Abstract
AIM
To investigate the pattern of diurnal variations of choroidal thickness of macular region of healthyindividuals.
METHODS
A prospective study of 32 healthy female subjects was conducted. Each subject underwent 1) a questionnaire on daily schedule, 2) the Pittsburgh Sleep Quality Index questionnaire (PSQI), and 3) ocular examinations including an eye dominance test, fundus photography, and sequential optical coherence tomography (OCT) imaging, on two separate days at five fixed 3h time intervals. Choroidal thickness was measured by two masked graders.
RESULTS
A significant diurnal variation of choriodal thickness at fovea (P<0.001), at 500 µm nasal (P<0.001), temporal to fovea (P=0.01) or 1500 µm nasal to fovea (P=0.001) was observed. The median choroidal thickness peaked at 11:00 at fovea (P=0.01), at 500 µm nasal (P=0.009) and temporal (P=0.03) to fovea. The median amplitude of foveal choroidal thickness was 20.5 µm (13, 31) and 20.0 µm (12.5, 28.2) for the first and second series of measurements, respectively. The greater amplitude of foveal choroidal thickness was associated with thickner initial foveal choroidal thickness [0.05 (0.03, 0.08), P=0.01], dominant eye [10.51 (4.02, 14.60), P=0.04] in the multivariate linear regression.
CONCLUSION
Our data show a significant diurnal variation of the choroidal thickness at fovea, at 500 µm nasal and temporal to fovea and 1500 µm nasal to fovea. Thicker initial foveal choroidal thickness and being dominant eye may influence the amplitude of foveal choroidal thickness.
Keywords: choroidal thickness, diurnal variation, optical coherence tomography
INTRODUCTION
Spectral-domain optical coherence tomography (SD-OCT) with technique of “enhanced depth imaging” (EDI) has allowed the full layer of choroid to be assessed in vivo[1]. With the EDI technique, abnormal choroidal thickness (CT) has been observed in several common macular diseases, such as central serous chorioretinopathy (CSC)[2], polypoidal choroidal vasculopathy (PCV)[3], and age-related macular degeneration (AMD)[4]. Compared with the eyes of healthy individuals or healthy contralateral eyes of the patients, the foveal choroid is reported to be thicker in eyes with CSC and PCV, but thinner in eyes with AMD[5]–[6]. The decreased CT after treatment has also been reported in eyes with CSC[7], PCV[8], and AMD[9]. These observations suggested that CT could be an important parameter in evaluating macular diseases. To explore the potential role of CT variations in macular diseases, the value of CT of healthy subjects and factors which might influence the CT would be of importance.
CT could be influenced by several other factors, such as age[10]–[11], axial length of eye[10],[12], high myopia[13], and ocular perfusion pressure[14].In addition, an animal studies has demonstrated a diurnal modulation of the CT, wherein the choroid being at its thickest at midnight and thinnest at noon, with a peak-to-peak amplitude of only 40 µm[15]. The diurnal variation of the human foveal CT in vivo on healthy adults has also been shown by EDI-OCT[16]–[20] and optical biometry 21where the mean amplitude of the foveal CT has shown to be varied between 10 µm and 33.7 µm[17]–[22]. In the healthy eyes, the mean CT was reported to be thickest under the fovea and decreased more rapidly in the nasal direction than in the temporal direction[1],[10]. In high myopic eyes, however, the mean CT was reported to be thickest temporally and then subfoveally and thinnest in the nasal area[22]. Previous studies on the diurnal variation pattern of CT in humans focused only on the fovea. To our knowledge no studies have included measurements of the parafoveal regions, where macular diseases are often involved. In this current study, we investigated the patterns of diurnal variation of CT in both the foveal and parafoveal regions in a small group of female young healthy Chinese and explored the possible existence of any influencing factors. Diurnal variations of CT at both fovea and parafoveal regions, if of significant magnitude, could be of relevance to both normative data studies as well as studies on longitudinal changes in CT.
SUBJECTS AND METHODS
The healthy volunteers, all female, with no history of ocular disease participated in the study. The study protocol was approved by the Ethics Committee of Beijing Tongren Hospital and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from each participant before the enrollment.
Participants were instructed to maintain consistent sleep/awake cycles and to record their daily working and rest schedules for one week. They were then interviewed using a lifestyle questionnaire, which included sections on sleep/waking rhythm, exercise, usage of visual display terminal (VDT), and refractive status. The Pittsburgh Sleep Quality Index (PSQI) questionnaire[23] was also completed by each participant to assess their sleep quality. Anthropometric parameters were also measured, including weight, height, and three measurements, 5min apart, of systolic blood pressure (SBP) and diastolic blood pressure (DBP). Body mass index (BMI) was calculated as the ratio of weight and the square of the height.
All subjects underwent comprehensive ophthalmological examinations, including visual acuity testing, slit-lamp biomicroscopy, intraocular pressure (IOP) by non-contact tonometer (Topocon, CT-80A, Japan) and non-mydriatic 30° color fundus photograph with a digital fundus camera (Heidelberg Engineering, Heidelberg, Germany) centered on the fovea. IOP, SBP and DBP were measured at 8:00 before OCT scans at each series of measurements. The mean arterial pressure (MAP) and mean ocular perfusion pressure (MOPP) were calculated according to the formulas: MAP=DBP+1/3(SBP-DBP) and MOPP=2/3(MAP-IOP)[19].
Presenting visual acuity (PVA) with or without correcting glasses was measured using the standard Early Treatment Diabetic Retinopathy Study (ETDRS) chart and the best-corrected visual acuity was measured if PVA was <55 letters. The uncorrected refractive error was defined as PVA <20/40 with a ≥2-line improvement after refraction correction[24]. Ocular dominance was assessed using the hole-in-the-card test[25].
SD-OCT (Spectralis OCT; Heidelberg Engineering, Heidelberg, Germany) scans were performed on both eyesat five different time points in a single day, at 3h intervals (8:00, 11:00, 14:00, 17:00, and 20:00), under standardized mesopic lighting conditions without pupil dilation. For each eye, a 7-section raster scan centered on the fovea (with eye tracking on) was performed, with 100 frames averaged to improve the image quality. The enhanced depth imaging (EDI) technique with the zero delay line oriented to the choroidal side was used to optimize choroidal sensitivity and to enhance visualization of the full CT. The first scan was set as a reference for all subsequent repeated scans to ensure the same point scanning[1]. The participants were reexamined after 1 to 4wk, and a repeated series of OCT scans was performed using the exact same time schedule. BP and IOP were measured again before the repeated first scan at 8:00. Two independent trained graders graded all OCT scans. The CT was measured using the built-in caliper tool of the OCT machine. Five daily measurement points on the horizontal scan line crossing the fovea were used to measure the CT at the fovea, 500 µm and 1500µm nasal to the fovea, and 500µm and 1500 µm temporal to the fovea. CT was measured from the outer part of the hyper-reflective line (corresponding to the base of the retinal pigment epithelium) to the hypo-reflective line or margin (corresponding to the sclera-choroidal interface). Measurements from the two graders were compared to assess the reproducibility. Bland-Altman plot and univariate linear regression was used to assess the reproducibility.
Statistical analysis was performed using the R statistical analysis package (http://www.R-project.org). Mean and standard deviation (SD) were calculated for continuous variables with normal distribution. For variables with a non-normal distribution, the median with qualities was used. To explore the diurnal pattern of the CT variation at each measured point, a repeated measurement ANOVA test with Huynh-Feldt correction was performed using the time point as within group factor. Data from both eyes of each subject were used to explore the diurnal variation pattern. To study the rhythm of CT variation in each eye, the number of eyes at each time points that reached its peak CT was counted. To study the potential factors that may influence the amplitude of the foveal CT, the univariate and multivariate linear analyses were used to test the association of the amplitude of the foveal CT with potential influencing factors, including age, BMI, PSQI score, persistence of daily exercise, use of VDT, initial foveal CT, spherical equivalent refraction, status of refractive error correction, and eye dominance. The data from the foveal CT of the both eyes in the first series of measurements was used to calculate the association. The amplitude of the foveal CT was calculated as the difference between the maximum of foveal CT and minimum of foveal CT during the one series of measurements.
RESULTS
Of the 34 female participants, 2 participants were excluded due to the failed visualization of the sclera-choroidal junctions. Therefore, a total of 32 subjects were included in the subsequent data analysis (Table 1). The mean age of the subjects was 26.0±3.1y, ranging from 23.1 to 33 years old. The measurement series were repeated twice in 20 participants.
Table 1. Characteristics of 32 participants.
| Characteristics | n (%) |
| Age (a) | 26.0±3.1c |
| Body mass index (kg/m2) | 20.9±1.8c |
| Having daily regular exercise | 11 (34.4) |
| Daily use of visual display terminal ≥4h | 16 (50.0) |
| The Pittsburgh Sleep Quality Index score | 5 (4,6) |
| Eye dominancea | 8 (25.0) |
| The status of refractive error correctionb | 10 (31.3) |
| Spherical equivalent refraction (D) | -2.00 (-3.75,-0.625)d |
| Initial foveal CT(µm) | 279 (225, 336)d |
| Presenting visual acuity | 79.9±9.6c |
| MOPP (mmHg) of right eyes | 41.9±4.9 c |
| MOPP (mmHg) of left eyes | 42.2±4.3 c |
aThe percentage of subject whose right eye is the dominant eye; bThe percentage of subject who present with uncorrected refractive error;cData presented as mean±SD; dData presented as median (IQR ). CT:Choroidal thickness; MOPP:Ocular perfusion pressure.
There was good agreement between the two OCT graders, with an interclass correlation of 0.998 (P<0.001). In 2 eyes, the discrepancies between the two graders were >4 µm (1.96 standard deviation on the Bland-Altman plot by comparing the two graders' measurement); these discrepancies were further solved by graders meeting face to face.
The median CT of the reference OCT scan in 40 studied eyes was 250.0 µm (1st and 3rd quartiles: 205.4, 325.0) under the fovea, 266 µm (217, 312.5) at 500 µm temporal to the fovea, 263 µm (214.0, 300.0) at 1500 µm temporal to the fovea, 248 µm (201.0, 300.0) at 500 µm nasal to the fovea, and 196.0 µm (140.0, 294.0) at 1500 µm nasal to the fovea.
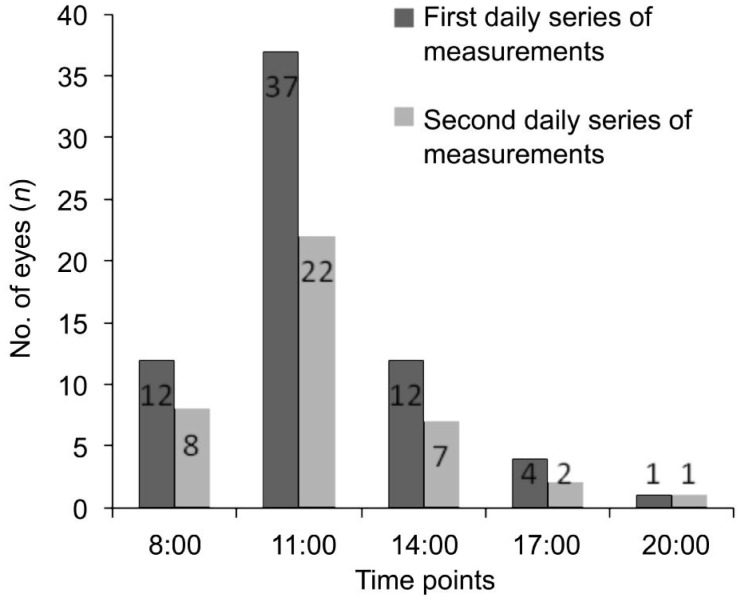
On the first daily series of measurements (Table 2), diurnal variation patterns were observed at fovea (P<0.001), at 500 µm nasal (P<0.001), temporal to fovea (P=0.01) or 1500 µm nasal to fovea (P=0.001); these patterns were analyzed using the repeated measurement ANOVA test with Huynh-Feldt correction. There was no significant diurnal variation at 1500 μm temporal to the fovea (P=0.160). The median CT peaked at 11:00 at fovea (P=0.01), at 500 µm nasal (P=0.009) and temporal (P=0.03) to fovea. The amplitude of the CT variation did not show significant difference at the fovea compared to 500 µm either nasal (P=0.24) or temporal to fovea (P=0.12). In 18 subjects, both eyes reached the peak foveal CT at the same time point. The number of eyes reaching the peak foveal CT at different time points was shown in Figure 1.
Table 2. The diurnal variation of mean CT at five measure points during the two day measurements.
| Time points | foveal CT |
500 µm nasal |
1500 µm nasal |
500 µm temporal |
1500µm temporal |
|||||
| Median (IQR)a | P | Median (IQR) | P | Median (IQR) | P | Median (IQR) | P | Median (IQR) | P | |
| The first daily measurement | ||||||||||
| 8:00 | 243.5 (190.0,312.5) | 241.0 (181.5,300.0) | 196.0 (138.1,279.5) | 262.8 (187.6,300.0) | 243 (197.1,297.9) | |||||
| 11:00 | 244.0 (183.8,325.0) | 0.01 | 246.5 (176.0,303.8) | 0.009 | 200 (140.5, 275.0) | 0.06 | 265.5 (187.6,300.0) | 0.03 | 244 (196.1,286.6) | 0.74 |
| 14:00 | 237.5 (194.0,323.8) | 0.09 | 244.0 (176.5,294.0) | 0.15 | 199.0 (134.0,274.8) | 0.05 | 263.0 (188.4.,299.2) | 0.1 | 240.0 (196.4,285.3) | 0.13 |
| 17:00 | 231.5 (186.0,312.9) | 0.99 | 237.2 (169.7, 287.9) | 0.28 | 190.8 (132.5,247.8) | 0.89 | 257.0 (186.0,297.8) | 0.46 | 239.0 (199.2285.8) | 0.29 |
| 20:00 | 228.5 (181.0,312.5) | 0.03 | 235 (166.2,298.2) | 0.16 | 195.0 (134.8,264.5) | 0.56 | 251.5 (181.5,283.2) | 0.55 | 237 (192.2,283.2) | 0.17 |
| Amplitude of CT | 20.5 (13.0,31.0) | 18.2 (10.3,28.5) | 15.0 (9.5,21.0) | 18.0 (10.2,28.0) | 14.0 (7.5,26.2) | |||||
| Pb | 0.81 | <0.001 | 0.80 | <0.001 | 0.59 | 0.001 | 0.63 | 0.01 | 0.55 | 0.160 |
| The second daily measurement | ||||||||||
| 8:00 | 226 (188.8,285.8) | 0.210 | 232.5 (185.8,275.2) | 0.005 | 194 (139.2,231) | 0.080 | 252.5 (214.2,284.8) | 0.870 | 243 (212,278) | 0.940 |
| 11:00 | 244 (197.8,291.8) | 0.110 | 234.5 (192.2,294) | 0.910 | 198.5 (144.2,241) | 0.400 | 261.5 (223.5,288.2) | 0.030 | 250.5 (218.5,280.2) | 0.170 |
| 14:00 | 233.5 (197.8,284.5) | 0.390 | 236.5 (179.8,289.8) | 0.880 | 195 (143,245.5) | 0.790 | 259.5 (210.5,284) | 0.310 | 246.5 (215.8,276.8) | 0.150 |
| 17:00 | 232 (191.2,289.2) | 0.080 | 237 (179.8,284.5) | 0.020 | 197.5 (141.2,237) | 0.140 | 253 (213.5,286.8) | 0.080 | 246.5 (211.2,274) | 0.910 |
| 20:00 | 227 (189.2,290) | 0.004 | 232 (183.5,286.5) | 0.010 | 191 (141.5,240.5) | 0.200 | 257 (211.8,288.2) | 0.150 | 244.5 (212.2,274.5) | 0.640 |
| Amplitude of CT | 20.0 (12.5,28.2) | 20.0 (12.7,29.5) | 14.0 (10.0,21.0) | 20.5 (10.7,30.2) | 20.5 (12.5,27.2) | |||||
| Pb | 0.72 | <0.001 | 0.970 | 0.010 | 0.790 | 0.003 | 0.680 | 0.010 | 0.520 | 0.260 |
aData are shown as median (IQR); bRepeated measurement ANOVA with Huynh-Feldt correction
Figure 1. The number of eyes reaching the peak foveal CT at each time points in the two series of measurements.
The number of eyes reaching peak foveal CT at different time points had a similar pattern compared to the first daily series of measurements (P=0.90).
On the second daily series of measurements (Table 2), the similar pattern of diurnal variation was observed at fovea (P<0.001), 500 µm nasal to fovea (P=0.01), 500 µm temporal to fovea (P=0.01), and 1500 µm nasal to fovea (P=0.003) (Table 2). Again, no significant diurnal variation was observed at 1500 µm temporal to fovea (P=0.260). Similar as the first measurement, there were 8 subjects who had reached peak foveal CT at the same time point in both eyes. As shown in Figure 1, the number of eyes reaching peak foveal CT at different time points had a similar pattern compared to the first daily series of measurements (P=0.90). The repeated measurement ANOVA test with Huynh-Feldt correction did not find a significant difference in the CT diurnal patterns between the first and second daily series of measurements (P=0.22).
The median amplitude of foveal CT diurnal variation was 20.5 µm (13, 31) for the first daily series of measurements and 20.0µm (12.5,28.2) for the second daily series of measurements. No significant difference on the amplitude of foveal CT was observed between the first and second daily series of measurements (P=0.06). No significant difference on MOPP was observed between the first and second daily measurements (P=0.39).
Based on the univariate linear regression results, the amplitude of foveal CT was positively associated with the initial foveal CT (R2=0.10, P=0.009), being dominant eye (R2=0.07, P=0.03), BMI (R2=0.08, P=0.02) but not associated with the spherical equivalent refraction (P=0.20), or status of refractive error correction (P=0.13), or ocular perfusion pressure (P=0.14).Variables with a P value ≤0.2 in the univariate linear analysis were further controlled in the multivariate regression model to test the association with the amplitude of foveal CT, including initial foveal CT, BMI, daily use of visual display terminal, eye dominance, status of refractive error correction, spherical equivalent refraction and ocular perfusion pressure. One variable was included or excluded in the multivariate linear regression model at each time until the least Akaike information criterion (AIC) was achieved. Based on the multivariate regression model, the amplitude of foveal CT was positively significant associated with initial foveal CT (P=0.01) and being dominant eye (P=0.04) (multiple R2=0.16, P=0.004) (Table 3).
Table 3. The association of characteristics with amplitude of fovea CT in univariable linear regression and multivariate linear regression.
| Characteristics | Univariate linear regression |
multivariate linear regression (AIC=567.4) |
||
| Coefficients (95%CI)c | P c | Coefficients (95%CI)d | Pd | |
| Age (a) | 0.94(0.177,1.71) | 0.22 | ||
| Body mass index (kg/m2) | 2.99(1.29,4.52) | 0.02 | ||
| Having daily regular exercise | -8.06(-11.98,-4.12) | 0. 85 | ||
| Daily use of visual display terminal≥4h | -8.82(-14.12,-3.52) | 0.12 | ||
| The Pittsburgh Sleep Quality Index score | -1.56(-2.96,-0.09) | 0.29 | ||
| Eye dominancea | 11.26(6.21,16.46) | 0.03 | 10.51(4.02, 14.60) | 0.04 |
| The status of refractive error correctionb | 7.61(2.71,12.51) | 0.13 | ||
| Spherical equivalent refraction (D) | 1.25(0.25,2.44) | 0.20 | ||
| Initial foveal CT(µm) | 0.06(0.04,0.080) | 0.009 | 0.05(0.03,0.08) | 0.01 |
| Ocular perfusion pressure | 0.24(0.08,0.31) | 0.14 | ||
aThe percentage of subject whose right eye is the dominant eye; bThe percentage of subject who present with uncorrected refractive error.cUnivariate linear association with amplitude of fovea CT; dVariables with P value≤0.2 in the univariate linear analysis were further controlled in the multivariate linear regression model to test the association with the amplitude of foveal CT. CT: Choroidal thickness.
The initial foveal CT was positively associated with the spherical equivalent refraction (R2=0.17, P<0.001). The eyes with uncorrected refractive error (mean -3.25) were likely to have deeper myopia compared to eyes with corrected refractive error (-2.0) (P=0.04). The initial foveal CT was not different significantly between eyes being dominant or eyes being non-dominant (P=0.41).
DISCUSSION
In this study, we measured CT by EDI-OCT at fovea and parafoveal areas. In two daily series of measurements, we found that the CT at fovea, 500 µm temporal, 500 µm nasal and 1500 µm nasal to the fovea had a similar diurnal variation pattern with the median amplitude of foveal CT diurnal variation being 20-20.5 µm. Although each subject had her own specific rhythm of diurnal variation of the CT, most subjects reached their peak CT at 11:00 a.m. The measurement series were repeated twice and the results could be reproduced. Thicker initial foveal CT, being dominant eye were associated with greater amplitude of foveal CT variation.
The rhythm of diurnal variation varied greatly among different studies. For example, Chakraborty et al[21] reported the diurnal variation of CT measured by an optical biometer on 30 healthy young subjects and found that CT was thickest at night and thinnest in the morning, with the mean amplitude of the CT variation being 29 µm. Among several studies using SD-OCT, two studies showed that CT was thickest in the morning, with the mean amplitude of CT being 19.52-22.7 µm[16]–[17], whereas Toyokawa et al[20] showed that the CT was thicker in the evening as compared to that in the morning on 12 healthy older aged subjects. Usui et al[19] showed that each subject reached the thickest CT at different time points, and most of the subjects reached the thickest CT between 3:00 and 6:00. This current study found CT diurnal variation peaking at 11:00 with similar median amplitude of CT variation. In reviewing the rhythmic pattern of each subject, as did in an earlier study[19], the subjects in our study reached the thickest CT at different time points, while most of the subjects reached the thickest CT at 11:00, which was further repeated in the second series of measurements. The findings of most subjects reached their peak CT at 11:00 a.m. may contribute to the median CT variation peaking at 11:00. The factors may influence the rhythm of individual's diurnal CT variation need to be further explored. It is suggested the time of OCT scan should be of importance when evaluating foveal CT variations in clinical practice and research.
An earlier study on Chinese healthy individuals showed that CT measured at the same time was thickest underneath the fovea, and the CT at 1 mm to the fovea temporally was thicker than that nasally[10]. Toyokawa et al[20] and colleagues measured the CT on 12 older healthy individuals at 9:00 and 19:30 and found that the mean change of CT at fovea and 1500 µm nasal to foveal were of significance. We measured CT at 500 µm and 1500 µm nasal or temporal to the fovea, as well as at fovea, and found that the diurnal variation pattern of CT presented not only underneath the fovea, but also at 500 µm nasal or temporal to the fovea, demonstrating a similar pattern of diurnal variation. In this study, the amplitude of CT variation did not differ significantly at fovea compared to that 500 µm temporal or nasal to fovea. The diurnal variation of CT was not found at 1500 µm temporal to fovea. Our results showed that the similar duriation variation pattern could be observed at fovea, 500 µm temporal or nasal to fovea. It was suggested that the time of SD-OCT examination should be taken into account when evaluating lesions at the parafoveal region as well as under the fovea.
The potential factors that may influence the amplitude of foveal CT variation as reported by previous studies include the initial CT and refraction status. For example, it has been reported that subjects with thinner initial CTs tend to have lower amplitude in the CT variation[16]–[17]. In this current study, the initial foveal CT was significantly associated with the amplitude of foveal CT based on both univariate linear regression and multivariate linear regression, which aligns with the findings of Colinset al[16]but not with those of Chakraborty et al[21]. The discrepancies among these studies regarding the association between the spherical equivalent refraction and the amplitude of foveal CT may be caused by sampling bias. For example, the current study included more myopic subjects than Colins' study[16]. In addition, we also found the amplitude of foveal CT was statistically significantly associated with being the dominant eye while the initial foveal CT was not affected by being dominant eye. The influence of eye dominance on the amplitude of foveal CT variation need to be further explored and conformed in further larger cohort. Frequent usage of dominant eye may cause fast circulation of choroidal vascular, which may in turn makes the variation of CT greater. Our data indicated a larger cohort could be required for further exploration of the potential factors that may influence the amplitude of foveal CT.
The participants in our study were of a similar age (23-27 years old) and were all female. It was reported that the choroidal thickness decresed in the mid-luteal phase of menstrual cycle in young healthy women[26]. MOPP was measured at the beginning of CTs measurements, which showed the difference between blood pressure and IOP. The CTs and MOPP were re-measured on 1-4wk later, at different phase of individual menstrual cycle. In regard to foveal CT and MOPP, the second daily series of measurements failed to show significant difference compared to the first daily series of measurements. We failed to find variation on the circulatory factor or CT on individuals at different phase of menstrual cycle. The role of menstruation on diurnal variation of CT would be explored in further study. Similar to the previous work[16]–[17],[21],[27], we did not measure the CTs from 20:00-8:00 in this study.
In summary, this study demonstrated a significant and similar CT diurnal variation in young healthy female subjects at fovea, 500 µm nasal or temporal to the fovea and 1500 µm nasal to the fovea. It is important to consider this observation when clinically assessing the CT. The thicker initial foveal CT, being dominant eye was associated with greater daily variation of foveal CT. The factors affecting the variation in CT must be further explored.
Acknowledgments
Conflicts of Interest: Zhao M, None; Yang XF, None; Jiao X, None; Lim A, None; Ren XT, None; Snellingen T, None; Liu NP, None.
REFERENCES
- 1.Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147(5):811–815. doi: 10.1016/j.ajo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Jirarattanasopa P, Ooto S, Tsujikawa A, Yamashiro K, Hangai M, Hirata M, Matsumoto A, Yoshimura N. Assessment of macular choroidal thickness by optical coherence tomography and angiographic changes in central serous chorioretinopathy. Ophthalmology. 2012;119(8):1666–1678. doi: 10.1016/j.ophtha.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 3.Jirarattanasopa P, Ooto S, Nakata I, Tsujikawa A, Yamashiro K, Oishi A, Yoshimura N. Choroidal thickness, vascular hyperpermeability, and complement factor H in age-related macular degeneration and polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2012;53(7):3663–3372. doi: 10.1167/iovs.12-9619. [DOI] [PubMed] [Google Scholar]
- 4.Chung SE, Kang SW, Lee JH, Kim YT. Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology. 2011;118(5):840–845. doi: 10.1016/j.ophtha.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Kim SW, Oh J, Kwon SS, Yoo J, Huh K. Comparison of choroidal thickness among patients with healthy eyes, early age-related maculopathy, neovascular age-related macular degeneration, central serous chorioretinopathy, and polypoidal choroidal vasculopathy. Retina. 2011;31(9):1904–1911. doi: 10.1097/IAE.0b013e31821801c5. [DOI] [PubMed] [Google Scholar]
- 6.Manjunath V, Goren J, Fujimoto JG, Duker JS. Analysis of choroidal thickness in age-related macular degeneration using spectral-domain optical coherence tomography. Am J Ophthalmol. 2011;152(4):663–668. doi: 10.1016/j.ajo.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maruko I, Iida T, Sugano Y, Furuta M, Sekiryu T. One-year choroidal thickness results after photodynamic therapy for central serous chorioretinopathy. Retina. 2011;31(9):1921–1927. doi: 10.1097/IAE.0b013e31822bf6b1. [DOI] [PubMed] [Google Scholar]
- 8.Maruko I, Iida T, Sugano Y, Saito M, Sekiryu T. Subfoveal retinal and choroidal thickness after verteporfin photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2011;151(4):594–603.e1. doi: 10.1016/j.ajo.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 9.Yamazaki T, Koizumi H, Yamagishi T, Kinoshita S. Subfoveal choroidal thickness after ranibizumab therapy for neovascular age-related macular degeneration: 12-month results. Ophthalmology. 2012;119(8):1621–1627. doi: 10.1016/j.ophtha.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 10.Ding X, Li J, Zeng J, Ma W, Liu R, Li T, Yu S, Tang S. Choroidal thickness in healthy Chinese subjects. Invest Ophthalmol Vis Sci. 2011;52(13):9555–9560. doi: 10.1167/iovs.11-8076. [DOI] [PubMed] [Google Scholar]
- 11.Chen FK, Yeoh J, Rahman W, Patel PJ, Tufail A, Da Cruz L. Topographic variation and interocular symmetry of macular choroidal thickness using enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(2):975–985. doi: 10.1167/iovs.11-8771. [DOI] [PubMed] [Google Scholar]
- 12.Ikuno Y, Kawaguchi K, Nouchi T, Yasuno Y. Choroidal thickness in healthy Japanese subjects. Invest Ophthalmol Vis Sci. 2010;51(4):2173–2176. doi: 10.1167/iovs.09-4383. [DOI] [PubMed] [Google Scholar]
- 13.Ikuno Y, Tano Y. Retinal and choroidal biometry in highly myopic eyes with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50(8):3876–3880. doi: 10.1167/iovs.08-3325. [DOI] [PubMed] [Google Scholar]
- 14.Kim M, Kim SS, Kwon HJ, Koh HJ, Lee SC. Association between choroidal thickness and ocular perfusion pressure in young, healthy subjects: enhanced depth imaging optical coherence tomography study. Invest Ophthalmol Vis Sci. 2012;53(12):7710–7717. doi: 10.1167/iovs.12-10464. [DOI] [PubMed] [Google Scholar]
- 15.Papastergiou GI, Schmid GF, Riva CE, Mendel MJ, Stone RA, Laties AM. Ocular axial length and choroidal thickness in newly hatched chicks and one-year-old chickens fluctuate in a diurnal pattern that is influenced by visual experience and intraocular pressure changes. Exp Eye Res. 1998;66(2):195–205. doi: 10.1006/exer.1997.0421. [DOI] [PubMed] [Google Scholar]
- 16.Colins S, Tan YO, Humberto Ruiz, SriniVas R. Sadda. Diurnal Variation of Choroidal Thickness in Normal,Healthy Subjects Measured by Spectral Domain Optical Coherence Tomography. Retina. 2012;25(53):261–266. doi: 10.1167/iovs.11-8782. [DOI] [PubMed] [Google Scholar]
- 17.Lee SW, Yu SY, Seo KH, Kim ES, Kwak HW. Diurnal variation in choroidal thickness in relation to sex, axial length, and baseline choroidal thickness in healthy Korean subjects. Retina. 2014;34(2):385–393. doi: 10.1097/IAE.0b013e3182993f29. [DOI] [PubMed] [Google Scholar]
- 18.Osmanbasoglu OA, Alkin Z, Ozkaya A, Ozpinar Y, Yazici AT, Demirok A. Diurnal choroidal thickness changes in normal eyes of Turkish people measured by spectral domain optical coherence tomography. J Ophthalmol. 2013;2013:687165. doi: 10.1155/2013/687165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Usui S, Ikuno Y, Akiba M, Maruko I, Sekiryu T, Nishida K, Lida T. Circadian changes in subfoveal choroidal thickness and the relationship with circulatory factors in healthy subjects. Invest Ophthalmol Vis Sci. 2012;53(4):2300–2307. doi: 10.1167/iovs.11-8383. [DOI] [PubMed] [Google Scholar]
- 20.Toyokawa N, Kimura H, Fukomoto A, Kuroda S. Difference in morning and evening choroidal thickness in Japanese subjects with no chorioretinal disease. Ophthalmic Surg Lasers Imaging. 2012;43(2):109–114. doi: 10.3928/15428877-20120102-06. [DOI] [PubMed] [Google Scholar]
- 21.Chakraborty R, Read SA, Collins MJ. Diurnal variations in axial length, choroidal thickness, intraocular pressure, and ocular biometrics. Invest Ophthalmol Vis Sci. 2011;52(8):5121–5129. doi: 10.1167/iovs.11-7364. [DOI] [PubMed] [Google Scholar]
- 22.Flores-Moreno I, Lugo F, Duker JS, Ruiz-Moreno JM. The relationship between axial length and choroidal thickness in eyes with high myopia. Am J Ophthalmol. 2013;155(2):314–319.e1. doi: 10.1016/j.ajo.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 24.Schneider J, Leeder SR, Gopinath B, Wang JJ, Mitchell P. Frequency, course, and impact of correctable visual impairment (uncorrected refractive error) Surv ophthalmol. 2010;55(6):539–560. doi: 10.1016/j.survophthal.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Linke SJ, Baviera J, Munzer G, Steinberg J, Richard G, Katz T. Association between ocular dominance and spherical/astigmatic anisometropia, age, and sex: Analysis of 10,264 Myopic Individuals. Invest Ophthalmol Vis Sci. 2011;52(12):9166–9173. doi: 10.1167/iovs.11-8131. [DOI] [PubMed] [Google Scholar]
- 26.Ulaş F, Doğan U, Duran B, Keleş A, Ağca S, Celebi S. Choroidal thickness changes during the menstrual cycle. Curr Eye Res. 2013;38(11):1172–1181. doi: 10.3109/02713683.2013.811258. [DOI] [PubMed] [Google Scholar]
- 27.Brown JS, Flitcroft DI, Ying GS, Francis EL, Schmid GF, Quinn GE, Quinn GE, Stone RA. In vivo human choroidal thickness measurements: evidence for diurnal fluctuations. Invest Ophthalmol Vis Sci. 2009;50(1):5–12. doi: 10.1167/iovs.08-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]