Abstract
AIM
To observe the presence and expression of indoleamine 2,3-dioxygenase (IDO) during the corneal immunity to Aspergillus fumigatus (A. fumigatus) in the murine models.
METHODS
The murine model of fungal keratitis was established by smearing with colonies of A. fumigatus after scraping central epithelium of cornea and covering with contact lenses in C57BL/6 mice. The mice were randomly divided into control group, sham group and A. fumigatus keratitis group. The cornea was monitored daily using a slit lamp and recorded disease score after infection. Corneal lesion was detected by immunofluorescence staining. IDO mRNA and protein were also detected by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and Western blot.
RESULTS
The disease score and slit lamp photography indicated that disease severity was consistent with corneal inflammation in the murine models, and the disease scores in A. fumigatus keratitis group were obviously higher than those in the sham group. By immunofluorescence staining, IDO was mainly localized in corneal epithelium and stroma in the murine corneal tissues with A. fumigatus keratitis. Compared with the sham group, IDO mRNA expression was significantly enhanced in corneal epithelium infected by A. fumigatus. Furthermore, IDO protein expression detected by Western blot was in accord with transcript levels of IDO mRNA measured by qRT-PCR. IDO protein expression was enhanced after A. fumigatus infection compared with the sham group.
CONCLUSION
IDO is detected in corneal epithelium and stroma locally, which indicates IDO takes part in the pathogenesis of A. fumigatus keratitis and plays a key role in immune regulation at the early stage.
Keywords: indoleamine 2,3-dioxygenase; corneal epithelium; fungal keratitis; Aspergillus fumigatus; innate immune response
INTRODUCTION
Fungal keratitis (FK) is among the most dangerous ocular infections of blindness and visual impairment in China and other developing countries, where trauma to the ocular surface is the primary risk factor, most commonly in connection with agricultural work[1]. Aspergillus (A. flavus, A. fumigates) and Fusarium (F. solani, F. oxysporum) species are the main pathogenic microorganism of FK[2]. Once the corneal epithelial barrier is destroyed, the conidia germinate, and hyphae spread throughout the corneal stroma and can penetrate into the anterior chamber[3]. Infected individuals trigger a prominent host response to those pathogenic microorganisms, which induces anti-fungi immunity and clearance by secretion of proinflammatory, chemotactic and regulatory cytokines, activation and recruitment of neutrophils, macrophages, and T lymphocytes [4].
Indoleamine 2,3-dioxygenase (IDO) plays a multifaceted role in induction of immune tolerance against Aspergillus fumigates (A. fumigatus)[5]–[6]. IDO were detected in the immune cells such as macrophages, polymorphonuclear neutrophils (PMNs)[7] and epithelial cells[8]. IDO and the downstream enzymes in the process of tryptophan degradation are the key elements in acute and chronic infection[7]–[10]. In pathogenic inflammation to fungi, IDO and kynurenines as immunoregulatory mechanism can control the balance between Th17 and regulatory T (Treg) cell subsets by inducing Treg and inhibiting Th17[9],[11]. Thus IDO contributes to immune regulation in the innate and acquired immune responses to infection.
In the study, we mainly assessed the contribution of IDO and related expression cells in murine models of A. fumigatus keratitis. In vitro, we also evaluated the antifungal function of IDO involved in this process in order to explore a novel therapeutic strategy for FK.
MATERIALS AND METHODS
Mice
Female C57BL/6 mice, 8-10 weeks old, were bought from the Chinese Academy of Medical Sciences (Beijing, China). All animals were conducted in line with the Chinese Ministry of Science and Technology Guidelines on the Humane Treatment of Laboratory Animals (vGKFCZ-2006-398) and the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
Aspergillus Fumigatus Strains
A. fumigatus strains (NO 3.0772) were purchased from China General Microbiological Culture Collection Center and cultivated in Sabouraud medium at 28°C for 5-7d. Spores were harvested from A. fumigatus cultures as previous described[12]–[13]. Spores were harvested in 5 mL phosphate buffer saline (PBS). Pure spore were suspended by passing the culture suspension through PBS-soaked sterile gauges placed at the tip of a 10 mL syringe. Spores were made a suspension of 5×104/µL in PBS by quantification using a hemacytometer.
Animal Models of Keratitis
C57BL/6 mice were randomly divided into 3 groups: 5 for control group (normal corneas were collected without any scrape or other treatment), 10 for sham group (corneal epithelium were only scraped about 3-4 mm in diameter) and 12 for A. fumigatus keratitis group. All corneas were examined under a slit lamp microscope before incorporating in experiments. The right corneas were used for model establishing while the left corneas were used as untreated controls. Mice were anaesthetized by intraperitoneal injection of 10% chloral hydrate 3 mL/kg, and 0.4% oxybuprocaine hydrochloride eyedrops for surface anesthesia. After cleaning conjunctival sac by 0.1% entoiodine, central epithelium of cornea were scraped about 3-4 mm in diameter using a 30-gauge needle, and then went on to scratch into stromal layer under microscope. Then the damaged region of cornea was smeared with colonies of A. fumigatus (about 3-4 mm in diameter), and covered with contact lenses to prevent the loss of fungi in the eyes. Finally, 5-0 black silk thread was used to suture and close eyelids. Nothing was done for the sham group, except for scraping central epithelium of cornea and laying a contact lens before closing the eyelids. The contact lenses were removed after 24h. The diagnoses of FK models were confirmed by corneal scraping and fungal culture. The corneas were examined daily by a slit lamp with a digital camera, and recorded disease score for each mouse after infection (1, 3, 5 and 7d) according to a 12-point scoring system[14]. In a word, the disease was evaluated and scored according to area and density of corneal opacity, and surface regularity, each of which was given a grade of 0-4. The highest score was recorded for total opacity in over three-quarters of the corneal area, perforation (never seen in this study), or descemetocele.
Quantitative Real-time Polymerase Chain Reaction
The corneas were collected and preserved at -80°C. The total RNA of isolated cells was extracted using RNAiso plus reagent (TaKaRa, Dalian, Liaoning Province, China) and rapidly quantified by spectrophotometry. Complementary DNA was generated by reverse transcription of 2 µg of total RNA and then used in the following quantitative polymerase chain reaction reactions with SYBR Green using specific primers: 95°C for 30s, followed by 40 cycles of 95°C for 5s, 60°C for 30s, and a final stage of 95°C for 15s, 60°C for 30s, and 95°C for 15s. The oligonucleotide primers were as follows: β-actin, Sense GATTACTGCTCTGGCTCCTAGC; Antisense GACTCATCGTACTCCTGCTTGC; IDO, Sense TCCTGGCAAACTGGAAGAAA; Antisense CACCAATAGAGAGACGAGGAAGAAG. Reverse transcription followed by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was performed using the housekeeping gene β-actin as an internal control and quantified using the 2−ΔΔCt method. Each experiment was repeated at least three separate times.
Immunofluorescence Staining
Corneal samples were fixed in 4% formaldehyde, embedded in paraffin, and cut into 3 µm-thick serial tissue sections. In order to block non-specific binding, we used normal goat serum diluted 1:100 with PBS. Tissue proteolysis was performed by treatment with 0.1% protease (protease XIV, EC 3.4.24.31, Sigma, Vienna, Austria) in 0.05 mol/L Tris-HCl, pH 7.6. After three wash with EDTA-buffered saline (pH 7.6), corneal sections were incubated by polyclonal rabbit antimouse IDO antibodies (Bioss, Beijing, China) diluted 1:200 overnight at 4°C. 1.5 hours' staining at room temperature with fluorescein isothiocyanate (FITC)-conjugated affinipure goat anti-rabbit secondary antibody (Bioss, Beijing, China; 1:100)was performed without light. Isotype IgG was used as the negative controls. The sections were viewed using a Zeiss Axiovert fluorescent microscope at 20× magnification.
Western Blot Analysis
Cell lysates were extracted via RIPA lysis buffer(Solarbio, Beijing, China) plus 1 mmol/L phenylmethylsulfonyl fluoride (PMSF, Solarbio, Beijing, China) at 4°C for 40min. The lysate was vibrated for 3 times, and centrifuged at 14 000 rpm for 15min at 4°C. Total protein was detected via bicinchoninic acid assay, and denatured with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample loading buffer at 95°C for 5min. Proteins (60 µg/well) were separated by 12% SDS-PAGE in Tris/glycine/SDS buffer and electroblotted onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). After immersing in 1% Bovine serum albumin (BSA) for 1h, membranes were incubated with 1:200 diluted rabbit anti-mouse IDO and mouse anti-β-Tubulin at 4°C overnight and then followed by secondary antibody for 1h. Blots of cells lysates were measured with BeyoECL Plus (Beyotime, Shanghai, China). Band intensity was assessed by Quantity One Software (Bio-Rad, CA, USA).
Statistical Analysis
The results were indicated as mean±standard deviation. One-way analysis of variance followed by Student-Newman-Keuls test was analyzed by GraphPad 5.0 software. Mann-Whitney U test was performed to test the difference in clinical score between two groups at each time in the A. fumigatus infected mice. P<0.05 was determined to be significant.
RESULTS
Progression of Aspergillus Fumigatus Keratitis in C57BL/6 Mice
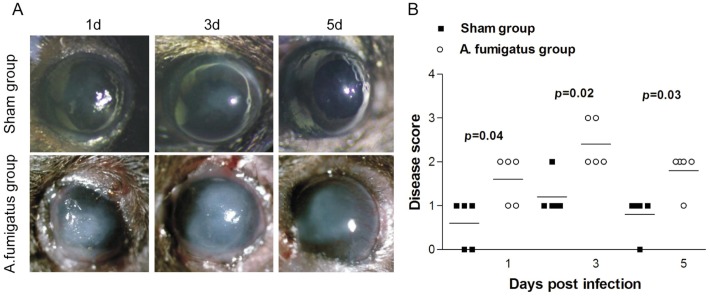
In order to observe the progression of A. fumigatus keratitis, we resorted to murine models to induce obvious A. fumigatus keratitis. Significant corneal edema and an irregular whitish mass even ulceration could be seen at 1d after fungal infection (Figure 1A). Thereafter, severity aggravated gradually and the highest disease symptom score appeared around 3d (Figure 1B). After 3d, the mice began to alleviate or recover even without therapeutic treatment. Obvious neovascularization was present in A. fumigatus keratitis group at 5d. In the sham group, mild opacity was also caused at the early stage after infection, but the corneas returned to the normal transparent status after 1-2d. As indicated by disease score in Figure 1B, A. fumigatus infection increased corneal severity at 1, 3 and 5d, and was obviously higher than that of sham group.
Figure 1. Progression of corneal infection by A. fumigatus in C57BL/6 murine model.
A: Corneal photographs taken under a slit lamp; B: Disease score evaluated with the scoring system in all corneas infected by A. fumigatus and sham-infected corneas. Disease score was shown as mean±standard deviation.
Indoelamine 2,3-dioxygenase Expression in the Cornea of C57BL/6 Mouse Infected by Aspergillus Fumigates
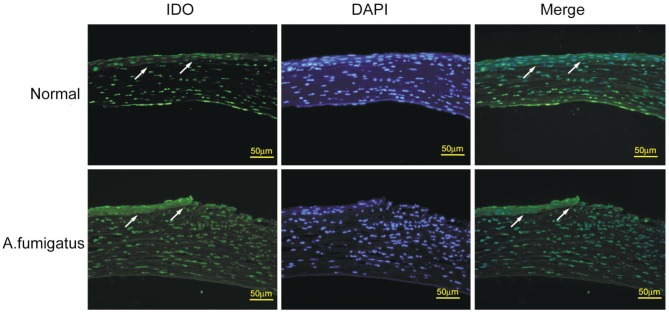
To investigate whether IDO is involved in pathological process of A. fumigatus keratitis, we tested the expression of IDO in the normal and infected corneas of C57BL/6 mouse by immunofluorescence staining. In the normal corneal epithelium, few immunoreactivity of IDO was detected, while IDO-positive cells with strong green fluorescence were mainly detected in the epithelium of infected corneal tissues (Figure 2).
Figure 2. Immunofluorescence staining of IDO in the normal and A. fumigatus-infected corneas in C57BL/6 murine models.
Serial sections of normal and A. fumigatus-infected corneas at 3d were stained using IDO antibody and FITC-conjugated rabbit antimouse second antibody (for IDO, green), and counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). As indicated by the arrows in the normal cornea arrayed in the first row, few green fluorescence was observed in corneal epithelium. However, in the A. fumigatus-infected corneas (second row), strong green fluorescence in corneal epithelium was detected. Scale bar in immunofluorescence images: 50 µm.
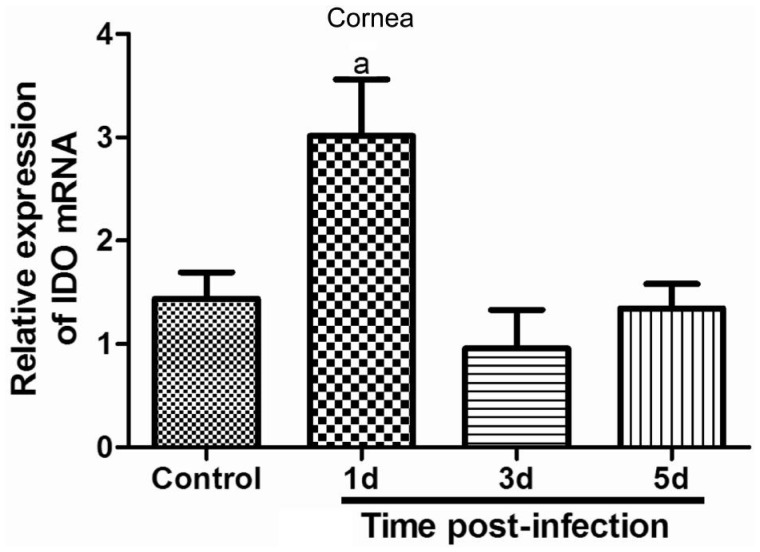
Furthermore, we investigated mRNA and protein levels of IDO in normal and infected C57BL/6 corneas by qRT-PCR and Western blot. The sham corneas were as the control group. Results indicated that relative IDO mRNA were detected in the control corneal tissues, significantly increased and reached the climax in mouse corneas at 1d after infection by A. fumigatus. Thereafter, IDO mRNA began to drop, and there was no significant difference between the control and infected corneas at 3 and 5d (Figure 3).
Figure 3. Expression of IDO mRNA in the sham and A. fumigatus-infected corneal tissues.
Relative expression of IDO mRNA in corneal epithelium was detected by qRT-PCR. A. fumigatus infection enhanced IDO mRNA expression at 1d and began to decline at 3 and 5d. The data were indicated as the mean±standard deviation of four independent experiments. (aP<0.05 compared with control)
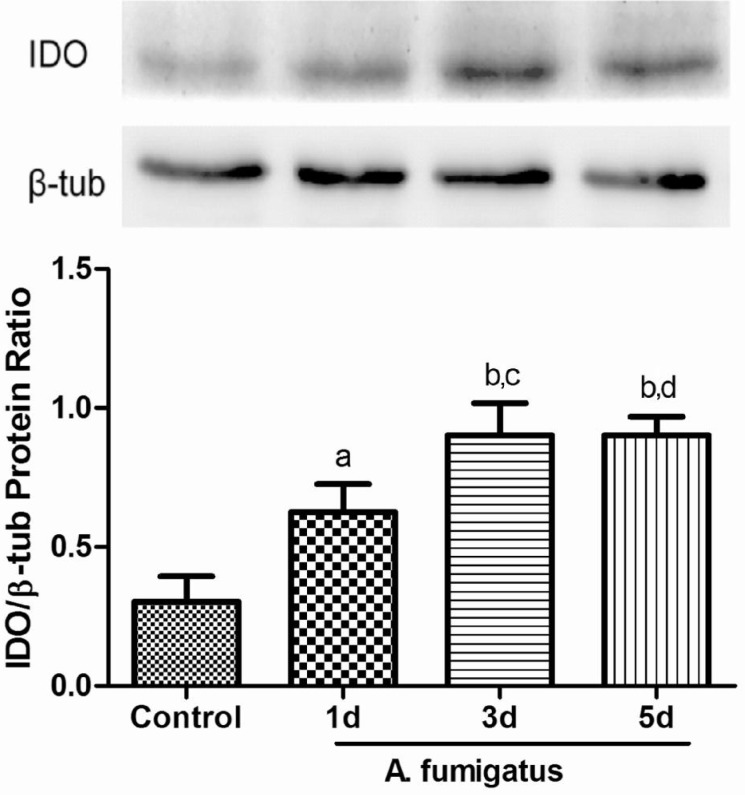
Expessions of IDO protein were further examined by Western blot (Figure 4). As shown in Figure 4, IDO protein began to elevate in the corneas with A. fumigatus keratitis at 1d after infection, continued to increase at 3d and declined at 5d, but was still much higher than 1d. There has a significant difference of IDO protein expression between 1 and 3d while no difference was observed between 3 and 5d.
Figure 4. IDO protein in the sham and A. fumigatus-infected corneal tissues.
Western blot analysis showed IDO protein was elevated at 1, 3, 5d after A. fumigatus infection compared with control group. At 3d and 5d, IDO protein was higher than that of 1d. The data were indicated as the mean±standard deviation of four independent experiments. (aP<0.01, bP<0.001 compared with control; cP<0.05, dP<0.01, compared with 1d group).
DISCUSSION
A. fumigatus, a termotolerant saprophyte, is associated with a wide spectrum of diseases in humans, ranging from severe infections to allergy[15]. The inherent resistance to A. fumigatus suggests the existence of the regulatory mechanisms that efficiently oppose both inflammatory and allergic responses to the fungi in order to protect the host from excessive damage[16]. IDO plays a pivotal role in induction of immune tolerance against to A. fumigates[5]–[6]. In murine cystic fibrosis, dysfunctional IDO activity was correlated with imbalanced Th17/Treg-cell responses to A. fumigatus and treatments enhancing IDO function or preventing pathogenic Th17 cell activation could restore protective immunity to the fungi and improved lung inflammation[17]. The stratified squamous epithelium covering of the cornea provides one of the first physical and immunological lines of defense, and human corneal epithelial cells (HCECs) become important participants in the innate immune responses of the ocular surface in defense against fungal invasion[18]. Interestingly, human corneal fibroblasts and epithelial cells can express IDO, with higher levels in the human corneal fibroblasts[19]. Up to now, the relationship between IDO and FK is still unknown. In our previous study, we discovered that IDO took part in the pathogenesis of FK. The results displayed that IDO mRNA expression was obviously enhanced in human corneal epithelium infected by A. fumigatus. Furthermore, the expression of IDO was consistent with the severity of keratomycosis[20]. In order to further evaluate the contribution of IDO to immune reaction to A. fumigatus and the possible mechanisms underlying FK, we resorted to the experimental murine models of FK.
In the present study, few IDO expressed in the normal corneal tissues, while significant enhancement of IDO expression was detected in the corneal epithelium and stroma infected with A. fumigatus. Furthermore, IDO expression was mainly localized in the epithelium. At the early stage of A. fumigatus inflammation, mRNA and protein of IDO were up-regulated significantly. Besides, our research showed blockage of IDO by 1-methyl-tryptophan further increased disease severity[20]. The data implied IDO was involved in the immune reaction of A. fumigatus keratitis at the early stage. Bozza et al[7] found IDO was present in the innate immune cells such as macrophages, PMNs and epithelial cells[8], which was an important element in the suppression of acute inflammatory responses. Moreover, IDO expression by plasmacytoid dendritic cells (pDC) activated the onset of tolerance in adaptive immune. Thus immune regulation induced by IDO is involved in the inductive and effector phases of the innate and acquired immune responses to infection. Recently, IDO activation and tryptophan degradation have appeared to play a significantly regulatory role in damping down the activation of the immune system and inducing tolerance is greatlyrealized[11]. IDO and tryptophan catabolites were initially recognized in the infection due to antimicrobial activity (“tryptophan starvation” of intracellular parasites)[21]. In addition to direct effector activities, IDO and the other enzymes in the metabolic pathway induce the generation of Treg cells with anti-inflammatory and tolerogenic activities by immunoactive molecules[17]. IDO acts an important immunosuppressive role. Besides, the IDO-kynurenine pathway can serve as a negative feedback loop for TH1 cells. IDO-induced Treg cells may act the negative feedback suppression of T cell response. IDO and the kynurenines can prevent the exaggerated immune response in order to maintain the homeostasis[22]–[24]. In experimental fungal infections, IDO blockade greatly aggravated the infection and associated inflammatory damage, and alleviated resistance to re-infection[16]. Moreover, in the mice with C. albicans infection, IDO inhibition greatly exacerbated the infection and associated inflammation as a result of deregulated innate and adaptive/regulatory immune responses[7]. Our study also indicated that IDO took part in the pathogenesis of FK. Based on those previous results, we hypothesized that IDO might at least partly contribute to the immune resistance and tolerance to A. fumigatus infections by regulation of the Th1/Treg versus Th17 pathway balance in FK. The elaborate mechanism underlying FK needs further testification in the murine model. Over the past few years, an increasing number of publications have emphasized the immunoregulatory role of IDO by tryptophan deprivation and production of kynurenines as a metabolic enzyme sustaining immune homeostasis. For example, in the context of corneal allograft, therapeutic induction of immune tolerance by endothelium-derived IDO/kynurenine in a human transplant might maintain a relative immune privilege in the ocular anterior chamber, thereby contributing to the prolonged graft survival[25]–[26]. Besides, inhibitory molecules IDO may act as an element of ocular immune privilege in corneal keratocytes, and can be used as a developing therapeutic agent for controlling ocular inflammation or immune diseases[27]. Interestingly, blockade of IDO2 results in antitumor activity in clinical trials[28], a finding implyinga possible role of tryptophan catabolism in tumor escape from immunosurveillance. In conclusion, we observed that IDO contributed to the inflammation in the cornea infected by A. fumigatus in murine models. We provide the experimental evidence that corneal epithelial cells may induce IDO to control the infection and associated inflammatory response properly. These results provide novel mechanistic insights into the fungus/pathogen interface, relating to the dynamics of host adaptation to the fungus. At this interface, IDO activation probably exerts a fine control over fungal morphology as well as inflammation and antifungal responses. Targeting the role of IDO and kynurenines in the immune responses may open up new areas for pharmacological research with therapeutical potential.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81170825, No.81470609); Specialized Research Fund for the Doctoral Program of Higher Education (No.20123706110003); The Youth Natural Science Foundation of Shandong Province (No.ZR2013HQ007); The Key Project of Natural Science Foundation of Shandong Province (No.ZR2012HZ001).
Conflicts of Interest: Jiang N, None; Zhao GQ, None; Lin J, None; Hu LT, None; Che CY, None; Li C, None; Wang Q, None; Xu Q, None; Zhang J, None; Peng XD, None.
REFERENCES
- 1.Thomas PA. Fungal infections of the cornea. Eye(Lond) 2003;17(8):852–862. doi: 10.1038/sj.eye.6700557. [DOI] [PubMed] [Google Scholar]
- 2.Xie L, Dong X, Shi W. Treatment of fungal keratitis by penetrating keratoplasty. Br J Ophthalmol. 2001;85(9):1070–1074. doi: 10.1136/bjo.85.9.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor PR, Leal SM, Jr, Sun Y, Pearlman E. Aspergillus and Fusarium corneal infections are regulated by Th17 cells and IL-17-producing neutrophils. J Immunol. 2014;192(7):3319–3327. doi: 10.4049/jimmunol.1302235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karthikeyan RS, Leal SM, Jr, Prajna NV, Dharmalingam K, Geiser DM, Pearlman E, Lalitha P. Expression of innate and adaptive immune mediators in human corneal tissue infected with Aspergillus or fusarium. J Infect Dis. 2011;204(6):942–950. doi: 10.1093/infdis/jir426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carvalho A, Cunha C, Bozza S, Moretti S, Massi-Benedetti C, Bistoni F, Aversa F, Romani L. Immunity and tolerance to fungi in hematopoietic transplantation: principles and perspectives. Front Immunol. 2012;3:156. doi: 10.3389/fimmu.2012.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Luca A, Bozza S, Zelante T, Zagarella S, D'Angelo C, Perruccio K, Vacca C, Carvalho A, Cunha C, Aversa F, Romani L. Non-hematopoietic cells contribute to protective tolerance to Aspergillus fumigatus via a TRIF pathway converging on IDO. Cell Mol Immunol. 2010;7(6):459–470. doi: 10.1038/cmi.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozza S, Fallarino F, Pitzurra L, Zelante T, Montagnoli C, Bellocchio S, Mosci P, Vacca C, Puccetti P, Romani L. A crucial role for tryptophan catabolism at the host/Candida albicans interface. J Immunol. 2005;174(5):2910–2918. doi: 10.4049/jimmunol.174.5.2910. [DOI] [PubMed] [Google Scholar]
- 8.Puccetti P, Grohmann U. IDO and regulatory T cells: a role for reverse signalling and non-canonical NF-kappaB activation. Nat Rev Immunol. 2007;7(10):817–823. doi: 10.1038/nri2163. [DOI] [PubMed] [Google Scholar]
- 9.Popov A, Schultze JL. IDO-expressing regulatory dendritic cells in cancer and chronic infection. J Mol Med. 2008;86(2):145–160. doi: 10.1007/s00109-007-0262-6. [DOI] [PubMed] [Google Scholar]
- 10.Romani L, Puccetti P. Protective tolerance to fungi: the role of IL-10 and tryptophan catabolism. Trends Microbiol. 2006;14(4):183–189. doi: 10.1016/j.tim.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4(10):762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 12.Bozza S, Gaziano R, Spreca A, Bacci A, Montagnoli C, di Francesco P, Romani L. Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus. J Immunol. 2002;168(3):1362–1371. doi: 10.4049/jimmunol.168.3.1362. [DOI] [PubMed] [Google Scholar]
- 13.Aimanianda V, Bayry J, Bozza S, Kniemeyer O, Perruccio K, Elluru SR, Clavaud C, Paris S, Brakhage AA, Kaveri SV, Romani L, Latge JP. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature. 2009;460(7259):1117–1121. doi: 10.1038/nature08264. [DOI] [PubMed] [Google Scholar]
- 14.Wu TG, Wilhelmus KR, Mitchell BM. Experimental keratomycosis in a mouse model. Invest Ophthalmol Vis Sci. 2003;44(1):210–216. doi: 10.1167/iovs.02-0446. [DOI] [PubMed] [Google Scholar]
- 15.Moss RB. Pathophysiology and immunology of allergic bronchopulmonary aspergillosis. Med Mycol. 2005;43(Suppl. 1):S203–206. doi: 10.1080/13693780500052255. [DOI] [PubMed] [Google Scholar]
- 16.Montagnoli C, Bozza S, Gaziano R, Zelante T, Bonifazi P, Moretti S, Bellocchio S, Pitzurra L, Romani L. Immunity and tolerance to Aspergillus fumigatus. Novartis Found Symp. 2006;279 [PubMed] [Google Scholar]
- 17.Iannitti RG, Carvalho A, Cunha C, De Luca A, Giovannini G, Casagrande A, Zelante T, Vacca C, Fallarino F, Puccetti P, Massi-Benedetti C, Defilippi G, Russo M, Porcaro L, Colombo C, Ratclif L, De Benedictis FM, Romani L. Th17/Treg imbalance in murine cystic fibrosis is linked to indoleamine 2,3-dioxygenase deficiency but corrected by kynurenines. Am J Respir Crit Care Med. 2013;187(6):609–620. doi: 10.1164/rccm.201207-1346OC. [DOI] [PubMed] [Google Scholar]
- 18.Hua X, Yuan X, Tang X, Li Z, Pflugfelder SC, Li DQ. Human corneal epithelial cells produce antimicrobial peptides LL-37 and beta-defensins in response to heat-killed Candida albicans. Ophthalmic Res. 2014;51(4):179–186. doi: 10.1159/000357977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryu YH, Kim JC. Expression of indoleamine 2,3-dioxygenase in human corneal cells as a local immunosuppressive factor. Invest Ophthalmol Vis Sci. 2007;48(9):4148–4152. doi: 10.1167/iovs.05-1336. [DOI] [PubMed] [Google Scholar]
- 20.Jiang N, Zhao GQ, Lin J, Hu L, Che C, Li C, Wang Q, Xu Q, Peng X. Indoleamine 2,3-dioxygenase is involved in the inflammation response of corneal epithelial cells to aspergillus fumigatus infections. PLoS One. 2015;10(9):e0137423. doi: 10.1371/journal.pone.0137423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunol Cell Biol. 2003;81(4):247–265. doi: 10.1046/j.1440-1711.2003.t01-1-01177.x. [DOI] [PubMed] [Google Scholar]
- 22.Hill M, Tanguy-Royer S, Royer P, Chauveau C, Asghar K, Tesson L, Lavainne F, Rémy S, Brion R, Hubert FX, Heslan M, Rimbert M, Berthelot L, Moffett JR, Josien R, Grégoire M, Anegon I. IDO expands human CD4+CD25high regulatory T cells by promoting maturation of LPS-treated dendritic cells. Eur J Immunol. 2007;37(11):3054–3062. doi: 10.1002/eji.200636704. [DOI] [PubMed] [Google Scholar]
- 23.Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9(10):1069–1077. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 24.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Santamaria P, Fioretti MC, Puccetti P. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176(11):6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 25.Serbecic N, Lahdou I, Scheuerle A, Höftberger R, Aboul-Enein F. Function of the tryptophan metabolite, L-kynurenine, in human corneal endothelial cells. Mol Vis. 2009;15:1312–1324. [PMC free article] [PubMed] [Google Scholar]
- 26.Beutelspacher SC, Pillai R, Watson MP, Tan PH, Tsang J, McClure MO, George AJ, Larkin DF. Function of indoleamine 2,3-dioxygenase in corneal allograft rejection and prolongation of allograft survival by over-expression. Eur J Immunol. 2006;36(3):690–700. doi: 10.1002/eji.200535238. [DOI] [PubMed] [Google Scholar]
- 27.Yang JW, Ham DS, Kim HW, Lee SG, Park SK, Seo SK. Expression of Stat3 and indoleamine 2, 3-dioxygenase in cornea keratocytes as factor of ocular immune privilege. Graefes Arch Clin Exp Ophthalmol. 2012;250(1):25–31. doi: 10.1007/s00417-011-1768-8. [DOI] [PubMed] [Google Scholar]
- 28.Metz R, Duhadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67(15):7082–7087. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]