Abstract
Purpose
Some studies report a role of leukotrienes in the pathogenesis of atopic dermatitis and suggest a rationale for the use of leukotriene receptor antagonist (LTRA) in the treatment of atopic dermatitis. This study aimed to evaluate the treatment effectiveness of montelukast in children with atopic dermatitis.
Methods
Fifty-four children between the ages of 2 and 6 years with moderate to severe atopic dermatitis were enrolled. Group A received montelukast for 8 weeks, followed by a crossover to 8 weeks of placebo after a 2-week washout period. Group B reversed the administration according to a randomized, double-blind, placebo-controlled, crossover design. The SCORing atopic dermatitis (SCORAD) index, urinary leukotriene E4 (LTE4), and eosinophil-derived neurotoxin (EDN) were assessed at every visit.
Results
Forty-three patients (21 males) completed the study. Although the SCORAD index was decreased in both groups, there was no statistically significant difference between montelukast and placebo (-3.0±11.2 vs -5.7±11.3, P=0.43). The level of urinary LTE4 was decreased after taking montelukast when compared to placebo, but there was no statistically significant difference (-65.9±556.2 vs 87.7±618.3, P=0.26). The changes in urinary EDN after taking montelukast and placebo had no significant difference (37.0±1,008.6 vs -195.8±916.7, P=0.10). When analyzing SCORAD indices, urinary LTE4, and EDN, we could not prove the effectiveness of montelukast in the atopic, non-atopic or high ECP (ECP ≥15 µg/L) subgroups.
Conclusions
There was no statistically significant difference in clinical improvement or biomarkers between montelukast and placebo treatment. Therefore, conventional treatments with skin care and infection control might be more important strategies in the treatment of atopic dermatitis.
Keywords: Atopic dermatitis, montelukast, treatment effectiveness
INTRODUCTION
Leukotriene receptor antagonists (LTRAs) are widely used in the treatment of asthma and allergic rhinitis. Recent experimental data revealed that both leukotriene B4 (LTB4) and cysteinyl-leukotrienes (LTC4, D4, and E4) play important roles in the pathogenesis of atopic dermatitis. LTB4 initiates the recruitment of inflammatory cells, particularly neutrophils, and Th2 cells, into the skin, and then cysteinyl-leukotrienes play roles in the alteration of skin structure, including skin fibrosis and keratinocyte proliferation.1 Therefore, there is a rationale for the use of LTRAs in the treatment of atopic dermatitis. However, there are only a few articles that investigate the efficacy of LTRAs for atopic dermatitis, and the results are inconsistent.2,3
Levels of LTE4 measured in urine may be a useful measure of whole-body cysteinyl-leukotriene production in vivo, as LTE4 is a stable urinary metabolite of LTC4 and LTD4. Several studies report that urinary LTE4 reflects disease severity in atopic dermatitis.4,5,6,7 It has been reported that measurements of eosinophil-derived neurotoxin (EDN and Eosinophil protein X) may be useful for identifying eosinophil activities in allergic diseases, including atopic dermatitis.8,9,10
This study aimed to evaluate the effectiveness of a LTRA (montelukast) in children with atopic dermatitis by measuring the SCORing Atopic Dermatitis (SCORAD) index,11,12 urinary LTE4, and EDN.
MATERIALS AND METHODS
Study design
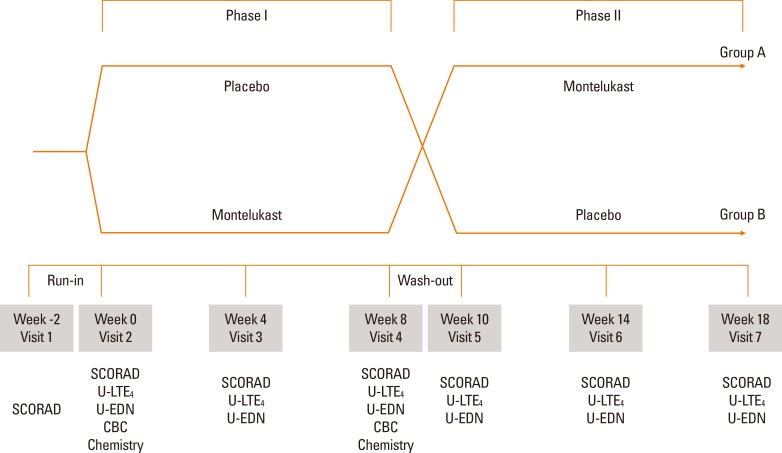
This study was a 20-week, randomized, double-blind, placebo-controlled, crossover trial. After a 2-week run-in period, children were randomized to one of two groups. Patients in group A were assigned to receive montelukast (Singulair®; Merck & Co, Whitehouse Station, NJ, USA) for 8 weeks (phase I) and, following a 2-week washout period, receive placebo for 8 weeks (phase II). Group B received placebo for 8 weeks then crossed over to montelukast (Fig. 1).
Fig. 1. Study scheme. The study was a 20-week, double-blind, randomized, placebo-controlled trial with a crossover design and a 2-week washout after visit 4. The SCORAD index, and urinary leukotriene E4 (LTE4) and eosinophil-derived neurotoxin (EDN) were assessed at every visit. CBC and blood chemistry were checked at visits 2 and 4.
Enrollment
Fifty-four children with moderate to severe atopic dermatitis diagnosed according to Hanifin and Rajka's diagnostic criteria13 were included in the study. The severity of atopic dermatitis was evaluated using the SCORAD index, and the study subjects were patients with SCORAD greater than 15.
Randomization
Patients were randomized to receive either montelukast (chewable tablet, 4 mg for those under the age of 6 years, 5 mg for those aged 6 years or over) or placebo (chewable ascorbic acid which was equal in size and color to montelukast) once daily. Randomization was performed by drawing 1 of the 2 sealed envelopes with either group A or group B written inside. Only the pharmacist preparing the study drug and placebo was aware of the coding.
Run-in period
At enrollment, patients and their parents were educated about proper skin care and the standardized application of topical steroids (0.1% hydrocortisone lotion twice daily) and emollients (3-4 times a day). All patients started topical steroid treatment after visit 1 and were instructed that topical steroids should be applied twice daily during the whole study period including the wash-out period.
Follow-up
Patients were monitored 7 times during the study period: at enrollment, at the beginning of phase I, 4 weeks after beginning phase I, at the end of phase I, at the beginning of phase II (2 weeks later), 4 weeks after beginning phase II, and at the end of phase II. The same physician recorded the SCORAD index of each patient at every visit.
Sample size calculation
Thus far, there have been only a few studies of montelukast use in pediatric atopic dermatitis patients. Friedmann et al.3 published a double-blind, placebo-controlled study of atopic eczema. A difference in the response rate was defined as 30% between treatment arms, at an alpha level of 0.05. Based on the review of published articles, a difference level of 30% was chosen. Standard deviation (SD) was defined as 60%, which is twice the mean difference in the SCORAD index for adjusting variability in the data.
σ: standard deviation (60%)
δ: estimated difference for 2 groups (30%)
α: 5%
β: 20%
According to the above equation, to detect a 30% reduction in the SCORAD index at a 5% significance level with 90% power, 27 children per group are required, including a 20% dropout rate.
Inclusion/exclusion criteria
Inclusion criteria
- Children aged 2 to 6 years old with moderate to severe atopic dermatitis diagnosed according to Hanifin and Rajka's criteria were included in the study.
- Volunteer children with atopic dermatitis were recruited from the Pediatric Allergy and Respiratory Center of Soonchunhyang University Hospital (Seoul, Korea). All submitted written consent.
Exclusion criteria
- Patients with very severe atopic dermatitis defined as a SCORAD index of ≥80.
- Patients with a history of liver disease, allergy to montelukast, or taking drugs that interact with montelukast (phenobarbital, phenytoin, or rifampicin).
- Patients who used systemic steroids, immunosuppressive drugs, or Korean herbal medicine within the previous 6 weeks.
Prestudy evaluation
Blood tests were performed for all patients for total immunoglobulin E (IgE), specific IgE (ImmunoCAP, ThermoFisher, Uppsala, Sweden) and eosinophil cationic protein (ECP) at the beginning of phase I (visit 2). Allergy skin prick tests for common food (egg white, cow milk, soy, peanut) and inhalant allergens (D. pteronyssinus, D. farinae, cat epithelium, and dog dander) were performed.
Efficacy assessment
(1) The SCORAD index was evaluated at each visit by the same physician.
(2) Urinary LTE4 and EDN were measured at each visit after the run-in period (from visit 2) with the first morning urine sample. Urinary LTE4 levels were measured using an enzyme-linked immunoassay (ELISA) (Cayman Chemical, MI, USA). The detection limit in the assay was <1,000 pg/mL, and the intra-assay and inter-assay variations were 7.4±2.1 and 12.4±7.8, respectively. Urinary EDN levels were measured using an ELISA (MBL, Woburn, MA, USA). The detection limit was <2,040 ng/mL, and the intra-assay and inter-assay variations were 3.0±0.5 and 7.7±1.5, respectively.
Safety evaluation
CBC and blood chemistry (AST/ALT, BUN/creatinine, and protein/albumin) were checked for the evaluation of the safety of treatment at the beginning and the end of phase I. The same physician examined and asked patients about adverse reactions at every visit.
Statistical analysis
The characteristics between the 2 groups (Groups A and B) were compared using Student's t test. Inter-visit differences in the 2 groups were compared using the Mann-Whitney U test.
Changes following treatment (montelukast and placebo) were compared using the Wilcoxon Signed Rank test. A P value of <0.05 was considered statistically significant.
All analyses were performed using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA).
Ethical consideration
The research protocol was approved by the Ethics Committees of Soonchunhyang University Hospital, and parents or guardians of all participating children signed informed consent (approved approval number: 2008-78).
RESULTS
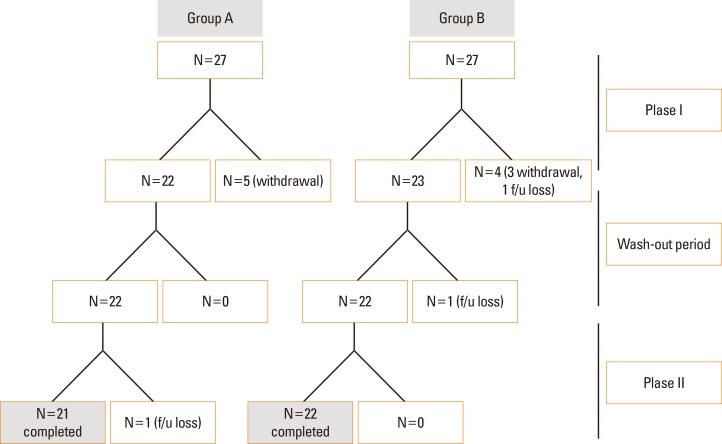
A total of 43 patients (21 in group A and 22 in group B) out of the 54 enrolled patients completed the study. The discrepancy is due to withdrawal (n=8) and follow-up loss (n=3) (Fig. 2). The mean age of the enrolled patients was 45.1±16.5 months (minimum age 23 months, maximum age 76 months). Baseline characteristics, including sex, age, severity of atopic dermatitis, and accompanied allergic diseases, were not significantly different between the 2 groups (Table 1).
Fig. 2. A total of 43 patients (21 patients in group A, 22 patients in group B) out of the 54 enrolled patients completed the study. The reasons for the difference were withdrawal (n=8) and follow-up loss (n=3).
Table 1. Characteristics of group A (montelukast-placebo group) and group B (placebo-montelukast group).
| Group A (n=22) |
Group B (n=21) |
P value | |
|---|---|---|---|
| Male (%) | 11 (50) | 10 (47.6) | 1.00 |
| Age (month) | 48.8±17.2 | 41.2±15.1 | 0.13 |
| Severity of atopic dermatitis (%) | 0.55 | ||
| Moderate | 9 (40.9) | 11 (52.4) | |
| Severe | 13 (59.1) | 10 (47.6) | |
| Accompanying allergic disease (%) | |||
| Asthma | 4 (18.2) | 2 (9.5) | 0.66 |
| Allergic rhinitis | 16 (72.7) | 13 (61.9) | 0.53 |
| Atopy (%) | 15 (68.2) | 16 (76.2) | 0.74 |
| Aeroallergen sensitization (%) | 11/21 (52.4) | 11 (52.4) | 1.00 |
| Polysensitization (%) | 2/14 (14.3) | 2/16 (12.5) | 1.00 |
| Total IgE (kU/L) | 871.1±2,208.3 | 435.2±601.1 | 0.39 |
| ECP (µg/L) | 22.8±22.8 | 15.9±16.9 | 0.27 |
IgE, immunoglobulin E; ECP, eosinophil cationic protein.
Inter-visit differences in the SCORAD index, urinary LTE4, and urinary EDN
SCORAD indices were significantly decreased in the whole subjects (between visits 1 and 7, 43.6±16.8 vs 30.3±15.4, P=0.00), but there were no statistically significant differences between the start and end of each phase (between visits 2 and 4, and visits 5 and 7) in groups A and B. Urin LTE4 and EDN had no statistically significant differences between the start and end of each phase in both groups (Tables 2 and 3).
Table 2. Inter-visit difference in group A (montelukast-placebo group).
| Phase I | P value | Phase II | P value | |||
|---|---|---|---|---|---|---|
| Visit 2 (Week 0) | Visit 4 (Week 8) | Visit 5 (Week 10) | Visit 7 (Week 18) | |||
| SCORAD index | 44.6±18.3 | 40.7±17.6 | 0.48 | 41.7±19.9 | 34.5±17.4 | 0.21 |
| U-LTE4 (pg/mL) | 585.3±428.1 | 542.7±429.5 | 0.74 | 526.7±412.2 | 690.2±385.9 | 0.18 |
| U-EDN (ng/mL) | 1,106.7±743.9 | 883.0±742.2 | 0.32 | 1,236.7±749.2 | 1,016.6±573.3 | 0.28 |
SCORAD, SCORing Atopic Dermatitis; U-LTE4, Urine leukotriene E4; U-EDN, Urine eosinophil-derived neurotoxin.
Table 3. Inter-visit difference in group B (placebo-montelukast group).
| Phase I | P value | Phase II | P value | |||
|---|---|---|---|---|---|---|
| Visit 2 (Week 0) | Visit 4 (Week 8) | Visit 5 (Week 10) | Visit 7 (Week 18) | |||
| SCORAD index | 34.7±13.7 | 30.5±12.5 | 0.31 | 28.0±14.1 | 25.9±11.9 | 0.60 |
| U-LTE4 (pg/mL) | 430.4±428.0 | 438.8±420.6 | 0.95 | 552.0±427.4 | 461.7±433.6 | 0.50 |
| U-EDN (ng/mL) | 755.2±787.7 | 584.8±722.7 | 0.47 | 671.3±657.1 | 981.3±788.1 | 0.17 |
SCORAD, SCORing Atopic Dermatitis; U-LTE4, Urine leukotriene E4; U-EDN, Urine eosinophil-derived neurotoxin.
Montelukast vs placebo on the SCORAD index, urine LTE4 and urin EDN
No statistically significant differences were detected between the changes of the SCORAD index after taking montelukast or placebo. Urinary LTE4 levels were decreased after taking montelukast rather than placebo, but there were no statistically significant differences between changes after taking montelukast and those after taking placebo. In addition, montelukast had no significant effect on urinary EDN (Table 4).
Table 4. Effectiveness of montelukast compared to placebo in children with atopic dermatitis.
| Changes after taking Montelukast (Mean±SD) |
Changes after taking Placebo drug (Mean±SD) |
P value | |
|---|---|---|---|
| SCORAD index | -3.0±11.2 | -5.7±11.3 | 0.43 |
| Urine LTE4 (pg/mL) | -65.9±556.2 | 87.7±618.3 | 0.26 |
| Urine EDN (ng/mL) | 37.0±1,008.6 | -1,95.8±916.7 | 0.10 |
SCORAD, SCORing Atopic Dermatitis; LTE4, leukotriene E4; EDN, eosinophil-derived neurotoxin.
Montelukast vs placebo on the SCORAD index, urinary LTE4, and urinary EDN according to atopy
We divided subjects into an atopic eczema group (n=26) and a non-atopic eczema group (n=17) according to the results of specific IgE. No statistically significant differences were detected in changes in the SCORAD index after taking montelukast or a placebo in either the atopic eczema or the non-atopic eczema groups. Montelukast had no significant effect on urine LTE4 or on EDN in either the atopic or the non-atopic groups (Tables 5 and 6).
Table 5. Effectiveness of montelukast compared to placebo in the atopic eczema group.
| Changes after taking Montelukast (Mean±SD) |
Changes after taking Placebo drug (Mean±SD) |
P value | |
|---|---|---|---|
| SCORAD index | -5.4±8.6 | -7.7±10.2 | 0.51 |
| Urine LTE4 (pg/mL) | 4.8±528.0 | 61.8±661.8 | 0.72 |
| Urine EDN (ng/mL) | 160.5±1,068.6 | -6.9±963.3 | 0.20 |
SCORAD, SCORing Atopic Dermatitis; LTE4, leukotriene E4; EDN, eosinophil-derived neurotoxin.
Table 6. Effectiveness of montelukast compared to placebo in the non-atopic eczema group.
| Changes after taking Montelukast (Mean±SD) |
Changes after taking Placebo drug (Mean±SD) |
P value | |
|---|---|---|---|
| SCORAD index | 0.7±13.7 | -2.7±12.6 | 0.62 |
| Urine LTE4 (pg/mL) | -174.1± 596.5 | 127.4±562.3 | 0.18 |
| Urine EDN (ng/mL) | -152.0±907.3 | -484.7±780.6 | 0.31 |
SCORAD, SCORing Atopic Dermatitis; LTE4, leukotriene E4; EDN, eosinophil-derived neurotoxin.
Montelukast vs placebo on the SCORAD index, urinary LTE4, and urinary EDN according to ECP levels
We divided subjects into the high ECP group (ECP ≥15 µg/L, n=19) and the low ECP group (ECP <15 µg/L, N=24), and analyzed the effect of montelukast in both groups. Montelukast had no significant effect on the SCORAD index or urinary LTE4 in either the high ECP or low ECP groups. But changes in urinary EDN showed a statistically significant difference in the low ECP group (348.1±1,026.2 vs -169.0±838.9, P=0.03), but not in the high ECP group (Tables 7 and 8).
Table 7. Effectiveness of montelukast compared with placebo in the high ECP group (ECP ≥15 µg/L).
| Changes after taking Montelukast (Mean±SD) |
Changes after taking Placebo drug (Mean±SD) |
P value | |
|---|---|---|---|
| SCORAD index | -2.2±10.5 | -7.8±11.2 | 0.21 |
| Urine LTE4 (pg/mL) | -8.8± 557.9 | 146.8±487.0 | 0.44 |
| Urine EDN (ng/mL) | -356.0±857.7 | -229.6±1,029.3 | 0.81 |
ECP, eosinophil cationic protein; SCORAD, SCORing Atopic Dermatitis; LTE4, leukotriene E4; EDN, eosinophil-derived neurotoxin.
Table 8. Effectiveness of montelukast compared to placebo in the low ECP group (ECP <15 µg/L).
| Changes after taking Montelukast (Mean±SD) |
Changes after taking Placebo drug (Mean±SD) |
P value | |
|---|---|---|---|
| SCORAD index | -3.7±11.9 | -4.1±11.4 | 0.93 |
| Urine LTE4 (pg/mL) | -111.2± 562.5 | 41.0±712.2 | 0.39 |
| Urine EDN (ng/mL) | 348.1±1,026.2 | -169.0±838.9 | 0.03 |
ECP, eosinophil cationic protein; SCORAD, SCORing Atopic Dermatitis; LTE4, leukotriene E4; EDN, eosinophil-derived neurotoxin.
Safety results
There were no abnormal findings of CBC or blood chemistry (AST/ALT, BUN/creatinine, and protein/albumin) either before or after treatment. No patient was withdrawn due to an adverse event with respect to montelukast.
DISCUSSION
Topical steroids have long been the treatment of choice for atopic dermatitis, but their use is limited by their adverse effects and patients' apprehensiveness toward steroid use. Leukotriene B4 (LTB4) and the cysteinyl-leukotrienes (LTC4, LTD4, and LTE4) are potent proinflammatory mediators derived from arachidonic acid through the 5-lipoxygenase pathway. They are secreted from eosinophils and other inflammatory cells, such as mast cells and macrophages. They play an important role in allergic diseases.4,5,6,7,8 LTRAs have been used with success in asthma and allergic rhinitis as steroid-sparing agents. This is the reason for increased interest in the potential utility of LTRAs in atopic dermatitis.14 Several studies have demonstrated that LTRAs are effective in the treatment of atopic dermatitis.15,16,17,18,19,20 Pei et al.15 reported a well-designed study that demonstrates the effectiveness of montelukast in children with atopic dermatitis. However, the sample size was inadequate, with only 15 children enrolled and only 11 children completing the study. The trial period was 10 weeks (each treatment phase was 4 weeks) which is not considered sufficient to evaluate the efficacy of a drug in the treatment of chronic relapsing disease, such as atopic dermatitis.15 Most studies compared the use of a severity symptom score, such as the SCORAD index. Only 1 study (a case series of 7 patients) analyzed objective data (serum soluble CD14 levels and urinary LTE4) simultaneously. The result was that montelukast increases sCD14 but has no effect on urinary LTE4.17 Studies that suggest the ineffectiveness of LTRAs in atopic dermatitis are fewer than those with positive outcomes. Friedmann et al.3 reported a double-blind, placebo-controlled trial of montelukast in adult atopic dermatitis. Sixty subjects were recruited and 54 completed the study. They compared the 6-area, 6-sign atopic dermatitis (SASSAD) score, and there were no statistically significant differences between treatment groups.3,21,22
This study evaluated the effectiveness of montelukast in children with atopic dermatitis using a double-blind, placebo-controlled, crossover approach. The sample size was relatively large compared to previous studies, and objective data (urinary LTE4 and EDN), which can reflect the severity of atopic dermatitis, was analyzed. One limitation of this study is that urinary LTE4 and EDN were not expressed as a quotient to creatinine concentration because urinary creatinine was not measured. However, participants submitted their first urine in the morning, so that bias due to differences in urine concentration were minimal.
When we compared the SCORAD indices of all patients before and after the study (visits 1 and 7, respectively), there was a significant difference (43.6±16.8 vs 30.3±15.4, P=0.00), as patients used topical corticosteroids with proper skin care during the study period. However, montelukast had no significant effect on the SCORAD index compared to the placebo drug. We saw the same results in the atopic eczema and non-atopic eczema groups, and montelukast had no effect on the SCORAD index in the high ECP group either. This result implies that the pathophysiology of atopic dermatitis is not fully explained by only a few inflammatory mediators. Degrees of skin barrier dysfunction, trigger factors, and aggravating factors of atopic dermatitis vary between individuals.23 In a previous study, the researchers presented their opinion that the occasional patients who show clinical response to treatment with LTRAs clearly form a minority subgroup with a different pathogenetic process from the majority.3 We could not prove the effectiveness of montelukast on urinary LTE4 and EDN in either the atopic or non-atopic subgroups or in the high ECP subgroup. If anything, in the low ECP group, urinary EDN was significantly lower after taking the placebo than after taking montelukast. When we compared the first urinary EDN before intervention in high ECP and low ECP patients without grouping, the urinary EDN level was significantly higher in high ECP patients than in low ECP patients (1,225.9±743.9 vs 704.7±744.7, P=0.03). However, when we divided the subjects into Groups A and B and compared the first urinary EDN level in high ECP patients and low ECP patients, there were no statistically significant differences (1,235.5±742.5 vs 881.3±738.1, P=0.29; 1,199.07±616.5 vs 616.5±755.7, P=0.15). This suggests that we may achieve different results if the study population is larger.
As a conclusion of this study, montelukast had no significant effect on atopic dermatitis, and conventional treatment with skin care and infection control seems to be a more important strategy in the management of atopic dermatitis. However, if we can analyze a larger number of subjects, we may have the chance to achieve positive results. Although there was no statistically significant difference, urinary LTE4 levels were decreased after taking montelukast compared to placebo. This suggests the possible utility of montelukast as an adjunctive agent in children with atopic dermatitis in selected cases, especially those who are limited in the use of steroids and those with concomitant asthma or allergic rhinitis. Further studies with large populations and consideration of other confounding factors are warranted.
Footnotes
This work was supported by the Soonchunhyang University Research Fund.
The study was registered at ClinicalTrials.gov. (NCT00903357).
References
- 1.Sadik CD, Sezin T, Kim ND. Leukotrienes orchestrating allergic skin inflammation. Exp Dermatol. 2013;22:705–709. doi: 10.1111/exd.12239. [DOI] [PubMed] [Google Scholar]
- 2.Holme H, Winckworth LC. Montelukast can reduce the severity and extent of atopic dermatitis. J Paediatr Child Health. 2013;49:412–415. doi: 10.1111/jpc.12202. [DOI] [PubMed] [Google Scholar]
- 3.Friedmann PS, Palmer R, Tan E, Ogboli M, Barclay G, Hotchkiss K, et al. A double-blind, placebo-controlled trial of montelukast in adult atopic eczema. Clin Exp Allergy. 2007;37:1536–1540. doi: 10.1111/j.1365-2222.2007.02811.x. [DOI] [PubMed] [Google Scholar]
- 4.Hon KL, Leung TF, Ma KC, Li AM, Wong Y, Li CY, et al. Urinary leukotriene E4 correlates with severity of atopic dermatitis in children. Clin Exp Dermatol. 2004;29:277–281. doi: 10.1111/j.1365-2230.2004.01512.x. [DOI] [PubMed] [Google Scholar]
- 5.Hishinuma T, Suzuki N, Aiba S, Tagami H, Mizugaki M. Increased urinary leukotriene E4 excretion in patients with atopic dermatitis. Br J Dermatol. 2001;144:19–23. doi: 10.1046/j.1365-2133.2001.03947.x. [DOI] [PubMed] [Google Scholar]
- 6.Adamek-Guzik T, Guzik TJ, Czerniawska-Mysik G, Korpanty G, Mastalerz L, Radwan J, et al. Urinary leukotriene levels are increased during exacerbation of atopic eczema/dermatitis syndrome. Relation to clinical status. Allergy. 2002;57:732–736. doi: 10.1034/j.1398-9995.2002.23688.x. [DOI] [PubMed] [Google Scholar]
- 7.Øymar K, Aksnes L. Increased levels of urinary leukotriene E4 in children with severe atopic eczema/dermatitis syndrome. Allergy. 2005;60:86–89. doi: 10.1111/j.1398-9995.2005.00658.x. [DOI] [PubMed] [Google Scholar]
- 8.Severien C, Artlich A, Jonas S, Becher G. Urinary excretion of leukotriene E4 and eosinophil protein X in children with atopic asthma. Eur Respir J. 2000;16:588–592. doi: 10.1034/j.1399-3003.2000.16d03.x. [DOI] [PubMed] [Google Scholar]
- 9.Goto T, Morioka J, Inamura H, Yano M, Kodaira K, Igarashi Y, et al. Urinary eosinophil-derived neurotoxin concentrations in patients with atopic dermatitis: a useful clinical marker for disease activity. Allergol Int. 2007;56:433–438. doi: 10.2332/allergolint.O-07-489. [DOI] [PubMed] [Google Scholar]
- 10.Oymar K, Bjerknes R. Urinary eosinophil protein X in children with atopic dermatitis: relation to atopy and disease activity. Allergy. 2000;55:964–968. doi: 10.1034/j.1398-9995.2000.00682.x. [DOI] [PubMed] [Google Scholar]
- 11.Kunz B, Oranje AP, Labrèze L, Stalder JF, Ring J, Taïeb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1997;195:10–19. doi: 10.1159/000245677. [DOI] [PubMed] [Google Scholar]
- 12.Charman C, Williams H. Outcome measures of disease severity in atopic eczema. Arch Dermatol. 2000;136:763–769. doi: 10.1001/archderm.136.6.763. [DOI] [PubMed] [Google Scholar]
- 13.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980;60(Suppl 92):44–47. [Google Scholar]
- 14.Chari S, Clark-Loeser L, Shupack J, Washenik K. A role for leukotriene antagonists in atopic dermatitis? Am J Clin Dermatol. 2001;2:1–6. doi: 10.2165/00128071-200102010-00001. [DOI] [PubMed] [Google Scholar]
- 15.Pei AY, Chan HH, Leung TF. Montelukast in the treatment of children with moderate-to-severe atopic dermatitis: a pilot study. Pediatr Allergy Immunol. 2001;12:154–158. doi: 10.1034/j.1399-3038.2001.012003154.x. [DOI] [PubMed] [Google Scholar]
- 16.Ehlayel MS, Bener A, Sabbah A. Montelukast treatment in children with moderately severe atopic dermatitis. Eur Ann Allergy Clin Immunol. 2007;39:232–236. [PubMed] [Google Scholar]
- 17.Hon KL, Leung TF, Ma KC, Wong Y, Fok TF. Brief case series: montelukast, at doses recommended for asthma treatment, reduces disease severity and increases soluble CD14 in children with atopic dermatitis. J Dermatolog Treat. 2005;16:15–18. doi: 10.1080/09546630510026328. [DOI] [PubMed] [Google Scholar]
- 18.Yanase DJ, David-Bajar K. The leukotriene antagonist montelukast as a therapeutic agent for atopic dermatitis. J Am Acad Dermatol. 2001;44:89–93. doi: 10.1067/mjd.2001.111352. [DOI] [PubMed] [Google Scholar]
- 19.Angelova-Fischer I, Tsankov N. Successful treatment of severe atopic dermatitis with cysteinyl leukotriene receptor antagonist montelukast. Acta Dermatovenerol Alp Pannonica Adriat. 2005;14:115–119. [PubMed] [Google Scholar]
- 20.Rahman ML, Choudhury AM, Islam MM. Effectiveness of montelukast in the treatment of atopic dermatitis. Mymensingh Med J. 2006;15:85–88. doi: 10.3329/mmj.v15i1.8. [DOI] [PubMed] [Google Scholar]
- 21.Silverberg NB, Paller AS. Leukotriene receptor antagonists are ineffective for severe atopic dermatitis. J Am Acad Dermatol. 2004;50:485–486. doi: 10.1016/s0190-9622(03)00758-8. [DOI] [PubMed] [Google Scholar]
- 22.Veien NK, Busch-Sørensen M, Stausbøl-Grøn B. Montelukast treatment of moderate to severe atopic dermatitis in adults: a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2005;53:147–149. doi: 10.1016/j.jaad.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Pyun BY. Natural history and risk factors of atopic dermatitis in children. Allergy Asthma Immunol Res. 2015;7:101–105. doi: 10.4168/aair.2015.7.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]