Abstract
Brain-derived neurotrophic factor (BDNF) expressed in the paraventricular hypothalamus (PVH) has been shown to play a key role in regulating energy intake and energy expenditure. BDNF is also expressed in other hypothalamic nuclei; however, the role in the control of energy balance for BDNF produced in these structures remains largely unknown. We found that deleting the Bdnf gene in the ventromedial hypothalamus (VMH) during embryogenesis using the Sf1-Cre transgene had no effect on body weight in mice. In contrast, deleting the Bdnf gene in the adult VMH using Cre-expressing virus led to significant hyperphagia and obesity. These observations indicate that the lack of a hyperphagia phenotype in the Sf1-Cre/Bdnf mutant mice is likely due to developmental compensation. To investigate the role of BDNF expressed in other hypothalamic areas, we employed the hypothalamus-specific Nkx2.1-Cre transgene to delete the Bdnf gene. We found that the Nkx2.1-Cre transgene could abolish BDNF expression in many hypothalamic nuclei, but not in the PVH, and that the resulting mutant mice developed modest obesity due to reduced energy expenditure. Thus, BDNF produced in the VMH plays a role in regulating energy intake. Furthermore, BDNF expressed in hypothalamic areas other than PVH and VMH is also involved in the control of energy expenditure.
Brain-derived neurotrophic factor (BDNF) is a small, secreted growth factor, and it potently regulates neuronal development and synaptic plasticity (1–3). Furthermore, BDNF and its receptor tropomyosin receptor kinase B (TrkB) are among a few ligand-receptor pairs crucial for the central control of energy balance. Mutations in either the Bdnf or Ntrk2 (encoding TrkB) gene have been shown to lead to marked hyperphagia and severe obesity in both mice and humans (4–10). BDNF is expressed in many hypothalamic regions, including the paraventricular hypothalamus (PVH), ventromedial hypothalamus (VMH), dorsomedial hypothalamus (DMH), and lateral hypothalamus (5, 7). BDNF expressed in the PVH has been shown to potently suppress energy intake and promote adaptive thermogenesis in brown adipose tissues (BATs) (11). However, the role in the control of energy balance for BDNF expressed in other hypothalamic regions has not been clearly established or examined.
Studies have obtained conflicting results with regard to the role of BDNF expressed in the VMH (termed VMH BDNF thereafter) in the control of energy balance. Food deprivation was found to drastically and selectively reduce the Bdnf mRNA level in the VMH (7, 12, 13). Because administration of either a melanocortin analog or glucose into fasted mice increased the Bdnf mRNA level in the VMH, melanocortin and glucose are likely key mediators linking energy status to Bdnf gene expression in the VMH (7, 12). These Bdnf gene expression data suggest that VMH BDNF should play a role in the control of energy balance. Indeed, deleting the Bdnf gene in the DMH and VMH of adult mice via stereotaxic injection of Cre-expressing adeno-associated virus (AAV) was shown to result in modest hyperphagic obesity (12). However, normal body weight was found in mutant mice where the Bdnf gene was specifically deleted in the VMH during embryogenesis using a Cre transgene under the control of the promoter for steroidogenic factor-1 (SF1) (14, 15). Several causes may account for the conflicting results obtained from the 2 types of VMH Bdnf mutant mice. First, the Sf1-Cre transgene may not be able to completely abolish Bdnf gene expression in the VMH, because many BDNF neurons in the adult VMH do not express SF1 (16). Second, the obesity phenotype in mutant mice where Bdnf was deleted in the adult DMH and VMH could be the consequence of DMH BDNF ablation. Third, the genetic background and housing condition of mice used in the studies were different.
In this study, we abolished Bdnf gene expression in the VMH of mice using both Sf1-Cre and AAV-Cre. We also employed the Nkx2.1-Cre transgene to abolish Bdnf gene expression in the hypothalamus. Our study shows that VMH BDNF plays an important role in the control of energy intake and that BDNF produced in non-VMH and non-PVH hypothalamic neurons is involved in the control of energy expenditure.
Materials and Methods
Animals
Bdnflox/+ (stock number 004339), Sf1-Cre (stock number 012462), and Nkx2.1-Cre (stock number 008661) mouse strains were obtained from The Jackson Laboratory (6, 14, 17). BdnfLacZ/+ and Bdnfklox/+ mouse strains were described previously (18). All mouse strains were backcrossed to C57BL/6J mice for at least 5 generations before they were used in this study. Mice were maintained on a 12-hour light, 12-hour dark cycle with ad libitum access to water and a regular rodent chow (Harlan 2019 with metabolizable energy of 3.3 kcal/g). The Animal Care and Use Committees at Scripps Florida approved all animal procedures used in this study.
Physiological measurements
Measurement of body weight, body length, and food intake was conducted as described previously (18). Body composition was determined using a Minispec LF-50/mq 7.5 NMR analyzer (Brucker Optics), and it was divided into 3 parts: lean mass, fat mass, and body fluid. Oxygen consumption (VO2) and locomotor activity were assessed with a comprehensive lab animal monitoring system (Columbia Instrument). Locomotor activity was measured as light beam breaks in the XY horizontal plane.
In situ hybridization, immunoblotting, and immunohistochemistry
Radioactive in situ hybridization using 35S-labeled riboprobes and immunoblotting were performed as previously described (7, 19). Immunohistochemistry was performed as previously described (18). The following primary antibodies were used for immunoblotting: rabbit polyclonal antibody against tyrosine hydroxylase (1:1000; Millipore), rabbit polyclonal antibody against uncoupling protein 1 (UCP1) (1:1000; Thermo Scientific), and mouse monoclonal antibody against α-tubulin (1:8000; Sigma-Aldrich). The following primary antibodies were used for immunohistochemistry: rabbit polyclonal antibody against β-galactosidase (1:4000; Cappel) and rabbit polyclonal antibody against Cre recombinase (1:10 000; Millipore).
Stereotaxic injection of AAV
AAV-GFP and AAV-Cre-GFP viral vectors (serotype 2, UNC vector core) were administered bilaterally into the hypothalamus of 8-week-old female Bdnflox/lox mice using a 10-μL Hamilton syringe with a 33-gauge needle that was attached to a stereotaxic arm as described previously (11). Each viral vector (0.25 μL at 1012 viral particles/mL) was infused into a hypothalamic area at 1.5 μL/h. The coordinates (relative to the bregma) for the VMH and DMH were anteroposterior, −1.46 and −1.56 mm; mediolateral, ±0.46 and ±0.42 mm; and dorsoventral, −6.06 and −6.01 mm, respectively.
Cold exposure and temperature measurement
Measurement of core body temperature was obtained from mice that were exposed to 10°C for up to 6 hours by a rectal probe for mice and a thermometer (Thermo Fisher Scientific). The probe was inserted into the rectum to a depth of 2 cm. All experiments began at 10 am, and the temperature was measured once every hour.
Measurement of serum BDNF
Blood samples were collected from the mouse orbital sinus. The blood samples were allowed to clot at room temperature for 30 minutes and then centrifuged at room temperature for 20 minutes at 13 000 rpm. The serum was collected from each sample and stored at −80°C until used. Serum BDNF levels were measured using a BDNF ELISA kit (Abcam) according to the manufacturer's instruction.
Statistical analysis
All data are expressed as mean ± SEM. The significance of differences was tested using Student's t test, linear regression, or two-way ANOVA with post hoc Bonferroni correction (*, P < .05; **, P < .01; and ***, P < .001).
Results
Normal body weight in mice with deletion of the Bdnf gene in the embryonic VMH
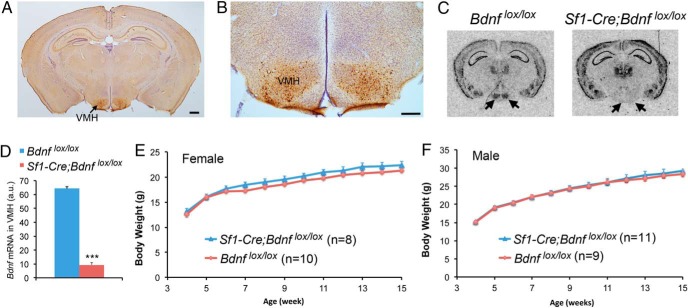
We asked whether the lack of an obesity phenotype in mice where the Bdnf gene was deleted using the BAC Sf1-Cre transgene (14) was due to incomplete BDNF ablation in the VMH, because many BDNF neurons in the mature VMH do not express SF1 (16). To this end, we crossed Sf1-Cre mice to Bdnfklox/+ mice to generate Sf1-Cre;Bdnfklox/+ mice, in which β-galactosidase is expressed in otherwise BDNF-expressing neurons once the floxed Bdnfklox allele is deleted by Cre-mediated recombination (18). We detected β-galactosidase-expressing cells only in the VMH (Figure 1A). Furthermore, the distribution of β-galactosidase-expressing cells in the nucleus (Figure 1B) was nearly identical to that found in BdnfLacZ/+ mice (7). These observations indicate that the Sf1-Cre transgene is capable of ablating Bdnf gene expression specifically and completely in the VMH. To confirm the completeness of BDNF ablation, we crossed Sf1-Cre mice to Bdnflox/lox mice (6) to generate Bdnflox/lox(control) and Sf1-Cre;Bdnflox/lox (mutant) mice. In situ hybridization showed that Bdnf mRNA nearly disappeared in the VMH of Sf1-Cre;Bdnflox/lox mice (Figure 1, C and D). This result indicates that nearly all BDNF neurons in the VMH express SF1 at some point during their development, which is consistent with a critical role of SF1 in the formation of the VMH (20).
Figure 1.
Deletion of the Bdnf gene in SF1-expressing cells does not alter body weight in mice. A, A representative immunohistochemistry image showing β-galactosidase expression in the Sf1-Cre;Bdnfklox/+ brain. The brain section was counterstained with Nissl. Scale bar, 500 μm. B, An immunohistochemistry image showing β-galactosidase-expressing neurons in the Sf1-Cre;Bdnfklox/+ VMH. Scale bar, 250 μm. C, In situ hybridization of Bdnf mRNA revealing abolishment of Bdnf gene expression in the Sf1-Cre;Bdnflox/lox VMH. The arrows denote the VMH. D, Quantification of Bdnf mRNA levels in the VMH of Bdnflox/lox and Sf1-Cre;Bdnflox/lox mice (n = 3 mice per genotype). E, Body weight of female Bdnflox/lox and Sf1-Cre;Bdnflox/lox mice. Two-way ANOVA indicates a significant effect of genotypes on body weight: F1,192 = 19.3, P < .0001; however, post hoc Bonferroni tests do not find significant difference in body weight between genotypes at any time point. F, Body weight of male Bdnflox/lox and Sf1-Cre;Bdnflox/lox mice. Two-way ANOVA does not find a significant effect of genotypes on body weight: F1,216 = 0.7174, P = .398. Error bars indicate SEM.
In agreement with the previous observation (14, 15), neither female nor male Sf1-Cre;Bdnflox/lox mice were significantly heavier than sex-matched Bdnflox/lox mice at any time point when body weight was monitored (Figure 1, E and F). Therefore, deleting the Bdnf gene in the VMH during early development does not affect energy balance in mice.
Hyperphagia and obesity in mice with BDNF ablation in the adult VMH
We previously found that deleting the Bdnf gene in the adult PVH using stereotaxic injection of AAV-Cre led to much more marked hyperphagia and more severe obesity than deleting the same gene in the embryonic PVH using the Sim1-Cre transgene (11). This observation raises the possibility that the lack of an obesity phenotype in Sf1-Cre;Bdnflox/lox mice could be due to compensatory changes in the appetite-controlling neural network induced by early Bdnf deletion in the VMH. To test this possibility, we deleted the Bdnf gene by injecting AAV-Cre-GFP into the VMH of 8-week-old Bdnflox/lox mice bilaterally. Because female mice develop more severe obesity than male mice when TrkB signaling is impaired (6, 7, 18), deleting the Bdnf gene in VMH would produce more robust obesity in female mice than in male mice if VMH BDNF plays a role in the control of energy balance. Thus, we conducted this experiment using female mice.
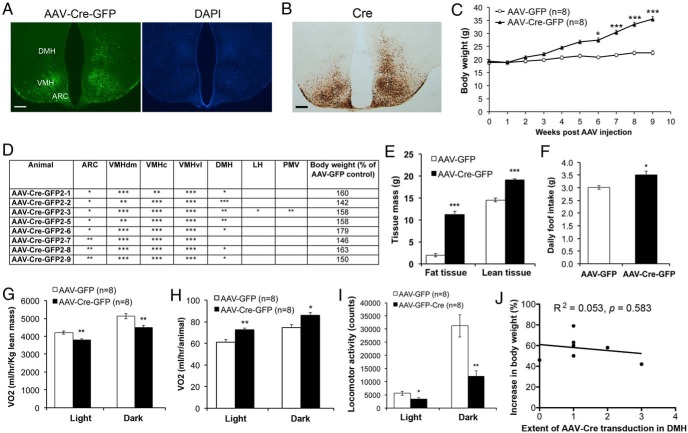
Injected AAV-Cre-GFP transduced neurons throughout the whole VMH, as indicated by the expression of GFP (Figure 2, A and D). Because the AAV-Cre-GFP vector expresses a Cre-GFP fusion protein, Cre recombinase immunoreactivity covered the whole VMH in mice injected with the virus as expected (Figure 2B). Injected AAV also frequently transduced neurons in the arcuate nucleus (ARC) and DMH to various extents (Figure 2, A and D). We termed these mice as mediobasal hypothalamus Bdnf knockout (MBH-BDNF KO). MBH-BDNF KO mice developed obesity and were 57% heavier on average at 9 weeks after AAV injection, compared with the control mice injected with AAV-GFP (Figure 2, C–E). The obesity was associated with increased linear growth (10.2 ± 0.1 cm for AAV-Cre-GFP mice vs 9.6 ± 0.1 cm for AAV-GFP mice, P < .0001) and increased levels of serum BDNF (703 ± 141 pg/mL for AAV-Cre-GFP mice vs 301 ± 45 pg/mL for AAV-GFP mice, P < .05).
Figure 2.
Deletion of the Bdnf gene in the VMH of adult mice leads to obesity. A, Representative images showing AAV-Cre-GFP transduction in the Bdnflox/lox hypothalamus. DAPI staining was used to reveal anatomic structures. Scale bar, 250 μm. B, Cre immunoreactivity of brain sections from Bdnflox/lox mice injected with AAV-Cre-GFP into the VMH. Scale bar, 250 μm. C, Body weight of female Bdnflox/lox mice injected with either AAV-GFP (n = 8) or AAV-Cre-GFP (n = 8). Two-way ANOVA indicates a significant effect of AAV injection on body weight: F1,140 = 61.14, P < .0001. D, Analysis of injection sites in mice with AAV-Cre-GFP targeted to the VMH. The number of the * symbol indicates the extent of AAV transduction in different hypothalamic regions. E, Body composition of mice at 10 weeks after AAV injection (n = 8 mice for each treatment). Fat tissues were 8.6% and 29.8% of body weight in mice injected with AAV-GFP and mice injected with AAV-Cre-GFP, whereas lean tissues were 63.6% and 50.6% of body weight in mice injected with AAV-GFP and mice injected with AAV-Cre-GFP, respectively. F, Daily food intake of mice at 6 weeks after AAV injection (n = 8 mice for each treatment). G–I, VO2 and locomotor activity of mice at 10 weeks after AAV injection. J, Correlation between the extent of AAV-Cre-GFP transduction in the DMH and body weight at 9 weeks after injection. Error bars indicate SEM. LH, lateral hypothalamus; PMV, ventral premammillary nucleus; VMHc, central part of VMH; VMHdm, dorsomedial part of VMH; VMHvl, ventrolateral part of VMH.
To find out the cause for obesity in MBH-BDNF KO mice, we monitored daily food intake during the sixth week after AAV injection and measured energy expenditure at 10 weeks after AAV injection. The mutant mice ate significantly more food than control mice (Figure 2F). When normalized to lean mass, VO2 of the mutant mice was lower than the control mice during both the light and dark cycles (Figure 2G). However, the lean mass was significant higher in the mutant mice than in the control mice at 10 weeks after AAV injection (Figure 2E), when the mutant mice were obese (Figure 2C). Individual mutant mice actually used more energy than individual control mice (Figure 2H), although their locomotor activity was reduced (Figure 2I). These results indicate that increased energy intake is the main cause for the development of obesity in the mutant mice.
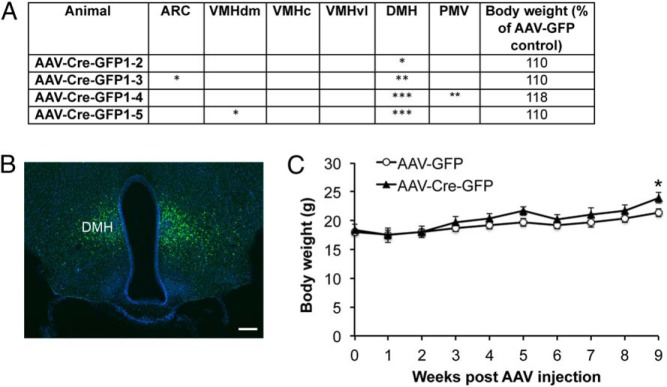
We next determined in which hypothalamic area Bdnf deletion led to hyperphagic obesity in MBH-BDNF KO mice. Because the ARC expresses little or no BDNF (see figure 4 below) (7), it is unlikely that the obesity phenotype results from Bdnf deletion in the ARC. Linear regression analysis also did not find significant correlation between the body weight of MBH-BDNF KO mice and the extent of AAV transduction in either the DMH (Figure 2J) or the ARC (R2 = 0.083, P = .489). To further assess the role of DMH BDNF in the control of energy balance, we analyzed a cohort of Bdnflox/lox mice in which AAV was targeted to a site slightly more dorsal, more posterior, and more medial than in MBH-BDNF KO mice. Some of these mice had AAV-Cre-GFP transduction concentrated in the DMH (Figure 3, A and B; termed DMH-BDNF KO). DMH-BDNF KO mice only became significantly heavier by 12% than control mice at 9 weeks after AAV injection (Figure 3C). These results indicate that obesity developed in MBH-BDNF KO mice largely results from deletion of the Bdnf gene in the VMH.
Figure 3.
Deletion of the Bdnf gene in the DMH of adult mice. A, Analysis of injection sites in mice with AAV targeted to the DMH. B, A representative image showing AAV-Cre-GFP transduction in the DMH. The image was from animal AAV-Cre-GFP1-5. Scale bar, 250 μm. C, Body weight of female Bdnflox/lox mice injected with either AAV-GFP (n = 5) or AAV-Cre-GFP (n = 4) into the DMH. Two-way ANOVA indicates a significant effect of AAV injection on body weight: F1,70 = 16.4, P < .001. Error bars indicate SEM.
BDNF produced in non-PVH neurons in the control of energy expenditure
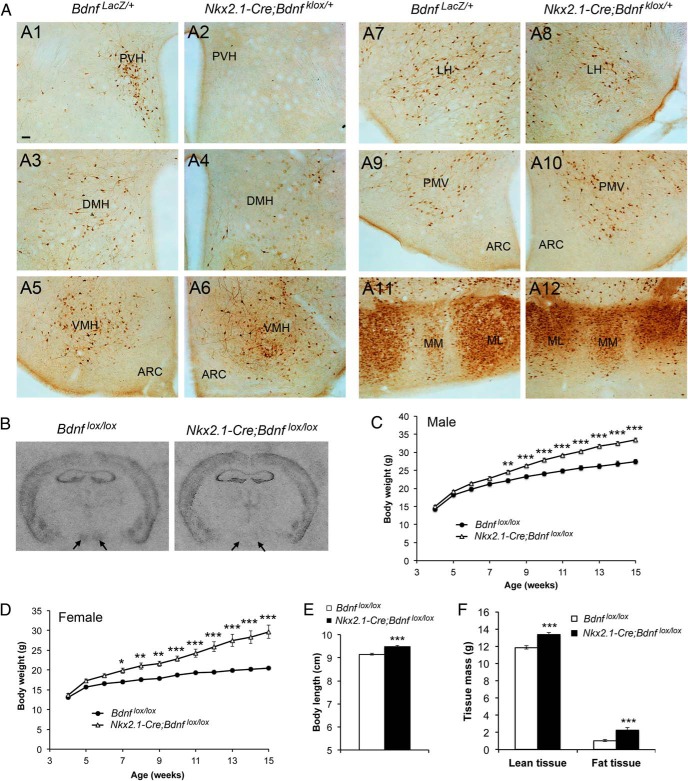
We have shown that BDNF expressed in the PVH plays a critical role in regulating both energy intake and energy expenditure (11). The results described above show that BDNF expressed in the VMH is also involved in the control of energy intake. We employed the Nkx2.1-Cre transgene, which starts to express Cre recombinase at embryonic day 10.5 and is specific to the hypothalamus and interneurons in the brain (17), to investigate if BDNF expressed in other hypothalamic areas plays any role in the regulation of energy balance. We first determined in which brain regions Nkx2.1-Cre could ablate BDNF by comparing β-galactosidase immunoreactivity between BdnfLacZ/+ mice and Nkx2.1-Cre;Bdnfklox/+ mice (Figure 4A). Except the PVH where no β-galactosidase-expressing cells were observed in Nkx2.1-Cre;Bdnfklox/+ mice (Figure 4, A1 and A2), the patterns of β-galactosidase expression in the 2 genotypes of mice were comparable in the DMH (Figure 4, A3 and A4), VMH (Figure 4, A5 and A6), lateral hypothalamus (Figure 4, A7 and A8), ventral premammillary nucleus (Figure 4, A9 and A10), and medial mammillary nucleus (Figure 4, A11 and A12). In agreement with the fact that interneurons do not express BDNF (21), we did not detect β-galactosidase-expressing cells in any brain region outside the hypothalamus in Nkx2.1-Cre;Bdnfklox/+ mice (data not shown). These results indicate that the Nkx2.1-Cre transgene is capable of ablating BDNF in non-PVH hypothalamic neurons. Indeed, in situ hybridization revealed that Bdnf mRNA was not detectable in the VMH of Nkx2.1-Cre;Bdnflox/lox mutant mice (Figure 4B).
Figure 4.
BDNF expressed in non-PVH hypothalamic cells is involved in the control of body weight. A, Immunohistochemistry images showing β-galactosidase expression in various hypothalamic areas of BdnfLacZ/+ and Nkx2.1-Cre;Bdnfklox/+ mice. B, Representative images of in situ hybridization for Bdnf mRNA. Arrows denote the VMH. C, Body weight of male Bdnflox/lox (n = 8) and Nkx2.1-Cre;Bdnflox/lox (n = 13) mice. Two-way ANOVA indicates a significant effect of genotypes on body weight: F1,240 = 280.4, P < .0001. D, Body weight of female Bdnflox/lox (n = 11) and Nkx2.1-Cre;Bdnflox/lox (n = 8) mice. Two-way ANOVA indicates a significant effect of genotypes on body weight: F1,204 = 272.6, P < .0001. E, Body length of 12-week-old female control (n = 8) and mutant (n = 6) mice. F, Body composition of 10-week-old female mice (n = 8 and 7 for control and mutant mice, respectively). Fat tissues were 5.8% and 11.3% of body weight in control mice and mutant mice, whereas lean tissues were 67.3% and 66.0% of body weight in control mice and mutant mice, respectively. Scale bar, 50 μm. Error bars indicate SEM. LH, lateral hypothalamus; ML, lateral part of medial mammillary nucleus; MM, medial part of medial mammillary nucleus; PMV, ventral premammillary nucleus.
Both male and female Nkx2.1-Cre;Bdnflox/lox mice developed modest obesity, and their body weights on average were 22% and 46% heavier than sex-matched Bdnflox/lox control mice at 15 weeks of age, respectively (Figure 4, C and D). The obesity was associated with increased linear growth (Figure 4E), increased lean mass (Figure 4F), and elevated levels of serum BDNF (444 ± 41 pg/mL for female Nkx2.1-Cre;Bdnflox/lox mice vs 223 ± 88 pg/mL for female Bdnflox/lox mice, P < .05).
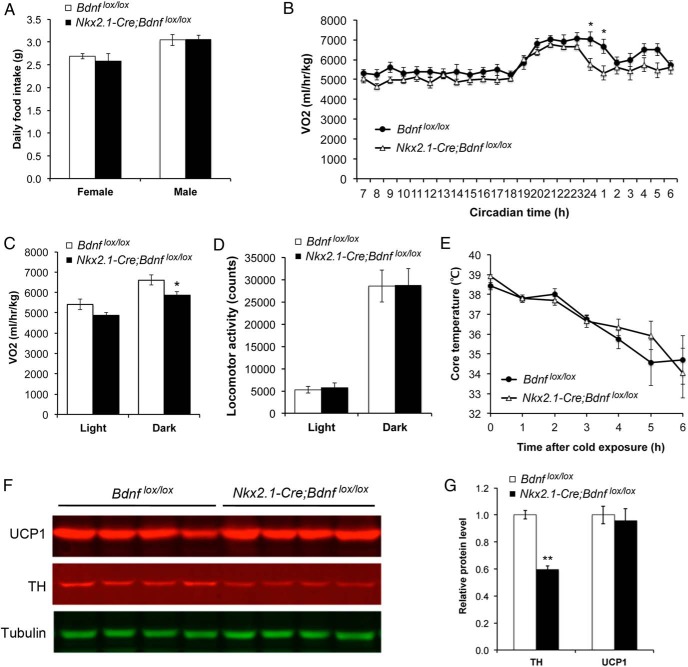
Nkx2.1-Cre;Bdnflox/lox mice had similar daily food intake to control mice (Figure 5A). However, these mutant mice had significantly lower VO2 than control mice at 6 weeks of age when these 2 genotypes of mice had comparable body weights (Figure 5, B and C). These results indicate that Nkx2.1-Cre;Bdnflox/lox mice develop obesity mainly due to reduced energy expenditure.
Figure 5.
Nkx2.1-Cre;Bdnfklox/+ mice show reduced energy expenditure. A, Daily food intake was measured when mice were 8 weeks old (n = 5, 6, 4, and 5 mice for female Bdnflox/lox, female Nkx2.1-Cre;Bdnflox/lox, male Bdnflox/lox, and male Nkx2.1-Cre;Bdnflox/lox, respectively). B, Distribution of VO2 over a 24-hour period in female mice at 6 weeks of age. Two-way ANOVA for the effect of genotype: F1,288 = 31.10 (n = 6–8 per genotype), P < .0001. C and D, VO2 and locomotor activity of female mice at 6 weeks of age (n = 6–8 mice per genotype). E, Rectal temperature of 8-week-old female mice after exposure to 10°C (n = 6–8 mice per genotype). F and G, Immunoblotting analysis and quantification of UCP1 and tyrosine hydroxylase (TH) in the iBAT of 12-week-old female mice. Levels of UCP1 and TH were normalized to those of α-tubulin in the same samples (n = 4 mice per genotype). Error bars indicate SEM.
Mice consume energy in many biological processes such as adaptive thermogenesis, physical activity, and basal metabolism. In adaptive thermogenesis, UCP1 in BATs allows the energy generated from β-oxidation of fatty acids to dissipate as heat in response to physiological and environmental stimuli such as overeating and cold by uncoupling the proton gradient from ATP synthesis in mitochondria (22, 23). Nkx2.1-Cre;Bdnflox/lox mice had normal locomotor activity (Figure 5D), normal core body temperature (Figure 5E), and normal body temperature response to cold exposure (Figure 5E), indicating that physical activity and adaptive thermogenesis are not significantly altered in these mutant mice. In agreement with the observation of normal adaptive thermogenesis, expression of UCP1 was normal in the interscapular BAT (iBAT) of Nkx2.1-Cre;Bdnflox/lox mice (Figure 5, F and G). We did find that the level of tyrosine hydroxylase in iBAT was drastically reduced in Nkx2.1-Cre;Bdnflox/lox mice, compared with Bdnflox/lox mice (Figure 5, F and G). Tyrosine hydroxylase in iBAT is inside the innervating axons of sympathetic neurons, and it is the rate-limiting enzyme of the norepinephrine synthesis pathway and its amount is indicative of sympathetic activity. Thus, Nkx2.1-Cre;Bdnflox/lox mice could have lower sympathetic outflows than control mice, leading to reduced resting metabolic rate and thereby lower energy expenditure.
Discussion
In this study, we demonstrate that BDNF ablation in the adult VMH leads to significant hyperphagic obesity, indicating an important role of VMH BDNF in the control of food intake. Furthermore, we found that BDNF expressed in non-PVH and non-VMH hypothalamic neurons is involved in the control of energy expenditure.
Deletion of the Bdnf gene in the adult DMH and VMH using AAV-Cre has been found to cause modest hyperphagic obesity (12). We were able to reproduce this finding and further narrow down the appetite-controlling BDNF neurons to the VMH. Our MBH-BDNF KO mice developed more severe obesity than the mutant mice reported by Unger et al (12). This probably results from a difference in the completeness of Bdnf deletion in the VMH between the 2 strains of mutant mice. In addition, the gender difference in mice used in the 2 studies should contribute to the difference in obesity severity. The current study used female mice, whereas the previous study used male mice (12). It has been documented that female Bdnf mutant mice develop more severe obesity than male counterparts (6, 18). The genetic evidence on the role of VMH BDNF in the control of energy intake is consistent with the pharmacological observation that administration of recombinant BDNF into the VMH suppressed food intake (24).
Our study also confirms the observation that Sf1-Cre;Bdnflox/lox mice have a normal body weight (14, 15). The lack of an obesity phenotype in these mice is not the result of incomplete BDNF ablation, because we found that Bdnf mRNA was basically gone in the VMH of Sf1-Cre;Bdnflox/lox mice. The phenotype discrepancy between Sf1-Cre;Bdnflox/lox mice and MBH-BDNF KO mice should be due to the difference in the timing of Bdnf deletion between the 2 mouse mutant strains. SF1 is expressed during embryogenesis (20), and thereby the Bdnf gene in Sf1-Cre;Bdnflox/lox mice is deleted in the VMH during early development. After early Bdnf deletion, neural network reorganization may compensate for the effect of VMH BDNF loss on food intake. In support of this explanation, we previously found that deleting the Bdnf gene in the adult PVH produced much more marked hyperphagia than deleting the Bdnf gene in the embryonic PVH (11). Similarly, it has been demonstrated that although specific ablation of neurons expressing agouti-related protein in adult mice results in loss of appetite, ablation of the same neurons during early postnatal life has no impact on body weight (25).
It appears that developmental compensation only works for the regulation of energy intake. Although the Nkx2.1-Cre transgene is expressed in embryos (17), Nkx2.1-Cre;Bdnflox/lox mice still develop obesity due to reduced energy expenditure. Because we found that Nkx2.1-Cre did not ablate BDNF in the PVH and that BDNF ablation in the embryonic VMH does not alter body weight, reduced energy expenditure in Nkx2.1-Cre;Bdnflox/lox mice should be the consequence of Bdnf deletion in non-PVH and non-VMH hypothalamic neurons. Daily energy expenditure of a sedentary mouse is composed of 4 major components: the thermic effect of feeding, spontaneous physical activity, adaptive thermogenesis, and resting metabolic rate (26). Because daily food intake, locomotor activity, UCP1 expression, and cold exposure response were comparable in Nkx2.1-Cre;Bdnflox/lox mice and control mice, the first 3 components of energy expenditure should be normal in Nkx2.1-Cre;Bdnflox/lox mice. This suggests that reduced energy expenditure in Nkx2.1-Cre;Bdnflox/lox mice be due to a decrease in resting metabolic rate. In support of this explanation, we found that the level of tyrosine hydroxylase in sympathetic axons, an indicator of sympathetic tone, was reduced in the iBAT of Nkx2.1-Cre;Bdnflox/lox mice. Together with the finding that BDNF expressed in distinct groups of PVH neurons is required for the regulation of locomotor activity and adaptive thermogenesis (11), these findings suggest that hypothalamic BDNF could regulate various aspects of energy expenditure through distinct neural circuits.
This study and our previous study (11) have identified that the VMH and PVH are 2 key brain regions that produce BDNF to regulate food intake. Because the 2 studies were done in mice with the same gender and genetic background, using the same viral vectors, and in the same animal facility, it is possible to determine the relative importance of the 2 groups of BDNF neurons in suppressing food intake by comparing the results from the 2 studies. Deletion of the Bdnf gene in the VMH and PVH of adult female mice increased food intake by 17% and 60%, respectively. Thus, BDNF neurons in the PVH could be more potent than BDNF neurons in the VMH in suppressing food intake.
Estrogens have antiobesity effects in women and female mammals (27, 28), and there are many estrogen-responsive neurons in the VMH (29). It is tempting to speculate that estrogens and BDNF interact in the VMH to regulate body weight. Although deletion of estrogen receptor-α in the embryonic VMH using the Sf1-Cre transgene does not lead to hyperphagia in mice (30), it remains possible that the receptor in the mature VMH does regulate food intake in light of our finding that BDNF ablation in the adult VMH, but not embryonic VMH, causes hyperphagia. Because estrogens have been shown to increase BDNF levels in the prefrontal cortex and hippocampus (31), it would be interesting to investigate whether estrogens control food intake in part by regulating the expression of VMH BDNF in future studies.
The relationship between serum BDNF levels and obesity is unclear. As expected, serum BDNF levels are reduced in obese human subjects with 1 nonfunctional Bdnf allele (9, 10); however, serum BDNF levels in obese subjects without obvious mutations in the BDNF gene were found to be increased (32, 33) or unchanged (34, 35), compared with nonobese subjects. We found that serum BDNF levels were significantly elevated in obese mice in which the Bdnf gene was deleted in the adult mediobasal hypothalamus or with the Nkx2.1-Cre transgene. In the future studies it would be interesting to find out whether the elevated serum BDNF level results from BDNF up-regulation in peripheral tissues (eg, adipose tissue) and/or in the PVH neurons, some of which are magnocellular neurosecretory cells (11) and could release BDNF into the circulation system.
In conclusion, several populations of hypothalamic neurons produce BDNF to regulate energy balance. These include neurons in the PVH and VMH, which produce BDNF to suppress food intake, and neurons in the PVH and an unidentified hypothalamic nucleus, which produce BDNF to promote locomotor activity, thermogenesis, and resting metabolic rate.
Acknowledgments
This work was supported by the United States National Institutes of Health Grants R01 DK103335 and R01 DK089237 (to B.X.) and by a scholarship from the China Scholarship Council (H.Y.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AAV
- adeno-associated virus
- ARC
- arcuate nucleus
- BAT
- brown adipose tissue
- BDNF
- brain-derived neurotrophic factor
- DMH
- dorsomedial hypothalamus
- iBAT
- interscapular BAT
- MBH-BDNF KO
- mediobasal hypothalamus Bdnf knockout
- PVH
- paraventricular hypothalamus
- SF1
- steroidogenic factor-1
- TrkB
- tropomyosin receptor kinase B
- UCP1
- uncoupling protein 1
- VMH
- ventromedial hypothalamus
- VO2
- oxygen consumption.
References
- 1. Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Waterhouse EG, Xu B. New insights into the role of brain-derived neurotrophic factor in synaptic plasticity. Mol Cell Neurosci. 2009;42(2):81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Na Rev Neurosci. 2013;14(1):7–23. [DOI] [PubMed] [Google Scholar]
- 4. Lyons WE, Mamounas LA, Ricaurte GA, et al. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci USA. 1999;96(26):15239–15244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19(6):1290–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rios M, Fan G, Fekete C, et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15(10):1748–1757. [DOI] [PubMed] [Google Scholar]
- 7. Xu B, Goulding EH, Zang K, et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6(7):736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yeo GS, Connie Hung CC, et al. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat Neurosci. 2004;7(11):1187–1189. [DOI] [PubMed] [Google Scholar]
- 9. Gray J, Yeo GS, Cox JJ, et al. Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes. 2006;55(12):3366–3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han JC, Liu QR, Jones M, et al. Brain-derived neurotrophic factor and obesity in the WAGR syndrome. N Engl J Med. 2008;359(9):918–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. An JJ, Liao GY, Kinney CE, Sahibzada N, Xu B. Discrete BDNF neurons in the paraventricular hypothalamus control feeding and energy expenditure. Cell Metab. 2015;22(1):175–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Unger TJ, Calderon GA, Bradley LC, Sena-Esteves M, Rios M. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J Neurosci. 2007;27(52):14265–14274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tran PV, Akana SF, Malkovska I, Dallman MF, Parada LF, Ingraham HA. Diminished hypothalamic bdnf expression and impaired VMH function are associated with reduced SF-1 gene dosage. J Comp Neurol. 2006;498(5):637–648. [DOI] [PubMed] [Google Scholar]
- 14. Dhillon H, Zigman JM, Ye C, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49(2):191–203. [DOI] [PubMed] [Google Scholar]
- 15. Kamitakahara A, Xu B, Simerly R. Ventromedial hypothalamic expression of Bdnf is required to establish normal patterns of afferent GABAergic connectivity and responses to hypoglycemia. Mol Metab. 2016;5(2):91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tran PV, Lee MB, Marín O, et al. Requirement of the orphan nuclear receptor SF-1 in terminal differentiation of ventromedial hypothalamic neurons. Mol Cell Neurosci. 2003;22(4):441–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu Q, Tam M, Anderson SA. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J Comp Neurol. 2008;506(1):16–29. [DOI] [PubMed] [Google Scholar]
- 18. Liao GY, An JJ, Gharami K, et al. Dendritically targeted Bdnf mRNA is essential for energy balance and response to leptin. Nat Med. 2012;18(4):564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. An JJ, Gharami K, Liao GY, et al. Distinct role of long 3′ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell. 2008;134(1):175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luo X, Ikeda Y, Parker KL. The cell-specific nuclear receptor steroidogenic factor 1 plays multiple roles in reproductive function. Philos Trans R Soc Lond B Biol Sci. 1995;350(1333):279–283. [DOI] [PubMed] [Google Scholar]
- 21. Gorski JA, Zeiler SR, Tamowski S, Jones KR. Brain-derived neurotrophic factor is required for the maintenance of cortical dendrites. J Neurosci. 2003;23(17):6856–6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clapham JC. Central control of thermogenesis. Neuropharmacology. 2012;63(1):111–123. [DOI] [PubMed] [Google Scholar]
- 23. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277–359. [DOI] [PubMed] [Google Scholar]
- 24. Wang C, Bomberg E, Levine A, Billington C, Kotz CM. Brain-derived neurotrophic factor in the ventromedial nucleus of the hypothalamus reduces energy intake. Am J Physiol Regul Integr Comp Physiol. 2007;293(3):R1037–R1045. [DOI] [PubMed] [Google Scholar]
- 25. Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310(5748):683–685. [DOI] [PubMed] [Google Scholar]
- 26. Garland T, Jr, Schutz H, Chappell MA, et al. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. J Exp Biol. 2011;214(pt 2):206–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88(6):2404–2411. [DOI] [PubMed] [Google Scholar]
- 28. Gao Q, Mezei G, Nie Y, et al. Anorectic estrogen mimics leptin's effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med. 2007;13(1):89–94. [DOI] [PubMed] [Google Scholar]
- 29. Flanagan-Cato LM, Calizo LH, Daniels D. The synaptic organization of VMH neurons that mediate the effects of estrogen on sexual behavior. Horm Behav. 2001;40(2):178–182. [DOI] [PubMed] [Google Scholar]
- 30. Xu Y, Nedungadi TP, Zhu L, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14(4):453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Luine V, Frankfurt M. Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neuroscience. 2013;239:34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Monteleone P, Tortorella A, Martiadis V, Serritella C, Fuschino A, Maj M. Opposite changes in the serum brain-derived neurotrophic factor in anorexia nervosa and obesity. Psychosom Med. 2004;66(5):744–748. [DOI] [PubMed] [Google Scholar]
- 33. Suwa M, Kishimoto H, Nofuji Y, et al. Serum brain-derived neurotrophic factor level is increased and associated with obesity in newly diagnosed female patients with type 2 diabetes mellitus. Metabolism. 2006;55(7):852–857. [DOI] [PubMed] [Google Scholar]
- 34. El-Gharbawy AH, Adler-Wailes DC, Mirch MC, et al. Serum brain-derived neurotrophic factor concentrations in lean and overweight children and adolescents. J Clin Endocrinol Metab. 2006;91(9):3548–3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gajewska E, Sobieska M, Łojko D, Wieczorowska-Tobis K, Suwalska A. Obesity itself does not influence BDNF serum levels in adults. Eur Rev Med Pharmacol Sci. 2014;18(21):3246–3250. [PubMed] [Google Scholar]