Abstract
AIM
To demonstrate the cytotoxic effect and possible mechanisms of Tetracaine on human corneal epithelial (HCEP) cells in vitro.
METHODS
In vitro cultured HCEP cell were treated with Tetracaine hydrochloride at different doses for different times, and their morphology, viability, and plasma membrane permeability were detected by light microscopy, methyl thiazolyl tetrazolium (MTT) assay, and acridine orange (AO)/ethidium bromide (EB) staining, respectively. Their cell cycle progression, phosphatidylserine orientation in plasma membrane, and mitochondrial membrane potential (MTP) were assessed by flow cytometry. DNA fragmentation, ultrastructure, caspase activation, and the cytoplasmic apoptosis inducing factor (AIF) and cytochrome c (Cyt. c) along with the expression of B-cell lymphoma-2 (Bcl-2) family proteins were examined by gel electrophoresis, transmission electron microscope, enzyme linked immunosorbent assay (ELISA), and Western blot, respectively.
RESULTS
After exposed to Tetracaine at doses from 10.0 to 0.3125 g/L, the HCEP cells showed dose- and time-dependent morphological abnormality and typical cytopathic effect, viability decline, and plasma membrane permeability elevation. Tetracaine induced phosphatidylserine externalization, DNA fragmentation, G1 phase arrest, and ultrastructural abnormality and apoptotic body formation. Furthermore, Tetracaine at a dose of 0.3125 g/L also induced caspase-3, -9 and -8 activation, MTP disruption, up-regulation of the cytoplasmic amount of Cyt. c and AIF, the expressions of Bax and Bad, and down-regulation of the expressions of Bcl-2 and Bcl-xL.
CONCLUSION
Tetracaine above 0.3125 g/L (1/32 of its clinical applied dosage) has a dose- and time-dependent cytotoxicity to HCEP cells in vitro, with inducing cell apoptosis via a death receptor-mediated mitochondrion-dependent pathway.
Keywords: Tetracaine, cytotoxicity, human cornel epithelial cells, apoptosis, mitochondrion
INTRODUCTION
Human corneal epithelial (HCEP) cells, the uppermost protecting barrier of the cornea with 6-8 layers of cells, are essential for maintaining corneal transparency and our vision[1]. Once HCEP cells were damaged by trauma, infection and drugs, corneal epithelial dysfunction occurred and resulted in chronic inflammation, cornea ulcerations, edema, turbidity, and even blindness[2]–[4].
Local anaesthetics, such as Proparacaine, Lidocaine, Tetracaine, and Bupivacaine, are widely used in ocular diagnostic and ophthalmic surgery in eye clinic[5]–[6]. Some topical anaesthetics have been reported to have toxic side effects on corneal epithelial cells after repeated and prolonged usage[7]–[9]. Among these, Tetracaine, one of the most frequently used ester-type anaesthetic agents in ophthalmic surgeries for its fast onset of action and tissue penetration, has been reported to have side effects such as re-epithelialization retardation, epithelial cell membrane damage and microvillial rarefaction in clinical and animal studies[8]–[10]. However, the detailed cytotoxicity of Tetracaine and its possible cellular and molecular mechanisms have not yet been clearly clarified due to the lack of an in vitro model[11]. Recently, the established non-transfected HCEP cell line with normal phenotype[12] and functional potentials in corneal equivalent construction[13]–[14], makes it possible to study intensively the cytotoxicity of Tetracaine and its underlying mechanisms in vitro. Here, we aimed to investigate the cytotoxicity of Tetracaine to HCEP cells and its cellular and molecular cytotoxic mechanisms using an in vitro model of HCEP cells.
MATERIALS AND METHODS
Materials
HCEP cells, from a HCEP cell line established previously in our laboratory[12], were maintained and cultured in DMEM/F12 medium (Invitrogen, Carlsbad, CA, USA) containing 10% (v/v) fetal bovine serum (FBS; Invitrogen) at 37°C in 25-cm2 culture flasks (Nunc, Waltham, MA, USA). Tetracaine hydrochloride (C15H25ClN2O2; Cas No.: 136-47-0; Purity>98.0%) was purchased from Tokyo Chemical Industry (Tokyo, Japan). The 20.0 g/L stock solution of Tetracaine was prepared with serum-free DMEM medium, and step diluted into concentrations from 10.0 g/L (clinical applied dosage) to 0.15625 g/L (1/64 of its clinical applied dosage) dissolved in 10% (v/v) FBS-DMEM/F12 medium before usage.
Experimental Design
HCEP cells were cultured to logarithmic phase and treated with Tetracaine at concentrations from 10.0 g/L to 0.15625 g/L. For cytotoxicity evaluation, the cell morphology, viability, and cell cycle progression was checked by light microscopy, methyl thiazolyl tetrazolium (MTT) assay, and flow cytometry (FCM) using propidium iodide (PI) staining, respectively. For apoptosis verification, the plasma membrane permeability, phosphatidylserine (PS) orientation, DNA status, and ultrastructure was examined by acridine orange (AO)/ethidium bromide (EB) double-staining, FCM using Annexin-V/PI staining, DNA electrophoresis, and transmission electron microscopy, respectively. For apoptosis signaling pathway postulation, the caspase activation, mitochondrial transmembrane potential (MTP), and mitochondrial-released cytoplasmic apoptosis inducing factor (AIF) and cytochrome c (Cyt. c) along with the expression of B-cell lymphoma-2 (Bcl-2) family proteins was examined by enzyme linked immunosorbent assay (ELISA), FCM using 5,5′,6,6′-Tetrachloro-1′,1′,3,3′-Tetraethybenzimida (JC-1) staining, and Western blot, respectively. In all experiments, HCEP cells cultured in the same medium without any Tetracaine hydrochloride at the same time point were used as blank controls.
Methods
Light microscopy for growth and morphological observations
HCEP cells were cultured in a 24-well culture plate (Nunc) in 10% (v/v) FBS-DMEM/F12 medium at 37°C in a 5% (v/v) CO2 incubator. After the cells grown into logarithmic phase, the culture medium was replaced respectively with the same medium containing Tetracaine at concentrations 10.0-0.15625 g/L. The morphology and growing status of the cells were monitored successively with a TS100 inverted light microscope (Nikon, Tokyo, Japan).
Methylthiazolyl tetrazolium for cell viability assay
MTT assay of HCEP cells exposed to Tetracaine hydrochloride was performed as described previously[15]. Briefly, HCEP cells in 96-well plates (Nunc) (1×104 cells per well) were cultured and treated as described above. At a 2-4h interval, 20 µL of 1.1 mmol/L MTT (Sigma-Aldrich, St. Louis, MO, USA) was added into the medium and incubated for 4h at 37°C in the dark. Then 150 µL of dimethyl sulfoxide (DMSO, Sigma-Aldrich) was added to dissolve the formazan produced at 37°C in the dark for 15min, and the 490 nm absorbance was measured with a Multiskan GO microplate reader (Thermo Scientific, MA, USA).
Double fluorescent staining for plasma membrane permeability assay
AO/EB double-staining of HCEP cells exposed to Tetracaine was performed as described previously[15]. In brief, HCEP cells in 24-well culture plate were cultured and treated as described above. Then the cells were harvested every 1-4h by 2.5 g/L trypsin digestion (1-2min) and centrifugation (200 g, 10min). After stained with AO/EB solution (100 mg/L AO: 100 mg/L EB=1:1) (Sigma-Aldrich) for 1min, the stained cells were observed under a Ti-S fluorescent microscope (Nikon, Tokyo, Japan). At least 400 cells were counted in each group. HCEP cells with red or orange nuclei were designated as apoptotic cells while those with green nuclei as non-apoptotic cells, and their apoptotic ratio was calculated according to the formula: “apoptotic rate (%)=apoptotic cells/(apoptotic cells+non-apoptotic cells) ×100%” with at least 400 cells counted in each group.
Agarose gel electrophoresis for DNA fragmentation assay
DNA agarose gel electrophoresis of HCEP cells exposed to Tetracaine was performed following the method reported previously[16]. Briefly, HCEP cells in 25-cm2 flasks (Nunc) were cultured, treated and harvested as described above. After washed once with chilled phosphate-buffered saline (PBS), the genomic DNA was isolated with a Quick Tissue/Culture Cells Genomic DNA Extraction Kit (Dongsheng Biotech, Beijing, China). The DNA preparation from each group was electrophoresed on a 1% (w/v) agarose gel (200 mA, 260min), stained with 50 mg/L EB for 10min, and observed with an EC3 Imaging System (UVP, Upland, CA, USA).
Transmision electron microscopy for ultrastructure characterization
HCEP cells in 25-cm2 flasks were cultured, treated with 0.3125 g/L Tetracaine and harvested as described above. After fixed with 40 g/L glutaraldehyde and 10 g/L osmium tetroxide successively, the cells were dehydrated and embedded in epoxy resin. Ultrathin sections were stained with 20 g/L uranyl acetate-lead citrate and observed by an H700 transmission electron microscope (TEM, Hitachi, Tokyo, Japan).
Flow cytometry analysis
The cell cycle progression, PS orientation and MTP of Tetracaine-treated HCEP cells were detected and analyzed by FCM as described previously[15]. In brief, HCEP cells in 6-well plates were cultured, treated with 0.3125 g/L Tetracaine hydrochloride and harvested as described above. After washed twice with 1 mL PBS, the cells were fixed with 70% (v/v) alcohol overnight at 4°C. Then the cells were stained with PI for cell cycle assay, stained with annexin V/PI using FITC annexin V Apoptosis Detection Kit I for PS orientation assay, and stained with 10 µg/mL JC-1 for MTP assay, respectively. The stained cells were detected by a FACScan FCM (BD Biosciences, San Jose, CA, USA).
ELISA for caspase activation assay
The activation of caspase-3, -8, and -9 of Tetracaine-treated HCEP cells was performed as described previously[15]. Briefly, HCEP cells in 25 cm2 flasks were cultured, treated with 0.3125 g/L tetracaine and harvested as described above every 2h. Whole-cell protein extracts were prepared by lysing 1×106 cells in 500 µL RIPA lysis buffer (Biotime, Beijing, China) and coated into high-binding 96-well microtitre plates (Nunc), 100 µL per well, at 4°C overnight. After blocked with 5% (w/v) non-fat milk (BD Bioscience), the wells were incubated successively with 100 µL rabbit anti-human caspase-3/8/9 (active form) antibodies (1:500) (Biosynthesis Biotechnology, Beijing, China) and 100 µL HRP-labelled goat anti-rabbit secondary antibody (1:3000) (cwBiotech, Nanjing, Jiangsu Province, China) at 37°C for 2h. A colorimetric reaction was induced by addition of 100 µL chromogenic substrate (0.1 g/L tetramethylbenzidine, 100 mmol/L acetate buffer, pH 5.6 and 1 mmol/L urea hydrogen peroxide) for 25min in the dark at room temperature. Color development was stopped with 50 µL H2SO4 (0.5 mol/L), and the 490 nm absorbance of each well was measured, respectively, using a Multiskan GO microplate reader (Thermo Scientific).
Western blot analysis
The cytoplasmic amount of AIF and Cyt. c, and expression of Bcl-2 family proteins were detected by Western blot as described previously[17]. In brief, HCEP cells in 25-cm2 flasks were cultured, treated with 0.3125 g/L Tetracaine and harvested as described above every 4h. Whole-cell protein extract was prepared as described above for expression assay of Bcl-2 family proteins, and cytoplasmic extract was prepared using mitochondrial and cytoplasmic protein extraction kit (Sangon biological engineering, Shanghai, China) for mitochondrion release assay of AIF and Cyt. c. The protein extract from the same number of cells in each group was electrophoresed by 10% (w/v) SDS-PAGE, and transferred onto nitrocellulose blotting membranes with a semi-dry blot system. After blocked with 5% (v/v) nonfat milk, the membranes were incubated with rabbit anti-human IgG monoclonal antibody to Bad, Bax, Bcl-2, Bcl-xL, AIF, Cyt. c and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) (all in 1:1000 dilution) at 4°C overnight, and HRP-conjugated goat anti-rabbit IgG monoclonal antibody (1:5000) for 2h at room temperature, respectively. Finally, the membranes were immersed in chemiluminescence reagents (Pierce, Rorkford, IL, USA) (0.1 mL/cm2) and visualized in X-ray films. The optical density of each band was quantified using ImageJ analysis software (NIH, NY, USA) with β-actin as an internal control.
Statistical Analysis
Each experiment was repeated 3 times independently. All data were presented as mean±SD and analyzed for statistical significance by one-way analysis of variance (ANOVA) using SPSS for Windows version 17.0 (SPSS, Inc., Chicago, IL, USA). Differences to controls were considered statistically significant when P<0.05.
RESULTS
Morphological Abnormality
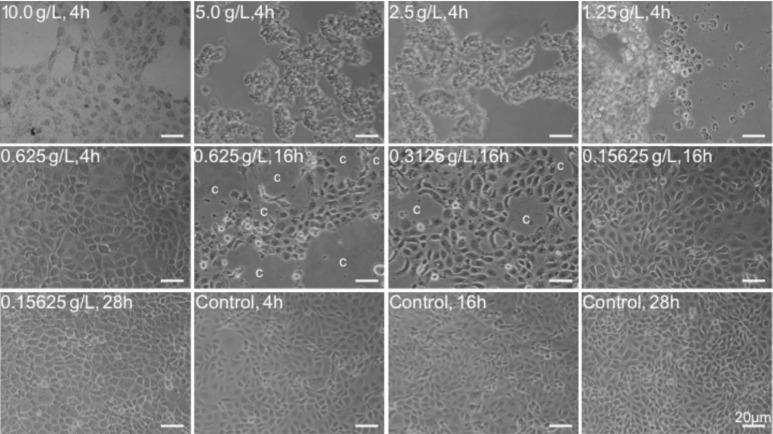
To evaluate the cytotoxicity of Tetracaine, the growth and morphology of HCEP cells was first checked by light microscopy. It was found that HCEP cells treated with 10.0-0.3125g/L Tetracaine exhibited growth retardation and abnormal morphological changes, such as cytoplasmic vacuolation, cellular shrinkage, turning round, detachment from culture matrix, appearance of cytopathic effect (CPE) and eventually death. Whereas, HCEP cells treated with Tetracaine below the concentration of 0.3125 g/L showed no obvious morphological changes compared to controls (Figure 1).
Figure 1. Morphological abnormality of Tetracaine-treated HCEP cells.
Cultured HCEP cells were treated with the indicated concentration and exposure time of Tetracaine, and their growth status and morphology were monitored by light microscopy. One representative photograph from three independent experiments was shown. c: CPE. Bar: 20 µm.
Viability Decline
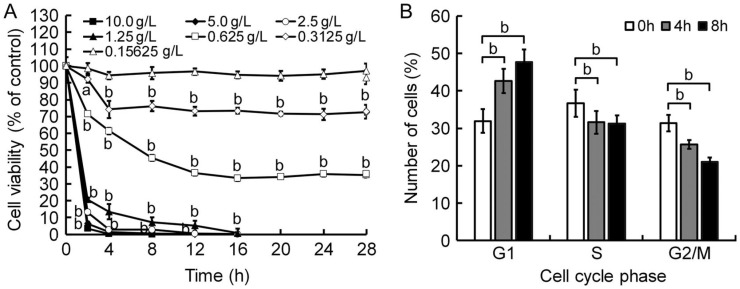
To verify the cytotoxicity of Tetracaine, the viability of HCEP cells was then measured by MTT assay. Results showed that the cell viability of HCEP cells treated with 10.0-0.15625 g/L Tetracaine decreased significantly (P<0.05 or P<0.01) in a dose- and time-dependent manner, while that of HCEP cells treated with Tetracaine hydrochloride below the concentration of 0.3125 g/L showed no significant difference to controls (Figure 2A).
Figure 2. Viability decline and cell cycle retardation of Tetracaine-treated HCEP cells.
A: MTT assay. The cell viability of Tetracaine-treated HCEP cells in each group was expressed as percentage (mean±SD) of 490 nm absorbance compared to its corresponding control (n=3). B: FCM with propidium iodide (PI) staining. G1 phase arrest of HCEP cells exposed to 0.3125 g/L Tetracaine was shown. The number of HCEP cells in different cell cycle phase in each group was expressed as percentage (mean±SD) of its total cell number (n=3), respectively. aP<0.05, bP<0.01 versus control.
Cell Cycle Arrest
To postulate the growth retardation mechanisms of Tetracaine, the cell cycle progression of HCEP cells was assayed by FCM assay using PI staining. It was found that the number of 0.3125 g/L Tetracaine-treated HCEP cells in G1 phase increased with time, while that in S and G2/M phase decreased with time, when compared with that of controls (Figure 2B).
Plasma Membrane Permeability Elevation
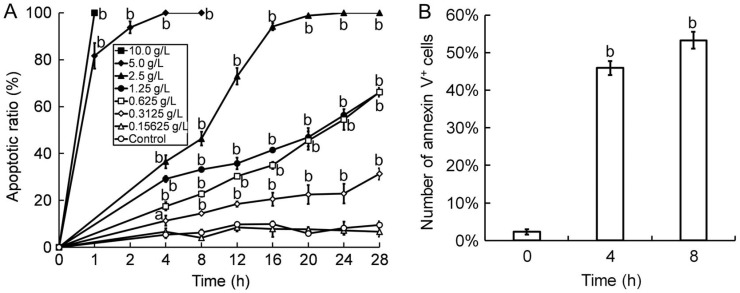
To determine the apoptosis inducing effect of Tetracaine, the plasma membrane permeability of HCEP cells was first detected by AO/EB double fluorescent staining. Results showed that the plasma membrane permeability of Tetracaine-treated HCEP cells elevated with dose and time (P<0.05 or P<0.01), while that of HCEP cells treated with Tetracaine below the concentration of 0.3125 g/L showed no significant difference to that of controls. The apoptotic ratio of HCEP cells was shown in Figure 3A.
Figure 3. Plasma membrane permeability elevation and PS externalization of Tetracaine-treated HCEP cells.
A: AO/EB double fluorescent staining. The apoptotic ratio was caculated as percentage (mean±SD) of the total number of cells based on the permeability elevation of plasma membrane of HCEP cells (n=3). B: FCM with annexin V/propidium iodide (PI) staining. The number of annexin V-positive (PS-externalized) HCEP cells exposed to 0.3125 g/L Tetracaine in each group was expressed as percentage (mean±SD) of its total cell number (n=3). aP<0.05, bP<0.01 versus control.
Phosphatidylserine Externalization
To validate the apoptosis inducing effect of Tetracaine, PS orientation in the plasma membrane of HCEP cells was then examined by FCM assay using annexin V/PI staining. It was found that HCEP cells treated with 0.3125 g/L Tetracaine exhibited significant increase of PS externalization, and in number of annexin V positive cells (PS externalized cells) increased with time (P<0.01) when compared to that of controls (Figure 3B).
DNA Fragmentation
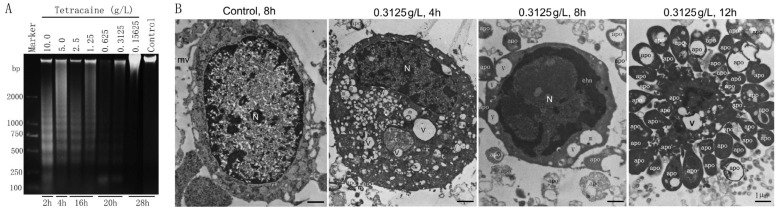
To verify the apoptosis inducing effect of Tetracaine, the genomic DNA of HCEP cells was also checked by agarose gel electrophoresis. Results revealed that genomic DNA extracted from HCEP cells treated 2-28h with 10.0-0.15625 g/L Tetracaine was damaged into a highly fragmented state and typical DNA ladders appeared, while no DNA fragmentation was found in HCEP cells treated with Tetracaine below the concentration of 0.3125 g/L even for 28h, which was similar as controls (Figure 4A).
Figure 4. DNA fragmentation and ultrastructural abnormality of HCEP cells.
A: 1% agarose gel electrophoresis of DNA. The dosage and exposure time of Tetracaine are shown in the top and bottom of each lane, respectively. The dispersed DNA ladders were shown. Marker, D2000 DNA marker. B: TEM photographs. The dosage and exposure time of Tetracaine are shown in the top of each photograph. chn: Condensed chromatin; m: Mitochondrion; mv: Microvillus; N: Nucleus; v: Vacuoles; apo: Apoptotic body. Bar: 1 µm.
The Ultrastructural Abnormality
To confirm the apoptosis inducing effect of Tetracaine, the ultrastructure of HCEP cells was further examined by TEM. Results showed that HCEP cells treated with 0.3125 g/L Tetracaine for 4h showed early apoptotic-like ultrastructural changes, such as structural disorganization, cytoplasmic vacuolization and mitochondrial swelling. Those treated for 8h exhibited middle-stage apoptotic-like ultrastructural changes including advanced mitochondrial swelling, chromatin condensation and intra-nuclear margination, and a few apoptotic body formation. Those treated for 12h exhibited late-stage apoptotic-like ultrastructural changes such as cell disintegration and a lot of apoptotic body formation (Figure 4B).
Caspase Activation
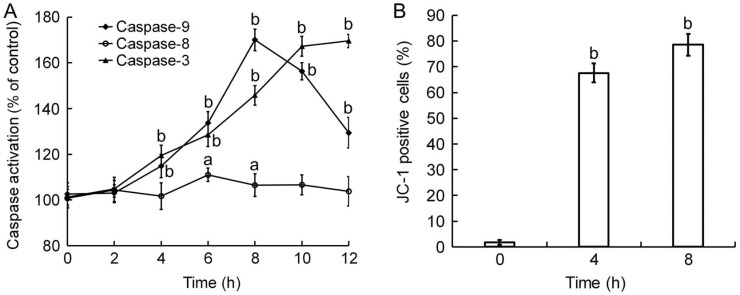
To postulated the triggering pathways of Tetracaine-induced apoptosis, the activation of caspase-3, -8, and -9 of HCEP cells was determined by ELISA using antibodies against their active forms. Results indicated that caspase-8 in 0.3125 g/L Tetracaine-treated HCEP cells was activated to a peak value at 6h (P<0.05), caspase-9 in the cells was activated to its peak value at 8h (P<0.01), and caspase-3 in the cells was activated continuously during the monitor period of 12h (P<0.01) (Figure 5A).
Figure 5. Caspase activation and MTP disruption of 0.3125 g/L Tetracaine-treated HCEP cells.
A: ELISA using monoclonal antibodies to the active forms of caspase-3, -8, and -9. The activation ratio of caspases in each group was expressed as percentage (mean±SD) compared to its corresponding control based on 490 nm absorbance (n=3). B: FCM with JC-1 staining. The cell number of JC-1 positive (MTP disrupted) HCEP cells in each group was expressed as percentage (mean±SD) of its total cell number (n=3). aP<0.05, bP<0.01 versus control.
Mitochondrial Membrane Potential Disruption
To verify the involvement of a mitochondrion-dependent pathway in Tetracaine-induced apoptosis, the MTP of HCEP cells was assayed by FCM using JC-1 staining. Results revealed that the MTP of HCEP cells treated with 0.3125 g/L Tetracaine was disrupted significantly with time. The number of monomer JC-1 positive cells increased from 1.73%±0.53% of control to 67.63%±3.75% at 4h and 78.61%±4.24% at 8h, respectively (Figure 5B).
Quantitive Changes of Apoptosis-triggering Proteins
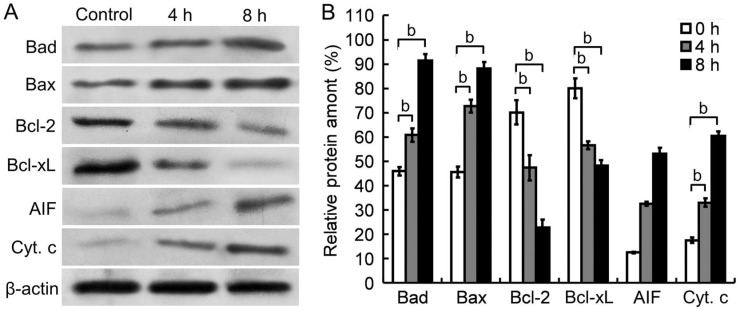
To confirm a mitochondrion-dependent pathway is involved in Tetracaine-induced apoptosis, the cytoplasmic amount of AIF and Cyt. c, and the expression of Bcl-2 family proteins in HCEP cells were further detected by Western blot. It was found that the expression level of Bax and Bad was up-regulated, that of Bcl-2 and Bcl-xL was down-regulated, and the amount of cytoplasmic AIF and Cyt. c was up-regulated in HCEP cells after exposed to 0.3125 g/L Tetracaine for 4 and 8h, respectively (Figure 6).
Figure 6. Western blots of apoptosis-triggering proteins in 0.3125 g/L Tetracaine-treated HCEP cells.
A: Western blot images. The cytoplasmic Cyt. c and AIF, and the expression pattern of Bcl-2 family proteins in HCEP cells were shown. B: Densitometry analysis. The relative level of protein amount was expressed as percentage (mean±SEM) of protein band density compared to an internal control of β-actin (n=3). aP<0.05, bP<0.01 versus control.
DISCUSSION
Tetracaine has been reported to have toxicity on rabbit corneal epithelial cells in vivo[7]–[8]. However, the cytotoxicity and its underlying mechanisms of tetracaine are not well understood till now. Here, we investigated the cytotoxicity of Tetracaine and its apoptosis-inducing mechanisms using an in vitro model of non-transfected HCEP cells for the first time.
To evaluate the cytotoxicity of Tetracaine, the morphology, viability, and cell cycle progression of HCEP cells were examined by light microscopy, MTT assay, and FCM using PI staining, respectively. Our results showed that Tetracaine at concentrations above 0.3125 g/L (1/32 of its clinical applied dosage) could induce growth retardation, apoptosis-like morphological changes with CPE, viability decline, and G1 phase arrest of HCEP cells in a time- and/or dose-dependent manner. All these indicate that Tetracaine has a dose- and time-dependent cytotoxicity to HCEP cells in vitro, which has been supported by previous reports on the cytotoxic effects of Proparacaine, Oxybuprocaine and Lidocaine[15],[18]–[20]. As reported, G1 phase arrested cells will permanently enter a senescent state that might induce apoptosis if they could not get through the G1/S checkpoint[21]. Therefore, the cell cycle arrest, combined with the morphological changes and viability decrease, imply that tetracaine might have an apoptosis-inducing effect on HCEP cells in vitro.
As well known, plasma membrane permeability elevation, PS externalization, DNA fragmentation (also known as DNA ladder) and apoptotic body formation are all hallmark features of apoptotic cell death[22]–[24]. To verify the apoptosis-inducing effect of Tetracaine, the plasma membrane permeability, PS orientation, DNA integrality, and ultrastructure of the Tetracaine-treated HCEP cells was then detected by AO/EB staining, FCM using Annexin-V/PI staining, agarose gel electrophoresis, and TEM, respectively. Our results displayed that Tetracaine at concentrations above 0.3125 g/L could induce elevation of the plasma membrane permeability and fragmentation of genomic DNA of HCEP cells in a dose- and time-dependent manner, Meanwhile, 0.3125 g/L Tetracaine could also induce PS externalization and typical apoptotic-like ultrastructural changes, such as structural disorganization, chromatin condensation and apoptotic body formation of HCEP cells. All these indicate that Tetracaine has an apoptosis-inducing effect on HCEP cells in vitro. The apoptosis-inducing effect of Tetracaine has also been supported by our previous reports on the apoptosis-inducing effects of Oxybuprocaine, Lidocaine and Proparacaine[15],[18]–[20].
Generally, apoptosis is triggered two main pathways, a death receptor mediated extrinsic pathway and an intrinsic mitochondrion-dependent pathway[25], both of which are related to the activation of different initiator caspases [26]. To postulate the possible pathways involved in Tetracaine-induced apoptosis, the activation of caspase-3/-8/-9 of HCEP cells was characterized by ELISA using monoclonal antibodies against the active form of caspase-3/-8/-9. We found that Tetracaine could activate caspase-8, -9 and -3, respectively. As well elucidated, activation of caspase-8 is mediated by death receptor of Fas/CD95 and activation of caspase-9 is mediated by Cyt. c released from the mitochondrion which is triggered by disruption of MTP[26]. Our results of the activation of both caspase-8 and -9 suggest that the Tetracaine-induced apoptosis is most probably mediated by both a death receptor pathway and a mitochondrion pathway. To verify the involvement of mitochondrion in the Tetracaine-induced apoptosis, we then examined the disruption of MTP by FCM using JC-1 staining, and found that Tetracaine could induce MTP disruption of HCEP cells. As known in the mitochondrion-dependent pathway, the disruption of MTP is a prerequisite for triggering mitochondrial release of Cyt. c (required for caspase-9 activation) and AIF (required for caspase-independent initial chromatin condensation and large-scale DNA fragmentation)[26]–[27]. Our results indicate that the Tetracaine-induced apoptosis is triggered by a mitochondrion-dependent pathway. To verify the mitochondrial release of Cyt. C and AIF, we finally examined the cytoplasmic amount of AIF and Cyt. c along with the expression level of Bcl-2 family proteins by Western blot. We found that Tetracaine could up-regulate the cytoplasmic amount of AIF and Cyt. c, up-regulate the expression level of Bax and Bad, and down-regulate the expression level of Bcl-2 and Bcl-xL. As well demonstrated in Bcl-2 family proteins, anti-apoptotic Bcl-2 and Bcl-xL function as a gatekeeper of mitochondria to prevent the release of Cyt. c and AIF, while pro-apoptotic Bax and Bad interact with the mitochondrial permeability transition pore to induce MTP disruption and release of Cyt. c and AIF from mitochondria into the cytoplasm[28]–[29]. Our results of up-regulation of Cyt. c and AIF, combined with MTP disruption and caspase-9 activation, indicates that the Tetracaine-induced apoptosis of HCEP cells is regulated by a mitochondria-dependent pathway. This conclusion is supported by our previous reports on the apoptosis triggered by local anaesthetics[15],[18]–[20].
To our knowledge, this is the first attempt of studying the cytotoxicity of Tetracaine to HCEP cells and its cytotoxic mechanisms at cellular and molecular levels in vitro. Even these findings are particularly relevant in deciding the optimal local anaesthetic to be applied in clinical situations, they do not allow us to predict clinical inferences directly without further investigations in vivo.
Tetracaine at concentrations above 0.3125 g/L (1/32 of its clinical applied dosage) has a dose- and time-dependent cytotoxicity to HCEP cells in vitro, which is realized by inducing apoptosis in these cells via a death receptor-mediated mitochondria-dependent pathway. Our findings provide new insights into the non-ignorable cytotoxicity and apoptosis-inducing effect of Tetracaine on HCEP cells.
Acknowledgments
We thank Mr. Ming-Zhuang Zhu, from Key Laboratory of Mariculture, for his guidance and support during flow cytometry analyses.
Foundation: Supported by National High Technology Research and Development Program (“863” Program) of China (No.2006AA02A132).
Conflicts of Interest: Pang X, None; Fan TJ, None.
REFERENCES
- 1.Suzuki K, Saito J, Yanai R, Yamada N, Chikama T, Seki K, Nishida T. Cell-matrix and cell-cell interactions during corneal epithelial wound healing. Prog Retin Eye Res. 2003;22(2):113–133. doi: 10.1016/s1350-9462(02)00042-3. [DOI] [PubMed] [Google Scholar]
- 2.Kinoshita S, Adachi W, Sotozono C, Nishida K, Yokoi N, Quantock AJ, Okubo K. Characteristics of the human ocular surface epithelium. Prog Retin Eye Res. 2001;20(5):639–673. doi: 10.1016/s1350-9462(01)00007-6. [DOI] [PubMed] [Google Scholar]
- 3.Dart J. Corneal toxicity: the epithelium and stroma in iatrogenic and factitious disease. Eye(Lond) 2003;17(8):886–892. doi: 10.1038/sj.eye.6700576. [DOI] [PubMed] [Google Scholar]
- 4.Patel M, Fraunfelder FW. Toxicity of topical ophthalmic anesthetics. Expert Opin Drug Metab Toxicol. 2013;9(8):983–988. doi: 10.1517/17425255.2013.794219. [DOI] [PubMed] [Google Scholar]
- 5.Verma S, Corbett MC, Patmore A, Heacock G, Marshall J. A comparative study of the duration and efficacy of tetracaine 1% and bupivacaine 0.75% in controlling pain following photorefractive keratectomy (PRK) Eur J Ophthalmol. 1997;7(4):327–333. doi: 10.1177/112067219700700404. [DOI] [PubMed] [Google Scholar]
- 6.Jackson T, McLure H. Pharmacology of local anesthetics. Ophthalmol Clin North Am. 2006;19(2):155–161. doi: 10.1016/j.ohc.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Grant RL, Acosta D. Comparative toxicity of tetreacaine, proparacaine and cocaine evalucated with primary cultures of rabbit corneal epithelial cells. Exp Eye Res. 1994;58(4):469–478. doi: 10.1006/exer.1994.1040. [DOI] [PubMed] [Google Scholar]
- 8.Bolika M, Kolar G, Vidensek J. Toxic side effects of local anaesthetics on the human cornea. Br J Ophthalmol. 1994;78(5):386–389. doi: 10.1136/bjo.78.5.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tappeiner C, Flueckiger F, Boehnke M, Goldblum D, Garweg JG. Effect of topical anesthetic agents and ethanol on corneoepithelial wound healing in an ex vivo whole-globe porcine model. J Cataract Refract Surg. 2012;38(3):519–524. doi: 10.1016/j.jcrs.2011.09.043. [DOI] [PubMed] [Google Scholar]
- 10.Grant RL, Acosta D., Jr A digitized fluorescence imaging study on the effects of local anesthetics on cytosolic calcium and mitochondrial membrane potential in cultured rabbit corneal epithelial cells. Toxicol Appl Pharmacol. 1994;129(1):23–35. doi: 10.1006/taap.1994.1225. [DOI] [PubMed] [Google Scholar]
- 11.Castro-Muñozledo F. Corneal epithelial cell cultures as a tool for research, drug screening and testing. Exp Eye Res. 2008;86(3):459–469. doi: 10.1016/j.exer.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Fan TJ, Xu B, Zhao J, Yang HS, Wang RX, Hu XZ. Establishment of an untransfected human corneal epithelial cell line and its biocompatibility with denuded amniotic membrane. Int J Ophthalmol. 2011;4(3):228–234. doi: 10.3980/j.issn.2222-3959.2011.03.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu B, Fan TJ, Yang HS, Sun A, Zhao J, Ma XY, Hu XZ. In vitro reconstruction and characterization of tissue-engineered human corneal epithelium with seeder cells from an untransfected human corneal epithelial cell line. Int J Ophthalmol. 2012;5(3):281–285. doi: 10.3980/j.issn.2222-3959.2012.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu B, Fan TJ, Zhao J, Sun A, Wang RX, Hu XZ, Yu HZ, Fan XY, Xu XH. Transplantation of tissue-engineered human corneal epithelium in limbal stem cell deficiency rabbit models. Int J Ophthalmol. 2012;5(4):424–429. doi: 10.3980/j.issn.2222-3959.2012.04.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen Q, Fan T, Bai S, Sui Y. Cytotoxicity of proparacaine to human corneal endothelial cells in vitro. J Toxicol Sci. 2015;40(4):427–436. doi: 10.2131/jts.40.427. [DOI] [PubMed] [Google Scholar]
- 16.Lee SV, Bahaman AR. Modified gel preparation for distinct DNA fragment analysis in agarose gel electrophoresis. Trop Biomed. 2010;27(2):351–354. [PubMed] [Google Scholar]
- 17.Tian CL, Wen Q, Fan TJ. Cytotoxicity of atropine to human corneal epithelial cells by inducing cell cycle arrest and mitochondrion-dependent apoptosis. Exp Toxicol Pathol. 2015;67(10):517–524. doi: 10.1016/j.etp.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Fan TJ, Wen Q, Yu MM, Ge Y, Miao Y, Wang DP. Experimental studies on the effect of oxybuprocaine hydrochloride on human corneal endothelial cells. Guoji Yanke Zazhi (Int Eye Sci) 2012;12(8):1442–1446. [Google Scholar]
- 19.Yu HZ, Li YH, Wang RX, Zhou X, Yu MM, Ge Y, Zhao J, Fan TJ. Cytotoxicity of lidocaine to human corneal endothelial cells in vitro. Basic Clin Pharmacol Toxicol. 2014;114(4):352–359. doi: 10.1111/bcpt.12186. [DOI] [PubMed] [Google Scholar]
- 20.Zhou X, Li YH, Yu HZ, Wang RX, Fan TJ. Local anesthetic lidocaine induces apoptosis in human corneal stromal cells in vitro. Int J Ophthalmol. 2013;6(6):766–771. doi: 10.3980/j.issn.2222-3959.2013.06.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao XY, Hao M, Yang XY, Ba Q, Li M, Ni SJ, Wang LS, Du X. Licochalcone A inhibits growth of gastric cancer cells by arresting cell cycle progression and inducing apoptosis. Cancer Lett. 2011;302(1):69–75. doi: 10.1016/j.canlet.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 22.Dallaporta B, Marchetti P, de Pablo MA, Maisse C, Duc HT, Métivier D, Zamzami N, Geuskens M, Kroemer G. Plasma membrane potential in thymocyte apoptosis. J Immunol. 1999;162(11):6534–6542. [PubMed] [Google Scholar]
- 23.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184(1):39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 24.Takemura G, Kato S, Aoyama T, Hayakawa Y, Kanoh M, Maruyama R, Arai M, Nishigaki K, Minatoguchi S, Fukuda K, Fujiwara T, Fujiwara H. Characterization of ultrastructure and its relation with DNA fragmentation in Fas-induced apoptosis of cultured cardiac myocytes. J Pathol. 2001;193(4):546–556. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH794>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 25.Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther. 2005;4(2):139–163. doi: 10.4161/cbt.4.2.1508. [DOI] [PubMed] [Google Scholar]
- 26.Fan TJ, Han LH, Cong RS, Liang J. Caspase family proteases and apoptosis. Acta Biochim Biophys Sin (Shanghai) 2005;37(11):719–727. doi: 10.1111/j.1745-7270.2005.00108.x. [DOI] [PubMed] [Google Scholar]
- 27.Lü CX, Fan TJ, Hu GB, Cong RS. Apoptosis-inducing factor and apoptosis. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 2003;35(10):881–885. [PubMed] [Google Scholar]
- 28.Fu YF, Fan TJ. Bcl-2 Family Proteins and Apoptosis. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 2002;34(4):389–394. [PubMed] [Google Scholar]
- 29.Chen Q, Lesnefsky EJ. Blockade of electron transport during ischemia preserves bcl-2 and inhibits opening of the mitochondrial permeability transition pore. FEBS lett. 2011;585(6):921–926. doi: 10.1016/j.febslet.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]