Abstract
AIM
To investigate the effects of posterior scleral reinforcement (PSR) in the treatment of pathological myopia.
METHODS
The study included 52 eyes in 43 patients with pathological myopia who underwent PSR (PSR group), and 52 eyes in 36 age- and myopia-matched patients who did not undergo such treatment as control group. Axial length, refraction error, best corrected visual acuity (BCVA), and macular scans by optical coherence tomography (OCT) were recorded at baseline, 6mo, 1, 3 and 5y after the surgery, and the complications were noted.
RESULTS
There were no statistical differences in axial length, refractive error, or BCVA between the PSR group and the control group at baseline. At the end of the follow-up, the mean axial length was 29.79±1.26 mm in the PSR group, which was significantly shorter than that in the control group (30.78±1.30 mm) (P<0.01), and the mean refractive error was -16.86±2.53 D in the PSR group, which was significantly lower than that in the control group (-19.18±2.12 D) (P<0.01). A statistically significant difference in BCVA was found between the PSR group (0.51±0.25 logMAR) and the control group (0.62±0.26 logMAR) at the postoperative 5-year follow-up (P<0.01). There were no serious complications during the 5-year follow-up period.
CONCLUSION
PSR can prevent axial elongation and myopia progression in eyes with pathological myopia.
Keywords: pathological myopia, posterior scleral reinforcement, axial length
INTRODUCTION
Pathological myopia is also known as degenerative myopia. It is one of the leading causes of blindness worldwide and is the most frequent cause of visual impairment in Asian countries[1]–[3]. The most obvious characteristic of pathological myopia is the gradual growth of the axial length, which is rapid during childhood and adolescence, with further continual increases in its dimensions occurring in the adult years of life[4]. Excessive axial elongation of the eyes with pathological myopia can cause scleral thinning and create shearing forces within the contiguous structures: the choroid, the retina and the vitreous body. The failure of these tissues has serious impacts on macular functioning and can also lead to various complications, such as macular schisis, macular hole, retinal tears and detachment, which may result in severe visual impairment in pathological myopic eyes[4]–[5].
However, the pathogenesis of pathological myopia is not clear so far. Genetic factors, environmental factors, and life-style factors may play a role together in the cause of pathological myopia[6]–[7]. There is no widely accepted medical or surgical treatment that can prevent myopic axial elongation and myopic progression as yet. Surgeons tried to prevent axial elongation and staphyloma progression by placing grafts over the posterior part of the eyes. Posterior scleral reinforcement (PSR), which was first proposed by Shevelev[8] and was later modified and simplified by Thompson[9], has the potential to prevent the progression of pathological myopia. After years of experience with their own variations of scleral reinforcement, Thompson and other scholars expressed satisfaction with the efficacy and safety of their series of cases[10]–[15]. In contrast, Karabatsas et al[16] and Zhang and Wu[17] had negative conclusions on the outcomes for the reinforcement surgery. Therefore, PSR is considered to be controversial, and more studies are needed to confirm its therapeutic benefits.
In the present study, we evaluated the effects of PSR in the treatment of pathological myopia.
SUBJECTS AND METHODS
Subjects
This study is a retrospective analysis of 52 eyes (43 patients) with pathological myopia treated with PSR (PSR group) at our hospital in the period between January 2005 and January 2010. As natural history controls (control group), we recruited 52 eyes (36 patients) who were matched for age and myopia. These patients did not want to undergo PSR surgery and were followed up at outpatient clinics. The study was approved by the Medical Ethics Committee of Zhengzhou University and conducted in accordance with the Declaration of Helsinki for research involving human subjects. Informed consent was obtained from all the patients after a thorough discussion on both the desired positive outcomes and the potential complications of PSR.
Inclusion criteria for this study included pathological myopia defined as a refractive error of -12.0 D or higher, an axial length ≥28 mm, as well as atrophic myopic macular degeneration, with RPE de-pigmentation and pigment clumping. Patients were excluded from the study if they had other ocular diseases that could affect the visual function (nystagmus, glaucoma, lens abnormality, ocular trauma, macula hole, retinal detachment, choroidal neovascularization and so on), and systemic disorders that could interfere with the results, or they had undergone ocular surgery such as refractive surgery, scleral buckle procedure, PSR or vitrectomy.
Methods
Surgical procedures
PSR was performed under general anesthesia. The procedure was performed as described before[18]. A 360° limbal conjunctiva incision was performed. Radial incisions of the conjunctiva were made in inferior nasal quadrant and superior temporal quadrant. Once the superior, inferior, medial and external rectus muscles were exposed and hooked, traction sutures were placed with 3-0 black silk. A scleral buckle of donor sclera with a width of 12 to 14 mm was made, and the superior end of the scleral buckle was sutured to the nasal side of the sclera near the attachment of superior rectus muscle with 6-0 absorbable sutures. With the assistance of the traction sutures and a muscle hook that lifted up the inferior oblique muscle completely, the scleral buckle was sequentially passed underneath the inferior oblique, external rectus and inferior rectus muscles. The inferior end of the buckle was fixed to the sclera at nasal side of the attachment of inferior rectus muscle with 6-0 absorbable sutures. Then another scleral belt of donor sclera with the size of 8×8-mm2 was made and placed between scleral buckle and posterior staphyloma. After that, the location of the scleral buckle was checked. The position relation between the buckle and optic nerve should be especially tested with a strabismus hook. The distance should be kept about 3 mm to ensure that the scleral buckle covered the posterior staphyloma without compressing the optic nerve. If the scleral buckle was too close to the optic nerve, the temporal border of it should be pulled forward and then fixed to the sclera near the attachment of inferior oblique muscle. The conjunctiva was closed with 8-0 absorbable sutures. After the surgery, 0.3% tobramycin and 0.1% dexamethasone eye drops were used four times a day and tapered off over 3wk.
Outcome measures
The main outcome measures were axial length, refractive error, best corrected visual acuity (BCVA), macular scans and the incidence of complications. All patients underwent a broad ophthalmologic examination at baseline and every postoperative follow-up visit (6mo, 1, 2, 3, 4 and 5y after PSR) including axial length measured with IOL Master (Carl Zeiss Meditec, Dublin, CA, USA), refractive error measured with streak retinoscopy (Heine Optotechnik GmbH & Co. KG, Herrsching, Germany), BCVA measured with Early Treatment Diabetic Retinopathy Study (ETDRS) charts at 4-meter distance, and macular scans measured with OCT (Heidelberg Engineering, Heidelberg, Germany). Refractive data were presented as the mean spherical equivalent refractive error. For statistical evaluation, the BCVA was converted into logarithm of the minimal angle of resolution (logMAR) format. An improvement or worsening of the postoperative BCVA was defined as a change of 0.2 logMAR units.
Statistical Analysis
The parameters were presented as mean±standard deviations. Comparisons of axial length, refractive error, and BCVA within groups were analyzed using paired t-tests. Comparisons between groups were performed using the group t-test at each follow-up visit. Statistical analyses were performed with SPSS for windows version 17.0 (IBM Corp., Armonk, NY, USA). A P value less than 0.05 was considered statistically significant.
RESULTS
Demographic and Clinical Data
The demographic and clinical characteristics of the patients in the PSR group and control group were presented in Table 1. There were no statistical differences in axial length, refractive error, or BCVA between the PSR group and the control group at baseline.
Table 1. Demographic and clinical characteristics of the patients.
| Characteristics | PSR group | Control group | P |
| M/F | 24/28 | 23/29 | |
| Age (a) | 41.03±2.27 | 40.68±3.32 | 0.62 |
| Axial length (mm) | 29.49±1.21 | 29.42±1.09 | 0.31 |
| Refractive error (D) | -16.12±2.87 | -15.98±2.84 | 0.48 |
| BCVA (logMAR) | 0.52±0.23 | 0.51±0.21 | 0.35 |
PSR: Posterior scleral reinforcement; BCVA: Best corrected visual acuity.
n=52
Axial Length
The difference in axial length between the PSR group and the control group was not significant at baseline (P=0.31). The statistically significant differences in axial length between the two groups were found at the 6-month (P=0.02), 1-year (P<0.01), 3-year (P<0.01) and 5-year (P<0.01) follow-up visit respectively after the surgery. The mean value of axial elongation in the control group was 1.35±0.56 mm over a 5-year follow-up, versus 0.30±0.21 mm in the PSR group. The study suggested that eyes with pathological myopia may show axial elongation even in adulthood, and the PSR surgery limited the axial elongation effectively (Table 2).
Table 2. Comparisons of axial length, refractive error, BCVA between the PSR group and the control group.
| Parameters | PSR group | Control group | P |
| Axial length (mm) | |||
| Baseline | 29.49±1.21 | 29.42±1.09 | 0.31 |
| 6-month | 29.49±1.16 | 29.57±1.13 | 0.02 |
| 1-year | 29.52±1.22 | 29.71±1.21 | <0.01 |
| 3-year | 29.65±1.23 | 30.24±1.25 | <0.01 |
| 5-year | 29.79±1.26 | 30.78±1.30 | <0.01 |
| Refractive error (D) | |||
| Baseline | -16.12±2.87 | -15.98±2.84 | 0.48 |
| 6-month | -16.13±2.36 | -16.27±2.65 | 0.11 |
| 1-year | -16.23±2.27 | -16.58±2.08 | 0.02 |
| 3-year | -16.54±2.87 | -17.85±2.39 | <0.01 |
| 5-year | -16.86±2.53 | -19.18±2.12 | <0.01 |
| BCVA (logMAR) | |||
| Baseline | 0.52±0.23 | 0.51±0.21 | 0.35 |
| 6-month | 0.51±0.27 | 0.52±0.24 | 0.14 |
| 1-year | 0.51±0.19 | 0.54±0.27 | 0.03 |
| 3-year | 0.51±0.26 | 0.58±0.30 | <0.01 |
| 5-year | 0.51±0.25 | 0.62±0.26 | <0.01 |
PSR: Posterior scleral reinforcement; BCVA: Best corrected visual acuity.
n=52
Refractive Error
There was no significant difference in refractive error between the PSR group and the control group at baseline (P = 0.48). The progression of refractive error was slower in the PSR group than that in the control group at 1-year (P=0.02), 3-year (P<0.01) and 5-year (P<0.01) follow-up visit respectively after the surgery. The mean change in refractive error from baseline to the end of follow-up was -0.72±0.41 D in the PSR group, versus -3.20±1.48 D in the control group. The data showed that the eyes in the PSR group had less myopia progression than that in the control group during the follow-up period (Table 2).
Best Corrected Visual Acuity
The data showed no differences in BCVA between the PSR group and the control group at baseline (P=0.35). After the surgery, there were statistical differences in BCVA between the PSR group and the control group at 1-year (P=0.03), 3-year (P<0.01) and 5-year (P<0.01) follow-up visit respectively after the surgery. In the PSR group, the changes in BCVA were not obviously at the end of the follow-up period (P=0.46). Among them, the BCVA improved in 12 eyes (23.08%), unchanged in 37 eyes (71.15%), and declined in 3 eyes (5.77%). However, the mean BCVA in the control group was obviously decreased at the end of the follow-up visit (P<0.01) (Table 2).
Anatomical Outcomes
All eyes in this study had atrophic myopic macular degeneration, with RPE de-pigmentation and pigment clumping. Myopic macular schisis was detected in 5 eyes in the PSR group. Twelve months after PSR, OCT images showed the relief of myopic vitreo-macular traction and a reattachment of the macular in the 5 eyes (Figure 1). In addition to increased visual acuity, the patients had a reduction in the distortion of straight lines. No recurrence of macular schisis was present on OCT during the 5y follow-up period. In the control group, myopic macular schisis was detected in 3 eyes. The retinal thicknesses in these eyes were stable during the 5-year follow-up period.
Figure 1. The OCT scans of the macula.
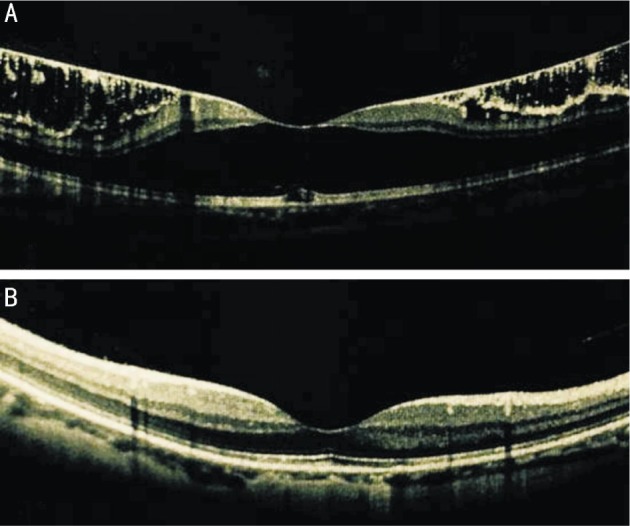
A: At baseline; B: At the 12-month follow-up visit after PSR surgery.
Three eyes in the control group have myopic macular schisis. They were inactivity and maintain stable retinal thickness.
Postoperative Complications
Conjunctival congestion and edema were found in all the patients who underwent PSR and alleviated two to three weeks later. The intraocular pressures (IOPs) of 32 eyes (61.54%) were 24-30 mm Hg after PSR, and dropped to 16-18 mm Hg after treatment with carteolol eye drops (twice a day) for about three weeks. Disorder of ocular movement and slight diplopia occurred in 16 eyes (30.77%) after PSR. The limitation in ocular motility was about 5°-10°. Eight eyes (15.38%) showed adduction deficiency and eight eyes (15.38%) showed abduction deficiency. The disorders of ocular movement were recovery two weeks later without any interventions. No other postoperative complications occurred during the 5-year follow-up period. None of the eyes lost visual acuity from the PSR surgery.
DISCUSSION
Pathological myopia is characterized by constant axial elongation and gradually thinning of the posterior sclera, which may be due to the weakening of the sclera mechanical properties[19]–[20]. PSR is believed to slow the axial elongation by the direct mechanical force of the reinforcement band surrounding the posterior pole and/or by the sclera remodeling and hyperplasia secondary to the non-specific inflammatory reaction between the posterior sclera and the reinforcement band. PSR may be the only effective method that can delay or stop the axial elongation in pathological myopia up to date[14].
We had favorable outcomes of PSR in our present study. Eyes with pathological myopia may show increments of axial elongation even in adulthood, as reflected in the data of the control eyes in our study. Our results showed that the mean axial elongation was 1.35 ±0.56 mm (approximately 0.27 mm per year) over 5y in the control group, equivalent to an increase in refractive error of -3.20±1.48 D (approximately 0.64 D per year). There was a greater increase in axial length of about 0.27 mm per year in our control group compared with the average axial elongation of 0.2 mm per year[4], whereas the relatively stable axial length was found in the PSR group in our study. In the PSR group, 52 eyes showed a mean increase in axial length of 0.30±0.21 mm (approximately 0.06 mm per year), equivalent to a mean increase in refractive error of -0.72±0.41 D (approximately 0.14 D per year) during the 5-year follow-up period. The current data supported the hypothesis that the axial elongation and myopia progression of pathological myopia may be limited by PSR. Furthermore, the ability to control the progression of pathological myopia has brought the hope of reducing the risk of myopic macular degeneration since any axial elongation may increase the risk of macular degeneration later in life.
In the current study, the results showed that the BCVA in the PSR group was significantly better (0.51±0.25 logMAR) than that in the control group (0.62±0.26 logMAR) at the end of follow-up. The mean BCVA improved in12 eyes (23.08%) in the PSR group, while the mean BCVA in the control group were obviously decreased. Possible reasons for the better BCVA in the PSR group may include two main factors: one factor was that the limitation of axial elongation and myopia progression may prevent the deterioration of vision; the other factor may be related to the microcirculation within the macula for studies revealed that scleral reinforcement towards the fovea could improve the microcirculation within the macula[21]. But the final BCVA was still unchanged in 37 eyes (71.15%), and declined in 3 eyes (5.77%) in the PSR group. We speculated that the stability of the BCVA may be because of the long-existing myopic chorioretinal lesions, and the decrease of the BCVA may be due to the progression of the various forms of myopic maculopathy induced by axial elongation and myopia progression.
Myopic macular schisis was detected in 5 eyes in our study. One year after PSR, OCT showed the relief of myopic macular schisis and a reattachment of the macular in the 5 eyes. In addition to the increased BCVA, the patients reported a reduction in the distortion of the lines. Our study results were similar to those of Zhu et al[18] and Ji et al[22]. They reported that PSR alone may be useful for treating myopic macular schisis for strengthening the macular structure and helping to maintain BCVA. Though vitrectomy and pre-retinal membrane peeling released myopic macular schisis[23]–[25], PSR has been suggested as a treatment option for myopic macular schisis. PSR can avoid further intraocular surgery, with the additional benefits of preventing axial elongation and myopia progression.
It has been reported that PSR may lead to some serious complications such as retinal detachment and cilioretinal artery occlusion[16]–[17]. The absence of any vision-threatening complications was a positive outcome of our present study. The most common complications were temporary: congestion and edema of the conjunctiva, increase of IOP, weakness of ocular movement and slight diplopia. Pre-operative counselling allowed the patients to be prepared for an uncertain period of weakness of ocular movement and slight diplopia, and created awareness of the need for a period of topical medication for IOP control. No serious complications occurred during the 5-year follow-up period in our study.
In conclusion, our findings suggested that PSR can prevent the axial elongation and myopia progression in eyes with pathological myopia. Further studies are needed to determine the long-term safety and effectiveness of this surgery.
Acknowledgments
Foundations: Supported by the Overseas Training Program for Medical Academic Leaders of Henan Province (No.2014005); the Project of Medical Science and Technology of Henan Province (No.201304007); the Development Plan of Science and Technology of Henan Province (No.142102310110).
Conflicts of Interest: Li XJ, None; Yang XP, None; Li QM, None; Wang YY, None; Wang Y, None; Lyu XB, None; Jia H, None.
REFERENCES
- 1.Zhang Z, Xu Y, Liu J, Wong DW, Kwoh CK, Saw SM, Wong TY. Automatic diagnosis of pathological myopia from heterogeneous biomedical data. PLoS One. 2013;8(6):e65736. doi: 10.1371/journal.pone.0065736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buch H, Vinding T, La Cour M, Appleyard M, Jensen GB, Nielsen NV. Prevalence and causes of visual impairment and blindness among 9980 Scandinavian adults: the Copenhagen City Eye Study. Ophthalmology. 2004;111(1):53–61. doi: 10.1016/j.ophtha.2003.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Iwase A, Araie M, Tomidokoro A, Yamamoto T, Shimizu H, Kitazawa Y, Tajimi Study Group Prevalence and causes of low vision and blindness in a Japanese adult population: the Tajimi Study. Ophthalmology. 2006;113(8):1354–1362. doi: 10.1016/j.ophtha.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Ward B, Tarutta EP, Mayer MJ. The efficacy and safety of posterior pole buckles in the control of progressive high myopia. Eye (Lond) 2009;23(12):2169–2174. doi: 10.1038/eye.2008.433. [DOI] [PubMed] [Google Scholar]
- 5.Verkicharla PK, Ohno-Matsui K, Saw SM. Current and predicted demographics of high myopia and an update of its associated pathological changes. Ophthalmic Physiol Opt. 2015;35(5):465–475. doi: 10.1111/opo.12238. [DOI] [PubMed] [Google Scholar]
- 6.Li M, Zhai L, Zeng S, Peng Q, Wang J, Deng Y, Xie L, He Y, Li T. Lack of association between LUM rs3759223 polymorphism and high myopia. Optom Vis Sci. 2014;91(7):707–712. doi: 10.1097/OPX.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 7.Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012;379(9827):1739–1748. doi: 10.1016/S0140-6736(12)60272-4. [DOI] [PubMed] [Google Scholar]
- 8.Shevelev MM. Operation against high myopia and scleralectasia with aid of the transplantation of fascia lata on thinned sclera. Russ Oftalmol J. 1930;11:107–110. [Google Scholar]
- 9.Thompson FB. A simplified scleral reinforcement technique. Am J Ophthalmol. 1978;86(6):782–790. doi: 10.1016/0002-9394(78)90121-6. [DOI] [PubMed] [Google Scholar]
- 10.Thompson FB, Turner AF. Computed axial tomography on highly myopic eyes following scleral reinforcement surgery. Ophthalmic Surg. 1992;23(4):253–259. [PubMed] [Google Scholar]
- 11.Xue A, Bao F, Zheng L, Wang Q, Cheng L, Qu J. Posterior scleral reinforcement on progressive high myopic young patients. Optom Vis Sci. 2014;91(4):412–418. doi: 10.1097/OPX.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 12.Liu XD, Lü JH, Chu RY. Long-term studies on clinical therapeutic efficiency of posterior scleral reinforcement surgery. Zhonghua Yan Ke Za Zhi. 2011;47(6):527–530. [PubMed] [Google Scholar]
- 13.Xu Y, Liu H, Niu T, Zhu X. Long-term observation of curative effects of posterior scleral reinforcement surgery in patients with juvenile progressive myopia. Zhonghua Yan Ke Za Zhi. 2000;36(6):455–458. [PubMed] [Google Scholar]
- 14.Shen ZM, Zhang ZY, Zhang LY, Li ZG, Chu RY. Posterior scleral reinforcement combined with patching therapy for pre-school children with unilateral high myopia. Graefes Arch Clin Exp Ophthalmol. 2015;253(8):1391–1395. doi: 10.1007/s00417-015-2963-9. [DOI] [PubMed] [Google Scholar]
- 15.Chen M, Dai J, Chu R, Qian Y. The efficacy and safety of modified Snyder-Thompson posterior scleral reinforcement in extensive high myopia of Chinese children. Graefes Arch Clin Exp Ophthalmol. 2013;251(11):2633–2638. doi: 10.1007/s00417-013-2429-x. [DOI] [PubMed] [Google Scholar]
- 16.Karabatsas CH, Waldock A, Potts MJ. Cilioretinal artery occlusion following scleral reinforcement surgery. Acta Ophthalmol Scand. 1997;75(3):316–318. doi: 10.1111/j.1600-0420.1997.tb00784.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Wu N. Retinal detachment--severe complication after posterior scleral reinforcement operation. Zhonghua Yan Ke Za Zhi. 1997;33(3):210–212. [PubMed] [Google Scholar]
- 18.Zhu Z, Ji X, Zhang J, Ke G. Posterior scleral reinforcement in the treatment of macular retinoschisis in highly myopic patients. Clin Experiment Ophthalmol. 2009;37(7):660–663. doi: 10.1111/j.1442-9071.2009.02111.x. [DOI] [PubMed] [Google Scholar]
- 19.McBrien NA, Jobling AI, Gentle A. Biomechanics of the sclera in myopia: extracellular and cellular factors. Optom Vis Sci. 2009;86(1):E23–30. doi: 10.1097/OPX.0b013e3181940669. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, Li X, Yan N, Cai S, Liu X. Myopia: a collagen disease? Med Hypotheses. 2009;73(4):485–487. doi: 10.1016/j.mehy.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Gentle A, Liu Y, Martin JE, Conti GL, McBrien NA. Collagen gene expression and the altered accumulation of scleral collagen during the development of high myopia. J Biol Chem. 2003;278(19):16587–16594. doi: 10.1074/jbc.M300970200. [DOI] [PubMed] [Google Scholar]
- 22.Ji X, Wang J, Zhang J, Sun H, Jia X, Zhang W. The effect of posterior scleral reinforcement for high myopia macular splitting. J Int Med Res. 2011;39(2):662–666. doi: 10.1177/147323001103900236. [DOI] [PubMed] [Google Scholar]
- 23.García-Layana A, García-Arumí J, Ruiz-Moreno JM, Arias-Barquet L, Cabrera-López F, Figueroa MS. A Review of Current Management of Vitreomacular Traction and Macular Hole. J Ophthalmol. 2015;2015:809640. doi: 10.1155/2015/809640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uchida A, Shinoda H, Koto T, Mochimaru H, Nagai N, Tsubota K, Ozawa Y. Vitrectomy for myopic foveoschisis with internal limiting membrane peeling and no gas tamponade. Retina. 2014;34(3):455–460. doi: 10.1097/IAE.0b013e3182a0e477. [DOI] [PubMed] [Google Scholar]
- 25.Gohil R, Sivaprasad S, Han LT, Mathew R, Kiousis G, Yang Y. Myopic foveoschisis: a clinical review. Eye (Lond) 2015;29(5):593–601. doi: 10.1038/eye.2014.311. [DOI] [PMC free article] [PubMed] [Google Scholar]


