Abstract
AIM
To choose appropriate concentration of sodium hydroxide (NaOH) solution to establish a stable and consistent corneal alkali burn mouse model in grade II.
METHODS
The mice (n=60) were randomly divided into four groups and 15 mice each group. Corneal alkali burns were induced by placing circle filter paper soaked with NaOH solutions on the right central cornea for 30s. The concentrations of NaOH solutions of groups A, B, C, and D were 0.1 mol/L, 0.15 mol/L, 0.2 mol/L, and 1.0 mol/L respectively. Then these corneas were irrigated with 20 mL physiological saline (0.9% NaCl). On day 7 postburn, slit lamp microscope was used to observe corneal opacity, corneal epithelial sodium fluorescein staining positive rate, incidence of corneal ulcer and corneal neovascularization, meanwhile pictures of the anterior eyes were taken. Cirrus spectral domain optical coherence tomography was used to scan cornea to observe corneal epithelial defect and corneal ulcer.
RESULTS
Corneal opacity scores (x±s) were not significantly different between the group A and group B (P=0.097). Incidence of corneal ulcer in group B was significantly higher than that in group A (P=0.035). Incidence of corneal ulcer and perforation rate in group B was lower than that in group C. Group C and D had corneal neovascularization, and incidence of corneal neovascularization in group D was significantly higher than that in group C (P=0.000).
CONCLUSION
Using 0.15 mol/L NaOH can establish grade II mouse model of corneal alkali burns.
Keywords: cornea, alkali burn, mouse model, corneal neovascularization
INTRODUCTION
The alkali corneal burn model, an animal model of ocular surface diseases, is commonly used to study pathological and physiological changes induced by injuries. The basic method is putting circle filter paper (2-mm diameter) soaked NaOH solution on the central cornea, and different concentrations of NaOH solution cause different damages to the cornea. Reports show, most of grade I corneal burns can be healed well without treatments, most of grade II corneal burns irrigated timely can be healed well[1]–[6]. When being used to treat grade II corneal burns, diphoterine solution has significantly faster healing effect than physiological saline. However the two irrigating solutions have no significant difference in healing grade III corneal burn[7]–[9]. Besides, the grade I corneal burn can be complete restituted, so we should choose the animal model of corneal burn in grade II to study buffer solutions' therapeutic effect on corneal alkali burns. In order to establish a mouse model of corneal alkali burn for evaluating buffer capacity of irrigating solution, we compared corneal opacity, corneal ulcer and corneal neovascularization of mouse's cornea injured by NaOH solution in four different concentrations.
MATERIALS AND METHODS
Animals
All animal-based procedures were performed in accordance with the Association for Research in Vision and Ophthalmology Statement for the use of Animals in Ophthalmic and Vision Research, and the National Institutes of Health Guidance for the Care and Use of Laboratory Animals. This study was approved by the Animal Care Committee of Second Military Medical University.
Methods
Alkali burns were inflicted in right eye of C57 mice of both sexes (n=60, male: female=1:1), 6 to 8 weeks old, using the following protocol: mice anesthetized with 5% chloralhydrate (0.1 mL/10 mg) intraperitoneally (IP) were randomly divided into four groups, and were placed under the surgical dissecting microscope in a laterally recumbent position. A single topical pontocaine 1% (ophthalmic solution) eye drop was applied to the right cornea, followed by the filter paper (2-mm diameter) soaked with 1.5 μL NaOH solution for 30s. The concentrations of NaOH solutions for group A, group B, group C, group D were 0.1 mol/L, 0.15 mol/L, 0.2 mol/L and 1.0 mol/L respectively. After the filter paper was removed from the cornea, the eye was thoroughly irrigated with 20 mL sterilized physiological saline. Mice were housed with plastic cages, normal diet, with 12/12h day and night. The slit lamp microscope (SLM-3, Chongqing Kang Hua technology co., Chongqing, China) was used to observe cornea, conjunctiva, the anterior chamber every day and the results of observation were recorded. On day 7, mice were anesthetized and we used camera attached to the slit lamp microscope to watch corneas and take pictures, meanwhile, Cirrus spectral domain optical coherence tomography (Germany Zeiss Co., Germany) was used to scan the anterior of the eye. Mice were sacrificed with overdose 5% chloral hydrate at the end of experiment.
Mesurement
After corneal alkali burn, two researchers (Bai JQ and Qin HF) used the slit-lamp microscope (magnify: ×10, angle: 45°, brightness: 5, Kanghua SLE imaging soft V1.1) to observe corneas, conjunctivas, anterior chambers and symblepharon, and recorded the results every day. On day 7, the researchers recorded cornea opacity scores, incidence of corneal ulcer and corneal neovascularization, used the slit lamp microscope to take pictures, and used Cirrus spectral domain optical coherence tomography (Anterior Segment 5 Line Raster, spacing: 0.25 mm, lenth: 3 mm ) to scan the anterior of eyes. As previously described[10], the degree of cornea opacity was scored clinically on a numerical scale of 0-4: 0, clear cornea; 1, mild stromal opacity; 2, moderate stromal opacity; 3, severe corneal opacity with visible iris; 4, opaque cornea with iris not visible.
Exclusion Criteria
Once corneal infection and perforation occurred, the model was considered as a failure and excluded from statistics, corresponding cases were added subsequently.
Statistical Analysis
Statistical analysis was performed using the statistical package SPSS v16.0 (SPSS Inc., Chicago, USA). Wilcoxon signed-rank test was used to compare corneal opacity score (x±s). Wilcoxon signed-rank test was also used to test corneal epithelial fluorescein staining positive rate, incidence of corneal ulcer and neovascularization. The P value <0.05 was considered statistically significant.
RESULTS
Morphological Observation
Immediately after central cornea injured by alkali: groups A, B, C, D had mixed conjunctival congestion, and 2-mm diameter circular epithelial defects with clear border can be found on the central cornea of four groups. What's more, corneal edema could be found in all groups. On day 1 postburn: all the four groups still had mixed conjunctival congestion and corneal epithelial defect of group A was partly healed, and defect of the other three groups still clearly visible. Meanwhile, all of the four groups still had corneal edema and group D was most edematous. On day 3, groups C, D had neovascularization in the limbic cornea. The neovascularization of groups C, D grew from limbic to the central cornea from day 4 to day 7 postburn.
Corneal Opacity Score
On day 7 postburn, cornea opacity scores were recorded and shown in Figure 1, Table 1. The four groups' corneal opacity scores (M±QR) had same QR (QR=1). Corneal opacity score (x±s) among four groups were significantly different (P=0.000): corneal opacity scores (x±s) was not significantly different between the group A and group B (P=0.097); corneal opacity scores (x±s) of group C and group D significantly higher than that of group A (P=0.008, and P=0.000, respectively ); corneal opacity scores (x±s) was not significantly different between group B and group C (P=0.124); corneal opacity scores (x±s) of group D significantly higher than that of group B (P=0.000); corneal opacity scores (x±s) of group D significantly higher than that in group C (P=0.000).
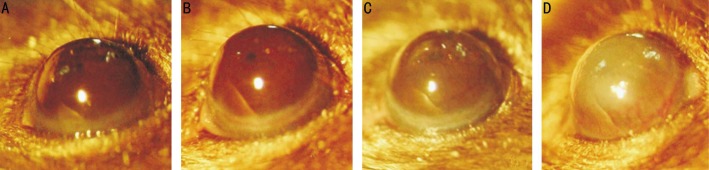
Figure 1. The anterior imaging using the slit lamp microscope on day 7.
Presence of corneal opacity and corneal neovascularization: corneal opacity scores of A, B, C and D are 0, 1, 2 and 4 respectively, and D has corneal neovascularization.
Table 1. Corneal opacity scores on day 7.
| Groups | Corneal opacity scores |
||||||
| 0 | 1 | 2 | 3 | 4 | M±QR | x±s1 | |
| A | 4 | 8 | 3 | 0 | 0 | 1±1 | 0.87±0.726 |
| B | 0 | 10 | 5 | 0 | 0 | 1±1 | 1.33±0.477 |
| C | 0 | 6 | 8 | 1 | 0 | 2±1 | 1.67±0.477 |
| D | 0 | 0 | 3 | 9 | 3 | 3±1 | 2.87±0.726 |
1Wilcoxon signed-rank test.
n=15
Corneal Epithelial Sodium Fluorescein Staining Positive Rate, Incidence of Corneal Ulcer and Corneal Neovascularization
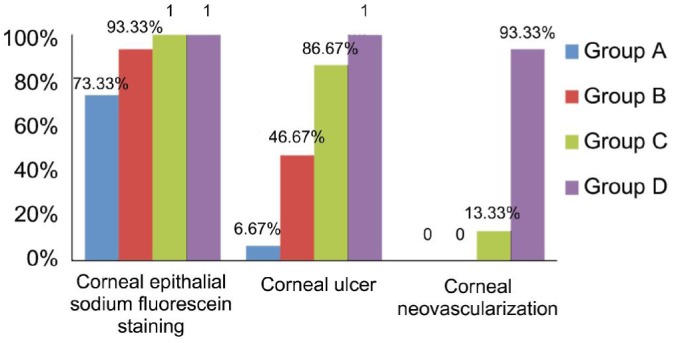
Results of corneal epithelial sodium fluorescein staining positive rate, incidence of corneal ulcer and corneal neovascularization on day 7 were showed in Figures 1, 2, 3, and 4. Corneal epithelial sodium fluorescein staining positive rates were not significantly different between four groups (P>0.05). Incidences of corneal ulcer in four groups were significantly different (P=0.000): incidence of corneal ulcer in group B was significantly higher than that in group A (P=0.035, Fisher); incidences of cornea ulcer in group C and group D were significantly higher than that in group A (P=0.001, and P=0.000, respectively), incidence of corneal ulcer in group C was significantly higher than in group B (P=0.020), incidence of corneal ulcer in group D was significantly higher than that in group B (P=0.002, Fisher); incidence of corneal ulcer in group D was significantly higher than that in group C (P=0.0483). Incidence of corneal neovascularization in group D was significantly higher than that in group C (P=0.000).
Figure 2. Corneal fluorescein staining image using the slit lamp microscope on day 7.
Presence of corneal epithelial defect and corneal ulcer: pictures of A and B have corneal epithelial defect, pictures of C and D have corneal ulcer.
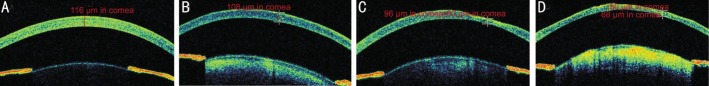
Figure 3. Cirrus spectral domain optical coherence tomography (spacing: 0.25 mm, lenth: 3 mm ) scan cornea of mouse eye on day 7.
Presence of corneal epithelial defect and corneal ulcer: pictures of A and B have corneal epithelial defect, pictures of C and D have corneal ulcer. Central corneal thickness of A, B, C and D are 116, 108, 96 and 80 μm respectively; and corneal thickness in ulcer of C and D are 60 and 68 μm.
Figure 4. Corneal epithelial sodium fluorescein staining positive rate, incidence of corneal ulcer and corneal neovascularization on day 7.
DISCUSSION
Mouse is an ideal animal model, breeding economically and fast, with genome similar to human being's. Mouse corneal alkali burns can result in corneal epithelial defect, corneal ulcer, perforation of cornea, corneal opacity and corneal neovascularization. Mouse corneal alkali burns model is an animal model used to study pathology of corneal chemical, thermal burns, corneal neovascularization and many other corneal diseases. According to different experiment purposes, we choose different NaOH solutions' concentrations, and the most commonly chosen concentrations are 0.01, 0.1, 0.15, 1.0 mol/L [11]–[20]. The place putting filter paper is the central of cornea; and corneal alkali burning times are 10, 20, 30, 40, 45 and 50s[11]–[20]. Filter paper sizes reported are different, but the most often used is 2-mm diameter circle filter paper, so filter paper used in this experiment was 2-mm filter diameter. In some experiment, the volume of alkali used was 2 μL [13], however, in our experiment, if 2 μL NaOH solution was dropped to 2-mm filter paper, the excess alkali would overflow, while 1.5 μL would not. So we used 1.5 μL in this experiment finally.
According to the system's summary: tap water, normal saline, lactated Ringer's, normal saline with sodium bicarbonate added, phosphate buffer solution, diphoterine buffer can improve the prognosis of corneal burn in grades I, II (Reim 1987, 1990)[5]–[6]. Corneal neovascularization are caused by alkali burn in grade III. Cornea opacity score and ulcer incidence of group C were significantly higher than that of B group (P<0.05) on day 7, but group C has neovascularization, which is caused by alkali burn in grade III. So we should choose 0.15 mol/L NaOH solution to study the buffer capacity of solution and choose 1 mol/L NaOH solution to study corneal neovascularization.
In addition, we still need to pay attention to the following details in the process: 1) filter paper should be placed on the central cornea, otherwise it may cause alkali burn in non-experimental place; 2) the volume of NaOH should be appropriate, otherwise excessive alkali may also cause alkali burn in non-experimental place; 3) anesthetic eye drops should be used to stop blinking; 4) the conjunctival sac should be washed thoroughly, otherwise the occurrence of symblepharon can cause corneal injures which make it difficult to recognize the reason for the corneal defect and corneal ulcer.
Acknowledgments
Foundation: Supported by National Science and Technology Major Project of China (No. 2011ZXJ09104-10C).
Conflicts of Interest: Bai JQ, None; Qin HF, None; Zhao SH, None.
REFERENCES
- 1.Beare JD. Eye injuries from assault with chemicals. Br J Ophthalmol. 1990;74(9):514–518. doi: 10.1136/bjo.74.9.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagoner MD. Chemical injuries of the eye: current concepts in pathophysiology and therapy. Surv Ophthalmol. 1997;41(4):275–313. doi: 10.1016/s0039-6257(96)00007-0. [DOI] [PubMed] [Google Scholar]
- 3.Kuckelkorn R, Schrage N, Keller G, Redbrake C. Emergency treatment of chemical and thermal eye burns. Acta Ophthalmol Scand. 2002;80(1):4–10. doi: 10.1034/j.1600-0420.2002.800102.x. [DOI] [PubMed] [Google Scholar]
- 4.Fish R, Davidson RS. Management of ocular thermal and chemical injuries, including amniotic membrane therapy. Curr Opin Ophthalmol. 2010;21(4):317–321. doi: 10.1097/ICU.0b013e32833a8da2. [DOI] [PubMed] [Google Scholar]
- 5.Schrage NF, Struck HG, Gerard M. Recommendations for acute treatment for chemical and thermal burns of eyes and lids. Ophthalmologe. 2011;108(10):916–920. doi: 10.1007/s00347-010-2252-2. [DOI] [PubMed] [Google Scholar]
- 6.Chau JP, Lee DT, Lo SH. A systematic review of methods of eye irrigation for adults and children with ocular chemical burns. Worldviews Evid Based Nurs. 2012;9(3):129–138. doi: 10.1111/j.1741-6787.2011.00220.x. [DOI] [PubMed] [Google Scholar]
- 7.Cho WK, Kang S, Choi H, Rho CR. Topically administered gold nanoparticles inhibit experimental corneal neovascularization in mice. Cornea. 2015;34(4):456–459. doi: 10.1097/ICO.0000000000000343. [DOI] [PubMed] [Google Scholar]
- 8.Iakimenko SA, Buznyk OI, Rymgayllo-Jankowska B. Amniotic membrane transplantation in treatment of persistent corneal ulceration after severe chemical and thermal eye injuries. Eur J Ophthalmol. 2013;23(4):496–503. doi: 10.5301/ejo.5000243. [DOI] [PubMed] [Google Scholar]
- 9.Merle H, Donnio A, Ayeboua L, Ayeboua L, Michel F, Thomas F, Ketterle J, Leonard C, Josset P, Gerard M. Alkali ocular burns in Martinique (French West Indies) Evaluation of the use of an amphoteric solution as the rinsing product. Burns. 2005;31(2):205–211. doi: 10.1016/j.burns.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Yoon KC, Heo H, Kang IS, Lee MC, Kim KK, Park SH, Cho KO. Effect of topical cyclosporine A on herpetic stromal keratitis in a mouse model. Cornea. 2008;27(4):454–460. doi: 10.1097/ICO.0b013e318160602d. [DOI] [PubMed] [Google Scholar]
- 11.Yan J, Zeng Y, Jiang J, Zhou J, Yin Z, Wang Z, Zhu P. The expression patterns of vascular endothelial growth factor and thrombospondin 2 after corneal alkali burn. Colloids Surf B Biointerfaces. 2007;60(1):105–109. doi: 10.1016/j.colsurfb.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Xiao O, Xie ZL, Lin BW, Yin XF, Pi RB, Zhou SY. Minocycline inhibits alkali burn-induced corneal neovascularization in mice. PLoS One. 2012;7(7):e41858. doi: 10.1371/journal.pone.0041858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kubota M, Shimmura S, Kubota S, Miyashita H, Kato N, Noda K, Ozawa Y, Usui T, Ishida S, Umezawa K, Kurihara T, Tsubota K. Hydrogen and N-acetyl-L-cysteine rescue oxidative stress-induced angiogenesis in a mouse corneal alkali-burn model. Invest Ophthalmol Vis Sci. 2011;52(1):427–433. doi: 10.1167/iovs.10-6167. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Q, Yang L, Qu M, Wang Y, Chen P, Wang Y, Shi W. Role of senescent fibroblasts on alkali-induced corneal neovascularization. J Cell Physiol. 2012;227(3):1148–1156. doi: 10.1002/jcp.22835. [DOI] [PubMed] [Google Scholar]
- 15.Cade F, Paschalis EI, Regatieri CV, Vavvas DG, Dana R, Dohlman CH. Alkali burn to the eye: protection using TNF-α inhibition. Cornea. 2014;33(4):382–389. doi: 10.1097/ICO.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 16.Giacomini C, Ferrari G, Bignami F, Rama P. Alkali burn versus suture-induced corneal neovascularization in C57BL/6 mice: an overview of two common animal models of corneal neovascularization. Exp Eye Res. 2014;121:1–4. doi: 10.1016/j.exer.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Sakimoto T, Sugaya S, Ishimori A, Sawa M. Anti-inflammatory effect of IL-6 receptor blockade in corneal alkali burn. Exp Eye Res. 2012;97(1):98–104. doi: 10.1016/j.exer.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Zhou WJ, Liu GQ, Li LB, Zhang XG, Lu PR. Inhibitory effect of CCR3 signal on alkali-induced corneal neovascularization. Int J Ophthalmol. 2012;5(3):251–257. doi: 10.3980/j.issn.2222-3959.2012.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Yin H, Chen P, Xie L, Wang Y. Inhibitory effect of canstatin in alkali burn-induced corneal neovascularization. Ophthalmic Res. 2011;46(2):66–72. doi: 10.1159/000322804. [DOI] [PubMed] [Google Scholar]
- 20.Chen P, Yin H, Wang Y, Mi J, He W, Xie L, Wang Y. Multi-gene targeted antiangiogenic therapies for experimental corneal neovascularization. Mol Vis. 2010;16:310–319. [PMC free article] [PubMed] [Google Scholar]