Abstract
Purpose
Allergic rhinitis (AR) is a common and increasing disease in which Dermatophagoides (D.) farinae is one of the most common causative allergens. The aims of this study were to confirm the presence of locally produced antibodies to D. farinae in nasal secretions between nasal provocation test (NPT)-positive and -negative groups of AR patients, to evaluate their relationships with the levels of inflammatory mediators, and to determine adaptive and innate immune responses in nasal mucosa.
Methods
Sixty AR patients sensitive to house dust mites confirmed by skin prick test or serum specific IgE to D. farinae underwent NPT for D. farinae. Nasal packs were placed in both nasal cavities of the patients for 5 minutes to obtain nasal secretions after NPT. The levels of total IgE, specific IgE to D. farinae, eosinophil cationic protein (ECP), and tryptase in nasal secretions were detected by using ImmunoCAP. The levels of specific IgE, IgA, and secretory IgA antibodies to D. farinae in nasal secretions were measured by using ELISA. The levels of IL-8, VEGF, IL-25, and IL-33 were also measured by using ELISA.
Results
High levels of total IgE, specific IgE, specific IgA, and secretory IgA to D. farinae, as well as inflammatory mediators, such as ECP, IL-8, VEGF and tryptase, were detected in nasal secretions, although the differences were not statistically significant between the NPT-positive and NPT-negative groups. Levels of all immunoglobulins measured in this study significantly correlated with ECP, IL-8, and VEGF (P<0.05), but not with tryptase (P>0.05). IL-33 and IL-25 were also detected, and IL-25 level significantly correlated with IL-8 (r=0.625, P<0.001).
Conclusions
These findings confirmed the presence of locally produced specific antibodies, including D. farinae-specific IgE and IgA, in nasal secretions collected from D. farinae-sensitive AR patients in both the NPT-positive and NPT-negative groups, and close correlations were noted between antibodies and nasal inflammatory mediators, including such as ECP, IL-8 and VEGF, indicating that locally produced antibodies may be involved in the nasal inflammation of AR.
Keywords: Allergic rhinitis, nasal mucosa, local specific antibody
INTRODUCTION
Allergic rhinitis (AR) is a chronic inflammatory condition in the mucosa of the nasal cavity and paranasal sinuses characterized by rhinorrhea, sneezing, itching, and nasal congestion.1 The prevalence of AR is increasing, emerging as a global problem that affects approximately 10% to 30% of the adult population. Since AR not only interferes with the quality of life, but also attributes to risk factors for asthma and rhinosinusitis, early diagnosis and intervention of AR are essential in preventing asthma and further sensitization to other allergens.2,3
House dust mites, including Dermatophagoides (D.) farinae and D. farinae, are one of the most common causative inhalant allergens responsible for AR.1,4 Exposure to house dust mites induces specific antibody production and nasal inflammation by various inflammatory cells, including mast cells, eosinophils, and structural cells, such as nasal epithelial cells.5,6,7
The innate immune response has been identified to augment inflammation in nasal airway mucosa.8 IL-33 and IL-25 produced by airway epithelial cells are important Th2-augmenting cytokines to affect eosinophilic homeostasis and airway inflammation.9,10 These 2 cytokines are involved in the development of allergic disease as a link between innate and adaptive airway mucosal immunity.11,12
Nasal secretion is the first-line defense mechanism that produces cytokines and biologic factors, such as protein, cells, and mediators, to protect airway epithelium from inoculation with allergens and pathogens like bacteria and virus and to regulate immune responses in nasal inflammation.13 The most important point in the collection of nasal secretions is the method of collection and storage which requires careful and coordinated efforts. Various methods, such as blowing of the nose, suction, use of absorbent cotton wool samplers, and collection of nasal lavage fluid, are applied to collect nasal secretions in order to investigate upper airway mucosa.14,15 However, there has been no consensus on a standardized method for the collection of nasal secretion.
In this study, we applied a simple, non-invasive method using a cotton-ball nasal pack to collect nasal secretions in the nasal provocation test (NPT)-positive and NPT-negative groups of AR patients in order to confirm the presence of locally produced specific antibodies to D. farinae and also evaluated their correlation with inflammatory mediators in nasal secretions between the 2 groups. Moreover, we investigated the link between innate and adaptive immune responses in the nasal inflammation of AR.
MATERIALS AND METHODS
Study patients
Sixty patients were diagnosed with AR sensitized to D. farinae based on either positive results of skin prick test (A/H ratio >3) or elevated serum specific IgE (>0.35 kU/L). The study patients were divided into 2 groups according to the NPT results: the NPT-positive (n=39) and NPT-negative (n=21) groups. The patients stopped receiving antihistamines, leukotriene receptor antagonists, or intranasal steroids prior to NPT for at least 3 days. The results of skin prick test, levels of serum total/specific IgE to D. farinae and D. pteronyssinus, and eosinophil cationic protein (ECP) levels were obtained, which are summarized in Table 1. The study was approved by the Institutional Review Board of Ajou Medical Center, Suwon, Korea. Informed consent was obtained from each study patient.
Table 1. Clinical characteristics of the study subjects.
| NPT-positive group (n=39) | NPT-negative group (n=21) | P value | |
|---|---|---|---|
| Age (year) | 30.92±10.94 | 36.67±12.78 | 0.073 |
| Sex (male/female) | 15/24 | 13/8 | 0.107 |
| Positive SPT result to D. farinae (%) | 38/39 (97.4) | 17/21 (80.1) | 0.002 |
| AR vs AR with BA | 27/12 | 18/3 | 0.218 |
| Serum total IgE* (IU/mL) | 370.18±412.33 | 478.00±1,095.68 | 0.585 |
| Serum specific IgE to D. farinae* (kU/L) | 29.79±37.27 | 2.63±8.03 | <0.001 |
| Total nasal symptom scores before NPT | 11.59±9.49 | 6.81±7.33 | 0.043 |
| Total nasal symptom scores after NPT | 19.92±8.1 | 7.52±8.27 | <0.001 |
All data are expressed as mean±SD.
*Detected by using the ImmunoCAP method.
SPT, skin prick test; D., Dermatophagoides; AR, allergic rhinitis; BA, bronchial asthma; NPT, nasal provocation test.
Nasal provocation test
NPT with D. farinae was performed according to previously described methods.16,17,18 Patients were first placed at room temperature for 30 minutes to minimize the effects of daily-life stimuli. They underwent saline challenge tests to exclude nasal hyperreactivity. NPT was performed by applying an 8-mm filter paper disk impregnated with an allergen solution (5,000 BU/µL, D. farinae; prepared by Allergopharma, Reinbek, Germany) to the anterior tip of the inferior turbinate on the wider side of the nose for 10 minutes or until allergic symptoms appear. Nasal symptoms of rhinorrhea, nasal itching, nasal obstruction, and sneezing were determined on a Visual Analogue Scale (VAS) from 0 to 10 (subjective symptoms reported by patients) at baseline and 30 minutes after NPT. A positive response to NPT with D. farinae was defined as a 3 point or more increase in the total nasal symptom score, which is the sum of rhinorrhea, nasal itching, nasal obstruction, and sneezing during the test.
Sample collection and processing
The cotton ball nasal packing method suggested by Ghent University Hospital19 which was adjusted according to circumstances was applied. In brief, a Falcon tube with the cotton ball (Wejae Sangsa, Chungju, Korea) was weighed. Cotton ball nasal packs were placed on the middle turbinate for 5 minutes after NPT to collect nasal secretions. The cotton ball was placed back to the Falcon tube and weighed again to obtain the volume of nasal secretions. After 3 mL of normal saline were added to the pack and incubated at 4℃ for 2 hours, the pack was put in the shaft of a syringe 10 mL (Kovax-Syringe; Korea Vaccine Co. LTD, Ansan, Korea). The syringe with the pack and no piston was placed in a Falcon tube. The Falcon tube was centrifuged at 1,500 g for 10 minutes at 4℃. Aliquots of 500 µL were prepared and stored at -70℃ for analysis.
Measurement of local antibodies, inflammatory mediators, and inflammatory markers in nasal secretion samples
The levels of total IgE and specific IgE to D. farinae and D. pteronyssinus in the nasal secretion were detected by using ImmunoCAP® (ThermoFisher Scientific, Uppsala, Sweden). The protein concentrations were determined by the Bradford assay. The levels of specific IgE, IgA, and secretory IgA to D. farinae in the nasal secretion were measured by using enzyme-linked immunosorbent assay (ELISA) as previously described.5,16 In brief, 96-well microtiter plates (Corning, Corning, NY, USA) were coated with D. farinae (2 µg/well, homemade antigen)20 and incubated overnight at 4℃. After washing, the plates were blocked with 10% fetal bovine serum (FBS) in phosphate buffered saline (PBS) and incubated for 2 hours. Fifty microliters of the nasal secretion was incubated for 2 hours. Then, biotin-labeled goat anti-human IgE antibody (Vector Lab, Burlingame, CA, USA) at 1:1,000 dilution, biotinylated anti-human IgA (Sigma Co., St. Louis, MO, USA) at 1:2,000 dilution, and monoclonal anti-secretory IgA (Sigma Co.) at 1:2,000 dilution were added and incubated for 1 hour to detect specific IgE, IgA, and secretory IgA to D. farinae. After washing, the plates were incubated with streptavidine-peroxidase (Sigma Co.) at 1:1,000 dilution for 30 minutes, anti-goat IgG-AP (Sigma Co.) at 1:10,000 dilution, and anti-mouse IgG-AP (Sigma Co.) at 1:10,000 dilution for 1 hour at room temperature. After washing, O-phenylene diamine was added as a substrate solute, and optical densities were measured by using an ELISA reader (Bio-Tek Instruments, Winooski, VT, USA) at 405 nm.
ECP and tryptase were detected by using ImmunoCAP® (ThermoFisher Scientific). The level of IL-8 and vascular endothelial growth factor (VEGF) in the nasal secretion samples were measured by using ELISA kits (Endogen, Woburn, MA, USA and R&D Systems Inc., Minneapolis, MN, USA, respectively). To evaluate the role of innate immune responses, IL-25 and IL-33 levels in the nasal secretion samples were measured by using ELISA kits (R&D systems).
Calculation of concentrations and statistical analysis
After 3 mL of normal saline was added to each sample, concentrations of local antibodies and inflammatory mediators were recalculated using protein concentration for each measurement to correct the dilution effect of the samples. All the values were shown as the protein ratio.
All analyses were carried out by using SPSS 20.0 (SPSS Inc., Chicago, IL, USA). The values are presented as mean±SD. The levels of antibodies in the nasal mucosa were compared by using Student's t test between the NPT-positive and NPT-negative groups. Symptom scores were compared by using Wilcoxon's signed-ranks test. Pearson's correlation coefficients were applied to examine the correlation between local antibodies and inflammatory mediators. A P value of <0.05 was considered statistically significant.
RESULTS
Characteristics of the study patients
There was no significant difference in age or sex between the NPT-positive and NPT-negative groups. Twelve of the 39 patients in the NPT-positive group and 3 of the 18 patients in the NPT-negative group had bronchial asthma. The baseline total nasal symptom scores were 11.59±9.49 in the NPT-positive group and 6.81±7.33 in the NPT-negative group (P=0.043), and after NPT they were 19.92±8.1 in the NOT-positive group and 7.52±8.27 in the NPT-negative group (P<0.001). The symptom scores increased significantly in the NPT-positive group (P<0.001), while no significant change was noted in the NPT-negative group. The serum specific IgE level to D. farinae was significantly higher in the NPT-positive group than in the NPT-negative group. The clinical characteristics of the patients are summarized in Table 1.
Comparison of local antibody levels in nasal secretions between the NPT-positive and NPT-negative groups
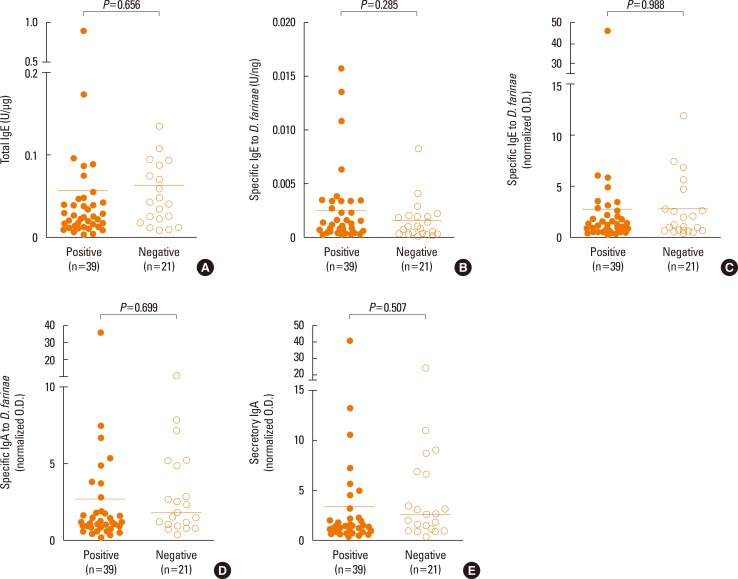
Fig. 1 shows comparison of total IgE, specific IgE, IgA, and secretory IgA to D. farinae between the NPT-positive and NPT-negative groups. Although some AR patients had high levels of local antibodies in nasal secretions, there was no significant difference in either variables between the NPT-positive and NPT-negative groups (P=0.656, 0.285, 0.988, 0.699, or 0.507, respectively).
Fig. 1. Detection of local specific antibodies in the nasal secretion of the NPT-positive and NPT-negative groups. (A) Total IgE, (B) specific IgE to D. farinae were detected by using the ImmunoCAP, (C) specific IgE to D. farinae, (D) specific IgA to D. farinae, and (E) secretory IgA to D. farinae were measured by using ELISA. The horizontal bars indicate positive cutoff values (mean+3 SD). All values were adjusted for the protein ratio. NPT, nasal provocation test.
Comparison of inflammatory markers in nasal secretions between the NPT-positive and NPT-negative groups
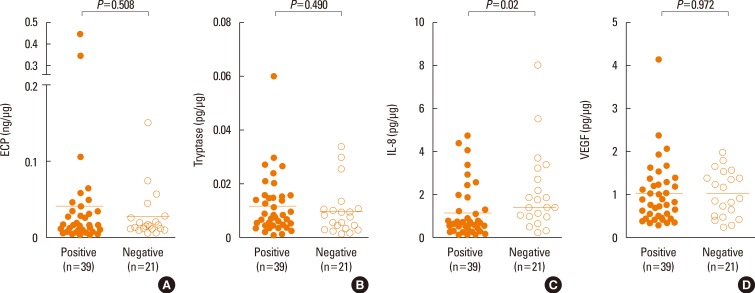
Fig. 2 shows comparison of the levels of ECP, tryptase, IL-8, and VEGF between the NPT-positive and NPT-negative groups. There was no significant difference in ECP, tryptase, or VEGF levels between the NPT-positive and NPT-negative groups (P=0.508, 0.490, or P=0.972, respectively), while the IL-8 level was significantly higher in the NPT-negative group than in the NPT-positive group (P=0.02).
Fig. 2. Detection of inflammatory mediators in the nasal secretion of the NPT-positive and NPT-negative groups. The levels of (A) ECP, (B) tryptase, (C) IL-8, and (D) VEGF were measured. The horizontal bars indicate positive cutoff values (mean+3 SD). All values were adjusted for the protein ratio. NPT, nasal provocation test; ECP, eosinophil cationic protein; IL, interleukin; VEGF, vascular endothelial growth factor.
Comparison of IL-33 and IL-25 levels in nasal secretions between the NPT-positive and NPT-negative groups
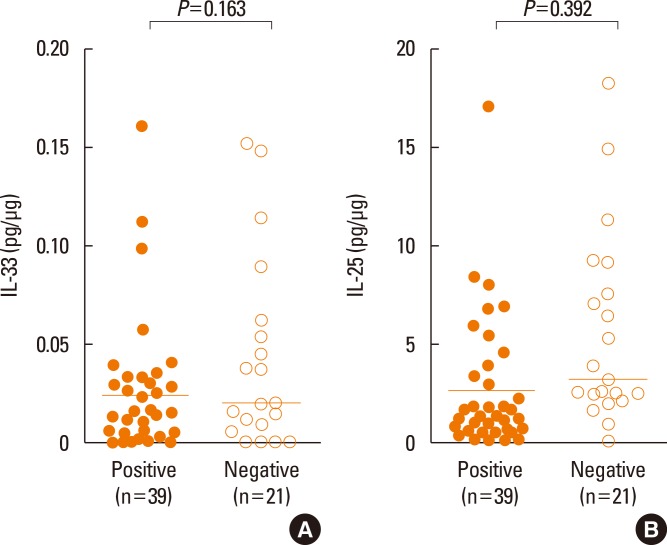
Fig. 3 shows comparison of IL-33 and IL-25 between the NPT-positive and NPT-negative groups. The level of IL-33 or IL-25 showed no significant difference between the NPT-positive and NPT-negative groups (P=0.163 or 0.392, respectively).
Fig. 3. Detection of (A) IL-33 and (B) IL-25 in the nasal secretion of the NPT-positive and NPT-negative groups. The horizontal bars indicate positive cutoff values (mean+3 SD). All values were adjusted for the protein ratio. IL, interleukin; NPT, nasal provocation test.
Correlation between local specific antibodies to D. farinae and inflammatory markers in nasal secretions
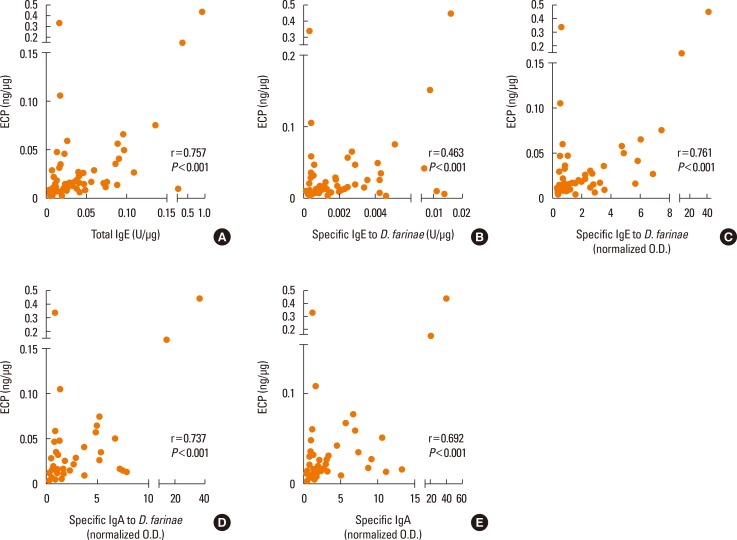
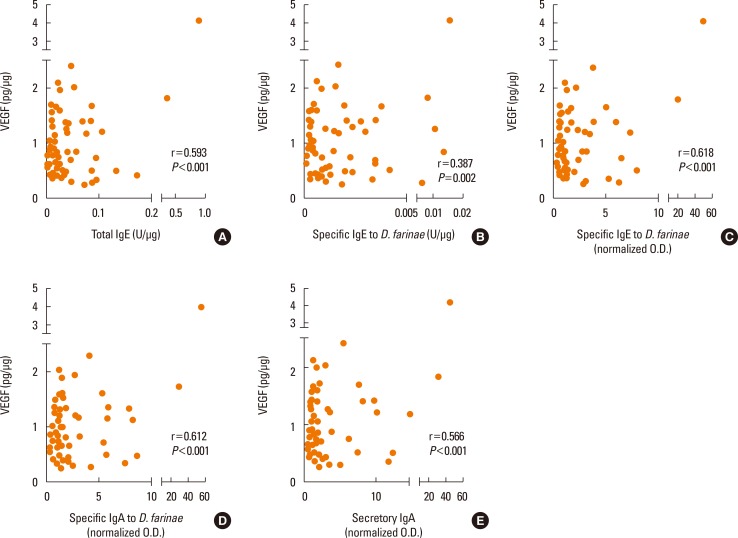
ECP correlated with total IgE, specific IgE detected by ImmunoCAP, specific IgE, IgA, and secretory IgA to D. farinae measured by ELISA (r=0.757, r=0.463, r=0.761, r=0.737 and r=0.692, respectively) with statistical significance (all P<0.001) (Fig. 4). However, there was no significant correlation between tryptase and local antibodies (r=0.179, r=0.208, r=0.219, r=0.169, or r=0.170, respectively; all P>0.05). IL-8 showed a significant correlation with local specific antibody levels (r=0.283, r=0.239, r=0.306, r=0.299, andr=0.295; P<0.05, except IL-8 to specific IgE to D. farinae P=0.065), and VEGF correlated with local specific antibody levels (r=0.593, r=0.387, r=0.618, r=0.612, and r=0.566; P<0.05) (Fig. 5). A significant correlation was noted between ECP and VEGF (r=0.520, P<0.001), but not between tryptase, IL-8, and VEGF.
Fig. 4. Correlation of ECP levels with local specific antibodies in nasal secretions. (A) Total IgE, (B) specific IgE to D. farinae, (C) specific IgE to D. farinae, (D) specific IgA to D. farinae, and (E) secretory IgA to D. farinae were measured. All values were adjusted for the protein ratio. Pearson's rank correlation coefficients were applied to the test. ECP, eosinophil cationic protein.
Fig. 5. Correlation of VEGF levels with local specific antibodies in nasal secretions. VEGF levels were measured by using ELISA. (A) Total IgE, (B) specific IgE to D. farinae, (C) specific IgE to D. farinae, (D) specific IgA to D. farinae, and (E) secretory IgA to D. farinae were measured. All values were adjusted for the protein ratio. Pearson's rank correlation coefficients were applied to the test. VEGF, vascular endothelial growth factor.
Correlation of local specific antibodies and inflammatory mediators with IL-25 and IL-33
The correlation of local specific antibodies and inflammatory mediators with IL-25 and IL-33 was evaluated (Table 2). No significant correlation was noted, except between IL-25 and IL-8 (r=0.625, P<0.001).
Table 2. Correlation of local specific antibodies to D. farinae and inflammatory mediators with IL-33 and IL-25.
| IL-33 | IL-25 | |||
|---|---|---|---|---|
| r | P value | r | P value | |
| Total IgE* | -0.171 | 0.191 | 0.079 | 0.546 |
| Specific IgE to D. farinae* | -0.162 | 0.216 | 0.218 | 0.095 |
| Specific IgE to D. farinae† | -0.0186 | 0.164 | 0.141 | 0.283 |
| Specific IgA to D. farinae† | -0.0186 | 0.156 | 0.086 | 0.516 |
| Secretory IgA to D. farinae† | -0.199 | 0.127 | 0.072 | 0.583 |
| ECP* | 0.017 | 0.900 | 0.025 | 0.850 |
| Tryptase* | -0.173 | 0.187 | 0.021 | 0.873 |
| IL-8† | -0.039 | 0.768 | 0.625 | <0.001 |
| VEGF† | 0.025 | 0.851 | 0.084 | 0.523 |
| IL-25† | -0.012 | 0.926 | ||
All values were adjusted by the protein ratio.
*Detected by using the ImmunoCAP method; †Measured by using ELISA; Pearson's rank correlation coefficients were applied to the test; All values were adjusted for the protein ratio.
D., Dermatophagoides; ECP, eosinophil cationic protein; IL, interleukin; VEGF, vascular endothelial growth factor.
DISCUSSION
IgE plays a key role in type I hypersensitivity that manifests various allergic diseases, such as AR, allergic asthma, and food allergy.21 IgA is an antibody produced mostly in the mucosal lining and plays a critical role in mucosal immunity.22 Secretory IgA, a dimeric form of IgA found in mucous secretions, such as nasal secretions and saliva, is associated with allergic diseases.23 Although allergen-specific IgA is known to be involved in allergen tolerance, few studies on physiologic functions of specific IgA have been conducted.24 In this study, the high levels of specific IgA and secretory IgA were noted in nasal secretions in both the NPT-positive and NPT-negative groups.
Various studies have revealed a role of IgA in Th2-related eosinophilic inflammation and innate immunity, suggesting that natural helper cells can stimulate B cells to produce IgA or the IgA-based immune complex against certain antigens.25,26,27,28 In our study, not only were locally produced specific IgE and IgA detected in the nasal secretions of both the NPT-positive and NPT-negative groups, but significant correlations were noted between specific IgE and IgA and between ECP and specific IgE/IgA. These findings suggest that locally produced specific and secretory IgA, as well as specific IgE, may play a role in the nasal inflammation of AR patients.
Eosinophilic inflammation plays a key role in the pathogenesis of AR; therefore, the ECP level in nasal secretions may be used to monitor the activity of eosinophilic inflammation and the efficacy of anti-inflammatory treatment.29,30 In this study, we confirmed a high level of ECP in the nasal secretion of AR patients. Moreover, significant correlations were found between ECP and local specific IgE/IgA antibodies, and between various inflammatory mediators, such as IL-8 and VEGF. These findings suggest that ECP plays a key role in nasal inflammation of AR patients in that locally produced specific antibodies, including IgE and IgA, could be involved in nasal eosinophilic inflammation of AR patients.
Mast cells contain cytokines, chemokines, and growth factors as well as mediators, such as histamine and tryptase. Tryptase has been used as a marker of mast cell activation, and numerous studies have proved the function of mast cells in type I hypersensitivity reaction associated with IgE.31,32 In this study, high tryptase levels were noted in the nasal secretion of AR patients in both the NPT-positive and NPT-negative groups, but there was no significant correlation between the tryptase levels and local antibodies/other inflammatory mediators. Tryptase shows no correlation with local antibodies; however, mechanisms other than local antibody response may be involved in the activation of mast cells.
Epithelial cells are positioned on the first-line exposure to allergens and pathogens, and regulate innate and adaptive immune systems in the nasal mucosa. IL-8 produced by epithelial cells, macrophages, and smooth muscle cells is released to the nasal fluid and recruits other inflammatory cells, such as lymphocytes and neutrophils.33 Zuyderdyun et al.34 have reported that IL-8 is released by airway smooth muscle cells in response to airway inflammation. Simpson et al.35 have suggested that airway disease is an innate immune response to neutrophilic inflammation caused by elevated IL-8. The results of their study showed a significantly higher level of IL-8 in the NPT-negative group compared to NPT-positive group as well as a significant correlation between specific antibodies. IL-25, a cytokine released from activated epithelial cells, is increased in nasal secretions after exposure to house dust mites and correlates with the IL-8 level. These findings suggest that activated epithelial cells may be involved in the nasal inflammation of AR patients via innate and adaptive immune responses. VEGF is a potent signal protein to induce vascular angiogenesis, permeability, remodeling, and wound protection/healing, which is derived from macrophages, neutrophils, eosinophils, epithelial cells, and fibroblasts in the airway mucosa. Recent studies have demonstrated that VEGF regulates immune responses by inhibiting T-cell development and dendritic-cell activity, and by stimulating eosinophil chemotaxis.5,36 Choi et al.37 have reported eosinophilic inflammation augmented by VEGF in the nasal lavage fluid in patients with AR sensitized to house dust mites. In this study, high VEGF levels were noted in AR patients in both the NPT-positive and NPT-native groups and showed a significant correlation with local specific antibodies, such as specific IgE, specific IgA, and secretory IgA. These findings suggest that the vascular factor may be another key component for nasal inflammation of AR patients regardless of positivity to NPT, which can be a target for anti-inflammatory treatment.
IL-25 and IL-33 both produced by sinonasal epithelial cells are reported to have critical roles in promoting Th2-mediated inflammation.21 Previous studies have shown a role of IL-25 in augmenting allergic inflammation by epithelial cell hyperplasia, mucus secretion, airway hyperresponsiveness, and production of specific eosinophilic chemokines and Th2 cytokines.38,39 In the present study, the innate immunity-related cytokines IL-25 and IL-33 were detected in the nasal secretion of AR patients, and IL-25 correlated strongly with IL-8, while no significant correlation was found between IL-33 and inflammatory mediators/local specific antibodies. These findings suggest innate immune responses induced by the nasal epithelial cells may augment nasal inflammation of AR patients.
According to our study, serum specific IgE is a key factor associated with NPT results (Table 1). However, the increased level of specific antibodies and inflammatory mediators in nasal secretions in both the NPT-positive and NPT-negative patients can explain active inflammation of nasal mucosa in AR patients. NPT is an effective method for the confirmative diagnosis of AR in patients without definitive history or clinical manifestation. Although NPT is time-consuming and has the potential for anaphylaxis, it has been widely used to confirm causative allergens in occupational asthma and aspirin-exacerbated respiratory disease in order to confirm nasal hyperreactivity.18 Furthermore, previous studies have mentioned a decrease in the hyperreactivity of airway mucosa by treatment with immunotherapy,40,41 and NPT results may be used to predict the efficacy of treatment modalities. Increased symptom scores may reflect the increased hyperreactivity of nasal mucosa in the NPT-positive group compared to the NPT-negative group. There is still no clear explanation of the insignificant difference in the level of local antibodies and the role of these antibodies between the NPT-positive and NPT-negative groups. We speculate that local antibodies may be involved in the nasal inflammation of AR patients, and increased target tissue sensitivity and nasal hypersensitivity may be more important in determining NPT results, although further studies are needed to confirm our results. Nasal secretion is a heterogeneous fluid composed of protein, various cells, plasma exudates, and mucus. Since considerable inter-individual variations exist in terms of amount, composition, biological activity, and cellular component, nasal secretions are difficult to obtain, so we need easy techniques to collect sufficient amounts of nasal secretions.15 In our study, a non-invasive and inexpensive method using a cotton ball nasal pack was introduced, and both ELISA and ImmunoCAP methods were applied to detect specific IgE to D. farinae in order to validate the consistency of our method with good results. Our method allows collection of sufficient amounts of nasal secretions, needs no special techniques or instruments, and does not cause patient discomfort. It was conducted on a total of 60 patients, and the specific antibodies and inflammatory mediators were detected, which suggests that its results are steady and highly reproducible.
Nasal secretion samples are usually insufficient in study subjects and normal controls. NPT provokes rhinorrhea, which allows collection of sufficient nasal secretion samples. Thus, nasal secretion samples are easy to collect after NPT, which allow investigators to elucidate mechanisms underlying nasal inflammation and therapeutic outcomes of pharmacologic treatment and allergen immunotherapy using nasal secretions.
In conclusion, we confirmed the presence of local specific antibodies and inflammatory mediators in the nasal secretion of AR patients by using a simple and non-invasive method for collecting nasal secretions. Specific IgE and IgA antibodies may be involved in nasal eosinophilic inflammation and innate immune responses via IL-25, and can be involved in nasal inflammation via epithelial-cell activation.
ACKNOWLEDGMENTS
This study was supported by a grant from the Korean Health Technology R&D Project funded by the Ministry of Health & Welfare, ROK (H14C2628).
Footnotes
This study was supported by a grant from the Korean Health Technology R&D Project funded by the Ministry of Health & Welfare, ROK (H14C2628).
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 2.Greiner AN, Hellings PW, Rotiroti G, Scadding GK. Allergic rhinitis. Lancet. 2011;378:2112–2122. doi: 10.1016/S0140-6736(11)60130-X. [DOI] [PubMed] [Google Scholar]
- 3.Cibella F, Ferrante G, Cuttitta G, Bucchieri S, Melis MR, La Grutta S, et al. The burden of rhinitis and rhinoconjunctivitis in adolescents. Allergy Asthma Immunol Res. 2015;7:44–50. doi: 10.4168/aair.2015.7.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jo EJ, Kim MY, Lee SE, Lee SY, Kim MH, Song WJ, et al. Eosinophilic airway inflammation and airway hyperresponsiveness according to aeroallergen sensitization pattern in patients with lower airway symptoms. Allergy Asthma Immunol Res. 2014;6:39–46. doi: 10.4168/aair.2014.6.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrara N. VEGF: an update on biological and therapeutic aspects. Curr Opin Biotechnol. 2000;11:617–624. doi: 10.1016/s0958-1669(00)00153-1. [DOI] [PubMed] [Google Scholar]
- 6.Rydell-Törmänen K, Johnson JR, Fattouh R, Jordana M, Erjefält JS. Induction of vascular remodeling in the lung by chronic house dust mite exposure. Am J Respir Cell Mol Biol. 2008;39:61–67. doi: 10.1165/rcmb.2007-0441OC. [DOI] [PubMed] [Google Scholar]
- 7.Shin SY, Choi SJ, Hur GY, Lee KH, Kim SW, Cho JS, et al. Local production of total IgE and specific antibodies to the house dust mite in adenoid tissue. Pediatr Allergy Immunol. 2009;20:134–141. doi: 10.1111/j.1399-3038.2008.00756.x. [DOI] [PubMed] [Google Scholar]
- 8.Gregory LG, Lloyd CM. Orchestrating house dust mite-associated allergy in the lung. Trends Immunol. 2011;32:402–411. doi: 10.1016/j.it.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reynolds JM, Angkasekwinai P, Dong C. IL-17 family member cytokines: regulation and function in innate immunity. Cytokine Growth Factor Rev. 2010;21:413–423. doi: 10.1016/j.cytogfr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cayrol C, Girard JP. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr Opin Immunol. 2014;31:31–37. doi: 10.1016/j.coi.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008;226:172–190. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kariyawasam HH, Rotiroti G. Allergic rhinitis, chronic rhinosinusitis and asthma: unravelling a complex relationship. Curr Opin Otolaryngol Head Neck Surg. 2013;21:79–86. doi: 10.1097/MOO.0b013e32835ac640. [DOI] [PubMed] [Google Scholar]
- 13.Jankowski R, Persoons M, Foliguet B, Coffinet L, Thomas C, Verient-Montaut B. Eosinophil count in nasal secretions of subjects with and without nasal symptoms. Rhinology. 2000;38:23–32. [PubMed] [Google Scholar]
- 14.Riechelmann H, Deutschle T, Friemel E, Gross HJ, Bachem M. Biological markers in nasal secretions. Eur Respir J. 2003;21:600–605. doi: 10.1183/09031936.03.00072003. [DOI] [PubMed] [Google Scholar]
- 15.Lü FX, Esch RE. Novel nasal secretion collection method for the analysis of allergen specific antibodies and inflammatory biomarkers. J Immunol Methods. 2010;356:6–17. doi: 10.1016/j.jim.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Oh JH, Hur GY, Ye YM, Kim JE, Park K, Park HS. Correlation between specific IgA and eosinophil numbers in the lavage fluid of patients with perennial allergic rhinitis. Allergy Asthma Proc. 2008;29:152–160. doi: 10.2500/aap.2008.29.3069. [DOI] [PubMed] [Google Scholar]
- 17.Kim YH, Jang TY. Proposed diagnostic standard using visual analogue scale and acoustic rhinometry in nasal provocation test in allergic patients. Auris Nasus Larynx. 2011;38:340–346. doi: 10.1016/j.anl.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Jang TY, Kim YH. Nasal provocation test is useful for discriminating allergic, nonallergic, and local allergic rhinitis. Am J Rhinol Allergy. 2015;29:e100–e104. doi: 10.2500/ajra.2015.29.4214. [DOI] [PubMed] [Google Scholar]
- 19.Watelet JB, Gevaert P, Holtappels G, Van Cauwenberge P, Bachert C. Collection of nasal secretions for immunological analysis. Eur Arch Otorhinolaryngol. 2004;261:242–246. doi: 10.1007/s00405-003-0691-y. [DOI] [PubMed] [Google Scholar]
- 20.Jeong KY, Choi SY, Lee JH, Lee IY, Yong TS, Lee JS, et al. Standardization of house dust mite extracts in Korea. Allergy Asthma Immunol Res. 2012;4:346–350. doi: 10.4168/aair.2012.4.6.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Schryver E, Devuyst L, Derycke L, Dullaers M, Van Zele T, Bachert C, et al. Local immunoglobulin e in the nasal mucosa: clinical implications. Allergy Asthma Immunol Res. 2015;7:321–331. doi: 10.4168/aair.2015.7.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fagarasan S, Honjo T. Intestinal IgA synthesis: regulation of front-line body defences. Nat Rev Immunol. 2003;3:63–72. doi: 10.1038/nri982. [DOI] [PubMed] [Google Scholar]
- 23.Corthésy B. Role of secretory IgA in infection and maintenance of homeostasis. Autoimmun Rev. 2013;12:661–665. doi: 10.1016/j.autrev.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Vazquez-Ortiz M, Pascal M, Juan M, Alsina L, Martín-Mateos MA, Plaza AM. Serum allergen-specific IgA is not associated with natural or induced tolerance to egg in children. Allergy. 2013;68:1327–1332. doi: 10.1111/all.12217. [DOI] [PubMed] [Google Scholar]
- 25.Hupin C, Rombaux P, Bowen H, Gould H, Lecocq M, Pilette C. Downregulation of polymeric immunoglobulin receptor and secretory IgA antibodies in eosinophilic upper airway diseases. Allergy. 2013;68:1589–1597. doi: 10.1111/all.12274. [DOI] [PubMed] [Google Scholar]
- 26.Bousquet J, Van Cauwenberge P, Bachert C, Canonica GW, Demoly P, Durham SR, et al. Requirements for medications commonly used in the treatment of allergic rhinitis. European Academy of Allergy and Clinical Immunology (EAACI), Allergic Rhinitis and its Impact on Asthma (ARIA) Allergy. 2003;58:192–197. doi: 10.1034/j.1398-9995.2003.00054.x. [DOI] [PubMed] [Google Scholar]
- 27.Wijburg OL, Uren TK, Simpfendorfer K, Johansen FE, Brandtzaeg P, Strugnell RA. Innate secretory antibodies protect against natural Salmonella typhimurium infection. J Exp Med. 2006;203:21–26. doi: 10.1084/jem.20052093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koyasu S, Moro K. Type 2 innate immune responses and the natural helper cell. Immunology. 2011;132:475–481. doi: 10.1111/j.1365-2567.2011.03413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suh KS, Park HS, Nahm DH, Kim YK, Lee YM, Park K. Role of IgG, IgA, and IgE antibodies in nasal polyp tissue: their relationships with eosinophilic infiltration and degranulation. J Korean Med Sci. 2002;17:375–380. doi: 10.3346/jkms.2002.17.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bystrom J, Patel SY, Amin K, Bishop-Bailey D. Dissecting the role of eosinophil cationic protein in upper airway disease. Curr Opin Allergy Clin Immunol. 2012;12:18–23. doi: 10.1097/ACI.0b013e32834eccaf. [DOI] [PubMed] [Google Scholar]
- 31.Wedemeyer J, Tsai M, Galli SJ. Roles of mast cells and basophils in innate and acquired immunity. Curr Opin Immunol. 2000;12:624–631. doi: 10.1016/s0952-7915(00)00154-0. [DOI] [PubMed] [Google Scholar]
- 32.Williams CM, Galli SJ. The diverse potential effector and immunoregulatory roles of mast cells in allergic disease. J Allergy Clin Immunol. 2000;105:847–859. doi: 10.1067/mai.2000.106485. [DOI] [PubMed] [Google Scholar]
- 33.Ohkubo K, Ikeda M, Pawankar R, Gotoh M, Yagi T, Okuda M. Mechanisms of IL-6, IL-8, and GM-CSF release in nasal secretions of allergic patients after nasal challenge. Rhinology. 1998;36:156–161. [PubMed] [Google Scholar]
- 34.Zuyderduyn S, Ninaber DK, Hiemstra PS, Rabe KF. The antimicrobial peptide LL-37 enhances IL-8 release by human airway smooth muscle cells. J Allergy Clin Immunol. 2006;117:1328–1335. doi: 10.1016/j.jaci.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 35.Simpson JL, Grissell TV, Douwes J, Scott RJ, Boyle MJ, Gibson PG. Innate immune activation in neutrophilic asthma and bronchiectasis. Thorax. 2007;62:211–218. doi: 10.1136/thx.2006.061358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 37.Choi GS, Park HJ, Hur GY, Choi SJ, Shin SY, Ye YM, et al. Vascular endothelial growth factor in allergen-induced nasal inflammation. Clin Exp Allergy. 2009;39:655–661. doi: 10.1111/j.1365-2222.2009.03216.x. [DOI] [PubMed] [Google Scholar]
- 38.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 39.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169:443–453. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 40.Calderón MA, Casale TB, Togias A, Bousquet J, Durham SR, Demoly P. Allergen-specific immunotherapy for respiratory allergies: from meta-analysis to registration and beyond. J Allergy Clin Immunol. 2011;127:30–38. doi: 10.1016/j.jaci.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 41.Peters AT, Spector S, Hsu J, Hamilos DL, Baroody FM, Chandra RK, et al. Diagnosis and management of rhinosinusitis: a practice parameter update. Ann Allergy Asthma Immunol. 2014;113:347–385. doi: 10.1016/j.anai.2014.07.025. [DOI] [PubMed] [Google Scholar]