Abstract
AIM
To investigate the association between collagen type I alpha 1 (COL1A1) gene and high myopia.
METHODS
In this Meta-analysis, we examined 5 published case-control studies that involved 1942 high myopia cases and 2929 healthy controls to assess the association between the COL1A1 rs2075555 polymorphism and high myopia risk. We calculated the pooled odds ratios (ORs) of COL1A1 rs2075555 polymorphism in high myopia cases vs healthy controls to evaluate the strength of the association.
RESULTS
Overall, there was no significant difference both in the genotype and allele distributions of COL1A1 rs2075555 polymorphism between high myopia cases and healthy controls: CC vs AA OR=1.10, 95% confidence interval (CI)=0.76-1.58; AC vs AA OR=0.98, 95%CI 0.80-1.20; CC/AC vs AA/OR=1.01, 95%CI 0.84-1.22; CC vs AC/AA OR=1.06, 95%CI=0.93-1.20; C vs A OR=1.06, 95%CI 0.91-1.23). In addition, in the stratified analyses by ethnicity, no significant associations were found in any genetic model both in European and Asia cohorts.
CONCLUSION
Our results indicate that the COL1A1 rs2075555 polymorphism may not affect susceptibility to high myopia.
Keywords: collagen type I alpha 1, polymorphism, high myopia, Meta-analysis
INTRODUCTION
Myopia is a common eye disorder that has been widely studied in recent years[1]–[3], particularly because of its increasing prevalence across populations worldwide[4]. According to epidemiological evidence, the incidence of myopia is increasing, especially in East Asia[3],[5]–[6]. Previous studies have indicated that the greatest contributor to myopic refraction is the axial length[7], and when the elongation of the eyeball is excessive, high myopia occurs. High myopia is an extreme form of myopia that is characterized by a spherical equivalent of less than -6.00 diopters or an axial length greater than 26 mm. Many serious complications are associated with high myopia, including retinal detachment, glaucoma, cataracts, macular degeneration, and scleral staphyloma[8]. High myopia is one of the major causes of blindness in many countries and is an important public health problem worldwide[9].
Unfortunately, the pathogenesis of high myopia remains unclear. Although environmental factors such as near work, higher educational levels, and poor economic development levels have been implicated in the occurrence of high myopia[10]–[11], they cannot explain all cases. However, many studies suggest that genetic factors may be responsible for high myopia[12]–[14]. Recent reports have demonstrated that interactions of multiple genetic and environmental factors may contribute to the development of high myopia[15]–[16], and several genes have been confirmed to have an association with susceptibility to high myopia[12],[14],[17]–[19]. However, other studies have not been able to replicate the original findings for these genes[20]–[22]. In particular, the collagen type I alpha 1 (COL1A1) gene has been studied.
The COL1A1 gene is located on chromosome 17 (17q21.23), which contains the myopia 5 (MYP517q21-22) locus[23]. This gene encodes the major component (pro-α1 chains) of type I collagen. Mutations or single nucleotide polymorphisms (SNPs) of the COL1A1 gene may affect the formation of COL1A1 products by altering COL1A1 gene expression, which as a result contributes to the susceptibility to collagen-related diseases such as osteoporosis, osteogenesis imperfecta, Ehlers-Danlos syndrome, and Marfan syndrome, as well as scleral thinning[24]. The COL1A1 gene also reportedly plays an important role in the development of animal myopia[25]–[26]. Recently, several studies performed in different regions investigated a genetic mutation in the COL1A1 sequence [rs2075555 (homosapiens), adenine to cytosine, A>C], as a candidate biomarker associated with high myopia[19],[21],[27]–[29]. However, the conclusions of these previous studies remain controversial and conflicting. To further investigate the role of COL1A1 rs2075555 polymorphism in high myopia, we performed a Meta-analysis involving all the relevant published studies available.
MATERIALS AND METHODS
Literature Search Strategy
Potential articles were identified by a systematic search on the ISI Web of Science, PubMed, EMBASE, Wiley Online Library, and Science Direct databases up to December 15, 2013, using a combination of search terms: “collagen type I alpha 1” OR “COL1A1”, “polymorphism” OR “variation” OR “mutation” AND ”myopia” without language or publication date restrictions. All relevant publications and their reference were manually screened to identify eligible studies.
Selection Criteria
Papers identified during the literature search had to meet the following inclusion criteria in order to be included in our study: 1) case-control or cohort design studies of humans; 2) evaluation of the association of COL1A1 rs2075555 polymorphism with high myopia; 3) sufficient published data available for our team to estimate odds ratios (ORs) of different genotype frequencies; 4) published original full-text literature. We excluded studies based on the following criteria: 1) insufficient reported data; 2) abstracts, review papers, and case-only studies; 3) duplication of previously published literature.
Data Extraction
Three investigators (Jin GM, Zhao XJ, and Chen YX) independently extracted the data. Discrepancies between different investigators were adjudicated by another 2 investigators (Chen AM and Li Q), who reached consensus. The collected data included: name of the first author, publication date, geographical location, ethnicity, source of control, genotyping methods, genotype frequencies, matching variables, and the numbers of cases and controls.
Quality Assessment
We employed the Newcastle-Ottawa scale (NOS), which has been described in detail in previous study[30] to evaluate the quality of the included studies by 2 investigators (Jin GM and Zhao XJ). A study scoring less than 3 stars was categorized as “low quality”, while 4 to 6 stars and 7 to 9 stars were categorized as “moderate quality” and “high quality”, respectively[31]. Discrepancies between investigators were resolved by discussion.
Statistical Analysis
The allelic frequency of COL1A1 rs2075555 was calculated, and Hardy-Weinberg equilibrium (HWE) was assessed for the control group in each included study. Pearson's χ2 test was used, and significant disequilibrium was defined as P<0.05. The strength of the association between COL1A1 rs2075555 polymorphism and high myopia susceptibility was assessed by ORs with 95% confidence intervals (CIs). We used Z-test to judge the significance of the pooled ORs, and statistical significance was considered when P<0.05. The pooled ORs were calculated for 5 genetic models: allele model (C vs A); homozygote model (CC vs AA); heterozygote model (AC vs AA); dominant genetic model (AC/CC vs AA); and recessive genetic model (CC vs AC/AA). Stratified analysis in different genetic models was performed by ethnicity. Heterogeneity among included studies was evaluated by the χ2-based Q test[32] and the I2 index[33]; P<0.10 or I2>50% were considered statistically significant. The random effects model was used to estimate the pooled ORs when obvious heterogeneity was present[34], otherwise the fixed-effects model was used[35]. The sources of the heterogeneity were identified by the Galbraith plot[36]. Sensitivity analyses that deleted 1 study at a time to reflect the effects of the individual study to the pooled ORs were used to estimate the stability of our results[37]. Publication bias of articles was assessed using Begg's funnel plots and Egger's test[38]. Significant publication bias was considered when P<0.05. All statistical analyses were performed with STATA 12.0 software (StataCorp LP, College Station, TX, USA).
RESULTS
Characteristics of Eligible Studies
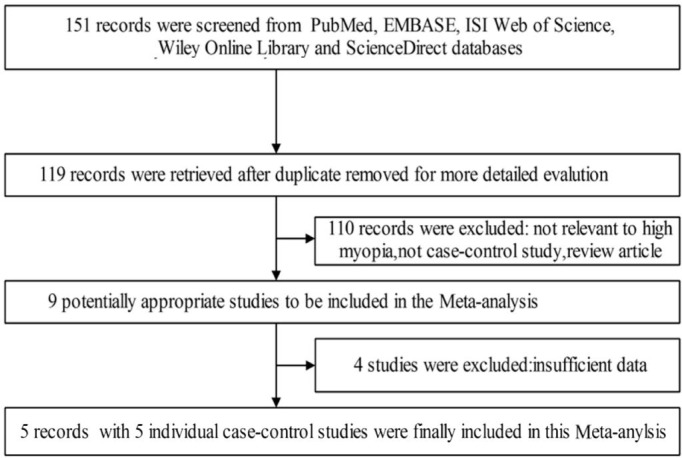
Overall, 151 published articles were retrieved by our literature search strategy. From this group we ultimately analyzed 5 case-control studies that included four Asian studies and one European study and in total involved 1942 cases and 2929 controls. Figure 1 shows the literature selection process. Genotype frequencies in the controls of one study showed significant deviation from the HWE, which required sensitivity analysis and evaluation of possible selection bias. As evaluated by the NOS, two studies were scored as “moderate quality” and three studies were scored as “high quality”, indicating that the quality of the included articles was acceptable for the Meta-analysis. Individual characteristics of the studies, patients, and the control groups are shown in Tables 1 and 2.
Figure 1. Flow chart for the selection of studies according to the criteria of this Meta-analysis.
Table 1. Main characteristics of eligible studies included in the Meta-analysis.
| First author | Year | Region | Ethnicity | Genotyping method | Source | Matching |
| Inamori Y[19] | 2007 | Japan | Asia | Probe method | HB | Age, sex and ethnicity |
| Liang CL[29] | 2007 | Taiwan | Asia | TaqMan | PB | Age, sex and ethnicity |
| Vatavuk Z[28] | 2009 | Croatia | European | BeadChip assay | PB | NA |
| Nakanishi H[21] | 2009 | Japan | Asia | TaqMan | PB | NA |
| Zhang D[27] | 2011 | China | Asia | SNaPshot method | HB | Age and sex |
HB: Hospital-based study; PB: Population-based study; NA: Not available.
Table 2. Distribution of COL1A1 rs2075555 genotypes among high myopia cases and controls included in the Meta-analysis.
| First author | No. |
Case |
Control |
P for HWE | |||||
| Case | Control | AA | AC | CC | AA | AC | CC | ||
| Inamori Y[19] | 330 | 330 | 34 | 166 | 128 | 52 | 176 | 98 | 0.07 |
| Liang CL[29] | 471 | 623 | 36 | 161 | 183 | 53 | 269 | 296 | 0.46 |
| Vatavuk Z[28] | 17 | 794 | 0 | 5 | 12 | 19 | 210 | 565 | 0.92 |
| Nakanishi H[21] | 427 | 420 | 65 | 194 | 167 | 72 | 189 | 158 | 0.23 |
| Zhang D[27] | 697 | 762 | 91 | 333 | 273 | 79 | 374 | 309 | 0.03 |
COL1A1: Collagen type I alpha 1; HWE: Hardy-Weinberg equilibrium.
Results of Meta-analysis
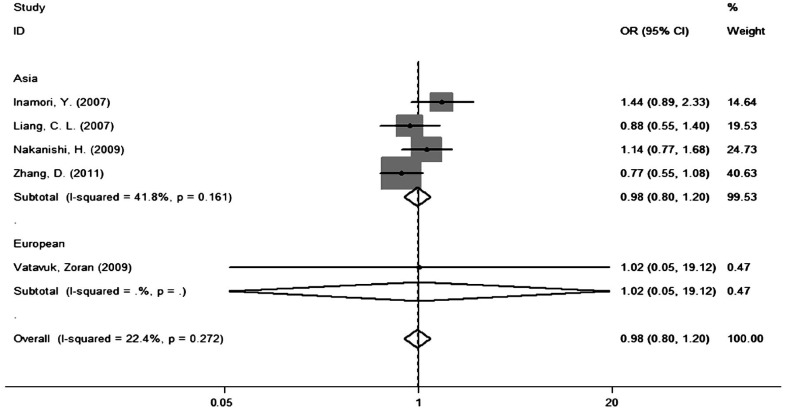
The primary results of the Meta-analysis regarding rs2075555 polymorphism and high myopia risk are shown in Table 3. Overall, no obvious associations between rs2075555 polymorphism and high myopia susceptibility were found in any genetic models (CC vs AA: OR=1.10; 95%CI 0.76-1.58); (AC vs AA: OR=0.98, 95%CI 0.80-1.20); (CC/AC vs AA: OR=1.01, 95%CI 0.84-1.22); (CC vs AC/AA: OR=1.06, 95%CI 0.93-1.20); (C vs A: OR=1.06, 95%CI 0.91-1.23). In the stratified analysis by ethnicity, we found no significant associations between rs2075555 polymorphism and high myopia for any genetic models (Figure 2).
Table 3. Meta-analysis of COL1A1 rs2075555 polymorphism and susceptibility to high myopia.
| Comparisons | Population | N | Test of association |
Test of heterogeneity |
|||||
| OR (95%CI) | Z | P | Model | χ2 | P | I2 (%) | |||
| CC vs AA | Overall | 5 | 1.10 (0.76-1.58) | 0.49 | 0.62 | R | 10.10 | 0.04 | 60.4 |
| (Homozygote) | Asian | 4 | 1.10 (0.75-1.62) | 0.50 | 0.62 | R | 10.08 | 0.11 | 70.3 |
| European | 1 | 0.86 (0.05-15.1) | 0.10 | 0.92 | R | - | - | - | |
| AC vs AA | Overall | 5 | 0.98 (0.80-1.20) | 0.16 | 0.87 | F | 5.15 | 0.27 | 22.4 |
| (Heterozygote) | Asian | 4 | 0.98 (0.80-1.20) | 0.17 | 0.87 | F | 5.15 | 0.16 | 41.8 |
| European | 1 | 1.02 (0.05-19.1) | 0.01 | 0.99 | F | - | - | - | |
| CC /AC vs AA | Overall | 5 | 1.01 (0.84-1.22) | 0.12 | 0.90 | F | 7.76 | 0.10 | 48.4 |
| (Dominant) | Asian | 4 | 1.01 (0.84-1.23) | 0.13 | 0.90 | F | 7.75 | 0.05 | 61.3 |
| European | 1 | 0.88 (0.05-15.2) | 0.09 | 0.93 | F | - | - | - | |
| CC vs AC/AA | Overall | 5 | 1.06 (0.93-1.20) | 0.86 | 0.39 | F | 5.55 | 0.24 | 27.9 |
| (Dominant) | Asian | 4 | 1.06 (0.93-1.20) | 0.88 | 0.38 | F | 5.52 | 0.14 | 45.7 |
| European | 1 | 0.97 (0.34-2.80) | 0.05 | 0.96 | F | - | - | - | |
| C vs A | Overall | 5 | 1.06 (0.91-1.23) | 0.73 | 0.47 | R | 8.62 | 0.07 | 53.6 |
| (Allele) | Asian | 4 | 1.06 (0.90-1.24) | 0.69 | 0.49 | R | 8.61 | 0.04 | 65.2 |
| European | 1 | 1.07 (0.41-2.80) | 0.14 | 0.89 | R | - | - | - | |
COL1A1: Collagen type I alpha 1; OR: Odds ratio; R: Random-effect model; F: Fixed-effect model.
Figure 2. Forest plot of the association between COL1A1 rs2075555 polymorphism and susceptibility to high myopia in the heterozygote model (AC vs AA) in different ethnicities.
Test of Heterogeneity
In our current Meta-analysis, significant heterogeneity was observed in 2 genetic models (CC vs AA and C vs A) (Table 3). The sources of the heterogeneity were assessed by the Galbraith plot of all included studies. Inamori et al's[19] study was identified as the main source of heterogeneity for the association between rs2075555 polymorphism and high myopia susceptibility both in the CC vs AA and the C vs A models. The pooled ORs in these 2 models were not significantly influenced when this study was removed.
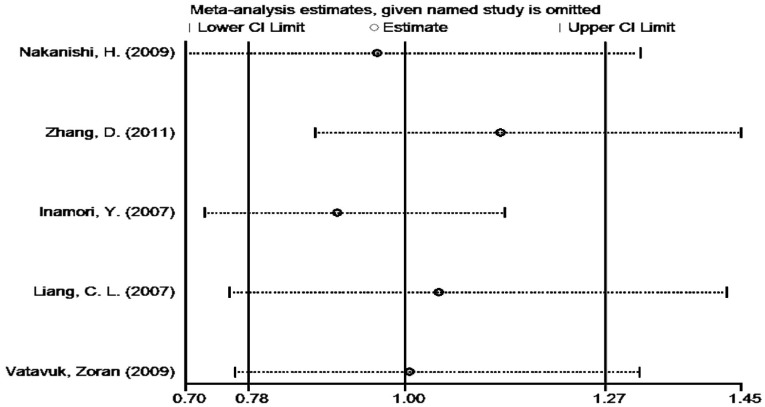
Sensitivity Analysis
A single study included in the current Meta-analysis was excluded at the time of sensitivity analysis. Similar results were revealed, and pooled ORs were not obviously altered in any of the genetic models (Figure 3) shows the sensitivity analysis results in the CA vs AA model), suggesting the reliability and stability of our results.
Figure 3. Sensitivity analysis performed to evaluate the influence of a single study on the pooled ORs in the heterozygote model (AC vs AA).
Publication Bias
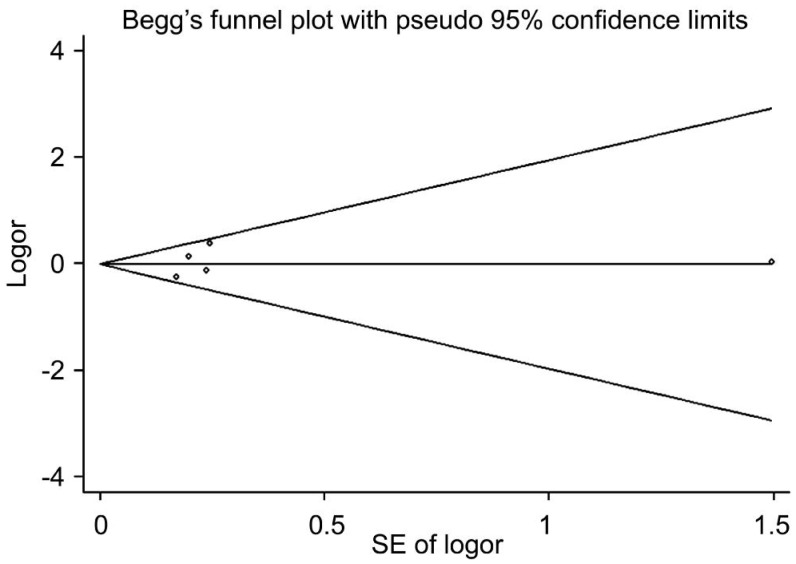
Publication bias was evaluated using Begg's funnel plot and Egger's test. As Figure 4 shows, the funnel plot shape for the heterozygote model (CA vs AA) seems approximately symmetrical, which suggests that publication bias in this study can be neglected. In addition, Egger's test provides further statistical evidence of the funnel plots' symmetry (P=0.712). Overall, neither Begg's funnel plot nor Egger's test suggested any statistically significant publication bias in any genetic model. These results indicate that there is no evidence of publication bias in our Meta-analysis.
Figure 4. Begg's funnel plot of COL1A1 rs2075555 polymorphism and high myopia susceptibility in the heterozygote model (AC vs AA).
DISCUSSION
Although the association between COL1A1 rs2075555 polymorphism and high myopia has been reported many times in several geographic locations, the results generally were conflicting rather than consistent[18],[20],[27]–[29]. Different genetic backgrounds, sources of controls, and study designs may be responsible for these inconsistencies among different studies. An individual study with a small sample size may be underpowered to evaluate the relationship between gene mutation and high myopia, but pooling all eligible data by Meta-analysis can possibly provide more powerful and credible evidence. The current Meta-analysis evaluating the possible effect of the COL1A1 rs2075555 polymorphism on high myopia susceptibility was based on a substantial amount of data from 5 individual case-control studies that included a total of 1942 high myopia cases and 2929 controls. Unfortunately, no valid association between COL1A1 rs2075555 polymorphism and high myopia was detected according to the pooled ORs in the different genetic contrast models. Population stratification is a factor that can lead to false evidence about the association between gene markers and high myopia. Previous studies have demonstrated that the prevalence of myopia varies according to ethnicity[39] and is much higher in the Asian population[40]–[41], especially in China[42]. Because of this, stratified analysis by ethnicity was performed but did not reveal a significant relation between the COL1A1 rs2075555 polymorphism and high myopia in either the Asian or European populations. The results of this study suggest that ethnicity is not a factor that influences the relationship between COL1A1 rs2075555 polymorphism and high myopia susceptibility.
A previous Meta-analysis performed by Nakanishi et al[21] showed a limited significance between COL1A1 rs2075555 polymorphism and high myopia susceptibility (OR=1.19; 95%CI 1.03-1.38, P<0.05) which is a result that is inconsistent with our findings. However, in their Meta-analysis, Nakanishi et al[21] combined data of their own with data from a previously published Japanese study that was the first reported positive association. As we know, the reported ORs in the first positive studies are usually higher than ORs reported in subsequent replication studies[43], so there was a possible correlation between COL1A1 rs2075555 polymorphism and high myopia susceptibility[19]. In addition, it is possible that publication bias affected the results in the first positive study, and the actual OR of the SNP was overestimated in their analysis, which included only 2 studies. Lastly, both of the studies included in their Meta-analysis were conducted in Japanese patients, and the sample size was too small to provide a powerful and precise estimate for COL1A1 rs2075555 polymorphism and high myopia risk. In contrast, our current Meta-analysis combined all the eligible published studies we could identify and used enhanced statistical methods to provide more reliable evidence that COL1A1 rs2075555 polymorphism does not contribute to high myopia susceptibility, even when different ethnicities are taken into consideration.
Several potential limitations of our Meta-analysis should be considered. Firstly, although a statistically significant publication bias was not found in all the genetic models, publication bias could still have occurred among the published articles meeting the inclusion criteria. Secondly, a lack of sufficient raw data such as age distribution and the sex of the patients included in the identified articles limited further exploration of potential interactions, and more precise and convincing analysis should be performed. Thirdly, all of the studies included in our study were conducted in Asians and Europeans cohorts, and, thus, conclusions from our Meta-analysis may be restricted to these two populations. However, some advantages of the current Meta-analysis can be highlighted. Firstly, based on the strict criteria of inclusion and exclusion, all the included studies were of acceptable quality, which significantly enhances the statistical power of our Meta-analysis. Secondly, we attempted to obtain adjusted estimates in different populations by performing a subgroup analysis between different ethnicities. Thirdly, no evidence of obvious publication bias was detected for any of the genetic models, which suggests that our results are unbiased and statistically reliable.
In summary, our Meta-analysis suggests that the COL1A1 rs2075555 polymorphism is not a risk factor for susceptibility to high myopia. Nevertheless, high myopia is a complex multifactorial eye disease that is influenced by both genetic and environmental factors as well as their interactions. Unfortunately, due to a lack of sufficient raw data from the included studies, interactions such as gene-gene, SNP-SNP, and gene-environment could not be evaluated. Further related research with larger sample sizes and enhanced designs are needed.
Acknowledgments
Conflicts of Interest: Jin GM, None; Zhao XJ, None; Chen AM, None; Chen YX, None; Li Q, None.
REFERENCES
- 1.He M, Xu J, Yin Q, Ellwein LB. Need and challenges of refractive correction in urban Chinese school children. Optom Vis Sci. 2005;82(4):229–234. doi: 10.1097/01.opx.0000159362.48835.16. [DOI] [PubMed] [Google Scholar]
- 2.Lam CS, Lam CH, Cheng SC, Chan LY. Prevalence of myopia among Hong Kong Chinese schoolchildren: changes over two decades. Ophthalmic Physiol Opt. 2012;32(1):17–24. doi: 10.1111/j.1475-1313.2011.00886.x. [DOI] [PubMed] [Google Scholar]
- 3.Pan CW, Ramamurthy D, Saw SM. Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol Opt. 2012;32(1):3–16. doi: 10.1111/j.1475-1313.2011.00884.x. [DOI] [PubMed] [Google Scholar]
- 4.Vitale S, Sperduto RD, Ferris FL., 3rd Increased prevalence of myopia in the United States between 1971-1972 and 1999-2004. Arch Ophthalmol. 2009;127(12):1632–1639. doi: 10.1001/archophthalmol.2009.303. [DOI] [PubMed] [Google Scholar]
- 5.Saw SM, Tong L, Chua WH, Chia KS, Koh D, Tan DT, Katz J. Incidence and progression of myopia in Singaporean school children. Invest Ophthalmol Vis Sci. 2005;46(1):51–57. doi: 10.1167/iovs.04-0565. [DOI] [PubMed] [Google Scholar]
- 6.He M, Zheng Y, Xiang F. Prevalence of myopia in urban and rural children in mainland China. Optom Vis Sci. 2009;86(1):40–44. doi: 10.1097/OPX.0b013e3181940719. [DOI] [PubMed] [Google Scholar]
- 7.Wickremasinghe S, Foster PJ, Uranchimeg D, Lee PS, Devereux JG, Alsbirk PH, Machin D, Johnson GJ, Baasanhu J. Ocular biometry and refraction in Mongolian adults. Invest Ophthalmol Vis Sci. 2004;45(3):776–783. doi: 10.1167/iovs.03-0456. [DOI] [PubMed] [Google Scholar]
- 8.Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Opt. 2005;25(5):381–391. doi: 10.1111/j.1475-1313.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 9.Buch H, Vinding T, La Cour M, Appleyard M, Jensen GB, Nielsen NV. Prevalence and causes of visual impairment and blindness among 9980 Scandinavian adults: the Copenhagen City Eye Study. Ophthalmology. 2004;111(1):53–61. doi: 10.1016/j.ophtha.2003.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Fan Q, Wojciechowski R, Kamran Ikram M, Cheng CY, Chen P, Zhou X, Pan CW, Khor CC, Tai ES, Aung T, Wong TY, Teo YY, Saw SM. Education influences the association between genetic variants and refractive error: a meta-analysis of five Singapore studies. Hum Mol Genet. 2014;23(2):546–554. doi: 10.1093/hmg/ddt431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegwart JT, Jr, Norton TT. Perspective: how might emmetropization and genetic factors produce myopia in normal eyes? Optom Vis Sci. 2011;88(3):E365–372. doi: 10.1097/OPX.0b013e31820b053d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khor CC, Fan Q, Goh L, Tan D, Young TL, Li YJ, Seielstad M, Goh DL, Saw SM. Support for TGFB1 as a susceptibility gene for high myopia in individuals of Chinese descent. Arch Ophthalmol. 2010;128(8):1081–1084. doi: 10.1001/archophthalmol.2010.149. [DOI] [PubMed] [Google Scholar]
- 13.Zhu G, Hewitt AW, Ruddle JB, Kearns LS, Brown SA, Mackinnon JR, Chen CY, Hammond CJ, Craig JE, Montgomery GW, Martin NG, Mackey DA. Genetic dissection of myopia: evidence for linkage of ocular axial length to chromosome 5q. Ophthalmology. 2008;115(6):1053–1057. doi: 10.1016/j.ophtha.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Hammond CJ, Andrew T, Mak YT, Spector TD. A susceptibility locus for myopia in the normal population is linked to the PAX6 gene region on chromosome 11: a genomewide scan of dizygotic twins. Am J Hum Genet. 2004;75(2):294–304. doi: 10.1086/423148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein AP, Duggal P, Lee KE, Klein R, Bailey-Wilson JE, Klein BE. Support for polygenic influences on ocular refractive error. Invest Ophthalmol Vis Sci. 2005;46(2):442–446. doi: 10.1167/iovs.04-0794. [DOI] [PubMed] [Google Scholar]
- 16.Morgan IG. The biological basis of myopic refractive error. Clin Exp Optom. 2003;86(5):276–288. doi: 10.1111/j.1444-0938.2003.tb03123.x. [DOI] [PubMed] [Google Scholar]
- 17.Liao X, Yang XB, Liao M, Lan CJ, Liu LQ. Association between lumican gene -1554 T/C polymorphism and high myopia in Asian population: a meta-analysis. Int J Ophthalmol. 2013;6(5):696–701. doi: 10.3980/j.issn.2222-3959.2013.05.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed I, Rasool S, Jan T, Qureshi T, Naykoo NA, Andrabi KI. TGIF1 is a potential candidate gene for high myopia in ethnic Kashmiri population. Curr Eye Res. 2013;39(3):282–290. doi: 10.3109/02713683.2013.841950. [DOI] [PubMed] [Google Scholar]
- 19.Inamori Y, Ota M, Inoko H, Okada E, Nishizaki R, Shiota T, Mok J, Oka A, Ohno S, Mizuki N. The COL1A1 gene and high myopia susceptibility in Japanese. Hum Genet. 2007;122(2):151–157. doi: 10.1007/s00439-007-0388-1. [DOI] [PubMed] [Google Scholar]
- 20.Park SH, Mok J, Joo CK. Absence of an association between lumican promoter variants and high myopia in the Korean population. Ophthalmic Genet. 2013;34(1–2):43–47. doi: 10.3109/13816810.2012.736591. [DOI] [PubMed] [Google Scholar]
- 21.Nakanishi H, Yamada R, Gotoh N, Hayashi H, Otani A, Tsujikawa A, Yamashiro K, Shimada N, Ohno-Matsui K, Mochizuki M, Saito M, Saito K, Iida T, Matsuda F, Yoshimura N. Absence of association between COL1A1 polymorphisms and high myopia in the Japanese population. Invest Ophthalmol Vis Sci. 2009;50(2):544–550. doi: 10.1167/iovs.08-2425. [DOI] [PubMed] [Google Scholar]
- 22.Pertile KK, Schache M, Islam FM, Chen CY, Dirani M, Mitchell P, Baird PN. Assessment of TGIF as a candidate gene for myopia. Invest Ophthalmol Vis Sci. 2008;49(1):49–54. doi: 10.1167/iovs.07-0896. [DOI] [PubMed] [Google Scholar]
- 23.Paluru P, Ronan SM, Heon E, Devoto M, Wildenberg SC, Scavello G, Holleschau A, Makitie O, Cole WG, King RA, Young TL. New locus for autosomal dominant high myopia maps to the long arm of chromosome 17. Invest Ophthalmol Vis Sci. 2003;44(5):1830–1836. doi: 10.1167/iovs.02-0697. [DOI] [PubMed] [Google Scholar]
- 24.Dalgleish R. The human type I collagen mutation database. Nucleic Acids Res. 1997;25(1):181–187. doi: 10.1093/nar/25.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Q, Zhao G, Xing S, Zhang L, Yang X. Role of bone morphogenetic proteins in form-deprivation myopia sclera. Mol Vis. 2011;17:647–657. [PMC free article] [PubMed] [Google Scholar]
- 26.Lin Z, Chen X, Ge J, Cui D, Wu J, Tang F, Tan J, Zhong X, Gao Q. Effects of direct intravitreal dopamine injection on sclera and retina in form-deprived myopic rabbits. J Ocul Pharmacol Ther. 2008;24(6):543–550. doi: 10.1089/jop.2008.0041. [DOI] [PubMed] [Google Scholar]
- 27.Zhang D, Shi Y, Gong B, et al. An association study of the COL1A1 gene and high myopia in a Han Chinese population. Mol Vis. 2011;17:3379–3383. [PMC free article] [PubMed] [Google Scholar]
- 28.Vatavuk Z, Skunca Herman J, Bencic G, et al. Common variant in myocilin gene is associated with high myopia in isolated population of Korcula Island, Croatia. Croat Med J. 2009;50(1):17–22. doi: 10.3325/cmj.2009.50.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang CL, Hung KS, Tsai YY, Chang WS, Wang HS, Juo SHH. Systematic assessment of the tagging polymorphisms of the COL1A1 gene for high myopia. J Hum Genet. 2007;52(4):374–377. doi: 10.1007/s10038-007-0117-6. [DOI] [PubMed] [Google Scholar]
- 30.Dou H, Ma E, Yin L, Jin Y, Wang H. The association between gene polymorphism of TCF7L2 and type 2 diabetes in Chinese Han population: a meta-analysis. PLoS One. 2013;8(3):e59495. doi: 10.1371/journal.pone.0059495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Carvalho TB, Suman M, Molina FD, Piatto VB, Maniglia JV. Relationship of obstructive sleep apnea syndrome with the 5-HT2A receptor gene in Brazilian patients. Sleep Breath. 2013;17(1):57–62. doi: 10.1007/s11325-012-0645-y. [DOI] [PubMed] [Google Scholar]
- 32.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 33.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 35.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 36.Galbraith RF. A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med. 1988;7(8):889–894. doi: 10.1002/sim.4780070807. [DOI] [PubMed] [Google Scholar]
- 37.Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37(5):1148–1157. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 39.Kempen JH, Mitchell P, Lee KE, Tielsch JM, Broman AT, Taylor HR, Ikram MK, Congdon NG, O'Colmain BJ. The prevalence of refractive errors among adults in the United States, Western Europe, and Australia. Arch Ophthalmol. 2004;122(4):495–505. doi: 10.1001/archopht.122.4.495. [DOI] [PubMed] [Google Scholar]
- 40.Sawada A, Tomidokoro A, Araie M, Iwase A, Yamamoto T. Refractive errors in an elderly Japanese population: the Tajimi study. Ophthalmology. 2008;115(2):363–370. doi: 10.1016/j.ophtha.2007.03.075. [DOI] [PubMed] [Google Scholar]
- 41.Pan CW, Wong TY, Lavanya R, Wu RY, Zheng YF, Lin XY, Mitchell P, Aung T, Saw SM. Prevalence and risk factors for refractive errors in Indians: the Singapore Indian Eye Study (SINDI) Invest Ophthalmol Vis Sci. 2011;52(6):3166–3173. doi: 10.1167/iovs.10-6210. [DOI] [PubMed] [Google Scholar]
- 42.Liu HH, Xu L, Wang YX, Wang S, You QS, Jonas JB. Prevalence and progression of myopic retinopathy in Chinese adults: the Beijing Eye Study. Ophthalmology. 2010;117(9):1763–1768. doi: 10.1016/j.ophtha.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 43.Lohmueller KE, Pearce CL, Pike M, Lander ES, Hirschhorn JN. Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet. 2003;33(2):177–182. doi: 10.1038/ng1071. [DOI] [PubMed] [Google Scholar]