Abstract
Epigenetic silencing of steroidogenic factor 1 (SF1) is lost in endometriosis, potentially contributing to de novo local steroidogenesis favoring inflammation and growth of ectopic endometrial tissue. In this study, we examine the impact of SF1 expression in the eutopic uterus by a novel mouse model that conditionally expresses SF1 in endometrium. In vivo SF1 expression promoted the development of enlarged endometrial glands and attenuated estrogen and progesterone responsiveness. Endometriosis induction by autotransplantation of uterine tissue to the mesenteric membrane resulted in the increase in size of ectopic lesions from SF1-expressing mice. By integrating the SF1-dependent transcriptome with the whole genome binding profile of SF1, we identified uterine-specific SF1-regulated genes involved in Wingless and Progesterone receptor-Hedgehog-Chicken ovalbumin upstream promoter transcription factor II signaling for gland development and epithelium-stroma interaction, respectively. The present results indicate that SF1 directly contributes to the abnormal uterine gland morphogenesis, an inhibition of steroid hormone signaling and activation of an immune response, in addition to previously postulated estrogen production.
Endometriosis is an estrogen-driven chronic disease defined by the presence of endometrial glands and stroma in the pelvic peritoneum and other extrauterine areas (1). Peritoneal fluid in women with endometriosis has been previously shown to contain elevated levels of cytokines, growth factors, and activated macrophages likely due to the presence of the ectopic tissue inherent in the disease process (2, 3). The heightened inflammatory response associated with endometriosis is thought to contribute to the severe pelvic pain frequently experienced by patients and to compromise multiple aspects of fertility, including sperm function, rates of embryo implantation, and embryo viability (4, 5). Currently, there is no consensus on the histological origin of endometriosis. However, Sampson has hypothesized that the endometriosis occurs as a result of retrograde menstruation (6). Although most women experience retrograde menstruation, only 10% of reproductive-aged women develop endometriosis. Epigenetic alterations of the chromatin landscape in endometrial tissue of some women are hypothesized to result in molecular abnormalities that subsequently functionally disrupt its normal responsiveness to steroid hormone regulation. These events collectively enhance the estrogen-driven survival, persistence, and growth of stromal and epithelial cells at the ectopic sites (7–9).
The ovary is the main site of steroid synthesis via the conversion of cholesterol to estrogen. Interestingly, it has been shown that endometriotic lesions aberrantly overexpress the entire repertoire of steroidogenic enzymes including STAR, CYP11A1, CYP17A1, and CYP19A1. This is believed to promote de novo local estradiol (E2) synthesis that could support the growth of lesions independent of ovarian E2 (10). Expression of the steroidogenic genes in the ovary is regulated by the nuclear receptor subfamily 5, group A, member 1 (NR5A1), commonly known as steroidogenic factor 1 (SF1). SF1 also regulates the transcription of many genes involved in gonadal development, sexual differentiation, and function of the hypothalamus and pituitary (11, 12). Expression of SF1 in the endometrium is silenced by heavy promoter methylation. Aberrant demethylation of the SF1 promoter in endometriosis results in the up-regulated expression of SF1 (13, 14). In turn, de novo SF1 activation is thought to orchestrate the expression of the steroidogenic enzymes and play a pivotal role in sustained survival of endometrial tissue at the ectopic sites by promoting a hyper estrogenic state (15). The primary mediator of estrogen action in the endometrium is estrogen receptor-α (ESR1) and is encoded by the ESR1 gene (10). However, stromal cells derived from ovarian endometriomas present elevated mRNA and protein expression levels of the estrogen receptor-β (ESR2) relative to the normal endometrium as a result of aberrant hypomethylation of the ESR2 promoter (11). The altered ratio of ESR1 to ESR2 is thought to also disrupt expression of the progesterone receptor (PGR). As a result of altered expression of the nuclear receptors, hormone signaling is altered in endometriosis. Collectively, these perturbations are proposed to increase estrogen-dependent proliferation and progesterone (P4)-resistance of endometriotic lesions (12–14).
The goal of this study is to investigate the expression of SF1 in endometriosis and to determine the transcriptional role of SF1 in endometrium in vivo in order to elucidate its functional role in endometriosis. To accomplish this goal, we developed a mouse model in which SF1 expression was activated in a Cre recombinase-dependent manner. We demonstrated that activation of uterine expression of SF1 promotes aberrant morphogenesis of the endometrial glands, functionally disrupting endometrial architecture and fertility, and promoting a cystic phenotype in an endometriosis model. Transcriptional targets of SF1 in the endometrium were defined by integrating microarray expression analysis with chromatin immunoprecipitation followed by deep sequencing (ChIP-Seq). This analysis identified the novel role of SF1 in the regulation of factors involved in glandular morphogenesis and in the deregulation of other members of the nuclear receptor family, including Pgr, Esr1, and Nr2f2 (Chicken Ovalbumin Upstream Promoter Transcription Factor II, COUP-TFII). Our data provides new insights into the role of SF1 in vivo beyond its role in steroidogenesis and improves our understanding of the etiology of endometriosis in a manner that can be used to improve the treatment of this complex disease.
Materials and Methods
Animal husbandry
Mice received irradiated Teklad global soy protein-free extruded rodent diet (Harlan Laboratories, Inc) and fresh water ad libitum. Breeding trials were performed by housing 6- to 8-week-old females individually and continuously with males of the B6D2F1 strain. Mating was confirmed by presence of vaginal plugs. Numbers of litters and litter sizes were recorded for a 6-month period. Females were killed 3 weeks after the last litter. At this time point, they were 8 months of age and cycling.
Mouse surgeries
All surgeries were performed using avertin anesthesia (2.5% [vol/vol] solution, 0.02-mL/g body weight) and postoperative pain was alleviated with the analgesic, ketoprofen (5–10 mg/kg of body weight). Ovariectomy was performed on 6- to 8-week-old female mice by exposing the reproductive tract via a dorsolateral incision. The ovaries were ligated with sterile absorbable suture and excised. The peritoneal opening was closed with absorbable suture and the skin closed with wound clips. Females were rested for 2 weeks to deplete endogenous hormones. For the artificial induction of decidualization, ovariectomized females were primed with 3 daily sc injections of 100-ng E2 and then rested for 2 days. Mice subsequently received 3 daily injections of 1-mg P4 and 6.7-ng E2 per mouse sc. One uterine horn was exposed by a dorsolateral incision and stimulated by instillation of sesame oil into the uterine lumen 6 hours after the third injection of P4 and E2. The reproductive tract was returned to the peritoneum and the opening closed as described above. Mice continued to receive daily injections of P4 and E2 until killed at day 5 after surgery.
Endometriosis was induced in 6-week-old females under endogenous cycling hormone conditions. Mice were anesthetized and the abdomen exposed by a midline incision. One uterine horn was longitudinally excised using a Geiger Thermal Cautery Unit. Using a 2-mm dermal biopsy punch, tissue samples was isolated and subsequently sutured to the mesenteric membrane in the same mouse through a midline incision. The peritoneal opening was closed with absorbable suture and the skin closed with wound clips. Mice were allowed to recover for 4 weeks before killing. Two hours before euthanasia, mice received an ip injection of 5-bromo-29-deoxyuridine (BrdU) (GE Healthcare Bio-Sciences) at a dose of 1 mg per 20-g body weight. The growth of ectopic lesions was quantified using the Vernier caliper. Tissues were fixed in 4% vol/vol paraformaldehyde in PBS for histological analysis.
Acute estrogen response
The effects of short-term estrogen exposure were measured in 8- to 10-week-old females 2 weeks after ovariectomy. Mice were administered a single injection of 100-ng E2 or sesame oil, as vehicle control, in a 100-μL sc injection volume. Mice were killed under anesthesia after 18 hours of E2 exposure. Uterine horns were flash frozen in liquid nitrogen for subsequent gene expression analysis.
Ovulation and fertilization study
Six females between 3 and 4 weeks of age were administered 5 IU of pregnant mare serum gonadotropin ip, followed 48 hours later by 5-IU human chorionic gonadotropin ip and housed with wild-type male mice of proven fertility. Mice were killed 24 hours later, and the oviducts were isolated and flushed to determine the number of ova ovulated and fertilized.
Serum hormone assay
Blood was collected from mice at the time of killing by retroorbital bleeding. Sera were isolated with BD Microtainer tubes with serum separator (Becton, Dickinson and Co). Concentration of P4 and E2 were measured by RIA and ELISA, respectively, by The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core.
RNA isolation
Uterine horns were dissected from anesthetized mice 2 weeks after ovariectomy surgery or after each specified hormone treatment as described above. The cervix was discarded. Tissue was homogenized in 1 mL of TRIzol (Thermo Fisher Scientific) 6 times for 10 seconds and rested intermittently for 10 seconds on ice. Homogenates were centrifuged 10 minutes at max speed at 4°C to pellet cellular debris. Supernatant was transferred to a new 1.5-mL centrifuge tube and 200 μL of chloroform was mixed by shaking the tubes. Samples were rested for 3 minutes at room temperature and subsequently centrifuged at max speed for 18 minutes at 4°C. Approximately 600 μL of aqueous layer were transferred to a new tube and mixed with equal parts of 70% ethanol. This mix was filtered in columns from the RNeasy Mini kit (QIAGEN, Inc). Columns were washed once with 700 μL of RWT buffer and 3 times with 500 μL of RPE buffer. RNA was eluted with RNase-free water. RNA from 2 mice was pooled for each control and experimental group for microarray gene expression analysis. Independent samples were used for RNA isolation in experiments validating the microarray results.
RT real-time PCR (RT-qPCR)
RT-qPCR was performed to validate microarray gene expression targets. mRNA was isolated by TRIzol (Thermo Fisher Scientific) extraction per the manufacturer's protocol as described above. RNA was reverse transcribed into cDNA with Moloney murine leukemia virus (Thermo Fisher Scientific) according to manufacturer recommendations. Expression levels of mRNA were determined by RT-qPCR on a QuantStudio 12K Flex Real-Time qPCR system (Thermo Fisher Scientific) using FastStart SYBR Green Master (Roche Diagnostics) and oligonucleotide primers synthesized by Sigma-Aldrich based on sequences deposited in PrimerBank. Gene expression was normalized to 18s rRNA.
Microarray analysis
Expression analysis was conducted in triplicate on samples containing RNA isolated from 2 mouse uteri for each genotype control (SF1LSL/+) and SF1-expressing (Pgrcre/+ SF1LSL/+) mice using the GeneChip Mouse Genome 430 2.0 Array (Affymetrix). CEL files were processed using dChip (PM-MM model, quantile normalization) (16). CEL files deposited to GEO under accession number GSE66963.
ChIP-deep sequencing
ChIP-Seq for SF1 was performed by Active Motif, Inc on uteri from 5 individual Pgrcre/+ SF1LSL/+ mice. The samples were pooled before sonication of chromatin. DNA library generation, sequencing, and mapping to the mouse genome (NCBI37/mm9) assembly was performed by Active Motif as previously described (17); 50-nt sequence reads identified by Illumina's Hi-Seq were mapped to the genome using the Burrows-Wheeler Aligner algorithm with default settings. Only reads that passed Illumina's purity filter, align with no more than 2 mismatches, and map uniquely to the genome were used in the subsequent analysis. The density of fragments (extended in silico with Active Motif software) along the genome was determined by dividing the genome in 32-nt binds. The number of fragments in each bin was determined and stored as BAR files. These peaks, with their associated metrics, were analyzed with model-based analysis of ChIP-Seq. Associated genes were called if SF1 intervals were located within +/− 10 kb of the gene boundaries. Data were deposited to GEO under accession number GSE69543.
Chromatin immunoprecipitation-qPCR
Six independent uterine samples from the Pgrcre/+ SF1LSL/+ mice were submitted to Active Motif for validation for ChIP-Seq binding intervals. The immunoprecipitation reaction was performed on 30 μg of mouse uterus chromatin and 4 μg of SF1 antibody (catalog number 07–618; EMD Millipore). ChIP-qPCR was performed using a positive control primer pair from a previously known SF1-binding site on Star and selected targets (18). Two negative control primer pairs were used to amplify regions in gene deserts on chromosome 6 and 17 (Untr6 and Untr17), with comparable results. Shown here are amplification data for Untr17. Binding levels greater than 2 times the negative control region was considered significant.
Immunohistochemistry
Fixed tissues were embedded in paraffin and sectioned at 5 μm. Slides were heated for 20 minutes at 60°C and cooled for 10 minutes. Sections were dehydrated in 3 washes of xylenes for 5 minutes each and hydrated in ethanol gradients (100%, 95%, and 70%). For eosin and hematoxylin staining, slides were incubated in hematoxylin for 3 minutes, washed with water, and developed in a 10% lithium carbonate solution. Slides were subsequently incubated with eosin for 1 minute, washed and dehydrated in an ethanol gradient and finally in xylene washes. For immunohistochemical staining, hydrated sections were boiled with unmasking solutions (Vector Laboratories) and washed 3 times with PBS. Endogenous peroxidases were quenched by incubating sections with 3% hydrogen peroxide in methanol for 10 minutes in the dark. Sections were washed and blocked with 10% normal goat serum, or 5% bovine serum albumin where appropriate. Sections were incubated over night at 4°C in a humidified chamber with primary antibodies against Nr5a1 (1:2000 dilution; EMD Millipore), MYC-tag (1:200 dilution; Cell Signaling Technology), FOXA2 (1:8000; Seven Hills Bioreagents), PGR (1:400 dilution; DAKO), ESR1 (1:1000 dilution; DAKO), β-catenin (1:100 dilution; Cell Signaling Technology), myeloperoxidase (1:100 dilution; Abcam), or BrdU (1:50 dilution; GE Healthcare Bio-Sciences). After washes in PBS, slides were incubated with corresponding biotinylated secondary antibodies (5 μL/mL) for 1 hour at room temperature in humidified chambers. Secondary antibody was removed and slides were washed trice with PBS. VECTASTAIN Elite ABC Reagent and Vector 3.3′-diaminobenzidine (DAB) peroxidase substrate kits (Vector Laboratories) were used for development of staining and optimized for each antibody condition. Slides were mounted with permount and glass coverslips.
Study approval
All animal studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health and animal protocols approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine under protocol number AN4203. All human subject tissues collected for this study were collected with Baylor College of Medicine, Institutional Review Board (H32749) approval. Written informed consent was received from participants before inclusion in the study. The patient population has been described previously (19).
Statistics
Statistical analysis of gene expression changes by RT-qPCR, serum E2 and P4 levels, ectopic lesion volume, gland number and size, and quantified epithelial proliferation were performed with GraphPad InStat version 3.06 employing an unpaired 2-tailed Student's t test and one-way ANOVA followed by Tukey multiple comparison post hoc test where appropriate.
Results
Uterine expression SF1
An SF1LSL/+ conditional allele was generated using established methods (20). The SF1LSL allele consists of a CAGGS promoter, a LoxP-STOP-LoxP (LSL) cassette, a cDNA encoding epitope-tagged mouse Nr5a1 (SF1) gene, and a polyadenylation signal. The ubiquitously active CAGGS hybrid promoter consists of the chicken β-actin promoter and the cytomegalovirus enhancer (21). The LSL cassette prevents expression of the downstream Nr5a1 gene unless excised by cre recombinase. The epitope-tag consists of a tandem FLAG-Myc tag fused in-frame to the 5′ end of the Nr5a1 gene. The mini gene contains a 3′ rabbit globin polyadenylation signal serving as the polyadenylation signal. This mini gene was targeted to the ROSA26 locus by homologous recombination (Supplemental Figure 1A).
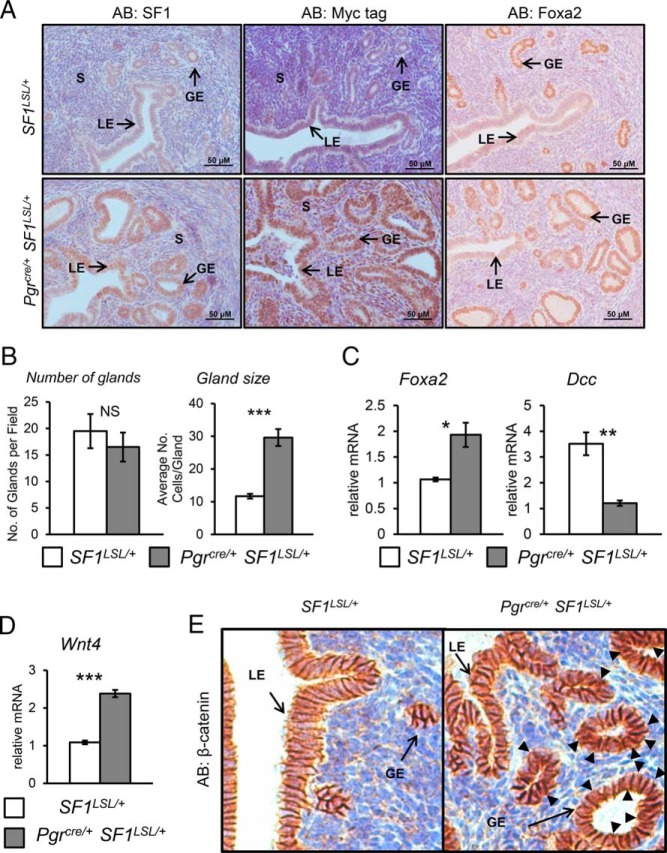
To generate a mouse with uterine expression of SF1, the Pgrcre/+ mouse (22) was crossed with the SF1LSL/+ mouse line. This breeding strategy allowed us to conditionally express SF1 in the Pgrcre/+ SF1LSL/+ background and use littermates carrying only the Pgrcre/+ or the SF1LSL/+ alleleas controls (Supplemental Figure 1A). Quantitative real-time PCR (qPCR) of ovariectomized 8- to 10-week-old endometrium shows that significantly elevated levels of Nr5a1 transcripts were generated in Pgrcre/+ SF1LSL/+ females as compared with SF1LSL/+ control females (Supplemental Figure 1B). Elevated levels of SF1 can also be detected in the luminal and glandular epithelium, as well as the stromal compartment of the uterus when visualized by immunohistochemical staining with antibodies for SF1 and the Myc epitope tag (Figure 1A). Notably, expression of SF1 resulted in an abnormal morphology of the uterine glands. Quantification of endometrial glands, marked by FOXA2 staining, determined that there was no difference in the total number of glands between genotypes. However, Pgrcre/+ SF1LSL/+ mice presented glands that were twice the size in cell number as the wild-type control (Figure 1B). Consistent with the increase in number of FOXA2-positive cells by immunohistochemistry, we determined an increase in Foxa2 message by qRT-PCR. To define the differentiation status of these glands, we compared the expression of the tumor suppressor gene deleted in colon carcinoma (Dcc) between genotypes. DCC has been proposed to be a negative regulator of maintenance of differentiated endometrial glandular cells during the menstrual cycle (23). Additionally, DCC and its ligand, netrin-1, are proposed to regulate the commitment of endometrial gland architecture and function in the transition from the proliferative to secretory phase (24). In the uteri of Pgrcre/+ SF1LSL/+ females, we observed a robust decrease in the expression of Dcc, suggesting that these glands were more differentiated (Figure 1C).
Figure 1.
Enhanced glandular development. A, Immunohistochemical staining for SF1, MYC-tag, and FOXA2 in representative uterine cross-sections of 8- to 10-week-old ovariectomized females. B, Quantification of glands and glandular size. C, RT-qPCR analysis for Foxa2 and Dcc message. D, RT-qPCR analysis for Wnt4 mRNA levels. E, Immunostaining of β-catenin. S, stroma; GE, glandular epithelium; LE, luminal epithelium. Two tailed t test significance indicated by ***, P < .001; **, P < .01; *, P < .05 and NS, not significant. SF1LSL/+ n = 8 and Pgrcre/+ SF1LSL/+ n = 6.
Aside from Foxa2 regulation of endometrial gland development, it has been demonstrated that Wnt4 and β-catenin are critical regulators or endometrial adenogenesis. Ablation of Wnt4 results in the loss of endometrial glands and conditional uterine expression of β-catenin with a deletion of exon 3 resulting in glandular hyperplasia (25, 26). To determine whether SF1 expression altered the β-catenin pathway, we assayed the expression of Wnt4 mRNA by real-time (RT-PCR) and β-catenin expression by immunohistochemistry. Expression of Wnt4 mRNA (Figure 1D) was elevated and immunohistochemical analysis (Figure 1E) shows that β-catenin protein was increased in the uterine epithelium. This would indicate that aberrant expression of SF1 in the uterus results in activation of the Wnt-β-catenin pathway and the promotion of adenogenesis.
The Pgrcre exhibits activity during a brief window in the corpus luteum in the ovary. This led us to evaluate key ovarian functions including ovulation and hormone production. To evaluate the ability of SF1 mice to produce oocytes, 3-week-old females were stimulated with gonadotropins and mated with wild-type males. At day 0.5 of pregnancy, ova were recovered from the oviduct. No difference was observed in the number of fertilized ova present between Pgrcre/+ control and Pgrcre/+ SF1LSL/+ overexpressing females (Supplemental Figure 1C). Serum concentrations of P4 and E2 after gonadotropin stimulation were not significantly different between genotypes (Supplemental Figure 1D). Additionally, uterine and ovarian E2 levels were not altered (Supplemental Figure 1, E and F). Collectively, these results indicate that Pgrcre/+ SF1LSL/+ overexpressing females retain normal ovarian and luteal function.
SF1 expression results in infertility
To determine the impact of SF1 expression on female fertility, SF1LSL/+ control and Pgrcre/+ SF1LSL/+ overexpressing females were mated with wild-type males of proven fertility in a 6-month fertility trial. Uterine expression of SF1 had a negative impact on fertility causing infertility in all 5 of the females tested (Figure 2A). Gross examination of uteri at the end of this breeding trial revealed abnormal morphology. The Pgrcre/+ SF1LSL/+ mice have enlarged and translucent uterine horns (Figure 2B). Quantification of uterine weight determined that these fluid filled uteri were significantly larger than controls (Figure 2C). Histological examination of uterine cross-sections stained with eosin and hematoxylin revealed a severely altered endometrial architecture. Pgrcre/+ SF1LSL/+ uteri contained numerous cystic glands (Figure 2D).
Figure 2.
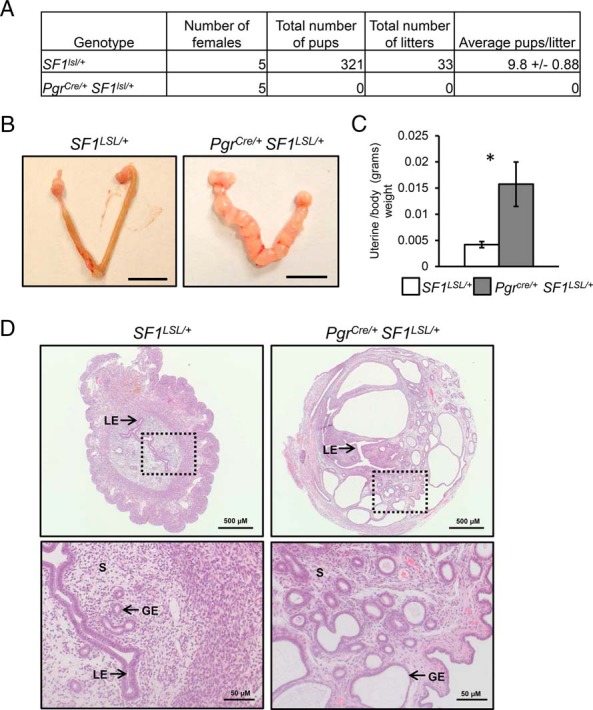
An aging uterine phenotype. A, Summary of litter production in a 6-month breeding period. B, Gross morphology of uterine horns in 8-month-old cycling females. Scale bar, 1 cm. C, Uterine to body weight ratio. Significance indicated by *, P < .05. D, Eosin and hematoxylin staining of uterine cross-sections. Lower panels are high magnification of the boxed area in top panel. Arrows indicate endometrial compartments: S, stroma; GE, glandular epithelium; LE, luminal epithelium. Two tailed t test significance indicated by *, P < .05. SF1LSL/+ n = 5 and Pgrcre/+ SF1LSL/+ n = 5.
Absent decidual response in SF1-expressing uteri
The endometrium undergoes a complex process of differentiation in response to cyclical stimulation by the ovarian hormones estrogen and P4 acting via their cognate nuclear receptors, the estrogen receptor and PGR. We first analyzed expression of Pgr and Esr1 mRNA and protein in the uterus of ovariectomized mice to determine the basal level of these receptors in the unstimulated state. We observed a significant down-regulation of Pgr and Esr1 message in Pgrcre/+ SF1LSL/+ uteri by qRT-PCR (Supplemental Figure 2A) in ovariectomized mice. Immunohistochemical staining for Pgr determined that the endometrial glands had absent expression of PGR and only cells in the mesometrial pole of luminal epithelium contained Pgr immunopositive cells. Immunohistochemical staining for Esr1 demonstrated that protein expression was retained in the luminal and glandular epithelium, but attenuated in the stromal compartment of the Pgrcre/+ SF1LSL/+ endometrium (Supplemental Figure 2B). In order to determine whether the reduction in expression of Esr1 in the uterine stroma was functional, ability of estrogen to stimulate the expression of the endometrial stroma ESR1 target gene, Igf1, was assayed (27). Analysis of Igf1 mRNA in ovariectomized mice treated with vehicle or estrogen showed that expression of SF1 affected the ability of Igf1 mRNA to be induced by estrogen. This evidence indicated a loss of stromal Esr1 function (Supplemental Figure 2C).
The aberrant expression of Pgr led us to evaluate the ability of uteri in Pgrcre/+ SF1LSL/+ in 8-week-old adult females to decidualize in response to a well-defined regimen of estrogen and P4 (28). We conducted a hormonal induced decidual response in ovariectomized Pgrcre/+ SF1LSL/+ and control SF1LSL/+ mice to determine whether these uteri were able to undergo this reaction. Deciduogenic stimulation of the right uterine horn in control mice resulted in the robust increase in horn biomass compared with the unstimulated horn. This was readily observable by gross morphology. However, this response was absent in Pgrcre/+ SF1LSL/+ uteri (Supplemental Figure 3A). Eosin and hematoxylin staining of uterine cross-sections confirmed this result at the histological level by revealing that the stimulated horn of control mice contained large decidual cells, infiltration of blood vessels, and a lack of luminal epithelium. In contrast, stimulated horns in the Pgrcre/+ SF1LSL/+ mice appeared indistinguishable from unstimulated control horns (Supplemental Figure 3B). Expression of molecular markers of decidualization Wnt4 and Bmp2 were evaluated by qRT-PCR. Induction of Bmp2 and Wnt4 with decidual stimulus was observed in the control mice. However, Pgrcre/+ SF1LSL/+ mice failed to induce Bmp2, and robustly expressed Wnt4 independently of decidual stimulus (Supplemental Figure 3C). Collectively, this evidence indicates that endometrial expression of SF1 inhibits the ability of the endometrium to undergo decidualization due to the altered expression of the Pgr, known to be critical for this process (29). Furthermore, SF1 expression induced a decidual independent expression of Wnt4.
SF1 favored the growth of ectopic endometrial lesion
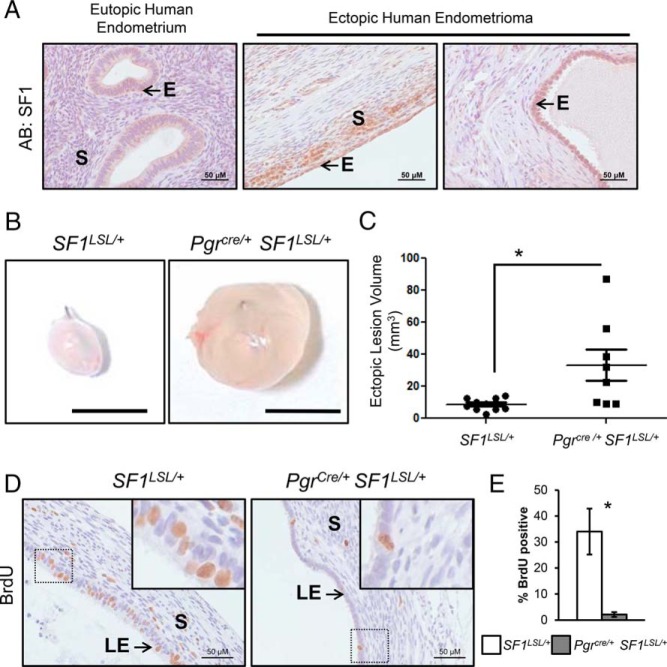
We sought to define the expression pattern of SF1 in healthy endometria and endometriotic tissue in humans. Consistent with published studies (30), expression of SF1 was not detected in the eutopic endometrium of healthy volunteers or the matched endometria of patients undergoing surgical resection of ovarian endometriomas. Expression of SF1 was observed in epithelial and stromal cells of these human ovarian endometriomas (Figure 3A). SF1 expression in endometriosis has been proposed to favor growth in ectopic endometriotic lesions (15). To evaluate this hypothesis in the Pgrcre/+ SF1LSL/+ mice, we surgically induced endometriosis in adult, ovary intact, females by auto transplantation of a 2-mm punch biopsy of endometrial tissue into the mesenteric membrane (31). The implants were allowed to develop in the mice without the administration of exogenous estrogen. Four weeks after surgery, we observed that endometrial tissue of both genotypes established spherical ectopic lesions (Figure 3B). Quantification of ectopic lesion volume revealed that the average volume of endometric lesions in Pgrcre/+ SF1LSL/+ mice was significantly larger than that of control lesions (Figure 3C). Histological staining of BrdU incorporation revealed lesions from control mice contained an extensive number of cells undergoing proliferation in the luminal epithelium and to a lesser extent in the stroma. SF1 ectopic lesions did not present the same pattern of proliferative activity (Figure 3D). Epithelial cell proliferation was quantified and indicated a significant decrease in the number of BrdU-positive cells in the Pgrcre/+ SF1LSL/+ lesions (Figure 3E). Interestingly, the luminal epithelial cells of Pgrcre/+ SF1LSL/+ lesions appeared cuboidal, as cells found in secretory glands. In contrast, the epithelial cells of control mice appeared to have a columnar morphology, as cells found in the luminal epithelium in eutopic healthy uteri. Most notably, most the lesion volume in Pgrcre/+ SF1LSL/+ mice was comprised by a large, fluid-filled central lumen. Analysis of the expression of PGR and ESR1 in these explants from Pgrcre/+ SF1LSL/+ and control SF1LSL/+ mice showed that expression of SF1 resulted in loss of PGR staining in the epithelial compartment of the lesion. However, both ESR1 and phospho-ESR1, an indicator of activated Estrogen receptor alpha (ERα), was not altered between Pgrcre/+ SF1LSL/+ and control SF1LSL/+explants (Supplemental Figure 4).
Figure 3.
SF1 in endometriosis. A, Immunohistochemical staining of SF1 in human eutopic endometrium and ovarian endometriomas. S, stroma; E, epithelium. B, Gross morphology of ectopic lesions in a murine endometriosis model. C, Quantification of ectopic lesion size. D, Immunohistochemical staining for BrdU incorporation in proliferating cells of ectopic lesions. E, Quantification of epithelial proliferation. Two tailed t test significance indicated by *, P < .05. SF1LSL/+ n = 9 and Pgrcre/+ SF1LSL/+ n = 7.
Microarray analysis of the SF1 transcriptome
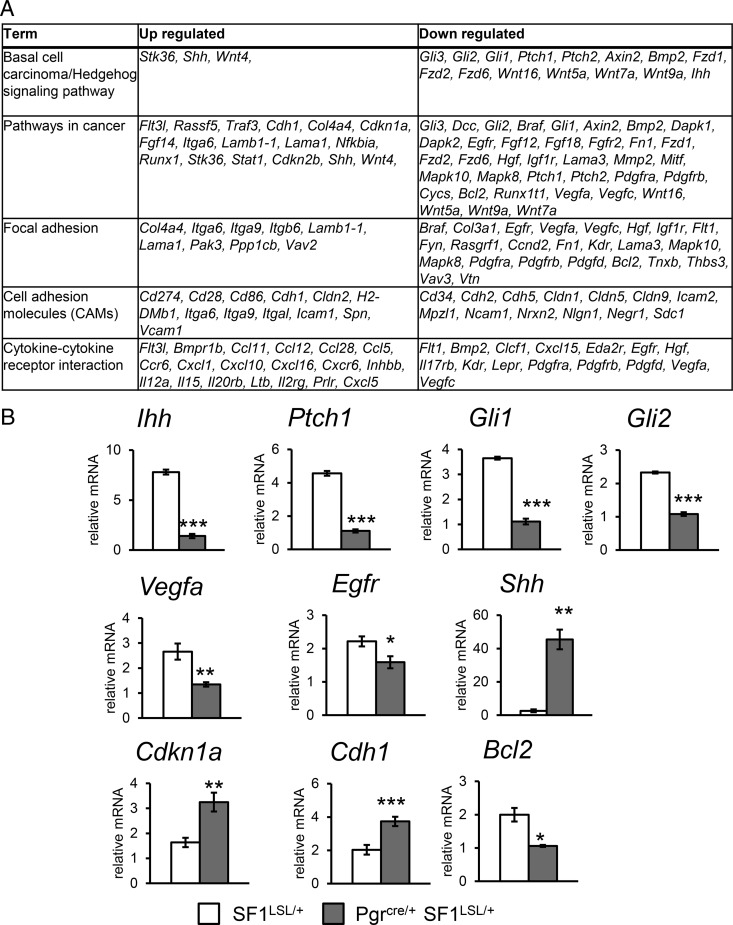
We sought to define the underlying mechanism for the SF1 endometrial phenotype. SF1 is an essential transcriptional regulator for many genes involved in steroidogenesis in the adrenal glands and the gonads. In endometrial tissue, SF1 is hypothesized to be sufficient to activate expression of the genes encoding for enzymes involved in the estrogen synthesis pathway. In order to define the uterine tissue-specific transcriptional targets of SF1 in an ovarian hormone independent basal state, we conducted a microarray analysis comparing control SF1LSL/+ uteri and Pgrcre/+ SF1LSL/+ uteri 2 weeks after ovariectomy. There were 2795 gene probes with P < .01 and fold change more than 1.4 (log-transformed data) between control and experimental groups, corresponding to 1992 unique genes (Supplemental Table 1). Figure 4A summarizes the top pathways enriched in the Kyoto Encyclopedia of Genes and Genome (KEGG) functional pathway analysis. This analysis identified SF1-regulated genes that were involved in pathways including hedgehog signaling, cancer, focal and cell adhesion, and cytokine-cytokine receptor interaction. These pathways are known to be involved in uterine development and endometrial function in early pregnancy. SF1 expression resulted in the down-regulation of the Pgr-regulated Ihh signaling pathway, Ihh Gli1, Gli2, and Ptch1, and the up-regulation of Shh. The Pgr-regulated Ihh pathway has been shown to inhibit endometrial gland formation (32, 33). Shh expression could be compensating for the loss of the Ihh expression. Bcl2, Egfr, and Vegfa were among the genes involved in cancer pathways that were down-regulated with SF1 expression by qRT-PCR validation. Other genes in this category, including Cdh1 and Cdkn1a were up-regulated with SF1 expression (Figure 4B).
Figure 4.
KEGG pathways enriched in genes differentially regulated by SF1 expression in murine endometrium. A, Top KEGG pathway terms. B, RT-qPCR validation of gene expression in uteri of 8- to 10-week-old ovariectomized females. Two tailed t test significance indicated by *, P < .05; **, P < .01; ***, P < .001. SF1LSL/+ n = 8 and Pgrcre/+ SF1LSL/+ n = 6.
SF1 expression disrupts regulators of uterine morphology and immune cell trafficking
We employed ingenuity pathway analysis (IPA) to identify up-stream molecular regulators and the targets they regulated among the genes that exhibited differential regulation in the Pgrcre/+ SF1LSL/+ uteri (Supplemental Table 2). We focused our analysis on transcription factors that were deregulated by SF1 expression and identified several downstream effectors of P4 signaling including the following: Hoxa11, Hoxa10, Hand2, Msx1, Klf4, and Klf9. Notably, we observed an inhibition of expression for the homologue of SF1, Nr5a2, commonly known as liver receptor homologue 1, and Nr2f2. We also observed a robust up-regulation of Hnf4a and Gata6 (Supplemental Figure 5A). Interestingly, although we observed a down-regulation of Esr1 in the stroma as well as the estrogen regulation of a stroma target gene, Igf1 (Supplemental Figure 2C), the expression of known ESR1 targets in the epithelial cells, including Greb1 (34) and Ltf (35), was up-regulated in the Pgrcre/+ SF1LSL/+ uteri. The elevated ESR1 targets in the epithelium reflect the loss of PGR that results in loss of induction of Hand2, which represses epithelial Esr1 signaling (36).
We followed this analysis with an IPA annotation of the SF1-regulated genes based on known diseases and functional categories. IPA predicted the activation of multiple developmental and morphological pathways with functions including the morphology of the central nervous system, as well as the morphology of the genital organs. Most notably, the SF1 gene signature indicated an activation of inflammatory-response pathways (Supplemental Table 3), which is supported by an increased presence of neutrophils in the Pgrcre/+ SF1LSL/+ uteri (Supplemental Figure 5B). IPA also predicted the decrease in activation state of functions including vasculogenesis, size, fertility, and proliferation of epithelial cells (Supplemental Table 3).
Evaluation of SF1-binding sites in the uterus
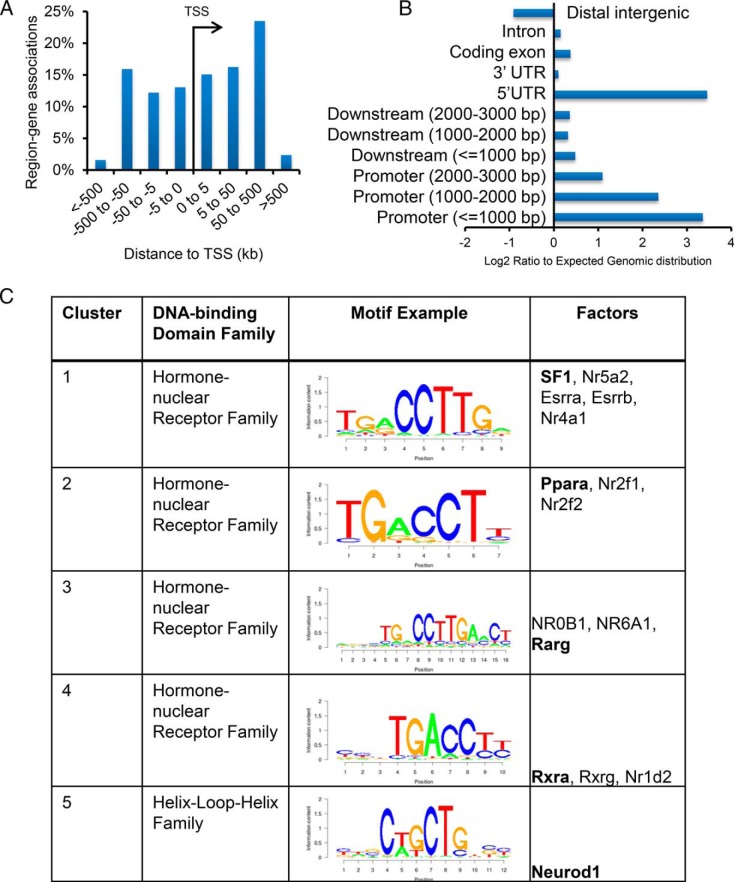
To define the global binding of SF1 and identify the direct genomic targets, ChIP-Seq for SF1 was performed in uterine samples from adult Pgrcre/+ SF1LSL/+ mice collected 2 weeks after ovariectomy. The number of tags (unique alignments without duplicate reads) was 11 million. Model-based analysis of ChIP-Seq algorithm (37) was run at default settings to identify 16 249 peaks with a P value of 10−7 and at an empirical False discovery rate of 0.13%. Peak distribution analyses indicated most intervals were localized within 500 kb of transcription start sites (Figure 5A). Cis-regulatory element annotation system enrichment analysis (38) determined these intervals showed significant enrichment for promoter regions and 5′-Untranslated Region relative to the genome reference (Figure 5B). Intervals within 10 kb of gene boundaries were associated with 10 842 genes (Supplemental Table 4). SF1-binding intervals were evaluated for enriched motifs near peak centers using the SeqPos tool in Cistrome (39). This analysis revealed a significant enrichment for motifs recognized by the hormone-nuclear receptor family, including the NR5A1 motif. Among the motifs enriched in the SF1-binding intervals were those recognized by the Peroxisome proliferator-activated receptor alpha (PPARA), NR2F1, NR2F2, and the retinoid X receptor (RXRs). The helix-loop-helix family was enriched in Cluster 3 and represented by the M01288 motif recognized by NEUROD1 (Figure 5C).
Figure 5.
Global genomic binding of SF1 in the endometrium determined by ChIP-Seq. A, Distribution of SF1-binding intervals around transcriptional start site of annotated genes in uteri of 8- to 10-week-old ovariectomized females. B, Enrichment of SF1-binding intervals relative to expected genomic distribution. C, SeqPos motif enrichments analysis of SF1-binding intervals identified the top enriched motifs with representative motif depicted by a graph of the position-specific scoring matrix.
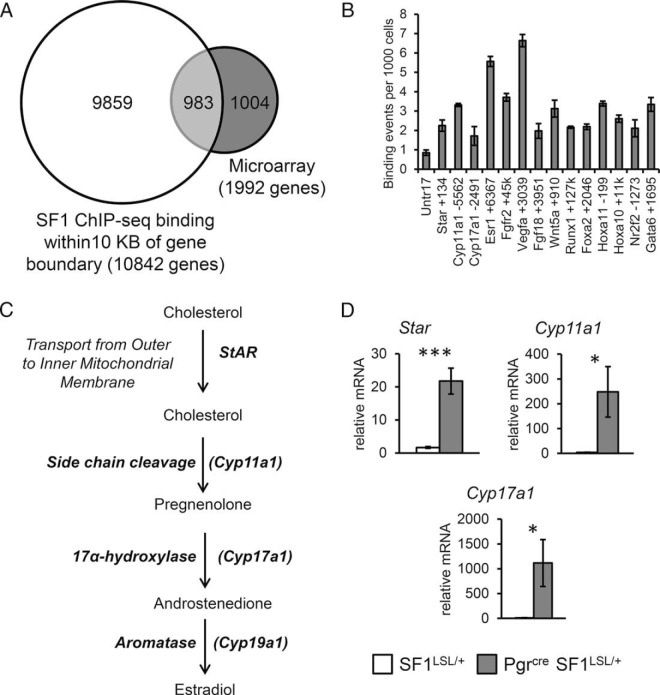
Validation of direct targets of SF1
Figure 6A shows a Venn diagram comparing the SF1-regulated genes according to the microarray and those that were bound by SF1 within 10 kb of genomic boundaries. Gene ontology analysis with DAVID bioinformatics resources identified these 983 direct gene targets were enriched for biological themes including tube, epithelium and gland development, patterning and tissue morphogenesis, and regulation of cell proliferation (Supplemental Table 5). Interestingly, this gene signature did not enrich for terms associated with steroidogenesis, as was expected for a transcriptional regulator of steroidogenic genes. This evidence indicated that endometrial expression of SF1 in vivo manifests a developmental gene signature characterized by altered expression of genes involved in epithelial development.
Figure 6.
Validation of direct SF1 targets. A, Venn diagram comparison of genes bound by SF1 within 10 kb of gene boundaries and genes with differential expression by microarray in uteri of 8- to 10-week-old ovariectomized females. B, ChIP-qPCR validation of direct targets in independent uterine samples from 8- to 10-week-old ovariectomized females. C, Schematic for genes encoding enzymes for synthesis of E2 from the cholesterol precursor. D, RT-qPCR validation of expression of steroidogenic genes in independent uterine samples from 8- to 10-week-old ovariectomized females. Two tailed t test significance indicated by *, P < .05 and ***, P < .001. SF1LSL/+ n = 8 and Pgrcre/+ SF1LSL/+ n = 6.
We followed this analysis with the validation of SF1 ChIP-Seq-binding sites by ChIP-qPCR performed on chromatin extracts from independent samples. Binding events per 1000 cells were confirmed for several canonical targets of SF1, including Cyp11a1, Cyp17a1, and Star (40). Novel SF1 targets include Esr1, Fgfr2, Vegfa, Fgf18, Wnt5a, Runx1, Foxa2, Hoxa11, Hoxa10, Nr2f2, and Gata6 (Figure 6B). Expression of SF1 in ovarian tissues or endometriotic HESC has been shown to be sufficient for the expression of the steroidogenic enzymes that synthesize E2 from the precursor cholesterol (Figure 6C) (41). We evaluated the expression of these enzymes by qRT-PCR and determined that Star, Cyp11a1 and Cyp17a1 were robustly expressed in Pgrcre/+ SF1LSL/+ uteri (Figure 6D). We were unable to detect binding of SF1 near the genomic locus for Cyp19a1 (aromatase) and expression of Cyp191a was at the limits of detection in Pgrcre/+ SF1LSL/+ uteri. This analysis demonstrates that although endometrial expression of SF1 is sufficient to up-regulate a subset of steroidogenic genes, it also functions to disrupt the expression of important endometrial-specific genes via direct interaction with regulatory gene regions.
Discussion
In this study, we have demonstrated that endometrial expression of SF1 in vivo manifests an epithelial dominant phenotype. Our murine model recapitulates the SF1 expression pattern observed in human endometriomas, where expression is seen in both the epithelial and stromal compartment. After activation of SF1 expression in the mouse, we observed a severely altered endometrial morphology in which the stromal component contribution to the overall endometrial architecture was reduced. We expected SF1 expression to promote a hyper estrogenic state characterized by increased levels of proliferation and uterine hyperplasia in the endometriosis model. However, ectopic lesions exhibited a significantly attenuated epithelial proliferation. Furthermore, we determined estrogen response to acute stimulus, measure by expression of the canonical estrogen target Igf1, was abolished in the SF1 uteri. This observation was partially explained by the decrease in the expression of stromal ESR1, paracrine regulator of epithelial proliferation (36).
In addition to the down-regulation of stroma ESR1, we observed a robust down-regulation in the expression of epithelial Pgr, which is known to play a critical role in sensitizing stromal cells to estrogen-induced proliferation (42). Although the loss of PGR expression did not cause an increase in endometrial epithelial proliferation, we did observe a significant impact on fertility. Loss of PGR expression caused a decrease in the Ihh-COUP-TFII-Hand2 signaling axis (43–45). This would explain the observed infertility and lack of uterine decidual response with a concurrent increase in the expression of uterine epithelial estrogen receptor target gene expression. In this regard, SF1 expression disrupts mechanisms underlying paracrine regulation and promotion of proliferation and fertility in the endometrial compartments.
The most striking phenotype exhibited with de novo endometrial expression of SF1 was the increase in glandular epithelial branching and glandular size. Onset of adenogenesis occurs in the neonate and has been found to be ovary-, adrenal-, and steroid-independent (46, 47). This process is sensitive to exogenous P4 stimulation in the neonatal period by altering the expression of Ihh and inhibition of epithelial cell proliferation, leading to a disruption of uterine gland development and resulting in an adult uterine gland knockout (48). After puberty, uterine gland maintenance is dependent on estrogen. Ovariectomy will result in loss of glands after 60 days in the mouse (49). In this study, we sought to evaluate the role of SF1 in the adult uterus by using Pgrcre-mediated activation of the conditional SF1 allele. With time, in intact female mice, we observed an increase in fluid filled uterine glands. Even in ovariectomized mice, where the removal of estrogen should result in diminution of uterine glands, we observed an increase in the glandular compartment of the uterus as judged by an increase in the proportion of FOXA2 negative cells surrounding a luminal space relative to the FOXA2-positive glandular cells. FOXA2 is a marker of glandular cells and a critical regulator of adenogenesis (50). The higher proportion of glandular to luminal cells indicated an increase differentiation of the epithelium to glandular type at the expense of the luminal epithelial.
Close histological examination of ectopic lesions in the endometriosis model revealed that SF1-expressing epithelial cells appeared cuboidal and not the expected columnar shape seen lining the lumen of the uterine endometrium. In a healthy endometrium, luminal epithelial cells deposit mucins and other glycoproteins to maintain an immune barrier during most of the reproductive cycle in the uterus. During the receptive phase, these columnar cells form an embryo permeable barrier that allows for tight regulation of trophoblast invasion. Therefore, this columnar structure has a very specific function in the uterine epithelium. In contrast, cuboidal cells are usually found lining ducts of glands, where they serve secretory functions (51). The morphological alteration observed in the SF1 lesions indicates a shift in epithelial identity. These epithelial cells possibly acquired a more secretory phenotype, consistent with the observed increase in fluid filled central lumen of the lesions.
Ontology analysis of direct targets of SF1 identified enrichment for terms related to epithelial development and included known regulators of adenogenesis including Foxa2 and Wnt4. It is possible that binding of SF1 on Foxa2, shown in Figure 6B, positively regulated its expression in epithelial cells and drives the morphological differentiation of luminal cells to glandular cells. SF1 binding was also found near the promoter of Wnt4 (52). The role of Wnt4 as a critical regulator of endometrial gland development and the maintenance of epithelial differentiation was demonstrated in the murine endometrium (25).
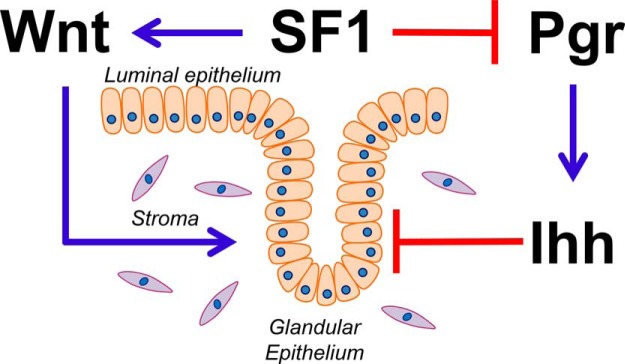
In addition to the activation of Wnt4, the ectopic expression of SF1 resulted in down-regulation of Pgr signaling and decreased signaling of the Ihh pathway. It has been shown that activation of Ihh signaling by epithelial expression of Pgr neonatally results in inhibition of gland formation (33). In addition, expression of a constitutively activated IHH receptor, Smoothened, in the uterus resulted in loss of endometrial glands (32). A model for the action of SF1 on uterine gland development is shown in Figure 7. SF1 activation of WNT signaling and inhibition of the PGR-IHH pathway result in activation of pathways promoting adenogenesis while inhibiting pathways that interfere with adenogenes. In addition to the increase in number and size of the endometrial glands, there is altered glandular differentiation as shown by the decrease in expression of Dcc. Expression of Dcc was inhibited in SF1-expressing endometrium. Endometrial glands express the tumor suppressor Dcc and its ligand Netrin1 in the proliferative and early secretory phase of the menstrual cycle. Glands in the late secretory phase silence Dcc expression. In endometrial cancer cell lines, Dcc expression is lost, and restored expression of Dcc in the absence of the ligand induces apoptosis (24, 53). Collectively, this evidence suggests that in the epithelium, SF1-dependent up-regulation of Foxa2 and Wnt4 accompanied with the down-regulation of Ihh and Dcc may promote the increased invasiveness and persistence of glandular epithelium, culminating in a cystic glandular phenotype.
Figure 7.
A model for the role of SF1-dependent regulatory network for uterine gland formation. SF1 promotes gland formation by increasing Wnt signaling and suppressing the Pgr-Ihh axis.
Annotation of the murine SF1 endometrial gene signature indicated an activation of developmental and inflammatory pathways. Among the pathways in the developmental categories were the central nervous system and morphology of the genital organ. This functional annotation is consistent with the known role of SF1 in the development and function of the entire reproductive axis, including the hypothalamic-pituitary-gonadal axis and sex determination in the reproductive track (12, 54). Most notably, we observed an activation of functions associated with inflammatory pathways. This evidence suggests that de novo expression of SF1 in the endometrium alters endometrial immune homeostasis and triggers a physiological inflammatory response. In this regard, this study is the first to provide in vivo evidence for the potential role of SF1 in the activation of inflammation and could provide novel insights into the inflammatory etiology of endometriosis.
Endometrial expression of SF1 resulted in the up-regulation of Gata6. Gata6 was demonstrated to exhibit up-regulation in endometriotic stromal cells where it is proposed to act as regulator of the endometriotic phenotype (55). SF1 regulation of Gata6 appears to be direct as suggested by the presence of an SF1-binding site near the Gata6 genomic boundary. Similarly, we observed SF1 binding and repression of 2 members of the Hox family of proteins, Hoxa10 and Hoxa11. Hox proteins are necessary for endometrial growth, differentiation, and implantation. Expression of these Hox proteins is normally regulated by the ovarian hormones and peak around the time of implantation in the healthy endometrium. However, HOXA10 hyper methylation in the endometrium of women with endometriosis results in the decreased expression of HOXA10 (56). This study provides first evidence for the SF1-dependent regulation of Gata6 and Hoxa10 in the endometrium in the context of endometriosis.
SF1 has been proposed to promote a hyper estrogenic state in endometrial cells at ectopic sites by up-regulating the expression of steroidogenic enzymes that catalyze the local production of E2. We observed that SF1 was bound near the promoters of Cyp11a1, Cyp17a1, and Star. Binding was accompanied by the robust up-regulation in mRNA expression. Interestingly, we did not observe binding on Cyp19a1, the final enzyme required for the aromatization of androgens to estrogens. Similarly, we were not able to detect an up-regulation in Cyp19a1 message. The lack of binding and regulation of Cyp19a1 in the SF1-expressing murine endometrium was an unexpected finding in this study. However, several explanations may be presented for this unexpected observation. It is known that in the healthy endometrium the promoter of aromatase is also hyper methylated and silenced. Hyper methylation of regulatory regions restricts accessibility of transcription factors and nuclear receptors to their genomic targets and is critical for limiting gene expression in cell-specific contexts. These epigenetic markers on regulatory regions can be revised by pioneer factors and chromatin modifying enzymes and result in the opening of chromatin. In endometriosis, the global methylation pattern undergoes major modifications (8). SF1 overexpression has been reported to result in substantial rearrangements in accessible sites of chromatin in H295R cells, an epithelial cell line established from an adrenocortical carcinoma tumor (57). Although we observed numerous binding sites of SF1 across the genome, including most of the canonical SF1 targets, it remains to be determined whether de novo endometrial SF1 expression is sufficient to modify the accessibility of chromatin. Previous reports have demonstrated that Cyp19a1 is expressed in the postimplantation mouse uterus (58). In the preimplantation uterus, the uterus may lack pioneer factors and methylation status that allows SF1 to bind and regulate Cyp19a1 as in the pregnant mouse uterus. The ability of SF1 to transcriptionally up-regulate the expression of Cyp19a1 may require additional alterations to the epigenetic landscape that were not present in this murine model.
When interpreted as a whole, these observations highlight several directions to pursue in the investigation of the role of SF1 in endometriosis. First, it will be critical to define the endometrial-compartment-specific roles of SF1 in ectopic lesions and how these affect paracrine regulation of cell proliferation and viability. Second, it will be critical to determine whether transcriptional SF1 activity acts synergistically with other epigenetic alterations to promote the endometriotic phenotype. Lastly, it will be beneficial to evaluate the therapeutic potential for targeting SF1 in endometriosis with small molecules that have been identified to modulate SF1 activity in other cellular contexts (59–61).
The goal of this study was to evaluate the transcriptional role of SF1 in endometrium in order to elucidate its functional role in endometriosis, specifically its role in the activation of steroidogenesis. This study provides key evidence to demonstrate that de novo endometrial SF1 expression is not sufficient to activate expression of the all the enzymes involved in the steroidogenic cascade. In endometriotic stromal cells, ablation of endogenous SF1 levels attenuates the expression of all the steroidogenic enzymes. This observation indicates that stromal cells from ectopic endometrial tissues might undergo additional genetic or epigenetic perturbations collectively contributing to aberrant activation of steroidogenesis. However, endometrial SF1 has roles beyond steroidogenesis and its expression disrupts multiple signaling pathways that alter epithelial morphology by promoting differentiation of cells to a glandular phenotype. Finally, SF1 expression alters steroid hormone response by directly deregulating expression of other members of the nuclear receptor family and their targets.
Acknowledgments
We thank Yiqun Zhang for technical assistance with the array analysis and Janet DeMayo for editing.
This work was supported by National Institutes of Health (NIH) Grants R01 HD042311 (to F.J.D.), U54 HD007495, (to F.J.D. and S.M.H.), and NCI P30 CA125123 (C.J.C.). Serum assays for progesterone and estradiol were performed by The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core which is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health (National Centers for Translational Research in Reproduction and Infertility) Grant P50-HD28934.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BrdU
- 5-bromo-29-deoxyuridine
- ChIP-Seq
- chromatin immunoprecipitation followed by deep sequencing
- COUP-TFII
- chicken ovalbumin upstream promoter transcription factor II
- Cyp19a1
- Cytochrome P450, family 19, subfamily A, polypeptide 1
- Dcc
- deleted in colon carcinoma
- E2
- estradiol
- ESR1
- estrogen receptor-α
- ESR2
- estrogen receptor-β
- Foxa2
- Forkhead Box A2
- Hand2
- Heart And Neural Crest Derivatives Expressed 2
- Hox
- Homeobox
- Ihh
- Indian hedgehog
- IPA
- ingenuity pathway analysis
- KEGG
- Kyoto Encyclopedia of Genes and Genome
- LSL
- LoxP-STOP-LoxP
- Myc
- myelocytomatosis oncogene
- NR5A1
- nuclear receptor subfamily 5, group A, member 1
- P4
- progesterone
- PGR
- P4 receptor
- qPCR
- Quantitative polymerase chain reaction
- SF1
- steroidogenic factor 1
- Wnt4
- wingless-type MMTV integration site family, 4member 4.
References
- 1. Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98(3):511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harada T, Iwabe T, Terakawa N. Role of cytokines in endometriosis. Fertil Steril. 2001;76(1):1–10. [DOI] [PubMed] [Google Scholar]
- 3. Iwabe T, Harada T, Terakawa N. Role of cytokines in endometriosis-associated infertility. Gynecol Obstet Invest. 2002;53(suppl 1):19–25. [DOI] [PubMed] [Google Scholar]
- 4. Ryan IP, Taylor RN. Endometriosis and infertility: new concepts. Obstet Gynecol Surv. 1997;52(6):365–371. [DOI] [PubMed] [Google Scholar]
- 5. Kao LC, Germeyer A, Tulac S, et al. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144(7):2870–2881. [DOI] [PubMed] [Google Scholar]
- 6. Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. 1927;3(2):93–110.43. [PMC free article] [PubMed] [Google Scholar]
- 7. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279. [DOI] [PubMed] [Google Scholar]
- 8. Guo SW. Epigenetics of endometriosis. Mol Hum Reprod. 2009;15(10):587–607. [DOI] [PubMed] [Google Scholar]
- 9. Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev. 2013;34(1):130–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bulun SE, Lin Z, Imir G, et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57(3):359–383. [DOI] [PubMed] [Google Scholar]
- 11. Ozisik G, Achermann JC, Jameson JL. The role of SF1 in adrenal and reproductive function: insight from naturally occurring mutations in humans. Mol Genet Metab. 2002;76(2):85–91. [DOI] [PubMed] [Google Scholar]
- 12. Ingraham HA, Lala DS, Ikeda Y, et al. The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes Dev. 1994;8(19):2302–2312. [DOI] [PubMed] [Google Scholar]
- 13. Xue Q, Lin Z, Yin P, et al. Transcriptional activation of steroidogenic factor-1 by hypomethylation of the 5′ CpG island in endometriosis. J Clin Endocrinol Metab. 2007;92(8):3261–3267. [DOI] [PubMed] [Google Scholar]
- 14. Utsunomiya H, Cheng YH, Lin Z, et al. Upstream stimulatory factor-2 regulates steroidogenic factor-1 expression in endometriosis. Mol Endocrinol. 2008;22(4):904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bulun SE, Utsunomiya H, Lin Z, et al. Steroidogenic factor-1 and endometriosis. Mol Cell Endocrinol. 2009;300(1–2):104–108. [DOI] [PubMed] [Google Scholar]
- 16. Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8(1):118–127. [DOI] [PubMed] [Google Scholar]
- 17. Rubel CA, Lanz RB, Kommagani R, Franco HL, Lydon JP, DeMayo FJ. Research resource: genome-wide profiling of progesterone receptor binding in the mouse uterus. Mol Endocrinol. 2012;26(8):1428–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reinhart AJ, Williams SC, Clark BJ, Stocco DM. SF-1 (steroidogenic factor-1) and C/EBP β (CCAAT/enhancer binding protein-β) cooperate to regulate the murine StAR (steroidogenic acute regulatory) promoter. Mol Endocrinol. 1999;13(5):729–741. [DOI] [PubMed] [Google Scholar]
- 19. Hawkins SM, Creighton CJ, Han DY, et al. Functional microRNA involved in endometriosis. Mol Endocrinol. 2011;25(5):821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu SP, Lee DK, Demayo FJ, Tsai SY, Tsai MJ. Generation of ES cells for conditional expression of nuclear receptors and coregulators in vivo. Mol Endocrinol. 2010;24(6):1297–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108(2):193–199. [DOI] [PubMed] [Google Scholar]
- 22. Soyal SM, Mukherjee A, Lee KY, et al. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41(2):58–66. [DOI] [PubMed] [Google Scholar]
- 23. Saegusa M, Hashimura M, Hara A, Okayasu I. Loss of expression of the gene deleted in colon carcinoma (DCC) is closely related to histologic differentiation and lymph node metastasis in endometrial carcinoma. Cancer. 1999;85(2):453–464. [DOI] [PubMed] [Google Scholar]
- 24. Kato HD, Kondoh H, Inoue T, et al. Expression of DCC and netrin-1 in normal human endometrium and its implication in endometrial carcinogenesis. Gynecol Oncol. 2004;95(2):281–289. [DOI] [PubMed] [Google Scholar]
- 25. Franco HL, Dai D, Lee KY, et al. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J. 2011;25(4):1176–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jeong JW, Lee HS, Franco HL, et al. β-Catenin mediates glandular formation and dysregulation of β-catenin induces hyperplasia formation in the murine uterus. Oncogene. 2009;28(1):31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhu L, Pollard JW. Estradiol-17β regulates mouse uterine epithelial cell proliferation through insulin-like growth factor 1 signaling. Proc Natl Acad Sci USA. 2007;104(40):15847–15851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Finn CA, Martin L. Endocrine control of the timing of endometrial sensitivity to a decidual stimulus. Biol Reprod. 1972;7(1):82–86. [DOI] [PubMed] [Google Scholar]
- 29. Fernandez-Valdivia R, Jeong J, Mukherjee A, et al. A mouse model to dissect progesterone signaling in the female reproductive tract and mammary gland. Genesis. 2010;48(2):106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bulun SE, Monsavais D, Pavone ME, et al. Role of estrogen receptor-β in endometriosis. Semin Reprod Med. 2012;30(1):39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Han SJ, Hawkins SM, Begum K, et al. A new isoform of steroid receptor coactivator-1 is crucial for pathogenic progression of endometriosis. Nat Med. 2012;18(7):1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Franco HL, Lee KY, Rubel CA, et al. Constitutive activation of smoothened leads to female infertility and altered uterine differentiation in the mouse. Biol Reprod. 2010;82(5):991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Franco HL, Rubel CA, Large MJ, et al. Epithelial progesterone receptor exhibits pleiotropic roles in uterine development and function. FASEB J. 2012;26(3):1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pellegrini C, Gori I, Achtari C, et al. The expression of estrogen receptors as well as GREB1, c-MYC, and cyclin D1, estrogen-regulated genes implicated in proliferation, is increased in peritoneal endometriosis. Fertil Steril. 2012;98(5):1200–1208. [DOI] [PubMed] [Google Scholar]
- 35. Pentecost BT, Teng CT. Lactotransferrin is the major estrogen inducible protein of mouse uterine secretions. J Biol Chem. 1987;262(21):10134–10139. [PubMed] [Google Scholar]
- 36. Li Q, Kannan A, DeMayo FJ, et al. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science. 2011;331(6019):912–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang Y, Liu T, Meyer CA, et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 2008;9(9):R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shin H, Liu T, Manrai AK, Liu XS. CEAS: cis-regulatory element annotation system. Bioinformatics. 2009;25(19):2605–2606. [DOI] [PubMed] [Google Scholar]
- 39. Liu T, Ortiz JA, Taing L, et al. Cistrome: an integrative platform for transcriptional regulation studies. Genome Biol. 2011;12(8):R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schimmer BP, White PC. Minireview: steroidogenic factor 1: its roles in differentiation, development, and disease. Mol Endocrinol. 2010;24(7):1322–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Attar E, Tokunaga H, Imir G, et al. Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis. J Clin Endocrinol Metab. 2009;94(2):623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vasquez YM, DeMayo FJ. Role of nuclear receptors in blastocyst implantation. Semin Cell Dev Biol. 2013;24(10–12):724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee K, Jeong J, Kwak I, et al. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet. 2006;38(10):1204–1209. [DOI] [PubMed] [Google Scholar]
- 44. Kurihara I, Lee DK, Petit FG, et al. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 2007;3(6):e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee DK, Kurihara I, Jeong JW, et al. Suppression of ERα activity by COUP-TFII is essential for successful implantation and decidualization. Mol Endocrinol. 2010;24(5):930–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bigsby RM, Cunha GR. Effects of progestins and glucocorticoids on deoxyribonucleic acid synthesis in the uterus of the neonatal mouse. Endocrinology. 1985;117(6):2520–2526. [DOI] [PubMed] [Google Scholar]
- 47. Ogasawara Y, Okamoto S, Kitamura Y, Matsumoto K. Proliferative pattern of uterine cells from birth to adulthood in intact, neonatally castrated, and/or adrenalectomized mice, assayed by incorporation of [125I]iododeoxyuridine. Endocrinology. 1983;113(2):582–587. [DOI] [PubMed] [Google Scholar]
- 48. Cooke PS, Ekman GC, Kaur J, et al. Brief exposure to progesterone during a critical neonatal window prevents uterine gland formation in mice. Biol Reprod. 2012;86(3):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nanjappa MK, Medrano TI, March AG, Cooke PS. Neonatal uterine and vaginal cell proliferation and adenogenesis are independent of estrogen receptor 1 (ESR1) in the mouse. Biol Reprod. 2015;92(3):78. [DOI] [PubMed] [Google Scholar]
- 50. Jeong JW, Kwak I, Lee KY, et al. Foxa2 is essential for mouse endometrial gland development and fertility. Biol Reprod. 2010;83(3):396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Filant J, Spencer TE. Uterine glands: biological roles in conceptus implantation, uterine receptivity and decidualization. Int J Dev Biol. 2014;58(2–4):107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dunlap KA, Filant J, Hayashi K, et al. Postnatal deletion of Wnt7a inhibits uterine gland morphogenesis and compromises adult fertility in mice. Biol Reprod. 2011;85(2):386–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kato H, Zhou Y, Asanoma K, et al. Suppressed tumorigenicity of human endometrial cancer cells by the restored expression of the DCC gene. Br J Cancer. 2000;82(2):459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ikeda Y. SF-1: a key regulator of development and function in the mammalian reproductive system. Acta Paediatr Jpn. 1996;38(4):412–419. [DOI] [PubMed] [Google Scholar]
- 55. Dyson MT, Roqueiro D, Monsivais D, et al. Genome-wide DNA methylation analysis predicts an epigenetic switch for GATA factor expression in endometriosis. PLoS Genet. 2014;10(3):e1004158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cakmak H, Taylor HS. Molecular mechanisms of treatment resistance in endometriosis: the role of progesterone-hox gene interactions. Semin Reprod Med. 2010;28(1):69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Doghman M, Figueiredo BC, Volante M, Papotti M, Lalli E. Integrative analysis of SF-1 transcription factor dosage impact on genome-wide binding and gene expression regulation. Nucleic Acids Res. 2013;41(19):8896–8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Das A, Mantena SR, Kannan A, Evans DB, Bagchi MK, Bagchi IC. De novo synthesis of estrogen in pregnant uterus is critical for stromal decidualization and angiogenesis. Proc Natl Acad Sci USA. 2009;106(30):12542–12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Madoux F, Li X, Chase P, et al. Potent, selective and cell penetrant inhibitors of SF-1 by functional ultra-high-throughput screening. Mol Pharmacol. 2008;73(6):1776–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Doghman M, Cazareth J, Douguet D, Madoux F, Hodder P, Lalli E. Inhibition of adrenocortical carcinoma cell proliferation by steroidogenic factor-1 inverse agonists. J Clin Endocrinol Metab. 2009;94(6):2178–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Del Tredici AL, Andersen CB, Currier EA, et al. Identification of the first synthetic steroidogenic factor 1 inverse agonists: pharmacological modulation of steroidogenic enzymes. Mol Pharmacol. 2008;73(3):900–908. [DOI] [PubMed] [Google Scholar]