Abstract
AIM
To investigate the serum levels of vascular endothelial growth factor receptor-2 (VEGFR-2) and adropin in age-related macular degeneration (AMD) patients.
METHODS
Ninety-eight AMD patients were included in the study. Seventy-eight age- and sex-matched healthy volunteers were recruited as the control group. Fundus florescein angiography and optical coherence tomography were performed to assess the posterior segment details. Serum VEGFR-2 and adropin levels were measured using enzyme-linked immunosorbent assays and compared between the study groups.
RESULTS
AMD group had significantly increased foveal retinal thickness, serum LDL and HDL levels and significantly decreased subfoveal choroidal thickness (P =0.01, 0.047, 0.025 and <0.001, respectively). Serum VEGFR-2 level revealed a significant decrease in AMD patients compared to controls (26.48±6.44 vs 30.42±7.92 ng/mL, P<0.001). There was an insignificant increase in serum adropin level in AMD patients (6.17±3.19 vs 5.79±2.71 ng/mL, P=0.4). Serum level of VEGFR-2 in AMD patients had a significant negative correlation with foveal retinal thickness (r=-0.226, P=0.025) and a significant positive correlation with subfoveal choroidal thickness (r=0.2, P=0.048).
CONCLUSION
The current study demonstrated that the decreased serum VEGFR-2 level may be considered in the development of AMD. Adropin does not seem to play a role in the pathogenesis of AMD.
Keywords: vascular endothelial growth factor receptor-2, adropin, age-related macular degeneration
INTRODUCTION
Age-related macular degeneration (AMD) is a chronic progressive disease leading to visual loss. Most patients with AMD have the dry type of the disease, however the dry type AMD can lead to the wet type. Although only about 10% of people with AMD develop the wet type, they make up the majority of those who have serious vision loss from the disease. Several pathways have been implicated in the pathogenesis of AMD. These are lipofuscin accumulation in retinal pigment epithelium (RPE), choroidal ischaemia and oxidative damage. In dry type, presence of yellow deposits (drusen) in the macula and progressive loss of the RPE, choriocapillaris and photoreceptors occur. In wet type AMD, choroidal neovascularization (CNV) breaks through to the neural retina, leaking fluid, lipids and blood and leading to fibrous scarring.
Recently, attention has been focused on vascular endothelial growth factor (VEGF) as a therapeutic target. In wet type AMD, subretinal neovascularization (NV) may originate from the retina or choroid[1]. The most common form of NV in AMD is CNV, in which vessels grow from the choroid into the subretinal space or retina. VEGF-A is a major factor in the development of CNV[2]. It has two main receptors, which are receptor tyrosine kinases, designated vascular endothelial growth factor receptor-1 (VEGFR-1) and vascular endothelial growth factor receptor-2 (VEGFR-2). VEGFR-2 is known as the major angiogenic receptor for VEGF-A on endothelial cells[3]. In addition, protective functions are mediated by VEGFR-2, as are autoregulatory functions of VEGF-A expression[4]–[5]. VEGFR-1 regulates VEGF activity in the vascular endothelium by preventing binding of VEGF to VEGFR-2[6].
Among the angiogenic factors, VEGF is the most important contributor to the angiogenesis in AMD patients; however, there is limited evidence about the role of anti-angiogenic molecules in AMD[7]–[8]. Recently, Uehara et al[9] found decreased serum VEGFR-1 levels in wet type AMD compared to dry type and Sharma et al[10] reported an association between the serum levels of VEGFR-2 and wet type AMD.
Adropin was found in 2008 as a secreted protein involved in energy homeostasis, metabolic adaptation to macronutrients and modulation of insulin sensitivity and diabetes[11]. Lovren et al[12] reported that adropin may also have non-metabolic properties including the regulation of endothelial function. Adropin was expressed in endothelial cells and improved angiogenesis-related responses. Authors concluded that adropin potently upregulates VEGFR-2 in endothelial cells and that gene silencing of VEGFR-2 significantly impaires the effects adropin had on modulating endothelial cell survival and function[12].
To date, VEGFR-2 and adropin in subtypes of AMD have not been investigated in one study. In order to contribute in the clarification of the involvement of both molecules in AMD, reviewing previous reports, in this study, we searched for a relation between the serum levels of VEGFR-2 and adropin in AMD patients.
SUBJECTS AND METHODS
The study included 98 (51 dry type, 47 wet type) AMD patients who were recruited from the Ophthalmology Department of Kirikkale University during 2013. Seventy-eight healthy age-matched volunteers were enrolled as control group. Local Ethics Committee approved the study protocol and informed consents were obtained from all participants. The study was done in adherence to the tenets of the Declaration of Helsinki.
The exclusion criteria were previous heart disease, renal/hepatic failure, acute infection, hematologic disorder, presence of any chronic inflammatory and autoimmune disease and any known malignancy. AMD group included patients with dry or wet type AMD. Patients who received laser photocoagulation or intravitreal drug injection during the last 6mo were not included into the study.
Each subject underwent a complete ophthalmological examination including best-corrected visual acuity, measurement of intraocular pressure and slit lamp examination. Fundus florescein angiography and optical coherence tomography were performed to assess the posterior segment details. Height and weight measurements were used to calculate the BMI. Peripheral blood samples were obtained from each participant and serum levels of cholesterol, triglyceride (TRG), high density lipoprotein (HDL), low density lipoprotein (LDL), glucose and HbA1c were measured.
Serum adropin levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Phoenix Pharmaceuticals, Burlingame, CA, USA) according to the manufacturer's instructions. The catalog number was 032-035. The detection range of the kit was 0.01 to 100 ng/mL and the sensitivity was 0.5 ng/mL. VEGFR-2 was also quantified using commercially available ELISA assays (eBioscience, San Diego, CA, USA) according to manufacturer's instruction. The lot number was 87412006 and the sensitivity was 7 pg/mL. Glucose was evaluated in serum by the glucoseoxidase method. TRG, cholesterol, LDL, and HDL concentrations were measured by an automated analyzer using commercially available kits.
Statistical Analysis
Statistical analyses were carried out using the SPSS statistical software (SPSS for windows 10.0, Inc., Chicago, USA). One way analysis of variance (ANOVA) and Student's t-test were used for the analysis. Correlations were performed using Pearson's correlation coefficient. All data were expressed as mean ± standard deviation (±SD). A P value less than 0.05 was considered statistically significant.
RESULTS
The demographic characteristics and retinal thickness measurements of the study groups are listed in Table 1. There was no statistically significant difference between both groups in terms of age (P=0.6) and sex (P=0.5). Compared to controls, AMD group had significantly increased foveal retinal thickness, serum LDL and HDL levels and significantly decreased subfoveal choroidal thickness (P=0.01, 0.047, 0.025 and <0.001, respectively). Table 1 lists the clinical background and biochemical characteristics of the two groups.
Table 1. Clinical background and biochemical characteristics of the study groups.
| Parameters | AMD group | Dry type AMD group | Wet type AMD group | Controls | 1P |
| Age (a) | 73.43±9.18 | 74.14±8.57 | 72.66±9.83 | 72.52±8.53 | 0.6 |
| Gender (F/M) | 51/47 | 22/29 | 28/19 | 38/40 | 0.5 |
| Weight (kg) | 76.84±14.11 | 75.13±13.22 | 78.72±14.94 | 80.35±12.71 | 0.09 |
| Height (cm) | 159.29±11.0 | 160.45±10.33 | 158.02±11.74 | 159.87±9.20 | 0.7 |
| BMI (kg/m2) | 30.49±5.95 | 29.33±5.50 | 31.75±6.21 | 31.71±6.14 | 0.1 |
| HbA1c (%) | 6.29±1.01 | 6.10±0.85 | 6.50±1.13 | 6.04±0.73 | 0.06 |
| Foveal retinal thickness (µm) | 306.80±115.92 | 261.01±47.17 | 356.60±145.12 | 268.81±61.92 | 0.01 |
| Subfoveal choroidal thickness (µm) | 196.23±69.90 | 195.37±72.44 | 197.17±67.81 | 261.41±61.83 | <0.001 |
| VEGFR-2 (ng/mL) | 26.48±6.44 | 26.82±5.45 | 26.10±7.41 | 30.42±7.92 | <0.001 |
| Adropin (ng/mL) | 6.17±3.19 | 5.57±2.60 | 6.81±3.65 | 5.79±2.71 | 0.4 |
| Total cholesterol (mg/dL) | 207.91±44.60 | 211.47±35.74 | 204.04±52.70 | 204.68±40.72 | 0.6 |
| Triglyceride (mg/dL) | 149.33±78.36 | 153.73±88.09 | 144.55±66.85 | 211.60±18.48 | 0.002 |
| High-density lipoprotein (mg/dL) | 52.03±15.51 | 54.88±18.89 | 48.94±10.04 | 47.40±10.42 | 0.025 |
| Low-density lipoprotein (mg/dL) | 127.57±37.95 | 128.96±32.14 | 126.06±43.70 | 117.19±30.76 | 0.047 |
| Glucose (mg/dL) | 118.95±48.85 | 115.65±52.36 | 122.53±45.03 | 108.29±27.93 | 0.08 |
AMD: Age-related macular degeneration. 1AMD group vs controls.
The study demonstrated that AMD patients had significantly lower mean serum VEGFR-2 level compared to controls (26.48±6.44 vs 30.42±7.92 ng/mL, P<0.001). Both dry and wet type AMD patients had significantly lower mean serum VEGFR-2 levels (26.82±5.45 vs 30.42±7.92 ng/mL, P=0.05 and 26.10±7.41 vs 30.42±7.92 ng/mL, P=0.003, respectively) compared to control group. There was not any significant difference in serum VEGFR-2 level between wet type and dry type AMD patients (26.10±7.41 vs 26.82±5.45 ng/mL, P=0.5).
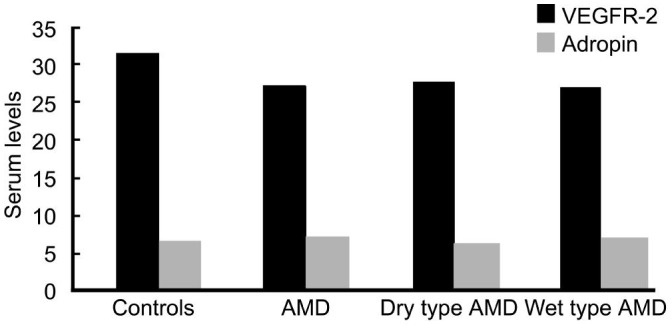
In comparison to controls, mean serum adropin level was higher in AMD patients (5.79±2.71 vs 6.17±3.19 ng/mL), but it did not show a significant difference (P=0.4). We observed a statistically insignificant decrease in dry type (5.57±2.60 vs 5.79±2.71 ng/mL, P=0.6) and a statistically insignificant increase in wet type AMD patients (6.81±3.65 vs 5.79±2.71 ng/mL, P=0.07) compared to controls. Comparison of wet type and dry type AMD patients did not reveal any significant difference in serum adropin levels (6.81±3.65 vs 5.57±2.60 ng/mL, P=0.055). Figure 1 presents the serum levels of VEGFR-2 and adropin in AMD patients versus controls.
Figure 1. Serum levels of VEGFR-2 and adropin in AMD patients versus controls.
Serum levels of VEGFR-2 in AMD patients had a significant negative correlation with foveal retinal thickness (r =-0.226, P=0.025) and a significant positive correlation with subfoveal choroidal thickness (r=0.2, P=0.048). Although the serum adropin concentration was higher in AMD patients than controls (P=0.4), there was no significant correlation between serum adropin level and retinal and choroidal thickness (r=0.059, P=0.5 and r=-0.127, P=0.2).
DISCUSSION
AMD is considered as the main cause of visual loss in the elderly. In a large epidemiologic study, a quarter of patients over the age of 75 were found to have some features of AMD, about 80% of whom have dry type AMD and are at risk of converting to wet type AMD. The onset of wet type AMD generally results in sudden vision loss and, if left untreated, can cause permanent loss of sight. Wet type AMD accounts for 90% of the blindness attributable to AMD[13]. In wet type AMD, a major factor in NV which may exert from the retina or from the choroid is the VEGF. It is present in a soluble and bound form and interacts with two receptors, VEGFR-1 and VEGFR-2, to promote angiogenesis and vascular integrity.
Endothelial cells produce several factors that regulate cellular adhesion, thromboresistance, smooth muscle proliferation and vessel wall inflammation. Therefore, endothelial dysfunction is associated with several pathophysiological conditions, including atherosclerosis, hypertension and diabetes[14]. Recent studies supported the emerging concept of endothelial dysfunction in the course of AMD. Clinical trials have found a link between endothelial dysfunction and drusen formation or NV in AMD patients[15]. The protective role of adropin on the endothelium has been shown previously[11],[15]–[17]. In this study, we investigated whether the serum levels of VEGFR-2 and adropin are related to the development of AMD.
We observed lower concentrations of serum VEGFR-2 in patients with AMD, particularly in the wet type. Serum level of adropin was found to be less important in the pathogenesis of AMD. VEGFR2 is the major positive signal transducer for both physiological and pathological angiogenesis. The lowering of circulating VEGFR-2 levels may be explained by a higher binding of the VEGF to its R2 receptors to form VEGF-receptor complexes. Soluble isoforms of VEGFR-1 and -2 are detected in blood circulation. These receptors are able to bind their ligands, thereby controlling their biodisponibility and inhibiting tumor or ischemia-induced angiogenesis[18]–[23]. Generally, circulating receptors inactivate their ligands by binding with them, since the soluble receptor does not possess the intracellular domain required to initiate signaling[24]. Both in vitro and in vivo studies have shown that elevated levels of VEGFR-2 has anti-angiogenic activity[25]–[26]. Therefore, a decrease of serum VEGFR-2 level, the major proangiogenic signal transducer for VEGF, may be a physiological response to promote angiogenesis in AMD eyes, particularly in the wet type. We found a significant negative correlation between serum levels of VEGFR-2 and foveal retinal thickness in AMD patients which is a measure of the disease activation.
In our study, serum VEGFR-2 levels in wet type AMD, though lower than in dry type, did not produce a statistical significance. Possible reasons for the insignificant difference for VEGR-2 levels could be in the ongoing, stepwise process in AMD pathogenesis. It could be proposed that serum levels of VEGFR-2 in dry type AMD in our study may be related to earlier steps of the disease process. Declining serum VEGFR-2 may be one of the the preliminary events allowing VEGF to activate the proangiogenic endothelial cell state and to induce permeability. The balance between angiogenic agent (like VEGF) and the anti-angiogenic factors (like VEGFR-2), which seems to be impaired in the AMD, and the degree to which the anti-angiogenic agents are decreased might have determined the stage of macular degeneration observed.
VEGFR-2 is activated by VEGF and is the initial pathway in modulating endothelial cell survival and function. Lovren et al[12] showed the functions of adropin including regulation of angiogenesis and increase in blood flow and capillary density and reported its potential endothelial protective role. Wu et al[27] found lower serum adropin levels in type 2 diabetes patients than in non-diabetic patients and reported that adropin level was inversely associated with angiographic severity of coronary atherosclerosis, suggesting that serum adropin may be a novel predictor of coronary atherosclerosis. It is hard to conclude that higher serum adropin levels in wet type AMD patients may be a response to pathological angiogenesis in these patients or an initial sign of endothelial dysfunction and atherosclerosis.
In comparison to genetic and environmental factors, serological biomarkers are less well known in AMD patients. Various immunological molecules and inflammatory mediators have been identified at the site of AMD lesions. Serum markers that have been associated with AMD development include elevated superoxide dismutase, C-reactive protein, homocysteine and LDL etc[28]–[31]. Lip et al[7] found increased plasma VEGF levels in AMD patients compared with age-matched controls. Interestingly, same Authors did not find any significant difference between dry and wet AMD cases when compared VEGF values. In a recent study, Tsai et al[8] found increased plasma VEGF levels in AMD patients compared with controls. They found significantly higher plasma VEGF levels in wet type AMD compared with dry type AMD. More recent studies have reported elevated serum VEGFR-2 and reduced serum VEGFR-1 in patients with neovascular AMD[9]–[10]. Serum level of adropin has not been studied in AMD patients yet.
Another issue is the relation of serum level of VEGFR-2 and adropin to the disease severity. Sydorova and Lee[32] measured high levels of VEGF in the serum and vitreous of proliferative diabetic retinopathy and found serum VEGF levels were higher only in advanced cases (proliferative vitreoretinopathy). As shown, there are still controversies regarding the behaviour of the serological biomarkers in AMD. Surely, an effective biomarker should be simple to analyze and clinically relevant. Unfortunately, many of the candidate markers have yet to be standardized. Maybe sooner, these biomarkers will be important tools for AMD researches by providing a scale for evaluating disease progression.
In summary, the decreased serum VEGFR-2 level seems to be related to the development of AMD. Future researches should study whether decreasing serum VEGFR-2 level promote the onset of wet type AMD and how VEGFR-2 levels decrease in these patients. Although adropin does not seem to play a role in the pathophysiology of AMD, it should be further studied at molecular levels in AMD patients. As future studies continue to unravel the molecular biology of AMD, both our understanding about this debilitating disease and the treatment approaches will improve.
Acknowledgments
Conflicts of Interest: Örnek N, None; Örnek K, None; Aydin S, None; Yilmaz M, None; Ölmez Y, None.
REFERENCES
- 1.Campochiaro PA. Ocular neovascularization. J Mol Med. 2013;91(3):311–321. doi: 10.1007/s00109-013-0993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res. 2008;27(4):331–371. doi: 10.1016/j.preteyeres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shibuya M. Vascular Endothelial growth factor (vegf) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer. 2011;2(12):1097–1105. doi: 10.1177/1947601911423031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byeon S, Lee SC, Choi SH, Lee HK, Lee JH, Chu YK, Kwon OW. Vascular endothelial growth factor as an autocrine survival factor for retinal pigment epithelial cells underoxidative stress via the VEGF-R2/PI3K/Akt. Invest Ophthalmol Vis Sci. 2010;51(2):1190–1197. doi: 10.1167/iovs.09-4144. [DOI] [PubMed] [Google Scholar]
- 5.Nishijima K, Ng YS, Zhong L, Bradley J, Schubert W, Jo N, Akita J, Samuelsson SJ, Robinson GS, Adamis AP, Shima DT. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007;171(1):53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lien S, Lowman HB. Therapeutic anti-VEGF antibodies. Handb Exp Pharmacol. 2008;181:131–50. doi: 10.1007/978-3-540-73259-4_6. [DOI] [PubMed] [Google Scholar]
- 7.Lip PL, Blann AD, Hope-Ross M, Gibson JM, Lip GY. Age-related macular degeneration is associated with increased vascular endothelial growth factor, hemorheology and endothelial dysfunction. Ophthalmology. 2001;108(4):705–710. doi: 10.1016/s0161-6420(00)00663-1. [DOI] [PubMed] [Google Scholar]
- 8.Tsai DC, Charng MJ, Lee FL, Hsu WM, Chen SJ. Different plasma levels of vascular endothelial growth factor and nitric oxide between patients with choroidal and retinal neovascularization. Ophthalmologica. 2006;220(4):246–251. doi: 10.1159/000093079. [DOI] [PubMed] [Google Scholar]
- 9.Uehara H, Mamalis C, McFadden M, Taggart M, Stagg B, Passi S, Earle P, Chakravarthy U, Hogg RE, Ambati BK. The reduction of serum soluble Flt-1 in patients with neovascular age-related macular degeneration. Am J Ophthalmol. 2015;159(1):92–100. doi: 10.1016/j.ajo.2014.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma NK, Gupta A, Prabhakar S, Singh R, Sharma S, Anand A. Single nucleotide polymorphism and serum levels of VEGFR2 are associated with age related macular degeneration. Curr Neurovasc Res. 2012;9(4):256–265. doi: 10.2174/156720212803530681. [DOI] [PubMed] [Google Scholar]
- 11.Kumar KG, Trevaskis JL, Lam DD, Sutton GM, Koza RA, Chouljenko VN, Kousoulas KG, Rogers PM, Kesterson RA, Thearle M, Ferrante AW, Jr, Mynatt RL, Burris TP, Dong JZ, Halem HA, Culler MD, Heisler LK, Stephens JM, Butler AA. Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab. 2008;8(6):468–481. doi: 10.1016/j.cmet.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lovren F, Pan Y, Quan A, Singh KK, Shukla PC, Gupta M, Al-Omran M, Teoh H, Verma S. Adropin is a novel regulator of endothelial function. Circulation. 2010;122(11):S185–192. doi: 10.1161/CIRCULATIONAHA.109.931782. [DOI] [PubMed] [Google Scholar]
- 13.Klein R, Klein BE, Knudtson MD, Meuer SM, Swift M, Gangnon RE. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007;114(2):253–262. doi: 10.1016/j.ophtha.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 14.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115(10):1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 15.Schaumberg DA, Christen WG, Buring JE, Glynn RJ, Rifai N, Ridker PM. High-sensitivity C-reactive protein, other markers of inflammation, and the incidence of macular degeneration in women. Arch Ophthalmol. 2007;125(3):300–305. doi: 10.1001/archopht.125.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Celik A, Balin M, Kobat MA, Erdem K, Baydas A, Bulut M, Altas Y, Aydin S, Aydin S. Deficiency of a new protein associated with cardiac syndrome X called adropin. Cardiovasc Ther. 2013;31(3):174–178. doi: 10.1111/1755-5922.12025. [DOI] [PubMed] [Google Scholar]
- 17.Gozal D, Kheirandish-Gozal L, Bhattacharjee R, Molero-Ramirez H, Tan HL, Bandla HP. Circulating adropin concentrations in pediatric obstructive sleep apnea: potential relevance to endothelial function. J Pediatr. 2013;163(4):1122–1126. doi: 10.1016/j.jpeds.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39(5):469–478. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- 19.Swendeman S, Mendelson K, Weskamp G, Horiuchi K, Deutsch U, Scherle P, Hooper A, Rafii S, Blobel CP. VEGF-A stimulates ADAM17-dependent shedding of VEGFR2 and crosstalk between VEGFR2 and ERK signaling. Circ Res. 2008;103(9):916–918. doi: 10.1161/CIRCRESAHA.108.184416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7(5):359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 21.Waldner MJ, Wirtz S, Jefremow A, Warntjen M, Neufert C, Atreya R, Becker C, Weigmann B, Vieth M, Rose-John S, Neurath MF. VEGF receptor signaling links inflammation and tumorigenesis in colitis-associated cancer. J Exp Med. 2010;207(13):2855–2868. doi: 10.1084/jem.20100438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acobi J, Tam BY, Wu G, Hoffman J, Cooke JP, Kuo CJ. Adenoviral gene transfer with soluble vascular endothelial growth factor receptors impairs angiogenesis and perfusion in a murine model of hindlimb ischemia. Circulation. 2004;110(16):2424–2429. doi: 10.1161/01.CIR.0000145142.85645.EA. [DOI] [PubMed] [Google Scholar]
- 23.Szentirmai O, Baker CH, Bullain SS, Lin N, Takahashi M, Folkman J, Mulligan RC, Carter BS. Successful inhibition of intracranial human glioblastoma multiforme xenograft growth via systemic adenoviral delivery of soluble endostatin and soluble vascular endothelial growth factor receptor-2: laboratory investiga:ion. J Neurosurg. 2008;108(5):979–988. doi: 10.3171/JNS/2008/108/5/0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Igarashi J, Michel T. S1P and eNOS regulation. Biochim Biophys Acta. 2008;1781(9):489–495. doi: 10.1016/j.bbalip.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O'Brien SM, Davis RB, Gowen LC, Anderson KD, Thurston G, Joho S, Springer ML, Kuo CJ, McDonald DM. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290(2):H560–576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- 26.Roeckl W, Hecht D, Sztajer H, Waltenberger J, Yayon A, Weich HA. Differential binding characteristics and cellular inhibition by soluble VEGF receptors 1 and 2. Exp Cell Res. 1998;241(1):161–170. doi: 10.1006/excr.1998.4039. [DOI] [PubMed] [Google Scholar]
- 27.Wu L, Fang J, Chen L, Zhao Z, Luo Y, Lin C, Fan L. Low serum adropin is associated with coronary atherosclerosis in type 2 diabetic and non-diabetic patients. Clin Chem Lab Med. 2014;52(5):751–758. doi: 10.1515/cclm-2013-0844. [DOI] [PubMed] [Google Scholar]
- 28.Anand A, Sharma NK, Gupta A, Prabhakar S, Sharma SK, Singh R. Superoxide dismutase1 levels in North Indian population with age-related macular degeneration. Oxid Med Cell Longev. 2013;2013:365046. doi: 10.1155/2013/365046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seddon JM, Gensler G, Milton RC, Klein ML, Rifai N. Association between C-reactive protein and age-related macular degeneration. JAMA. 2004;291(6):704–710. doi: 10.1001/jama.291.6.704. [DOI] [PubMed] [Google Scholar]
- 30.Ghosh S, Saha M, Das D. A study on plasma homocysteine level in age-related macular degeneration. Nepal J Ophthalmol. 2013;5(2):195–200. doi: 10.3126/nepjoph.v5i2.8728. [DOI] [PubMed] [Google Scholar]
- 31.Reynolds R, Rosner B, Seddon JM. Serum lipid biomarkers and hepatic lipase gene associations with age-related macular degeneration. Ophthalmology. 2010;117(10):1989–1995. doi: 10.1016/j.ophtha.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sydorova M, Lee MS. Vascular endothelial growth factor levels in vitreous and serum of patients with either proliferative diabetic retinopathy or proliferative vitreoretinopathy. Ophthalmic Res. 2005;37(4):188–190. doi: 10.1159/000086594. [DOI] [PubMed] [Google Scholar]