Abstract
Medical records of 139 tumors from 114 dogs that underwent surgery from May 2010 through March 2015 were retrospectively reviewed. Among 114 dogs, females (64.9%) were significantly more common than males (35.1%) (p<0.05). Dogs aged 6 to 10 years were more presented than non-tumor patients, however, there was no significant difference. The mean age (±SD) was 10.3±3.0 years. Although we found no significant difference of breed predisposition, the most common breed was Maltese (19.3%), followed by Shih-Tzu (14.0%), and Yorkshire terrier (13.2%). Proportional morbidity ratios (PMRs) of mammary gland, oral cavity, and skin tumors were high in Poodles, Yorkshire terriers, and Golden retrievers, respectively. Mammary gland (36.0%) was the most common site, followed by skin and soft tissues (12.2%), oral cavity (10.8%), and digestive organs (8.6%), but there was no significant difference. The objectives of surgery were curative surgery (86.2%), biopsy (4.9%), and palliative surgery (6.5%). In this study, 123 of 139 tumors had histopathological diagnoses. Adenocarcinoma was the most common type (n=24), followed by adenoma (n=17), soft tissue sarcoma (n=13), benign mixed tumor (n=5), and others (n=64). Recurrence or suspected metastasis was identified in 26 dogs. Median survival times of malignant mammary gland tumors, skin and subcutaneous tumors, and splenic tumors were 1,563.0±1,201.7, 469, and 128 days, respectively.
Keywords: Tumor, Surgical Outcome, Dog
INTRODUCTION
As the global burden of cancer continues to increase due to the population aging and adoption of cancer-causing life-styles, numerous reports describing cancer epidemiology not only the national aspect, but also the global aspect are published each year in human medicine (1,2). Since the small animal population is also aging like the human population, neoplasia is emerging as an important cause of death in the small animal (3,4).
Cancer epidemiology can help identify risk factors in carcinogenesis (5). Also, it is possible to identify fluctuation of tumor occurrence and to find out the association of environmental factor and tumor occurrence (1,6). Many cancer registries have yielded descriptive studies on the distribution of neoplastic diseases (7,8,9,10,11). However, a marked variation between countries and different breeds has been noted (6). Therefore, tumor distribution and relative incidence rate depending on breed, age, and sex would be valuable data for veterinary medicine in Korea.
According to previous studies, tumor incidence increases with age (10). Possibly due to the high incidence of sex-specific tumors, a higher incidence in females than in males has been reported (9,11). Although a significant difference in breed-related incidence has been found in many studies, marked variations between the geographic areas where the studies were performed have been noted (6). According to a previous retrospective investigation of skin and mammary tumors in Korea, Yorkshire terrier and Maltese were the most common breeds (12,13).
Tumors are usually classified by tumor site and histopathological diagnosis. The most common site of tumor development is also influenced by the region and the period. Although the most of studies reported that the skin and soft tissues and the mammary gland were most commonly observed tumors (7,8,11,14), a decreased incidence of mammary gland tumors and an increased incidence of lymphoma have been observed in other study (9). With respect to histopathology, lipoma, adenoma, mast cell tumors, and histiocytoma have been reported as the common tumor types (8,11).
For surgeons and clients, it is difficult to make decision of tumor surgery, especially for the geriatric patients who have underlying diseases like heart failure which threaten patient's life under the anesthesia. With the tumor easily visualized, fine needle aspiration (FNA) can be performed, and approximate differential diagnosis can be made. Using this information, surgeons can discuss with clients about suspected prognosis. However, according to anatomic sites, FNA may be hard to perform without anesthesia and can be a risky procedure with malignant tumor cell seeding. Therefore, knowledge of cancer epidemiology can be valuable information for surgeons to make surgical decision and discuss with client.
In Korea, there have been studies on the relative prevalence and distribution of canine skin and mammary tumors (12,13). However, there have been no studies on spontaneous tumors in client-owned dogs, especially surgically treated tumors, in Korea. Therefore, to identify the occurrence pattern of tumor and association with the pattern and surgery, we provide an overview of the tumor epidemiology managed with surgery in Korea and its clinical outcomes.
MATERIALS AND METHODS
Case selection
The medical records of 139 tumors from 114 patients that underwent surgery at the Veterinary Medical Teaching Hospital of Konkuk University from May 2010 through March 2015 were retrospectively reviewed.
Inclusion criteria
All 114 patients had tumors treated surgically. Data obtained from medical records included the followings: patient history (Initial or recurrence of tumor), signalment (age, sex, breed), anatomical site of tumor, modality of treatment (surgery, chemotherapy), histopathological diagnosis, survival time, and recurrences and metastasis.
Signalment and physical examination
The age, sex, reproductive status, and breed of patients were recorded. These data were compared with the data of other patients presented with non-tumor diseases. Patients were divided into five non-overlapping age groups (<2 years, 2 to <6 years, 6 to <10 years, 10 to <14 years, and >14 years). Complete physical examinations (such as palpation of enlarged superficial lymph nodes, and tumor size, number, and location if visualized) were done in all patients.
Diagnostic imaging
Three-view thoracic radiographs and abdominal radiographs were obtained to evaluate distant metastasis. If necessary, additional investigations, such as computed tomography (CT) or magnetic resonance imaging, were done with internal organ tumors.
Surgical treatment
Surgical removal of the tumors was performed under general anesthesia and perioperative pain control carried out. Client did informed consent to the surgery. Patients submitted for tumor treatment were stabilized with supportive treatment if necessary. Surgical procedures were performed for various objectives (e.g., for diagnosis, cure, and palliation) depending on tumor size and site. Incisional or excisional biopsy was performed in case of diagnostic surgery. Depending on the resection of tumor margins, intracapsular, marginal, wide, and radical surgeries were categorized as curative surgeries. In the intact patients with sex-specific tumors, sterilization was also performed with tumor resection to prevent recurrence of tumor. Postoperatively, according to histopathological diagnosis, chemotherapy was performed as an adjuvant therapy.
Histopathological examination
The surgically removed tumors were submitted for histopathological examination to the Institute for Veterinary Pathology of Konkuk University, Seoul, Korea. Immunohistochemistry was also performed to characterize poorly differentiated neoplasm. Cancer cases were classified according to their primary site and recorded according to the International Classification of Diseases for Oncology 3rd edition (15) to facilitate comparisons with existing animal registries.
Survival
Follow-up evaluations were recommended every 1~2 months in the initial 6 months or earlier if the owner observed clinical signs related to the tumor. Follow-up evaluations to detect local recurrence and metastasis of tumors were based on thoracic and abdominal radiography, ultrasonography, and CT, if necessary. Survival time was calculated from the date of surgical removal of the tumor to the date of death or the last date of follow-up. Survival analysis was done in patients performed the surgical resection of tumor except the patients undergone the chemotherapy.
Statistical analysis
The Pearson chi-square test was used to evaluate the relative distribution of tumor incidence across age groups. Within breeds, the Fisher exact test was applied to identify the relative incidence rates of all tumors. For all analyses, values less than 0.05 were considered significant. Commercial statistical software (IBM® SPSS® Statistics ver. 21.1; IBM, USA) was used to complete all data analyses. To quantify cancer occurrence, the proportional morbidity ratio (PMR) was used. The PMR for a specific tumor type for each breed was calculated as the incidence rate of the specific tumor type within breed (A) divided by the incidence rate of the specific tumor type within all other breeds (B); that is:
The Kaplan-Meier method of survival function estimation was used to calculate overall survival time. The data from the dogs that died of causes unrelated to their malignant tumors and from those that were alive at the end of the study were considered as censored data. Differences in survival distribution were compared using the log-rank test.
RESULTS
Age
The mean age at diagnosis was 10.3 years (SD, 3.0; range 3~18 years), and it was higher for malignant (10.6 years) than for benign (9.9 years) neoplasms (Table I). The age-specific tumor incidence rate was relatively low in younger animals, but it increased dramatically after the age of 6 to a peak in dogs aged 10 years and then decreased in animals older than this. Although we found no significant difference, tumor incidence rate was higher in the 6 to <10 years patients group and the 10 to <14 years group of tumor patients than non-tumor patients.
Table I. The signalments of the patients with tumor.
| Variables | Tumor patients | Non-tumor patients | ||
|---|---|---|---|---|
| Number | Percent (%) | Number | Percent (%) | |
| Age | ||||
| <2 years | 0 | 0 | 120 | 12.9 |
| 2~6 years | 5 | 4.4 | 125 | 13.5 |
| 6~10 years | 38 | 33.3 | 194 | 20.9 |
| 10~14 years | 54 | 47.4 | 351 | 37.8 |
| >14 years | 16 | 14.0 | 136 | 14.7 |
| Unknown | 1 | 0.9 | 2 | 0.2 |
| Sex | ||||
| Female* | 74 | 64.9 | 494 | 53.2 |
| Intact female | 47 | 41.2 | 271 | 29.2 |
| Spayed female | 27 | 23.7 | 223 | 24.0 |
| Male | 40 | 35.1 | 435 | 46.8 |
| Castrated male | 26 | 22.8 | 278 | 29.9 |
| Intact male | 14 | 12.3 | 157 | 16.9 |
| Breed | ||||
| Maltese | 22 | 19.3 | 147 | 15.8 |
| Shih-tzu | 15 | 13.2 | 131 | 14.1 |
| Yorkshire terrier | 16 | 14.0 | 81 | 8.7 |
| Cocker spaniel | 11 | 9.6 | 66 | 7.1 |
| Mongrel | 10 | 8.8 | 67 | 7.2 |
| Other breeds | 40 | 35.1 | 437 | 47.1 |
Differences for analysis are considered to be significany at *p<0.05.
Other breeds: Poodle, Schnauzer, Golden retriever, Alaskan malamute, Labrador retriever, Jindo, Doberman pinscher, American pitbull terrier, Beagle, Chihuahua, Collie, Dachshund, Flat coated retriever, Miniature pinsher, Pug, Setter, Siberian husky.
Breed
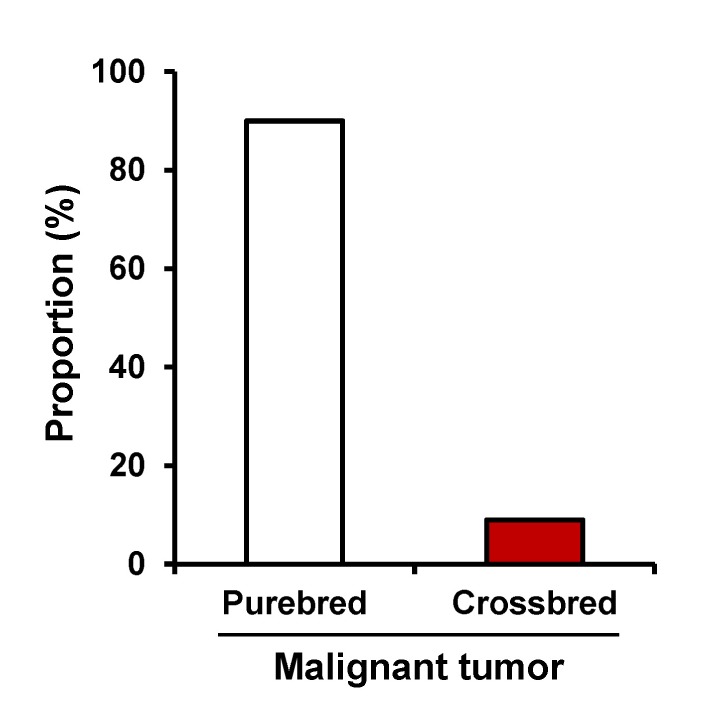
Tumors were diagnosed in 22 breeds, including mixedbreed dogs. Maltese (19.3%) were most frequent, followed by Shih-Tzu (14.0%), Yorkshire terrier (13.2%), Cocker spaniel (9.6%), and Mixed-breed (8.8%) (Table I). However, there was no significant difference in breed distribution. Other breeds (35.1%) consisted of the following: Poodle, Schnauzer, Golden retriever, Alaskan malamute, Labrador retriever, Jindo, Doberman pinscher, American Pitbull terrier, Beagle, Chihuahua, Collie, Dachshund, Flat-coated retriever, Miniature pinscher, Setter, and Siberian husky. Among the patients with malignant tumor, purebred constituted 91.4% of the tumor patients, while crossbred constituted 8.6% (Fig. 1).
Figure 1. The distribution of purebred and crossbred in the malignant tumor patients.
Sex
In the sex distribution of the tumor patients, 64.9% were female and 35.1% were male (Table I). The female patient group had a significantly higher incidence than the male group (p<0.05). The ratio of male to female dogs in the study was 0.541; after the exclusion of sex-specific neoplasms, the ratio was 0.95. In addition, while the incidence of all tumors was not highly influenced by neutering status, the incidence of sex-specific tumors was significantly influenced by neutering status (p<0.001).
Tumor distribution
Histopathological diagnosis of tumors
The histopathologic diagnosis was done in 123 (88.5%) specimens of total 139 tumor specimens. Seventy-two (51.8%) specimens were malignant with the benign tumors of 51 (36.7%) specimens (Table II). Adenoma was the most common tumor within benign tumors, followed by epithelioma and benign mixed tumors. In the case of malignant tumors, the most frequently observed tumor was adenocarcinoma, followed by soft tissue sarcoma, mast cell tumor, malignant melanoma, and osteosarcoma. While adenoma and benign mixed tumors were virtually exclusive to the mammary glands and skin and soft tissues, adenocarcinoma was found in the mammary glands, digestive organs, and respiratory systems.
Table II. The histopathologic diagnosis of the patients with tumor.
| Type of neoplasm | No. | % |
|---|---|---|
| Benign | 36.7 | |
| Adenoma | 17 | 33.3 |
| Epithelioma | 6 | 11.8 |
| Benign mixed tumor | 5 | 9.8 |
| Others | 23 | 45.1 |
| Subtotal | 51 | 100 |
| Malignant | 51.8 | |
| Adenocarcinoma | 24 | 33.4 |
| Soft tissue sarcoma | 13 | 18.1 |
| Mast cell tumor | 5 | 6.9 |
| Malignant melanoma | 5 | 6.9 |
| Osteosarcoma | 5 | 6.9 |
| Others | 20 | 27.8 |
| Subtotal | 72 | 100 |
| Not examined | 16 | 11.5 |
| Total | 139 | 100 |
Site of tumor development
The incidence rates of neoplasia, categorized by site, are shown in Fig. 2. The majority of the neoplasms in females were found in the mammary gland (51.5%) and in the skin (9.3%). Similarly, the majority of the neoplasms in males were found in the skin (19.0%), oral cavity (16.7%), and digestive organ (16.7%). As shown in Table III, the histopathologic diagnosis of tumor was classified by the sites of tumor development. In all, 67 different histopathologic types of tumor were identified. While the majority of tumors in the digestive and respiratory system were malignant, the majority of tumors in the ocular system were benign. In PMRs of breeds, marked differences were found as shown in Table IV. In mammary gland tumor, Poodles had PMR slightly over 2 which indicating that this breed developed mammary gland tumors about double times than other breeds. Likewise, PMR of Golden retrievers in skin tumor was more than 5 and Yorkshire terrier had PMR about 2.5.
Figure 2. The site of tumor development in the male and female patients.
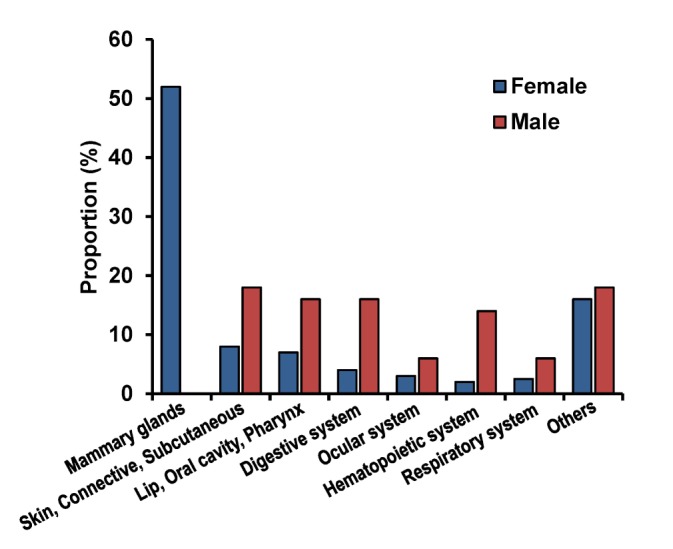
Table III. The histopathologic diagnosis classified by the sites of tumor development.
| ICD-O | Site of tumor development | Benign | Percent (%) | Malignant | Percent (%) |
|---|---|---|---|---|---|
| 000-14 | Lip, Oral cavity, Pharynx | Peripheral odontogenic fibroma | 25 | Malignant melanoma | 41.7 |
| Acanthomatous ameloblastoma | 16.7 | Squamous cell carcinoma | 8.3 | ||
| Epulis | 8.3 | ||||
| 15-26 | Digestive organs | Perianal gland epithelioma | 20 | Gastric adenocarcinoma | 10 |
| Perianal sebaceous adenoma | 10 | Hepatocellular carcinoma | 10 | ||
| Cholangiocarcinoma | 10 | ||||
| Small intestinal adenocarcinoma | 10 | ||||
| Perianal sebaceous adenocarcinoma | 10 | ||||
| Apocrine gland adenocarcinoma of anal sac | 10 | ||||
| Extraskeletal osteosarcoma | 10 | ||||
| 30-39 | Respiratory system | Nasal transitional carcinoma | 20 | ||
| Lung adenosquamous carcinoma | 40 | ||||
| Lung Adenocarcinoma | 40 | ||||
| 51-58 | Female genital | Leimyoma | 20 | Uterine carcinoma | 20 |
| Organs | Fibroma | 20 | Ovarian adenocarcinoma | 20 | |
| Vaginal polyp | 20 | ||||
| 50 | Mammary gland | Simple adenoma | 27.3 | Adenocarcinoma | 34.1 |
| Complex adenoma | 2.3 | Ductal carcinoma | 2.3 | ||
| Benign mixed tumor | 11.3 | Squamous cell carcinoma | 2.3 | ||
| Ductal adenoma | 2.3 | Liposarcoma | 2.3 | ||
| Lipoma | 4.5 | Anaplastic sarcoma | 2.3 | ||
| Osteosarcoma | 2.3 | ||||
| Carcinosarcoma | 4.5 | ||||
| Intraductal complex carcinoma | 2.3 | ||||
| 60-63 | Male genital organs | Seminoma | 50 | Sertoli cell tumor | 50 |
| 64-68 | Urinary tract | Transitional cell carcinoma | 50 | ||
| Renal cell carcinoma | 50 | ||||
| 42 | Spleen | Hemangioma | 14.3 | Hemangiosarcoma | 42.8 |
| Fibrosarcoma | 28.6 | ||||
| Malignant fibrous histiocytoma | 14.3 | ||||
| 69-72 | Ocular system | Sebaceous gland adenoma | 20 | ||
| Meibomian gland epithelioma | 20 | ||||
| Squamous papilloma | 20 | ||||
| Meibomian gland adenoma | 20 | ||||
| Uveal melanocytoma | 20 | ||||
| 44, 18 | Skin, Subcutaneous, | Lipoma | 11.7 | Basal cell carcinoma | 5.9 |
| Connective tissue | Sebaceous epithelioma | 17.6 | Aural squamous cell carcinoma | 5.9 | |
| Collagen nevus | 5.9 | Ceruminous gland adenocarcinoma | 5.9 | ||
| Mast cell tumor (Stage 2) | 5.9 | Mast cell tumor (Stage 3) | 29.4 | ||
| Ceruminous gland adenoma | 5.9 | ||||
| Ear polyp | 5.9 | ||||
| 40-41 | Bone | Osteosarcoma | 60 | ||
| Fibrosarcoma | 20 | ||||
| 75.4 | Carotid body | Carotid body tumor | 100 | ||
| 47 | Peripheral nerves and autonomic system | Hemangiopericytoma | 100 | ||
| 45 | Mesothelioma | Mesothelioma | 100 | ||
| 48 | Retroperitoneum | Hemangiosarcoma | 100 | ||
| 49 | Other soft tissue sarcoma | Fibrosarcoma | 50 | ||
| Rhabdomyosarcoma | 50 | ||||
| 81-96 | Malignant neoplasms of lymphoid, hematopoietic and related tissue | Lymphoma | 100 |
ICD-O, International Classification of Diseases for Oncology.
Table IV. The Proportional Morbidity Ratio of breeds in specific tumor site.
| Breeds | Mammary gland tumor | Skin tumor | Oral cavity tumor |
|---|---|---|---|
| Maltese | 1.111 | 0.870 | 1.111 |
| Yorkshire Terrier | 0.617 | 0.403 | 2.572 |
| Shih-tzu | 0.521 | 0.906 | - |
| Cocker spaniel | 1.389 | 1.610 | - |
| Mongrel | 0.833 | 1.449 | 1.852 |
| Poodle | 2.315 | 0.604 | - |
| Alaskan malamute | 0.556 | - | - |
| Golden Retriever | - | 5.435 | - |
Management of tumors
One-hundred twenty-three surgeries were performed in 114 patients with more than one tumor for the purposes of diagnosis, resection for cure, and palliation of symptoms. Most patients (106, 86.2%) received surgery for cure. Six patients (4.9%) received biopsy for diagnosis, and eight patients (6.5%) received surgery for palliation of symptoms. Concurrent sterilization with tumor resection was performed in 28 patients. After the surgery, chemotherapy was performed in four patients (3.5%).
Surgery
The surgical procedures included biopsy for diagnosis, resection for cure, and palliation of symptoms. Among the curative surgeries, wide resection (44, 41.5%) was the most common, followed by radical surgery (33, 31.1%), and marginal resection (29, 27.4%). Sterilization with tumor resection for intact patients was performed to prevent the recurrence of sex-specific tumor such as tumors of mammary gland, vagina, and perianal gland.
Chemotherapy
Four patients with three mast cell tumors and one fibrosarcoma were treated by chemotherapy after the surgery. Three patients with mast cell tumors had received treatments, including surgery and the administration of vinblastine and imatinib mesylate, and these patients received excisional biopsy, incisional biopsy, and radical limb excision, respectively. One patient with intrapelvic fibrosarcoma received carboplatin, ifosfamide, cyclophosphamide, and doxorubicin after palliative surgery.
Survival
Of all patients, 54% follow-up evaluations were not available. Twenty-two percent of patients had died because of malignant tumors, including squamous cell carcinoma, uterine carcinoma, osteosarcoma, hepatocellular carcinoma, nasal transitional cell carcinoma, malignant melanoma, and malignant fibrous histiocytoma. Roughly 5% died because of causes unrelated to the tumor, including chronic renal failure and postoperative shock, while 19% were alive at the end of the study. Recurrence or suspected metastasis was identified in 26 dogs. The recurrence or metastasis rate was identified in alimentary tract tumor (33%), followed by mammary gland tumor (26.5%), tumors of hematopoietic system (25%), and bone tumor (20%) (Table V).
Table V. The recurrence or suspected metastasis of tumor in the patients.
| Site of tumor development | Recurrence or suspected metastasis (%) |
|---|---|
| Alimentary tract tumor | 33 |
| Mammary gland tumor | 26.5 |
| Hematopoietic system tumor | 25 |
| Bone tumor | 20 |
The survival rate and recurrence or suspected metastasis rate of patients undergone curative surgery was assessed (Table VI). The patients lost during follow-ups were excluded, and the survival rate was calculated from the date of surgery. While the survival rate of patients with wide surgical margin was decreased to 45.5 percent, it was maintained 100 percent in the patients with marginal surgical margin. The half of the patients with radical surgical margin died at 6 months after the surgery. To find out factors that influence the survival rate, the site and histopathological diagnosis of tumor were classified as shown in Table VII.
Table VI. The survival rates of 6, 9, 12 months and local recurrence or suspected metastasis rate according to the surgical margin.
| Surgical margin | No. | 6 months survival rate (%) | 9 months survival rate (%) | 12 months survival rate (%) | Local recurrence or suspected metastasis rate (%) |
|---|---|---|---|---|---|
| Marginal | 3 | 100 | 100 | 100 | 67 |
| Wide | 11 | 72.7 | 63.6 | 45.5 | 45.5 |
| Radical | 7 | 50 | 50 | 33 | 67 |
Table VII. The histopathologic diagnosis and site of tumors classified according to the surgical margin.
| Surgical margin | Histopathologic diagnosis of tumors | ICD-O | Site of tumor |
|---|---|---|---|
| Marginal | Perianal sebaceous adenocarcinoma | 44.5 | Perianal |
| Fibrosarcoma | 40-41 | Bone | |
| Rhabdomyosarcoma | 49 | Thoracic wall | |
| Wide | Extraskeletal osteosarcoma | 17.1 | Small intestine |
| Malignant melanoma | 000-14 | Oral cavity | |
| Squamous cell carcinoma | 000-14 | Oral cavity | |
| Intraductal complex carcinoma | 50 | Mammary gland | |
| Adenocarcinoma | 50 | Mammary gland | |
| Ductal carcinoma | 50 | Mammary gland | |
| Radical | Hepatocellular carcinoma | 22 | Liver |
| Malignant fibrous histiocytoma | 42.2 | Spleen | |
| Hemangiosarcoma | 42.2 | Spleen | |
| Osteosarcoma | 40-41 | Bone | |
| Mammary gland adenocarcinoma | 50 | Mammary gland | |
| Mast cell tumor | 44 | Skin (Limb) |
ICD-O, International Classification of Diseases for Oncology.
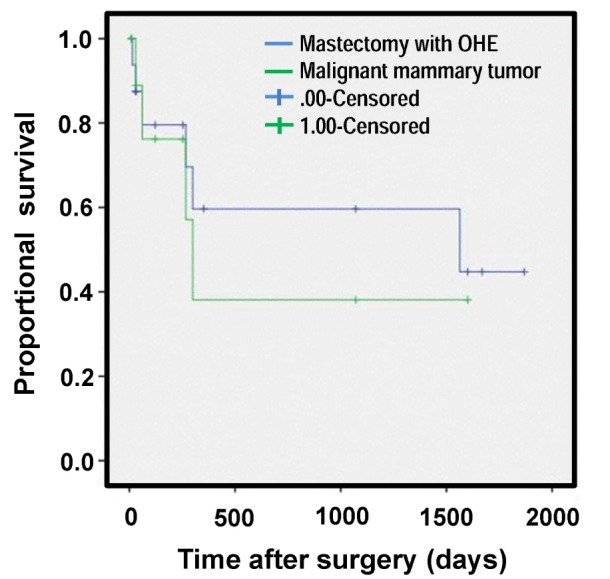
Data regarding the survival of dogs with mammary gland tumors after the surgery are presented in Fig. 3. Median survival times of malignant mammary gland tumor, skin and subcutaneous tumor, and splenic tumor were 1,563.0±1,201.7, 469, and 128 days, respectively.
Figure 3. Survival curve of the malignant mammary tumor. OHE, Ovariohysterectomy.
DISCUSSION
The findings about the tumor incidence between the sexes were similar to those of previous studies (9,11,16). It was significantly higher in female dogs than male dogs (p< 0.01). The fact that the male: female ratio was close to 1 after the sex-specific tumors were excluded may have been because of the sex-specific neoplasms, such as mammary gland tumors. The association between the incidence rate of mammary gland tumors and neutering was significantly high (p<0.01), while there was no significant difference in the association with the incidence of overall tumors. This finding was consistent with the previous studies (14).
Although making comparisons with the earlier studies performed around the world can be risky because of the different age structure of the population, age distribution was similar to that of previous studies (9,11). The mean age at diagnosis for benign tumors was lower than that for malignant tumors. This might be because a large proportion of the tumors were mammary gland tumors, which the malignancy of tumor has been reported to increase with aging (17).
The most frequently presented breeds were Maltese, Shih-Tzu, and Yorkshire terrier. These breeds were also commonly observed breeds in another study in Korea, and this may be mainly because these are the major populations in Korea (13). Because the study was conducted at a teaching hospital, these data did not represent the entire canine population in Korea. Therefore, to quantify cancer occurrence, the PMRs were used. Higher PMRs for mammary gland tumor, skin tumor, and oral cavity tumor were found in Poodles, Golden retrievers, and Yorkshire terriers, respectively.
The predisposition to mammary gland tumors have been found in several breeds, including Poodles, Chihuahuas, Dachshunds, Yorkshire terriers, Maltese, and Cocker spaniels (7,18,19). Like BRCA1 gene and BRCA2 genes in women breast cancer, studies on the English springer spaniels have proved that the same genes influence mammary cancer development (20). As the findings of studies performed in the U.S. and Europe are consistent with our own, the high occurrence of mammary gland tumors in Poodles may not be because of the different population structure but because of the fact that Poodles are influenced by genetic factors.
Similar to the studies from northern Italy, Tulsa (US), and California (US), the two most frequent tumor locations in female patients were mammary glands and skin and subcutaneous tissues (7,10,14). For male patients, skin, oral cavity, and digestive tissues were the most common tumor sites. Because the tumors of mammary gland, genital organs, and skin and subcutaneous tissues are easier to recognize by physical examination than the tumors of other internal organs that require specific examinations, these tumors may be easily found and overrepresented (10). According to the recent studies, skin tumors are more prevalent than mammary gland tumors when compared with the past studies (8,11). This may be attributable to the increasing frequency of spaying at a young age.
Adenoma and epithelioma comprised a large proportion of benign tumors in the present study. This finding with adenoma is similar to earlier observations (8,11), but different frequencies for the lipoma and the cutaneous histiocytoma were found, which have been cited as commonly observed tumors in many studies (8,11,12,13). The cutaneous histiocytoma is a benign tumor that undergoes spontaneous regression and, with respect to lipoma, marginal resection is recommended only when tumors interfere with normal function (21,22). Because only cases verified using histology were included in this study, and lipoma and cutaneous histiocytoma generally do not require surgery, they might have been underrepresented.
The most commonly observed malignant tumor types were adenocarcinoma and soft tissue sarcomas. The major proportional trends in terms of tumor types were quite similar to previous studies except for lymphosarcoma (11,16). Most patients with lymphosarcoma have multicentric diseases. Therefore they require systemic chemotherapy to effectively treat their disease (23). Because only surgically managed patients were involved in the study, lymphosarcoma might have been underrepresented.
Due to the small size of patients with complete follow-up, only tendency of outcomes from curative surgeries was investigated. According to the results of the study, no propensity of the recurrence rate classified by the surgery type was identified, and the wider the margin of surgery, the lower the survival rate of 6 and 12 months. While behavior of tumor excised by marginal surgery tends to progress slowly and present relatively low rate of metastasis, most of the tumors undertaken radical and wide surgery were highly malignant with aggressive biological behavior and rapid and widespread metastasis such as hemangiosarcoma, osteosarcoma, and splenic fibrous histiocytoma (24,25,26,27). Generally, surgical margin for curative surgery is determined by the propensity for local recurrence and microscopic metastasis (28). Considering that, the results of our study may be explained by tendency to choose surgeries of extensive margins with aggressive tumors. Also, there are a lot of reports describing micrometastasis (29,30,31). The intra-abdominal tumors can metastasize through the vessels, lymphatics, and peritoneal surface (30). Because of this, metastasis can occur in patients undergone tumor resection for cure (31). In human medicine, many reports describing shedding of malignant tumor cells into the blood according to surgical stress especially colorectal tumors were published, but there are still controversies (29,31,32). By contrast, animal studies have reported a lot of evidence about the tumor cells in the blood (33,34,35). Micrometastasis may more often develop with the tumors within the abdomen because of the relatively richer blood supply and lymphatics than external organs such as skin. Since the majority of tumors undertaken wide or radical surgery were tumors within the abdomen, the results of study may be influenced by stress from surgical manipulation and anatomic condition.
According to the previous studies, OHE before or at the time of mastectomy is not a prognostic factor in dogs with established mammary gland tumors (36,37). Contrary to these studies, a recent study suggested that OHE up to two years before mastectomy significantly influences survival time (38). Our findings also support that OHE may influence the survival time like the recent study.
Identifying the incidence rate and patterns of specific breeds, ages, and sex groups within specific geographic areas may help veterinary clinicians because the data are highly influenced by geographic areas (6). Although incidence is the most useful statistic for occurrence, the accurately calculated population at risk is needed to find out valid incidence (22). In this respect, this study has some limitations because the study was conducted in a teaching hospital, so the population might have been biased. Therefore, further investigations based on larger population will be needed to increase the accuracy of the population size assessment.
In this study, we focused on canine spontaneous tumors treated surgically and investigated the outcome from clinical perspective. The data obtained from this study may be also very useful for the translation study in human cancer field in future.
Abbreviations
- CT
computed tomography
- FNA
fine needle aspiration
- ICD-0
international classification of diseases for oncology
- OHE
ovariohysterectomy
- PMR
proportional morbidity ratio
Footnotes
CONFLICTS OF INTEREST: The authors have no financial conflict of interest.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Fleming JM, Creevy KE, Promislow DE. Mortality in North American dogs from 1984 to 2004: An investigation into age-, size-, and breed-related causes of death. J Vet Intern Med. 2011;25:187–198. doi: 10.1111/j.1939-1676.2011.0695.x. [DOI] [PubMed] [Google Scholar]
- 4.Inoue M, Hasegawa A, Hosoi Y, Sugiura K. A current life table and causes of death for insured dogs in Japan. Prev Vet Med. 2015;120:210–218. doi: 10.1016/j.prevetmed.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Misdorp W. Veterinary cancer epidemiology. Vet Q. 1996;18:32–36. doi: 10.1080/01652176.1996.9694610. [DOI] [PubMed] [Google Scholar]
- 6.Bronden LB, Flagstad A, Kristensen AT. Veterinary cancer registries in companion animal cancer: a review. Vet Comp Oncol. 2007;5:133–144. doi: 10.1111/j.1476-5829.2007.00126.x. [DOI] [PubMed] [Google Scholar]
- 7.MacVean DW, Monlux AW, Anderson PS, Jr, Silberg SL, Roszel JF. Frequency of canine and feline tumors in a defined population. Vet Pathol. 1978;15:700–715. doi: 10.1177/030098587801500602. [DOI] [PubMed] [Google Scholar]
- 8.Dobson JM, Samuel S, Milstein H, Rogers K, Wood JL. Canine neoplasia in the UK: estimates of incidence rates from a population of insured dogs. J Small Anim Pract. 2002;43:240–246. doi: 10.1111/j.1748-5827.2002.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 9.Merlo DF, Rossi L, Pellegrino C, Ceppi M, Cardellino U, Capurro C, Ratto A, Sambucco PL, Sestito V, Tanara G, Bocchini V. Cancer incidence in pet dogs: Findings of the animal tumor registry of Genoa, Italy. J Vet Intern Med. 2008;22:976–984. doi: 10.1111/j.1939-1676.2008.0133.x. [DOI] [PubMed] [Google Scholar]
- 10.Vascellari M, Baioni E, Ru G, Carminato A, Mutinelli F. Animal tumour registry of two provinces in northern Italy: Incidence of spontaneous tumours in dogs and cats. BMC Vet Res. 2009;5:39. doi: 10.1186/1746-6148-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bronden LB, Nielsen SS, Toft N, Kristensen AT. Data from the danish veterinary cancer registry on the occurrence and distribution of neoplasms in dogs in Denmark. Vet Rec. 2010;166:586–590. doi: 10.1136/vr.b4808. [DOI] [PubMed] [Google Scholar]
- 12.Pakhrin B, Kang MS, Bae IH, Park MS, Jee H, You MH, Kim JH, Yoon BI, Choi YK, Kim DY. Retrospective study of canine cutaneous tumors in Korea. J Vet Sci. 2007;8:229–236. doi: 10.4142/jvs.2007.8.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YH, Ahn NK, Roh IS, Yoon BI, Han JH. Retrospective investigation of canine skin and mammary tumors in Korea. J Vet Clin. 2009;26:556–562. [Google Scholar]
- 14.Dorn CR, Taylor DO, Schneider R, Hibbard HH, Klauber MR. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. II. Cancer morbidity in dogs and cats from Alameda County. J Natl Cancer Inst. 1968;40:307–318. [PubMed] [Google Scholar]
- 15.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin D, Whelan S. In: International Classification of Diseases for Oncology (ICD-O) 3rd ed. Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin D, Whelan S, editors. Geneva: World Health Organization; 2013. pp. 1–242. [Google Scholar]
- 16.Cohen D, Reif JS, Brodey RS, Keiser H. Epidemiological analysis of the most prevalent sites and types of canine neoplasia observed in a veterinary hospital. Cancer Res. 1974;34:2859–2868. [PubMed] [Google Scholar]
- 17.Mitchell L, De la Iglesia FA, Wenkoff MS, Van Dreumel AA, Lumb G. Mammary tumors in dogs: survey of clinical and pathological characteristics. Can Vet J. 1974;15:131–138. [PMC free article] [PubMed] [Google Scholar]
- 18.Moe L. Population-based incidence of mammary tumours in some dog breeds. J Reprod Fertil Suppl. 2001;57:439–443. [PubMed] [Google Scholar]
- 19.Todorova I. Prevalence and etiology of the most common malignant tumours in dogs and cats. Bulg J Vet Med. 2006;9:85–98. [Google Scholar]
- 20.Rivera P, Melin M, Biagi T, Fall T, Haggstrom J, Lindblad-Toh K, von Euler H. Mammary tumor development in dogs is associated with BRCA1 and BRCA2. Cancer Res. 2009;69:8770–8774. doi: 10.1158/0008-5472.CAN-09-1725. [DOI] [PubMed] [Google Scholar]
- 21.Pires I, Alves A, Queiroga FL, Silva F, Lopes C. Regression of canine cutaneous histiocytoma: reduced proliferation or increased apoptosis? Anticancer Res. 2013;33:1397–1400. [PubMed] [Google Scholar]
- 22.Butler M, Bonnett N, Page L. Epidemiology and the evidence-based medicine approach. In: Withrow SJ, Vail DM, Page RL, editors. Withrow & MacEwen's Small Animal Clinical Oncology. 5th ed. St. Louis, Missouri: Elsevier saunders; 2013. pp. 68–82. [Google Scholar]
- 23.Ettinger SN. Principles of treatment for canine lymphoma. Clin Tech Small Anim Pract. 2003;18:92–97. doi: 10.1053/svms.2003.36622. [DOI] [PubMed] [Google Scholar]
- 24.Spodnick GJ, Berg J, Rand WM, Schelling SH, Couto G, Harvey HJ, Henderson RA, MacEwen G, Mauldin N, McCaw DL, Moore AS, Morrison W, Norris AM, O'Bradovich J, O'Keefe DA, Page R, Ruslander D, Klausner J, Straw RC, Thompson JP. Prognosis for dogs with appendicular osteosarcoma treated by amputation alone: 162 cases (1978-1988) J Am Vet Med Assoc. 1992;200:995–999. [PubMed] [Google Scholar]
- 25.Kuntz CA, Dernell WS, Powers BE, Devitt C, Straw RC, Withrow SJ. Prognostic factors for surgical treatment of soft-tissue sarcomas in dogs: 75 cases (1986-1996) J Am Vet Med Assoc. 1997;211:1147–1151. [PubMed] [Google Scholar]
- 26.Spangler WL, Kass PH. Pathologic factors affecting postsplenectomy survival in dogs. J Vet Intern Med. 1997;11:166–171. doi: 10.1111/j.1939-1676.1997.tb00085.x. [DOI] [PubMed] [Google Scholar]
- 27.Wood CA, Moore AS, Gliatto JM, Ablin LA, Berg RJ, Rand WM. Prognosis for dogs with stage I or II splenic hemangiosarcoma treated by splenectomy alone: 32 cases (1991-1993) J Am Anim Hosp Assoc. 1998;34:417–421. doi: 10.5326/15473317-34-5-417. [DOI] [PubMed] [Google Scholar]
- 28.Jermyn K, Duncan B, Lascelles X. Principles of oncological surgery. In: Dobson M, Lascelles D, editors. BSAVA Manual of Canine and Feline Oncology. 3rd ed. Quedgeley, Gloucester: BSAVA; 2011. pp. 44–59. [Google Scholar]
- 29.Moore GE, Sandberg A, Schubarg JR. Clinical and experimental observations of the occurrence and fate of tumor cells in the blood stream. Ann Surg. 1957;146:580–587. doi: 10.1097/00000658-195710000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugarbaker PH. Overview of peritoneal carcinomatosis. Cancerologia. 2008;3:119–124. [Google Scholar]
- 31.Park SY, Choi GS, Park JS, Kim HJ, Ryuk JP, Choi WH. Influence of surgical manipulation and surgical modality on the molecular detection of circulating tumor cells from colorectal cancer. J Korean Surg Soc. 2012;82:356–364. doi: 10.4174/jkss.2012.82.6.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atkin G, Chopada A, Mitchell I. Colorectal cancer metastasis: in the surgeon's hands? Int Semin Surg Oncol. 2005;2:5. doi: 10.1186/1477-7800-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romsdahl MM, McGrath RG, Hoppe E, McGrew EA. Experimental model for the study of tumor cells in the blood. Acta Cytol. 1965;9:141–145. [PubMed] [Google Scholar]
- 34.Nishizaki T, Matsumata T, Kanematsu T, Yasuaga C, Sugimachi K. Surgical manipulation of VX2 carcinoma in the rabbit liver evokes enhancement of metastasis. J Surg Res. 1990;49:92–97. doi: 10.1016/0022-4804(90)90116-j. [DOI] [PubMed] [Google Scholar]
- 35.Abgral R, Valette G, Robin P, Rousset J, Keromnes N, Le Roux PY, Marianowski R, Salaun PY. Prognostic evaluation of percentage variation of metabolic tumor burden calculated by dual-phase 18FDG PET-CT imaging in patients with head and neck cancer. Head Neck. 2016;38:E600–E606. doi: 10.1002/hed.24048. [DOI] [PubMed] [Google Scholar]
- 36.Morris JS, Dobson JM, Bostock DE, O'Farrell E. Effect of ovariohysterectomy in bitches with mammary neoplasms. Vet Rec. 1998;142:656–658. doi: 10.1136/vr.142.24.656. [DOI] [PubMed] [Google Scholar]
- 37.Yamagami T, Kobayashi T, Takahashi K, Sugiyama M. Influence of ovariohysterectomy at the time of mastectomy on the prognosis for canine malignant mammary tumors. J Small Anim Pract. 1996;37:462–464. doi: 10.1111/j.1748-5827.1996.tb01740.x. [DOI] [PubMed] [Google Scholar]
- 38.Sorenmo KU, Shofer FS, Goldschmidt MH. Effect of spaying and timing of spaying on survival of dogs with mammary carcinoma. J Vet Intern Med. 2000;14:266–270. doi: 10.1892/0891-6640(2000)014<0266:eosato>2.3.co;2. [DOI] [PubMed] [Google Scholar]


