Abstract
AIM
To investigate the anti-inflammatory effect of an aqueous whole plant extract of Heliotropium indicum (HIE) on endotoxin-induced uveitis in New Zealand white rabbits.
METHODS
Clinical signs of uveitis including flares, iris hyperemia and miosis, were sought for and scored in 1.0 mg/kg lipopolysaccharide (LPS) -induced uveitic rabbits treated orally with HIE (30-300 mg/kg), prednisolone (30 mg/kg), or normal saline (10 mL/kg). The number of polymorphonuclear neutrophils infiltrating, the protein concentration, as well as levels of tumor necrosis factor-α (TNF-α), prostaglandin E2 (PGE2), and monocyte chemmoattrant protein-1 (MCP-1) in the aqueous humor after the various treatments were also determined. A histopathological study of the anterior uveal was performed.
RESULTS
The extract and prednisolone-treatment significantly reduced (P≤0.001) both the clinical scores of inflammation (1.0-1.8 compared to 4.40±0.40 in the normal saline-treated rabbits) and inflammatory cells infiltration. The level of protein, and the concentrations of TNF-α, PGE2 and MCP-1 in the aqueous humor were also significantly reduced (P≤0.001). Histopathological studies showed normal uveal morphology in the HIE and prednisolone-treated rabbits while normal saline-treated rabbits showed marked infiltration of inflammatory cells.
CONCLUSION
The HIE exhibits anti-inflammatory effect on LPS-induced uveitis possibly by reducing the production of pro-inflammatory mediators.
Keywords: monocyte chemotatic protein-1, prostaglandin E2, tumor necrosis factor-α, polymorphonuclear neutrophil, iris hyperemia, prednisolone, heliotropium, uveitis
INTRODUCTION
There are several unverified traditional health care practices in Ghana and elsewhere in Africa. One of such is the traditional use of Heliotropium indicum (H. indicum) in the treatment of several ailments of the eye. Fluids from the leaves of this plant are squeezed directly into the eye; but whole plant is taken orally as dietary ingredient, in managing local ocular inflammations and postpartum inflammatory reaction respectively. The popularity of H. indicum in traditional medicines across the globe has led to several scientific investigations into its pharmacological activity, some of which has resulted in the isolation of some alkaloids of pharmacological importance. Notable among these alkaloids are indicine, indicine-N-oxide, acetyl-indicine, indicinine, heleurine, heliotrine, supinine, supinidine and lindelofidine[1]–[6]. One of these bioactive compounds, indicine-N-oxide, is well known for its anti-cancer property.
Notwithstanding the growing interest of the scientific community in bio-prospecting for potential pharmaceuticals in H. indicum in managing various systems disorders, its traditional use as an antidote to ocular disorders such as uveitis remains largely unconfirmed[7]–[8]. This study therefore sought to evaluate the observed traditional use of H. indicum in the management of ocular inflammatory disorders[7],[9] in rabbits as an initial step to validate its potential usefulness as an anti-inflammatory agent. It is worthy to note that previous preclinical studies have indicated its anti-inflammatory effect in generalized inflammation however, its intraocular anti-inflammatory effect is yet to be established[10]. A number of animal models have been developed for in depth preclinical studies useful in guiding the development of novel therapeutic approaches to combat the menace of anterior uveitis, a potentially blinding eye disorder[11]. Popular among these models regarding anterior uveitis is endotoxin-induced uveitis[12]–[13]. This model seems ideal as it typifies intraocular inflammation in humans i.e. the exudation of flares, inflammatory cells and preponderance of inflammatory mediators leading to pain, photophobia and reduction in visual acuity[14]. These pathophysiological changes lead to symptoms worrisome enough to victims of uveitis causing them to seek desperate measures such as self medicating with unapproved herbals. Even though there is a low prevalence of uveitis group of disease worldwide (estimated at 0.73%) and accounts for only 0.8% outpatients visits[15]–[16], it affects people in their prime (averagely 30.7y). This makes it economically important due to it far reaching consequence on productivity and the individual sufferer's autonomy. Improperly managed anterior uveitis could lead to complications such as cataract, secondary glaucoma and macular edema[17]. However, conventional pharmacological management with steroid (the mainstay treatment) is bedeviled with a lot adverse effects equaling the complications of the disease entity[18]–[19]. Adjunctive therapy such as cycloplegics and mydratics are also not without adverse concerns such as induction of acute angle closure glaucoma and chemotatic neutrophil response despite their useful role in stabilization and restoration of the integrity of the blood-aqueous barrier[20]–[21]. There is therefore no doubt that alternatives and other unverified traditional practices with botanicals need to be explored to broaden treatment horizon for uveitis. Unlike the previous studies, this study aimed at evaluating the anti-inflammatory effect of aqueous extract of H. indicum (HIE) in endotoxin-induced uveitis, a specific intraocular inflammatory model and to predict its possible mechanism of action.
MATERIALS AND METHODS
Plant Collection
H. indicum was collected in November 2012, from the University of Cape Coast botanical gardens (5.1036° N, 1.2825° W), Cape Coast, Ghana. The plant was identified and authenticated by a botanist at the School of Biological Sciences, College Agricultural and Natural Sciences, University of Cape Coast, Cape Coast, Ghana, where a voucher specimen (Specimen number: 4873) has been deposited.
Preparation of the Heliotropium Indicum Aqueous Extract
Whole plants of H. indicum were washed thoroughly with tap water and shade-dried. The dry plants were milled into coarse powder by a hammer mill (Schutte Buffalo, New York, NY, USA). One kilogram of the plant powder was mixed with one liter of water. The mixture was Soxhlet extracted at 80°C, for 24h. The aqueous extract was freeze-dried (Hull freeze-dryer/lyophilizer 140 SQ, Warminster, PA, USA). The powder obtained (yield 12.2%), was labeled HIE, and stored at a temperature of 4°C. This was reconstituted in normal saline to the desired concentration for dosing in this study.
Experimental Animals and Husbandry
Ten-week-old New Zealand rabbits of either sex, weighing 1±0.2 kg were kept in the Animal House of the School of Biological Sciences, University of Cape Coast, Ghana, for use in this study. The animals were housed singly in aluminum cages (34×47×18-cm3) with soft wood shavings as bedding, under ambient laboratory conditions (temperature 28°C±2°C, relative humidity 60%-70%, and a normal light-dark cycle). They were fed with normal commercial pellet diet (Agricare Ltd., Kumasi, Ghana) and had access to water ad libitum.
Ethical and Biosafety Considerations
The study protocols were approved by the Institutional Review Board on Animal Experimentation, Faculty of Pharmacy and Pharmaceutical Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana (Ethical clearance number: FPPS/PCOL/0030/2013). All activities performed during the studies conformed to accepted principles for laboratory animal use and care (EU directive of 1986: 86/609/EEC), and Association for Research in Vision and Ophthalmology Statement for Use of Animals in Ophthalmic and Vision Research. Biosafety guidelines for protection of personnel in the laboratory were observed.
Preliminary Phytochemical Screening
Using standard procedures, described by Harborne[22] and Kujur et al[23], preliminary phytochemical screening was performed on HIE.
Drugs and Chemicals Used
Lipopolysaccharide (LPS) (Calbiochem, EMD Chemicals, San Diego, CA, USA) an endotoxin from Escherichia coli was used to induce uveitis. Bicinchoninic acid (BCA) protein assay reagent kit (Pierce, Rockford, IL. USA) was used to determine total protein in the aqueous humour. Prednisolone (M&G Pharmaceuticals Ltd., Accra, Ghana) was the reference anti-inflammatory agent in this study. Tumor necrosis factor alpha (TNF-α) ELISA kit (Genorise Scientific, Inc, USA), prostaglandin E2 (PGE2) ELISA kit (Wuhan Huamei Biotech Co., Ltd., Wuhan, China), monocyte chemotactic protein 1 (MCP-1) (Biotang Inc., USA) were used in assaying for marker of intraocular inflammation. Chloroform was used to euthanize the animals and normal saline solution (Claris lifesciences limited, Chacharwadi, India) was the vehicle in which other drugs were dissolved.
Induction of Anterior Uveitis
All rabbits were examined for clinical signs of inflammation prior to the induction of ocular inflammation to rule out any pre-existing inflammatory disorder. To induce uveitis, rabbits were injected with 1.0 mg/kg LPS through the marginal ear vein[24]. Two hours later, the anterior segment of the eye was evaluated by the diffuse and conical beam illumination technique using a slit lamp. Observation of infiltration of cells, flares, iris hyperemia, and miosis was indicative of uveitis.
Effect of Heliotropium Indicum Aqueous Extract on Lipopolysaccharide-induced Uveitis
LPS-induced uveitic rabbits were grouped into five (n=5), labelled I-V, and treated as follows: Groups I, II, and III were treated with 30, 100 or 300 mg/kg HIE respectively (selection of doses was based on reports from previous studies[25]). Groups IV and V were treated with 30 mg/kg prednisolone and 10 mL/kg normal saline respectively. All treatments were administered orally by means of an oral gavage and dosing was done once daily. Eighteen hours post-LPS injection, both eyes of the rabbits were examined again for vasodilation, and exudation of cells and proteins (clinical signs of uveitis) using the slit lamp (Lombart Instrument Company, Norfolk, VA, USA). The extent of uveitis per treatment groups was given clinical scores. The animals were euthanized and the aqueous humor from the anterior chamber of both eyes collected into sterile Eppendorf tubes using a 25 gauge needle. The aqueous humour was assessed for polymorphonuclear neutrophils (PMNs), total proteins, MCP-1, PGE2, TNF-α.
Clinical Score of Uveitis
The scoring was performed as follows: iris hyperemia/redness (0-2), flare (0-1), cells in the anterior chamber (0-2), hypopyon/pus in the anterior chamber (0-1), miosis (0-1). The maximum practicable score was 7[26].
Polymorphonuclear Neutrophil Count
Small quantities of a 1:10 dilution (Diluent: Türk's stain solution) of the aqueous humour collected was from the various treatment groups were pipetted onto the counting chamber of the Improved Neubauer Haemocytometer (Depth 0.1 mm, Area: 1/400 mm2; Yancheng Cordial Lab Glassware Co. Ltd., Jiangsu Province, China). PMNs were counted from four “large” squares (volume: 0.4 mm3) using a Ceti magnum-T/trinocular microscope for fluorescence (Medline Scientific limited, UK), under objective magnification of 40×. The number of PMNs was determined per mm3 of aqueous humour (taking into account the dilution dynamics).
Determination of Total Protein Concentration
A Bicinchoninic Acid (BCA) protein assay kit (Pierce, Rockford, IL, USA) was used to determine the total protein concentration in the aqueous humour. Fifty microliter (50 µL) quantities of aqueous humour collected from the various treatment groups as well as standard bovine serum albumin (BSA) were introduced into well on a 96-well microplate with a micropipette. A 200 µL quantity of working reagent, constituted according to the manufacturer's instructions, was mixed thoroughly with the content of each well and shaken for 30s. The microplate and its content was then incubated at 37°C for 30min and allowed to cool to room temperature. Absorbances of the mixtures were measured at 562 nm using an URIT-660 microplate reader (URIT Medical Electronic Co., Ltd, Guangxi, China). Each determination was done in triplicate.
Determination of Monocyte Chemotactic Protein 1 Concentration
The concentration of MCP-1 in the aqueous humour of each sample from the treatment groups was determined using a double-antibody sandwich ELISA kit per manufacturer's the procedure. All samples were assayed in duplicate and on the same plate. The detection limits for this assay was 15-4000 pg/mL. In brief, the samples were loaded along with the standards, and the biotinylated-MCP-1 antibody was then added. The wells were washed to remove unbound antibody-enzyme reagent, after which tetramethyl-benzidine (TMB) substrate was added. The addition of the TMB substrate produced blue coloration upon catalysis by horseradish peroxidase (HRP) enzyme. The reaction was terminated by adding the acidic stop solution which changed the solution from blue to yellow. Absorbances of the mixtures were measured at 450 nm using an URIT-660 microplate reader (URIT Medical Electronic Co., Ltd., Guangxi, China). The concentration of MCP-1 was calculated using a standard curve. Each determination was done in triplicate.
Determination of Prostaglandin E2 Levels
The ELISA kit had a minimum detectable dose of rabbit PGE2 of less than 7.8 pg/mL. The standards or samples were prepared according to the manufacturer's instruction which were pipette into the microtiter plate wells with a biotin-conjugated polyclonal antibody preparation specific for rabbit PGE2. Subsequently, avidin conjugated to HRP was introduced into each well and incubated. Following this, TMB (3,3′,5,5′ tetramethyl-benzidine) substrate solution was added to each well. Only wells which contain PGE2, biotin-conjugated antibody and enzyme-conjugated avidin showed a change in color. The enzyme-substrate reaction was stopped by the adding 50 µL of sulphuric acid solution. After which the color intensity was measured spectrophotometrically at a wavelength of 450 nm. The concentration of PGE2 in the samples was then determined by comparing the optical densities of the samples to the standard curve. Each determination was done in triplicate.
Determination of Tumor Necrosis Factor Alpha Levels in Aqueous Humour
A commercial ELISA kit for the estimation of TNF-α in the rabbit aqueous humour (detection range: 31-2000 pg/mL; sensitivity: 0.8 pg/mL) was used. It employs a quantitative sandwich enzyme immunoassay technique with improved performance owing to its biotin-streptavidine chemistry. Standards and samples (100 µL) were pipetted into a pre-coated microplate with antibody specific for rabbit TNF-α in which any TNF-α present was bound by the immobilized antibody. After washing away the unbound antibody substances, a detection antibody specific for rabbit TNF-α was added to the wells. A detection reagent was then added which led to color development in proportion to the amount of TNF-α bound in the initial step. The color development was stopped and the intensity of the color was measured at 540 nm using an URIT-660 microplate reader (URIT Medical Electronic Co., Ltd., Guangxi, China). All determinations were in triplicate.
Histopatholgical Assessment
The enucleated eyes of animals from the treatment groups were fixed in 4% phosphate-buffered paraformaldehyde, and embedded in paraffin. Sections of the anterior uvea of the treated (HIE, prednisolone or normal saline) rabbit were made and stained with hematoxylin and eosin[26] and fixed on glass slides for microscopic examination at the Pathology Department of the Komfo Anokye Teaching Hospital, Kumasi, Ghana for histopathological assessment by a specialist pathologist.
Statistic Analysis
Data obtained for control, test, and reference drug effects were analyzed by one-way analysis of variance followed by Dunnett's multiple comparisons test using GraphPad Prism (version 5.03; GraphPad, La Jolla, CA, USA). Values were expressed as the mean±standard error of the mean. P≤0.05 was considered to be statistically significant.
RESULTS
Phytochemical Screening
Preliminary phytochemistry showed that flavonoids, saponins, cyanogenic glycosides, sterol, tannins and alkaloids were present in HIE (Table 1).
Table 1. Results obtained after preliminary phytochemical screening of HIE.
| Phytochemical tested for | Results obtained |
| Anthraquinones | - |
| Tannins | + |
| Flavonoids | + |
| Alkaloids | + |
| Sterols | + |
| Glycosides | + |
| Saponnins | + |
| Triterpenoids | - |
+: Present; -: Absent.
Effect of Heliotropium Indicum Aqueous Extract on Lipopolysaccharide-induced Uveitis
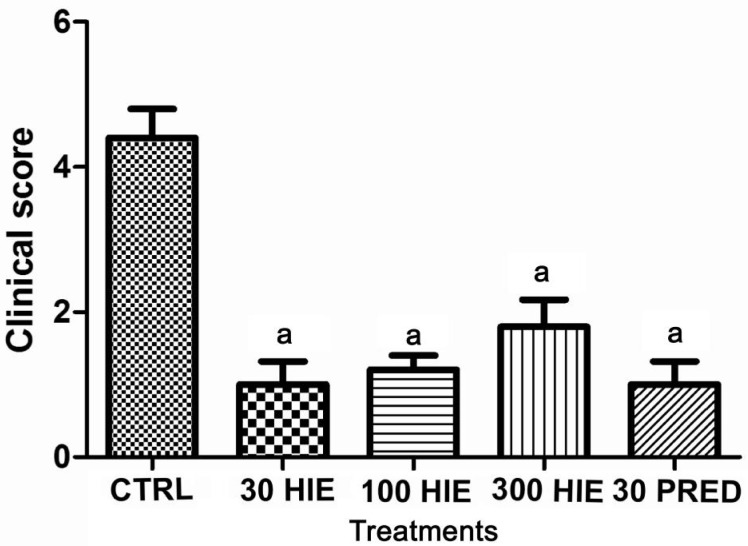
There was remarkable infiltration of cells and flares (protein exudates) in the aqueous humour, as well as intense iris hyperemia and vasodilation (clinical score: 4.40±0.40) of uveitic rabbits treated with normal saline. However, HIE- and prednisolone-treated groups showed no infiltration, or significantly reduced (P≤0.001) infiltration of cells and flares, iris hyperemia and vasodilation; reducing clinical scores to between 1.0 to 1.8 (Figure 1).
Figure 1. The clinical score of inflammation in LPS-induced uveitic rabbits treated with 30, 100 and 300 mg/kg HIE, 30 mg/kg prednisolone (PRED), and 10 mL/kg normal saline (CTRL).
aP≤0.001 (n=5), ANOVA followed by Dunnet's post hoc test.
Polymorphonuclear Neutrophil Count and Total Protein Concentration
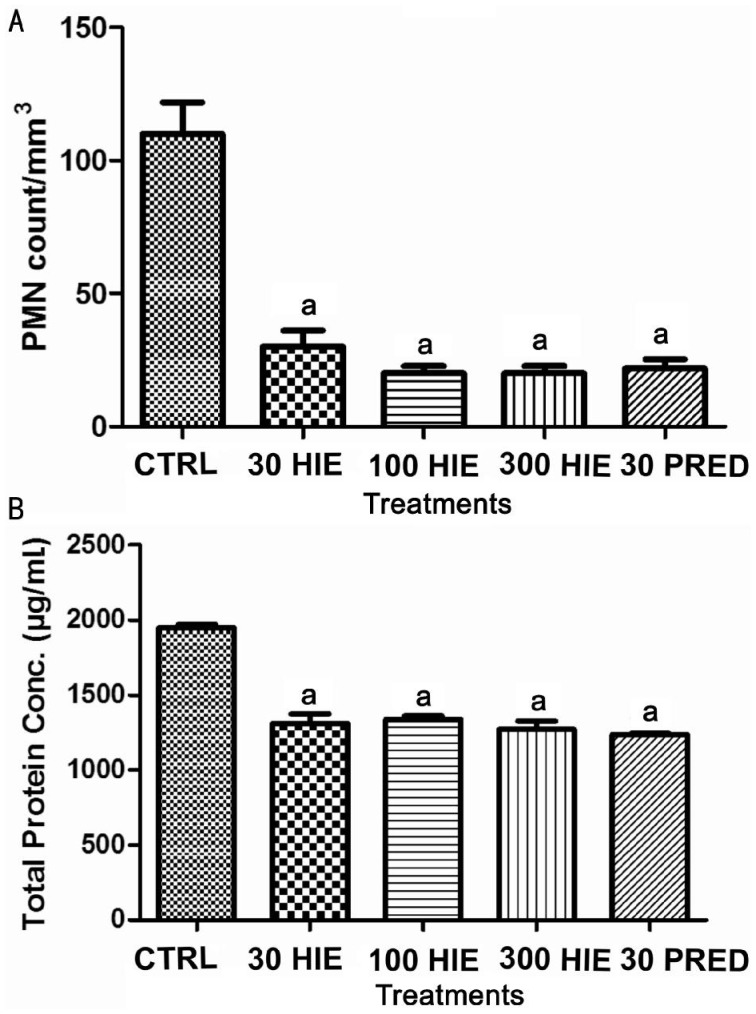
There was significant (P≤0.001) reduction of PMNs and protein exudation into the aqueous humour of the HIE and prednisolone-treated animals compared to the normal saline-treated group (Figure 2A, 2B).
Figure 2. The effect of 30, 100 and 300 mg/kg HIE, 30 mg/kg prednisolone (PRED), and 10 mL/kg normal saline (CTRL) on: (A) PMN count, (B) total protein concentration in the aqueous humour of the eyes of LPS-induced uveitic rabbits.
aP≤0.001 (n=5), ANOVA followed by Dunnet's post hoc test.
Monocyte Chemotactic Protein 1, Prostaglandin E2, Tumor Necrosis Factor Alpha Levels in Aqueous Humour
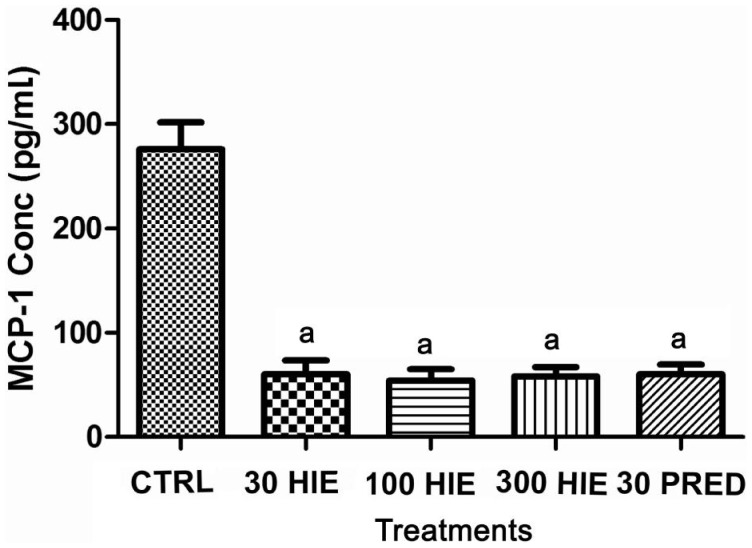
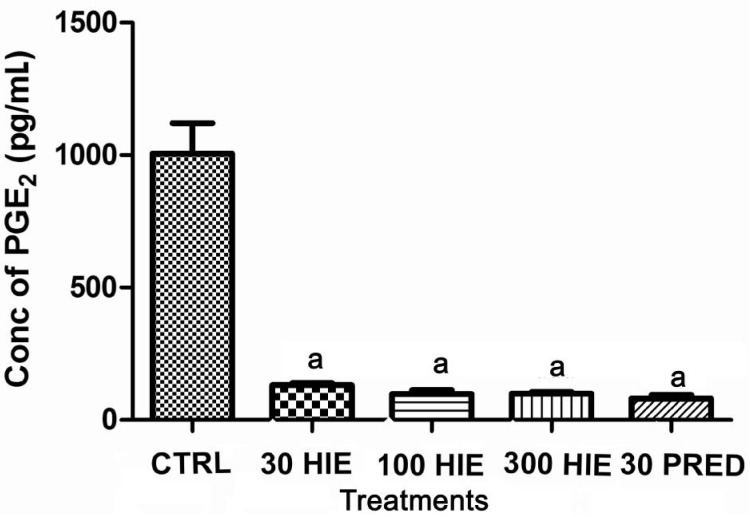
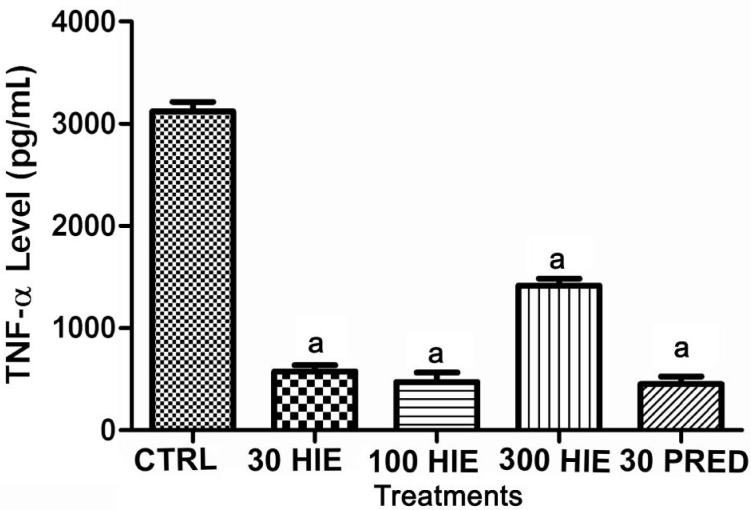
Treatment with HIE or prednisolone caused anti-inflammation as it significantly (P≤0.001) reduced the levels of MCP-1, PGE2, TNF-α, in the aqueous humour nevertheless the normal saline-treated rabbits had higher concentrations of these chemokine and cytokines in their aqueous humour (Figures 3, 4 and 5).
Figure 3. The effect of 30, 100 and 300 mg/kg HIE, 30 mg/kg prednisolone (PRED), and 10 mL/kg normal saline (CTRL) on MCP-1 in the aqueous humour of LPS-induced uveitis rabbits.
aP≤0.001 (n=5), ANOVA followed by Dunnet's post hoc test.
Figure 4. The effect of 30, 100 and 300 mg/kg HIE, 30 mg/kg prednisolone (PRED), and 10 mL/kg normal saline (CTRL) on PGE2 in the aqueous humour of LPS-induced uveitis rabbits.
aP≤0.001 (n=5), ANOVA followed by Dunnet's post hoc test.
Figure 5. The effect of 30, 100 and 300 mg/kg HIE, 30 mg/kg prednisolone (PRED), and 10 mL/kg normal saline (CTRL) on TNF-α level in the aqueous humour of LPS-induced uveitis rabbits.
aP≤0.001 (n=5), ANOVA followed by Dunnet's post hoc test.
Histopathological Assessment
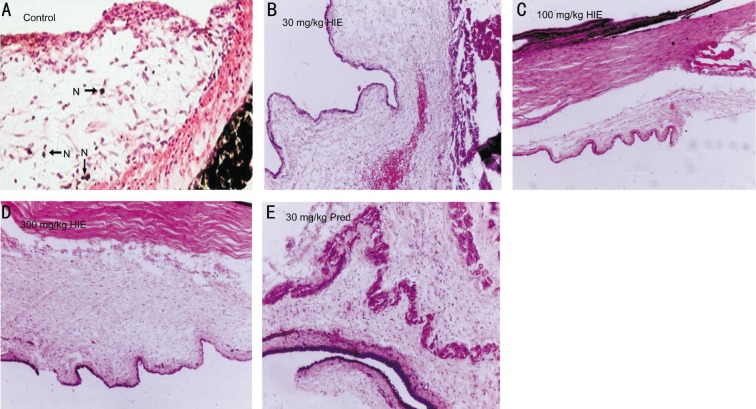
The histopathological assessment did not reveal any signs of inflammation in anterior uvea in rabbits treated with HIE or prednisolone. However, there were histopathological signs of inflammation characterized by neutrophil infiltration into the uveal tissues in the normal saline treated rabbits (Figure 6).
Figure 6. Photomicrographs of anterior uvea.
A: Marked infiltration of neutrophils in iris tissue of 10 mL/kg normal saline treatment (Control) rabbit; B: 30 mg/kg HIE treated rabbit with normal histology of anterior uvea and well defined margins; C: 100 mg/kg HIE treated rabbit with normal histology of the anterior chamber angle and uveal; D: 300 mg/kg HIE treated with normal histology of the anterior uvea and no inflammatory cells. N: Neutrophils. E: 30 mg/kg prednisolone treated with normal arhitecture of the anterior uveal tissue.
DISCUSSION
In this study, the anti-inflammatory property of an aqueous whole plant HIE was studied in lipopolysaccharide-induced uveitis in rabbits. The lipid A portion of LPS, a component of a gram negative bacterial cell wall, is responsible for the explicit uveitogenic tendency in rodents[27]. Injection of LPS results in the breakdown of the blood-aqueous barrier via a series of mechanisms beginning with the activity of the systemically injected LPS on local macrophages which in turn provoke the production of pro-inflammatory cytokines such as TNF-α and interleukin-1[28]. Chemokines such as MCP-1/CCL2 are released in response to signals from these pro-inflammatory cytokines where they regulate trafficking of inflammatory cells such as monocytes and neutrophils into the anterior uvea[29]. Under the auspices cytokines, macrophages and neutrophils secrete prostaglandin E2 and nitric oxide, which actually causes the breakdown of the blood-aqueous barrier leading to the observable infiltration of cells and flare (protein exudation) in the anterior chamber during the clinical stage of the disease process[11],[30]. The noticeable miosis especially in rabbit with uveitis can be explained by the release substance P from the peripheral nerve endings in reaction to deleterious stimuli[31].
Results from this study indicate that the aqueous whole plant HIE has potent anti-inflammatory effect in endotoxin-induced uveitis which gives credence to its traditional use in managing inflammatory eye disorders[7],[9]. TNF-α is one of the earliest cytokine involved in the pathogenesis of LPS-induced uveitis. Other researchers have reported that symptoms of Behcet's disease uveitis could be relieved by anti-TNF-α antibody treatment[32]. It is an established fact that the transcription of TNF-α is under the influence of nuclear factor-kappa B (NF-κB) and a positive loop exist between amplification of cytokine cascade during inflammation (i.e. TNF-α) and activation of NF-κB[33]–[34]. In this study HIE presumably down-regulated TNF-α in the aqueous humor, by obstructing the generation of the positive loop between TNF-α and NF-κB.
MCP-1 which is also transcriptionally controlled by NF-κB and protease activated receptor 1 (PAR1) signaling[35]. It has been found be an important inflammatory marker both in human and animal models of uveitis[30]. The low concentration of MCP-1, in the aqueous humour of the extract treated rabbits compared to the normal saline treated (control) further elucidate the therapeutic importance of the extract in obstructing classical NF-κB pro-inflammatory and protease activated receptor 1 (PAR1) signaling mechanism[35]–[36]. This is also supported by the normal histology of the anterior uveal tissue which was compromised in the control group.
PGE2, a major player in the causation of blood-aqueous barrier breakdown[11], leading to protein exudation into the anterior chamber, was found to be reduced. This significantly prevented protein exudation into the aqueous humour of the extract-treated rabbits, which was comparable to the prednisolone-treated rabbits. The expression of the inducible enzyme, involved in the synthesis of COX-2, is also illicited by NF-κB signaling or TAK1-IKK-NF-κB signaling pathway[37]. Again, cAMP signalling pathway co-operates with LPS in the induction of COX-2 and mPGES-1 transcriptional activation[38]. The findings suggest that the attenuated ocular inflammation can be ascribed in part to the down-regulation of these mediators by HIE.
Reactive oxygen species (ROS) are considered second messengers in NF-κB activation and cytokine expression[39]–[40]. A probable mechanism of inhibiting NF-κB activation and inflammatory cascade propagation by HIE is the scavenging of ROS. Several chemo-preventive phytoconstituents such as alkaloids, flavonoids and sterols in natural products[41]–[42], have been shown to inhibit COX-2 and iNOS expression by blocking improper NF-κB activation. There is convincing evidence that indicate extracellular-regulated protein kinase and p38 mitogen-activated protein kinase are important factors of the intracellular signaling cascades accountable for NF-κB activation in reaction to a number of external stimuli[43]. Prednisolone on the other hand, like all other corticosteroids are potent inhibitors of inflammatory processes and are widely used in the treatment of uveitis and other inflammatory disorders. It exerts its effect by directly binding of the glucocorticoid/glucocorticoid receptor complex to glucocorticoid receptive essentials in the promoter region of genes, or by an interaction of this complex with other transcription factors, more so activating protein-1 or NF-κB. It inhibits several inflammation-associated molecules such as cytokines, chemokines, arachidonic acid metabolites, and adhesion molecules[44].
By inhibiting multiple pro-inflammatory cytokines, the aqueous whole plant HIE exerts potent anti-inflammatory effect on LPS-induced uveitis in New Zealand white rabbits. This therefore provides scientific support for the traditional use as an ocular anti-inflammatory agent.
Acknowledgments
The authors are grateful to the management and staff of Life Science Diagnostic Centre, Cape Coast for permitting us to use their facility for aspects of the this study. We are also thankful to Ms. Carrin Martins for proof reading this manuscript.
Author Kyei S conceived the idea, designed the study, wrote the protocol, managed the literature searches, data collection and wrote the first draft of the manuscript. Asumeng Koffuor G and Ramkissoon P were involved in the conception and design of the study, managed the analyses of the study. Ofori Ameyaw E was involved in the design of the study, managed literature search and critically revised the content. Akomanin Asiamah E performed the histological evaluations, interpretation of data. All authors read and approved the final manuscript.
This article results from research towards a PhD (Optometry) degree in the Discipline of Optometry at the University of KwaZulu Natal under the supervision of Dr. George A. Koffuor and co- supervision of Prof. Paul Ramkissoon.
Foundation: Partly supported by University of Cape Coast.
Conflicts of Interest: Kyei S, None; Koffuor GA, None; Ramkissoon P, None; Ameyaw EO, None; Asiamah EA, None.
REFERENCES
- 1.Kone WM, Kande B. Qualitative analysis of the pyrrolidine alkaloids from 11 Asteraceae and Boraginaceae used in traditional medicine in Cote d' Ivoire. Research J of Phytochemistry. 2012;6(3):75–83. [Google Scholar]
- 2.Sharma RA, Singh B, Singh D, Chandrawat P. Ethnomedicinal, pharmacological properties and chemistry of some medicinal plants of Boraginaceae in India. J Med Plant Res. 2009;3(13):1153–1175. [Google Scholar]
- 3.Rahman MA, Mia MA, Shahid IZ. Pharmacological and phytochemical screen activities of roots of Heliotropium indicum. Pharmacologyonline. 2011;1:185–192. [Google Scholar]
- 4.Dash GK, Abdullah MS. A Review on Helitropium Indicum L. (Boraginaceae) International Journal of Pharmceutical Sciences and Research. 2013;4(4):1253–1258. [Google Scholar]
- 5.El-Shazly A, Wink M. Diversity of pyrrolizidine alkaloids in the Boraginaceae structures, distribution, and biological properties. Diversity. 2014;6(2):188–282. [Google Scholar]
- 6.Costa RS, Camelo SRP, Ribeiro-Costa RM, Barbosa WLR, Vasconcelos F, Silva JOC., Junior Physical, chemical and physic-chemical control of Heliotropium indicum Linn, Boraginaceae powder and tincture. International Journal of Pharmceutical Sciences and Research. 2011;2(7):2211–2216. [Google Scholar]
- 7.Rahmatullah M, Das AK, Ariful Md. AH, Mollik H, Jahan R, Khan M. An ethnomedicinal survey of Dhamrai sub-district in Dhaka District, Bangladesh. Eurasian J Sustain Agric. 2009;3(3):881–888. [Google Scholar]
- 8.Mollik AH, Hossan S, Paul AK, Rahman TU, Jahan R, Rahmatullah M. A comparative analysis of medicinal plants used by folk medicinal healers in three districts of Bangladesh and inquiry as to mode of selection of medicinal plants. Ethnobotany Research and Applications. 2010;8:195–218. [Google Scholar]
- 9.Roeder E, Wiedenfeld H. Plants containing pyrrolizidine alkaloids used in the traditional Indian medicine-including ayurveda. Pharmazie. 2013;68(3):83–92. [PubMed] [Google Scholar]
- 10.Srinivas K, Rao MEB, Rao SS. Anti-inflammatory activity of Heliotropium indicum Linn and Leucas aspera spreng. in albino rats. Ind J Pharmacol. 2000;32(1):37–38. [Google Scholar]
- 11.Bansal S, Barathi VA, Iwata D, Agrawal R. Experimental autoimmune uveitis and other animal models of uveitis: an update. Indian J Ophthalmol. 2015;63(3):211–218. doi: 10.4103/0301-4738.156914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenbaum JT, McDevitt HO, Guss RB, Egbert PR. Endotoxin-induced uveitis in rats as a model for human disease. Nature. 1980;286(5773):611–613. doi: 10.1038/286611a0. [DOI] [PubMed] [Google Scholar]
- 13.Kogiso M, Tanouchi Y, Mimura Y, Nagasawa H, Himeno K. Endotoxin-induced uveitis in mice. 1. Induction of uveitis and role of T lymphocytes. Jpn J Ophthalmol. 1992;36(3):281–290. [PubMed] [Google Scholar]
- 14.Camilo EN, Moura GL, Arantes TE. Clinical and epidemiological characteristics of patients with uveitis in an emergency eye care center in Brazil. Arq Bras Oftalmol. 2014;77(1):30–33. doi: 10.5935/0004-2749.20140009. [DOI] [PubMed] [Google Scholar]
- 15.Dandona L, Dandona R, John RK, McCarty CA, Rao GN. Population based assessment of uveitis in an urban population in southern India. Br J Ophthalmol. 2000;84(7):706–709. doi: 10.1136/bjo.84.7.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.London NJ, Rathinam SR, Cunningham ET., Jr The epidemiology of uveitis in developing countries. Int Ophthalmology Clin. 2010;50(2):1–17. doi: 10.1097/IIO.0b013e3181d2cc6b. [DOI] [PubMed] [Google Scholar]
- 17.Schlaegel TF., Jr . Essentials of uveitis. Boston: Little, Brown & Co; 1969. [Google Scholar]
- 18.James ER. The etiology of steroid cataract. J Ocul Pharmacol Ther. 2007;23(5):403–420. doi: 10.1089/jop.2006.0067. [DOI] [PubMed] [Google Scholar]
- 19.Clark AF, Wordinger RJ. The role of steroids in outflow resistance. Exp Eye Res. 2009;88(4):752–759. doi: 10.1016/j.exer.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Foster CS, Vitale A. Diagnosis and treatment of uveitis. 2nd Ed. Jaypee Highlights Medical Publishers Inc; 2012. [Google Scholar]
- 21.Agrawal RV, Murthy S, Sangwan V, Biswas J. Current approach in diagnosis and management of anterior uveitis. Indian J Ophthalmol. 2010;58(1):11–19. doi: 10.4103/0301-4738.58468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harborne JB. Phytochemical Methods: A Guide to modern techniques of plant analysis. 3rd ed. London, UK: Chapman and Hall; 1998. [Google Scholar]
- 23.Kujur RS, Singh V, Ram M, Yadava HN, Singh KK, Kumari S, Roy BK. Antidiabetic activity and phytochemical screening of crude extract of Stevia rebaudiana in alloxan-induced diabetic rats. Pharmacognosy Res. 2010;2(4):258–263. doi: 10.4103/0974-8490.69128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Touchard E, Omri S, Naud MC, Berdugo M, Deloche C, Abadie C, Jonet L, Jeanny JC, Crisanti P, de Kozak Y, Combette JM, Behar-Cohen F. A peptide inhibitor of c-Jun N-terminal kinase for the treatment of endotoxin-induced uveitis. Invest Ophthalmol Visl Sci. 2010;51(9):4683–4693. doi: 10.1167/iovs.09-4733. [DOI] [PubMed] [Google Scholar]
- 25.Boye A, Koffuor GA, Amoateng P, Ameyaw EO, Abaitey AK. Analgesic activity and safety assessment of Heliotropium indicum Linn. (Boraginaceae) in rodents. International Journal of Pharmacology. 2012;8:91–100. [Google Scholar]
- 26.Iba T, Saitoh D. Efficacy of antithrombin in preclinical and clinical applications for sepsis associated disseminated intravascular coagulation. J Intensive Care. 2014;2(1):66. doi: 10.1186/s40560-014-0051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allensworth JJ, Planck SR, Rosenbaum JT, Rosenzweig HL Investigation of the differential potentials of TLR agonists to elicit uveitis in mice. J Leukoc Biol. 2011;90(6):1159–1166. doi: 10.1189/jlb.0511249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zarogoulidis P, Yarmus L, Darwiche K, Walter R, Huang H, Li Z, Zaric B, Tsakiridis K, Zarogoulidis K. Interleukin-6 cytokine: a multifunctional glycoprotein for cancer. Immunome Res. 2013;9(62):16535. doi: 10.1186/2090-5009-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolattukudy PE, Niu J. Inflammation, endoplasmic reticulum stress, autophagy, and the monocyte chemoattractant protein-1/ccr2 pathway. Cir Res. 2012;110(1):174–189. doi: 10.1161/CIRCRESAHA.111.243212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chao SC, Vagaggini T, Nien CW, Huang S-C, Lin HY. Effects of lutein and zeaxanthin on lps-induced secretion of il-8 by uveal melanocytes and relevant signal pathways. J Ophthalmology. 2015;2015:152854. doi: 10.1155/2015/152854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishida T, Inui M, Nomizu M. Peptide therapies for ocular surface disturbances based on fibronectin-integrin interactions. Prog Ret Eye Res. 2015;47:38–63. doi: 10.1016/j.preteyeres.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Chen FT, Liu YC, Yang CM, Yang CH. Anti-inflammatory effect of the proteasome inhibitor bortezomib on endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2012;53(7):3682–3694. doi: 10.1167/iovs.12-9505. [DOI] [PubMed] [Google Scholar]
- 33.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49(11):1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DiDonato JA, Mercurio F, Karin M. NF-κB and the link between inflammation and cancer. Immunological Rev. 2012;246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 35.Lee H, Hamilton JR. Physiology, pharmacology, and therapeutic potential of protease-activated receptors in vascular disease. Pharmacol Ther. 2012;134(2):246–259. doi: 10.1016/j.pharmthera.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Heiligenhaus A, Thurau S, Hennig M, Grajewski RS, Wildner G. Anti-inflammatory treatment of uveitis with biologicals: new treatment options that reflect pathogenetic knowledge of the disease. Graefes Arch Clin Exp Ophthalmol. 2010;248(11):1531–1551. doi: 10.1007/s00417-010-1485-8. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, Lei T, Chen X, Peng Y, Long H, Zhou L, Huang J, Chen Z, Long Q, Yang Z. Resistin up-regulates COX-2 expression via TAK1-IKK-NF-kappaB signaling pathway. Inflammation. 2010;33(1):25–33. doi: 10.1007/s10753-009-9155-x. [DOI] [PubMed] [Google Scholar]
- 38.Díaz-Muñoz MD, Osma-García IC, Fresno M, Iñiguez MA. Involvement of PGE2 and the cAMP signalling pathway in the up-regulation of COX-2 and mPGES-1 expression in LPS-activated macrophages. Biochem J. 2012;443(2):451–461. doi: 10.1042/BJ20111052. [DOI] [PubMed] [Google Scholar]
- 39.Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21(1):103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Izzi V, Masuelli L, Tresoldi I, Sacchetti P, Modesti A, Galvano F, Bei R. The effects of dietary flavonoids on the regulation of redox inflammatory networks. Front Biosci (Landmark Ed) 2012;17:2396–2418. doi: 10.2741/4061. [DOI] [PubMed] [Google Scholar]
- 41.Jayakumar T, Thomas PA, Geraldine P. In-vitro antioxidant activities of an ethanolic extract of the oyster mushroom, Pleurotus ostreatus. Inn Food Sci Emerg Tech. 2009;10(2):228–234. [Google Scholar]
- 42.Pragada RR, Rao Ethadi S, Yasodhara B, Praneeth Dasari VS, Mallikarjuna Rao T. In-vitro antioxidant and antibacterial activities of different fractions of Heliotropium indicum L. Journal of Pharmacy Research. 2012;5(2):1051–1053. [Google Scholar]
- 43.Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75(1):50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ismael H, Horst M, Farooq M, Jordon J, Patton JH, Rubinfeld IS. Adverse effects of preoperative steroid use on surgical outcomes. Am J Surg. 2011;201(3):305–308. doi: 10.1016/j.amjsurg.2010.09.018. [DOI] [PubMed] [Google Scholar]