-HCV-positive people who are diagnosed and to reduce the high rates of loss to follow-up. Reflex testing involves administering an anti-HCV test concurrently with a venous blood draw to test for HCV-RNA if patients are found to be anti-HCV reactive. For community-based organizations that lack medical capacity, such as syringe services programs, myriad logistical issues exist with implementing reflex testing. For example, an on-site phlebotomist is needed to conduct blood draws for HCV RNA tests (i.e., preferably a person with experience drawing blood from PWID, as many PWID have compromised or collapsed veins) and diagnostic tests need to be conducted in the same facility where anti-HCV tests are performed, or through a reference laboratory to assist in analyzing specimens.12
Because PWID are often wary of utilizing health-care services because of stigma, shame, and/or discrimination,26 reflex capacity needs to be developed in community-based settings accustomed to serving this vulnerable and hard-to-reach population (e.g., syringe services programs, buprenorphine providers, and methadone maintenance treatment programs). Implementing reflex testing in settings with low threshold services can better ensure that PWID are tested and accurately diagnosed for HCV infection at point-of-care at a single testing appointment.
Limitations
Our analysis was subject to several limitations. First, because of the assignment of unique identifications by agency, one person could have been tested at multiple sites hosted by different grantees. Yet, because of the geographic distribution of participating grantees, this possibility was unlikely. Second, participants may have received an HCV RNA test following an anti-HCV-positive result but, because of loss to follow-up, grantees may have been unable to locate all of those who received the follow-up test or those who were lost before documentation of attending their first appointment; data on HCV RNA testing and first medical appointments may therefore be underreported. Lastly, because HepTLC was a demonstration project to increase testing among PWID, our results may not be replicated in other regions and/or settings and the sample population may not be representative of the total population of PWID.
CONCLUSION
The prevalence of HCV infection is higher among PWID than among the general U.S. population. Because PWID are not successfully transitioning through every stage in the HCV care continuum (from testing to diagnosis to linkage-to-care to cure), strategies to better test and treat the population are needed to reduce the morbidity and mortality associated with the disease. Achieving higher treatment rates among PWID will require overcoming individual and structural barriers to testing and linkage to care. Public health practitioners, community-based organizations, health clinics, and treatment providers may want to implement reflex HCV RNA testing in their facilities to reduce loss to follow-up care among PWID. Administering anti-HCV and HCV RNA tests during a single testing event can increase the number of PWID who are properly diagnosed and initiate treatment.
Footnotes
The National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention determined, in lieu of institutional review board review, that this study was a public health program activity and was not considered research. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charles E, Dustin L, Rice C. Natural history, pathogenesis, and prevention of HCV infection. In: Dammacco F, editor. HCV infection and cryoglobulinemia. Milan: Springer; 2012. pp. 11–9. [Google Scholar]
- 3.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007 [published erratum appears in Ann Intern Med 2012;156:840] Ann Intern Med. 2012;156:271–8. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 4.Lansky A, Finlayson T, Johnson C, Holtzman D, Wejnert C, Mitsch A, et al. Estimating the number of persons who inject drugs in the United States by meta-analysis to calculate national rates of HIV and hepatitis C virus infections. PLoS One. 2014;9:e97596. doi: 10.1371/journal.pone.0097596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–14. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 6.Klevens RM, Hu DJ, Jiles R, Holmberg SD. Evolving epidemiology of hepatitis C virus in the United States. Clin Infect Dis. 2012;55(Suppl 1):S3–9. doi: 10.1093/cid/cis393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34–41. doi: 10.1111/j.1365-2893.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 8.Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Cost-effectiveness of treatment for chronic hepatitis C infection in an evolving patient population. JAMA. 2003;290:228–37. doi: 10.1001/jama.290.2.228. [DOI] [PubMed] [Google Scholar]
- 9.Hagan H, Campbell J, Thiede H, Strathdee S, Ouellet L, Kapadia F, et al. Self-reported hepatitis C virus antibody status and risk behavior in young injectors. Public Health Rep. 2006;121:710–9. doi: 10.1177/003335490612100611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith BD, Jewett A, Burt RD, Zibbell JE, Yartel AK, DiNenno E. “To share or not to share?” Serosorting by hepatitis C status in the sharing of drug injection equipment among NHBS-IDU2 participants. J Infect Dis. 2013;208:1934–42. doi: 10.1093/infdis/jit520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Getchell JP, Wroblewski KE, DeMaria A, Jr, Bean CL, Parker MM, Pandori M, et al. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep. 2013;62(18):362–5. [PMC free article] [PubMed] [Google Scholar]
- 12.Spradling PR, Tong X, Rupp LB, Moorman AC, Lu M, Teshale EH, et al. Trends in HCV RNA testing among HCV antibody–positive persons in care, 2003–2010. Clin Infect Dis. 2014;59:976–81. doi: 10.1093/cid/ciu509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barocas JA, Brennan MB, Hull SJ, Stokes S, Fangman JJ, Westergaard RP. Barriers and facilitators of hepatitis C screening among people who inject drugs: a multi-city, mixed-methods study. Harm Reduct J. 2014;11:1. doi: 10.1186/1477-7517-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med. 2013;368:1859–61. doi: 10.1056/NEJMp1302973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Department of Health and Human Services (US) Prevention and Public Health Fund. 2014 [cited 2016 Mar 25] Available from: https://www.whitehouse.gov/sites/default/files/omb/budget/fy2014/assets/health.pdf.
- 16.Alter MJ, Margolis HS, Bell BP, Bice SD, Buffington J, Chamberland M, et al. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR-19):1–39. [PubMed] [Google Scholar]
- 17.Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Ward JW. Hepatitis C virus testing of persons born during 1945–1965: recommendations from the Centers for Disease Control and Prevention. Ann Intern Med. 2012;157:817–22. doi: 10.7326/0003-4819-157-9-201211060-00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luther Consulting, LLC. Carmel (IN): Luther Consulting, LLC; 2013. EvaluationWeb®: Version 5. [Google Scholar]
- 19.SAS Institute, Inc. Cary (NC): SAS Institute, Inc.; 2011. SAS®: Version 9.3. [Google Scholar]
- 20.Zeremski M, Zibbell JE, Martinez AD, Kritz S, Smith BD, Talal AH. Hepatitis C virus control among persons who inject drugs requires overcoming barriers to care. World J Gastroenterol. 2013;19:7846–51. doi: 10.3748/wjg.v19.i44.7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yehia BR, Schranz AJ, Umscheid CA, Lo Re V., III The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS One. 2014;9:e101554. doi: 10.1371/journal.pone.0101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Day C, Ross J, Dolan K. Hepatitis C-related discrimination among heroin users in Sydney: drug user or hepatitis C discrimination? Drug Alcohol Rev. 2003;22:317–21. doi: 10.1080/0959523031000154463. [DOI] [PubMed] [Google Scholar]
- 23.Darke S, Ross J. Polydrug dependence and psychiatric comorbidity among heroin injectors. Drug Alcohol Depend. 1997;48:135–41. doi: 10.1016/s0376-8716(97)00117-8. [DOI] [PubMed] [Google Scholar]
- 24.Morrill JA, Shrestha M, Grant RW. Barriers to the treatment of hepatitis C: patient, provider and system factors. J Gen Intern Med. 2005;20:754–8. doi: 10.1111/j.1525-1497.2005.0161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGowan CE, Monis A, Bacon BR, Mallolas J, Goncales FL, Goulis I, et al. A global view of hepatitis C: physician knowledge, opinions, and perceived barriers to care. Hepatology. 2013;57:1325–32. doi: 10.1002/hep.26246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grebely J, Oser M, Taylor LE, Dore GJ. Breaking down the barriers to hepatitis C virus (HCV) treatment among individuals with HCV/HIV coinfection: action required at the system, provider, and patient levels. J Infect Dis. 2013;207(Suppl 1):S19–25. doi: 10.1093/infdis/jis928. [DOI] [PMC free article] [PubMed] [Google Scholar]