Abstract
Background
Delays in delivering endovascular stroke therapy adversely affect outcomes. Time-sensitive treatments such as stroke interventions benefit from methodically developed protocols. Clearly defined roles in these protocols allow for parallel processing of tasks, resulting in consistent delivery of care.
Objective
To present the outcomes of a quality-improvement (QI) process directed at reducing stroke treatment times in a tertiary level academic medical center.
Methods
A Six-Sigma-based QI process was developed over a 3-month period. After an initial analysis, procedures were implemented and fine-tuned to identify and address rate-limiting steps in the endovascular care pathway. Prospectively recorded treatment times were then compared in two groups of patients who were treated ‘before’ (n=64) or ‘after’ (n=30) the QI process. Three time intervals were measured: emergency room (ER) to arrival for CT scan (ER–CT), CT scan to interventional laboratory arrival (CT–Lab), and interventional laboratory arrival to groin puncture (Lab–puncture).
Results
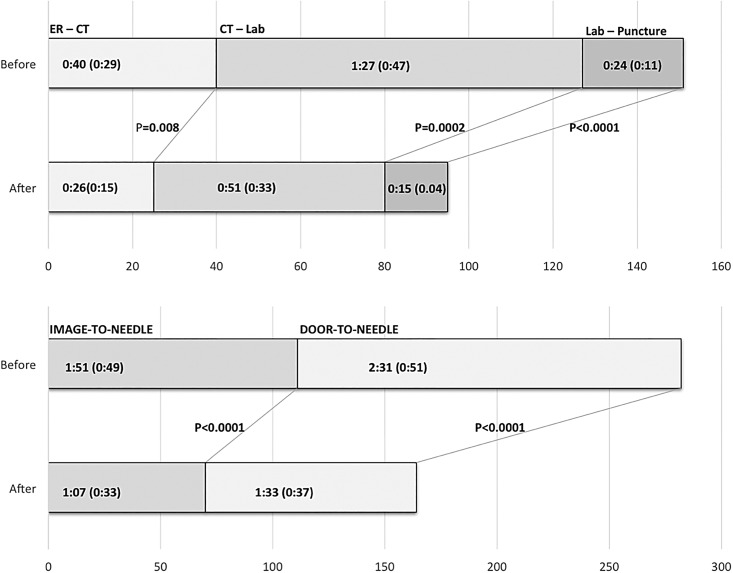
The ER–CT time was 40 (±29) min in the ‘before’ and 26 (±15) min in the ‘after’ group (p=0.008). The CT–Lab time was 87 (±47) min in the ‘before’ and 51 (±33) min in the ‘after’ group (p=0.0002). The Lab–puncture time was 24 (±11) min in the ‘before’ and 15 (±4) min in the ‘after’ group (p<0.0001). The overall ER–arrival to groin-puncture time was reduced from 2 h, 31 min (±51) min in the ‘before’ to 1 h, 33 min (±37) min in the ‘after’ group, (p<0.0001). The improved times were seen for both working hours and off-hours interventions.
Conclusions
A protocol-driven process can significantly improve efficiency of care in time-sensitive stroke interventions.
Keywords: Stroke, Standards
Introduction
Endovascular treatment of large vessel acute ischemic strokes in appropriately selected patients has been endorsed as evidence-based care.1 2 The next steps in advancing this therapy are to develop systems of care that can be divided into prehospital and intra-hospital pathways. Other time-dependent treatments, such as trauma and acute myocardial infarction, provide a valuable model for endovascular stroke therapy. The value of an initial ‘golden hour’ in improving outcomes has been shown for treatment of trauma3 to neonatal resuscitation.4 Early reperfusion for large vessel strokes is a critical determinant of endovascular therapy outcomes.5 6 Emergency medicine publications provide useful insight into developing checklists and protocols geared towards seamless resuscitation of injured or sick patients.7 These ‘pit-crew’-type protocols clearly define the role of each team member, allowing for synchronized, parallel delivery of care.
Our center has performed endovascular stroke interventions for the past 15 years and participated in several clinical trials. We had noticed a fall in the volume of patients in the past 3 years as an after effect of negative stroke trials8 9 and also because we had decided to offer stroke interventions only in the setting of a randomized clinical trial. This slowdown in cases adversely affected our treatment times. Towards the end of 2014, we instituted a quality improvement (QI) process to reduce our door-to-needle times and develop a process that might consistently reduce times to treatments at all hours of the day and all days of the week. The QI process was based on Six-Sigma which, although used by corporations for years, has only recently been adopted in medicine.10 This paper provides the results of those efforts, comparing the treatment times before and after implementation of the QI process.
Methods
Objective
To determine whether our times for different steps in endovascular stroke care improved as a result of the QI process; the null hypothesis stating that there would be no difference in these times.
Patient population
Our setting was a rural, tertiary level, academic medical center with almost 700 beds, which is also the regional level-1 trauma center. Prospectively recorded treatment times before and after the QI implementation provided the data for this analysis. The pre-QI treatment times included all endovascular patients from 2011 to 2014 who had presented to our emergency room (ER). Since the project was specifically targeted at improving efficiency between the ER and interventional neuroradiology (INR), the following patients were excluded: in-house patients undergoing an intervention for stroke, patients undergoing another procedure in the hospital with a stroke and patients treated with unknown symptom onset. The post-QI treatment times were those for all patients undergoing endovascular therapy between January 2015 and October 2015. The following treatment times were compared:
ER to imaging (CT) (ER–CT)
imaging to INR lab (CT–Lab)
INR lab to groin puncture (Lab–puncture)
imaging to needle (groin puncture) (CT–puncture)
door to needle (ER–puncture).
The QI process
The QI process was started in the fall of 2014 and incrementally implemented over almost 3 months. The impetus to initiate the process was treatment delays in stroke interventions, inconsistencies in times and care between working hours and on-call hours, ad hoc roles of different members, and suboptimal handover of patients between different services. The QI team was led by two Six-Sigma trained engineers and comprised physicians, allied staff, and management from interventional neuroradiology, neurology, emergency medicine, and anesthesia. Meetings were held to develop a baseline understanding of the existing practice, followed by observance of ‘real’ stroke interventions and patient flow pathways. Subsequent weekly meetings modified the process with each stroke therapy. A final protocol was signed off towards the end of 2014 and in 2015 all cases followed the updated protocol.
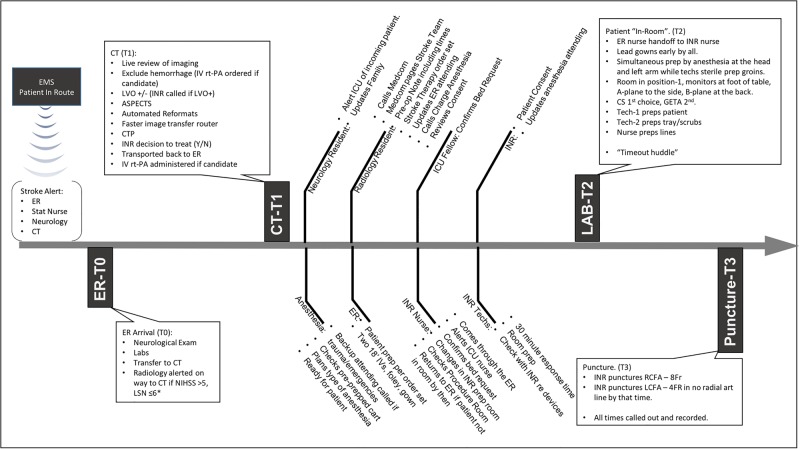
Important features of the protocol (figure 1) included identification of key personnel in the team, definition of a clear role for every member, and emphasis on parallel processing of assigned tasks. The protocol contained explicit details, such as how a nurse enters the hospital, where a technician would stand, and the position of the detectors in the INR laboratory. The protocol focused on the three main time-points along the stroke pathway: ER–CT, CT–Lab, and Lab–puncture.
Figure 1.
An overview of the protocol is presented along a timeline from patient arrival to arterial puncture. The role of different team members is listed along the timeline. ER, emergency room; GETA, general endotracheal anesthesia, off hours treatment: stroke intervention performed before 7:00 or after 17:00 or at the weekend; ICU, intensive care unit; INR, neurointerventionalist; LCFA, left common femoral artery; LSN, last seen normal; LVO, large vessel occlusion; NIHSS, National Institutes of Health Stroke Scale; onset-ER, time from symptom onset to ER arrival; RCFA, right common femoral artery; rt-PA, recombinant tissue plasminogen activator.
ER–CT
The emergency medical services evaluate stroke patients using the Cincinnati Prehospital Stroke Scale11 and alert the stroke team (neurology, radiology, emergency medicine, and laboratory staff) by paging the group. On arrival, the ER staff and on-call neurology residents evaluate the patient to confirm the stroke diagnosis. At least one IV line is placed while the patient is in the ER for the purpose of drawing laboratories and for contrast administration. After a point-of-care creatinine test (iSTAT; Abbot Laboratories, Abbott Park, Illinois), the stroke patient is transferred to the CT scanner by a dedicated stroke nurse and the neurology team. The CT technologists already alerted by the emergency medical services have the scanner ready for the patient. The radiology resident meets the stroke team in the scanner control room for live review of imaging for all patients presenting within 6 h with a National Institutes of Health Stroke Scale (NIHSS) score ≥6.
Before implementation of the QI process any CT scanner was used, but after the QI process a designated scanner (Aquilion-One, Toshiba America Medical Systems, Tustin, California, USA) was used for all stroke patients. The scanner is equipped with 320×0.5 mm detector rows covering 16 cm of volume per rotation, allowing for a uniform protocol comprising non-contrast CT (NCCT), volumetric CT perfusion (CTP) imaging, and CT angiography (CTA). In addition, the sequence of scanning was changed from NCCT–CTP–CTA to NCCT–CTA–CTP. This switch was crucial in allowing earlier detection of a large vessel occlusion (LVO) and alerting the attending neurointerventionalist—who could then review the NCCT and CTA (onsite or offsite), while CTP imaging was being carried out and processed. Another critical component was installation of an image router to simultaneously receive and disseminate images while they were being acquired. This reduced the scanner to transfer time of all images from 12 min to just over 5 min.
The CT stroke scan is performed with a gantry speed of 3 rotations/s. After the NCCT, the CTA is initiated from the aortic arch to the cranial vertex with an injection of 40–60 mL of Optiray 350 (Covidien, Hazelwood, Missouri, USA) through an 18–20 g antecubital IV line at a rate of 4–5 mL/s, followed by a similar volume of saline chaser. Images acquired are automatically sent via the router for immediate review. A volumetric whole brain, time-resolved CTP sequence follows. The imaging router enables transfer of 6080 (19×320) images to a picture archiving and communication system and the perfusion post-processing software (Vital Images, Minnetonka, Minnesota, USA). IV recombinant tissue plasminogen activator (rt-PA; Activase, Genentech Inc, San Francisco, California, USA) is administered if indicated after exclusion of hemorrhage on the NCCT.
CT–Lab
A collaborative decision about endovascular treatment is made based on clinical presentation, comorbidities, and imaging. If the decision is to treat, the radiology resident places a single order set. This was designed to simultaneously trigger multiple tasks with one click of the button—namely, anesthesia (in our view, use of anesthesia makes the procedure safer), intensive care unit (ICU) bed request, INR order set, Foley placement, peripheral IV line, etc. A single paging system was implemented allowing the resident to contact the neurointerventional team (one nurse, and two technicians) with one phone call. The paging system ‘Medcom’ provides the relevant patient information and location in the ER. If within 5 min the staff has not responded a repeat page is sent. The system also documents all paging times, which can be reviewed for QI purposes. The radiology resident places a preoperative note in the chart documenting the decision to treat. The neurology resident's duty running in parallel with this is to notify the ICU service of the eventual admission of the stroke patient. The INR nurse arrives through the ER, checks the patient, and alerts the ER staff to initiate transfer to the INR suite.
Lab–puncture
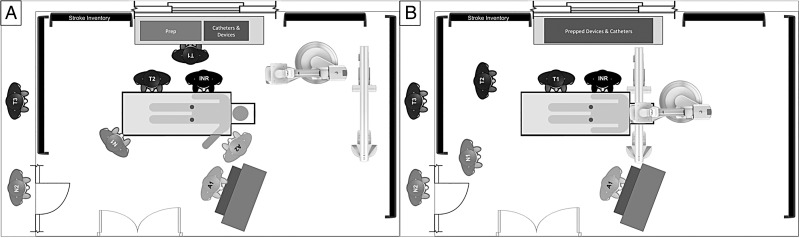
Our setup is illustrated in figure 2. The minimum staffing requirement for all neurovascular procedures is set at two technicians and one nurse. One technician is scrubbed and one floats. During working hours, a third technician is available for complex elective cases. When two emergent cases occur simultaneously after hours, the technicians are split and the backup nurse is called in. Improvement in groin puncture times upon patient arrival in the angiography suite required planning to ensure parallel preparation of patient, tray, and anesthesia. The ER stroke nurse hands over the patient to the INR nurse. The INR technicians prepare the room for patient arrival by opening pre-packaged stroke trays and devices as indicated by the INR attending. A separate stroke cart is stocked with the catheters, wires, thrombectomy devices, and syringes to hold everything in one place for this purpose. The biplane detectors are positioned in such a way as to facilitate patient transfer to the angiography table. Once the patient is placed on the table, a coordinated effort is made between the anesthestist working at the head of the patient and left arm (which is positioned out), and one of the INR technicians working at the groins for sterile preparation. The second technician continues with catheter, lines, and device preparation. The INR nurse assists the anesthesiologist, if required, prepares the flushes, and performs documentation. An 8 Fr right common femoral arterial sheath is placed as soon as the groin is prepped. If by that time a radial arterial line has not been established by the anesthesiologist, a second 4 Fr sheath is immediately placed in the left common femoral artery for invasive blood pressure monitoring. The 8 Fr sheath is removed at the end of the procedure and, if placed, the second 4 Fr sheath is sutured in place to be used in the ICU. An anesthesia cart and ventilator is permanently stationed in each of two adjacent biplane angiography suites, allowing performance of parallel emergent cases. Small coordinated steps in the patient preparation process were designed to emulate defined roles and parallel tasks seen in trauma resuscitation.
Figure 2.
INR: neurointerventionalist, T1: technician-1, T2: technician-2, T3: technician-3, N1: nurse-1, N2: nurse-2, A1: anesthesiologist/certified registered nurse anesthetist (CRNA)-1, A2: anesthesiologist/CRNA-2. During the patient preparation stage (A), T1 sets up the procedure trays and prepares the devices and catheters. T2 prepares the patient and helps the attending technician, who punctures the right femoral artery and typically places an 8 Fr sheath. The patient's left arm is extended out on an arm board for simultaneous access to anesthesia for placement of lines and administration of drugs. If there is no radial arterial access by the time the right femoral sheath is placed, the INR punctures the left femoral artery and places a 4 Fr sheath for invasive blood pressure monitoring. Even though it is possible to obtain arterial tracing via the 8 Fr right femoral sheath, placement of the 4 Fr sheath allows removal of the larger right femoral sheath at the end of the procedure. The patient is transferred to the intensive care unit with the 4 Fr sheath in place for pressure monitoring. The nurse takes a report, prepares the continuous flush lines, assists the anesthesiologist, and charts all times. The A-plane detector is stationed in such a way as to allow easy positioning over the groin in case fluoroscopy is required. For the interventional stage (B), T2 scrubs up and functions as the float. One anesthesiologist (A1) stays to cover the case, assisted by the nurse. This setup with stocked anesthesia cart is duplicated in an immediately adjacent second interventional biplane room. During working hours an additional technician (T3) and nurse (N2) are available. If two simultaneous emergent cases occur after hours, the technicians split and the backup nurse (N2) is called in.
Data analysis
The patient demographics and stroke severity are descriptively presented and compared. The significance of simple bivariate associations was assessed using the Fisher exact test for categorical variables. A Shapiro–Wilk W test demonstrated a non-normal distribution of the continuous time-point data. Non-parametric Wilcoxon rank sum tests were thus performed to compare the time-point means ‘before’ and ‘after’ the QI process. All statistical analysis was performed using the JMP Pro 12.0.1 software package (SAS Institute Inc, Cary, North Carolina, USA).
Results
A total of 94 patients were divided into a ‘before’ (n=64) and ‘after’ (n=30) group based on the implementation of the QI process. There were no differences in baseline demographics, stroke severity, symptom onset, comorbidities, and the use of IV rt-PA or general endotracheal anesthesia (GETA) between the two groups (table 1). A comparison of the time intervals showed a significant reduction in times in the ‘after’ QI implementation group across all parameters (figure 3). The time intervals were separately compared for interventions performed during working hours —that is, 7:00 to 17:00 on weekdays (table 2), and for off-hours and at weekends (table 3). There was significant reduction in times for both working hours and off-hours interventions. We found no correlation between age, NIHSS, onset to ER or any of the comorbidities and the door-to-needle time. We also looked at the impact of GETA on the patient preparation and puncture time after the implementation of the QI process. The mean time from INR room arrival to groin puncture was 15 (±4) min in patients who received GETA versus 15 (±3) min in patients treated without GETA (p=0.87), indicating that GETA caused no delays.
Table 1.
Comparison of baseline demographics, comorbidities and treatment variables
| Before, (n=64) | After, (n=30) | p Value | |
|---|---|---|---|
| Age, mean (SD) | 66.6 (15.8) | 66.2 (18.1) | 0.9 |
| Women, n (%) | 33 (51.6) | 16 (53.3) | 0.87 |
| NIHSS, median (IQR) | 16 (10–21) | 18 (11–24) | 0.2 |
| Onset–ER, mean h:min (SD) | 3:00 (2:08) | 3:03 (4:04) | 0.2 |
| IV rt-PA, n (%) | 26 (40.6) | 13 (43.3) | 0.8 |
| DM, n (%) | 22 (34.4) | 7 (23.3) | 0.27 |
| HTN, n (%) | 45 (70.3) | 21 (70) | 0.97 |
| HPL, n (%) | 30 (46.9) | 16 (53.3) | 0.56 |
| AFIB, n (%) | 19 (29.7) | 9 (30) | 0.97 |
| SMK, n (%) | 10 (15.6) | 8 (26.7) | 0.2 |
| Off-hours treatment, n (%) | 28 (43.8) | 16 (53.3) | 0.5 |
| GETA, n (%) | 39 (60.9) | 17 (56.7) | 0.7 |
AFIB, atrial fibrillation; DM, diabetes mellitus; ER, emergency room; GETA, general endotracheal anesthesia, off hours treatment: stroke intervention performed before 7:00 or after 17:00 or at the weekend; HPL, hyperlipidemia; HTN, hypertension; NIHSS, National Institutes of Health Stroke Scale; onset-ER, time from symptom onset to ER arrival; rt-PA, recombinant tissue plasminogen activator; SMK, smoking.
Figure 3.
Graphic comparison of the treatment times ‘before’ and ‘after’ implementation of the quality-improvement process.
Table 2.
Comparison of time parameters during working hours on weekdays
| Working hours—7:00–17:00 (n=50) | |||
|---|---|---|---|
| Before QI (n=36) | After QI (n=14) | p Value | |
| ER–CT, min | 42 (±28) | 27 (±17) | 0.011 |
| CT–Lab, min | 67 (±41) | 33 (±9) | 0.0008 |
| Lab–puncture, min | 24 (±16) | 16 (±3) | 0.0006 |
| CT–puncture, min | 90 (±45) | 49 (±10) | 0.0002 |
| Door–puncture, min | 132 (±53) | 75 (±18) | <0.0001 |
ER, emergency room; QI, quality improvement.
Table 3.
Comparison of time parameters after hours or on weekends
| Off hours and weekends (Sat–Sun) (n=44) | |||
|---|---|---|---|
| Before QI (n=28) | After QI (n=16) | p Value | |
| ER–CT, min | 38 (±30) | 26 (±13) | 0.35 |
| CT–lab, min | 113 (±40) | 67 (±38) | 0.0025 |
| Lab–puncture, min | 24 (±13) | 15 (±4) | 0.0035 |
| CT–puncture, min | 138 (±40) | 82 (±39) | 0.0002 |
| Door–puncture, min | 175 (±36) | 108 (±42) | <0.0001 |
ER, emergency room; QI, quality improvement.
Discussion
The Six-Sigma process, developed at Motorola in the 1980s because quality was lagging behind that of Japanese companies, was adopted by many corporations as a QI practice. An ideal healthcare environment would be efficient and free from errors. However, healthcare is neither of those and preventable errors cause 44 000–98 000 deaths a year.12 Six-Sigma has been applied in medicine to improve many processes from catheter-related10 and surgical-site infections13 to ER,14 surgery,15 and behavioral health systems.16 Six-Sigma achieves higher levels of quality by understanding and improving a process and decreasing, if not eliminating, variability. This is achieved using the DMAIC framework for ‘define’, ‘measure’, ‘analyze’, ‘improve’, and ‘control’.17 Each step in the DMAIC chain is fundamental to the success of the QI process.18
In our setting, the ‘process’ of endovascular stroke therapy required improvement of treatment times. The first step was to define specific goals —for example, door-to-needle time of no more than 120 min, split into key intervals such as ER–CT, CT–Lab, and Lab–puncture. Different members of the team were clearly identified for each stage and unambiguous roles assigned to each of them. The process was refined over a period of almost 3 months at weekly meetings, where all stroke interventions performed during that week were dissected, with iterations based on feedback from all involved. The performance of the different subprocesses and of the overall process was measured and incrementally improved. The new processes were then implemented, tested, and further enhanced. Our protocols mandated that processes should be performed simultaneously and not sequentially. For example, once it is decided to proceed with an intervention, the radiology and neurology residents perform their tasks independently and concurrently. Likewise, once the patient is in the INR suite, the nurse, the technicians, the attending physician, and the anesthesia team move at the same time. This has resulted in a 62% reduction in time to puncture from room arrival. Furthermore, this reduction in time is consistent regardless of the type of anesthesia used.
Our overall door-to-needle times are now at just over 90 min and the reduction in times is significant for both working hours (7:00–17:00 on weekdays) and off-hours interventions (tables 2 and 3). Our goal is to reduce this to 60 min. This may require the presence of an in-house interventional team, which could bring down the technicians/nurses’ response time from 30 min to <5 min. That alone will allow treatment to start within an hour of patient arrival. The other rate-limiting step is in imaging. We have significantly reduced our times for image acquisition, processing, and dissemination through a separate informatics project and it now takes about 10 min from placing the patient on the table to display of images; this includes NCCT, CTA, and CTP. A NCCT is the minimum imaging assessment that is required in stroke patients. By eliminating CTA and CTP we will gain, at most, 5–8 min. We do not think it is worth losing the large amount of information obtained from CTA/CTP merely to reduce the time by an additional 5–8 min. Information about clot location and burden, vascular anatomy, tandem lesions, access, and state of collaterals can make the subsequent intervention safer and faster.
Endovascular stroke therapy is entering its next phase of growth, which will require streamlining of both prehospital and intra-hospital care processes. Delivery of endovascular stroke therapy is resource intensive and costly,19 requiring round-the-clock readiness. Similar processes have been developed for acute coronary syndromes, specifically ST segment elevation myocardial infarctions (STEMIs).20 However, the number of current and projected future endovascular stroke interventions is much lower than STEMI interventions,21 owing to a higher prevalence of ischemic heart disease than stroke.22 This means that while both STEMI and LVO interventions require similar resources and investments to operate a round-the-clock service, the cost of LVO interventions will be lower owing to the smaller number of procedures performed. This is an important factor to consider when defining regional stroke care. It may be beyond the capability of smaller hospitals (defined as ≤300 beds) to invest in high-quality round-the-clock stroke therapy but the temptation could be there from a marketing perspective and a perceived financial benefit. Add to that an oversupply of physicians21 and we might find small regional hospitals with one to two physicians offering endovascular stroke care, not realizing that the postoperative care by dedicated neurospecialists and allied staff is as important as the procedure itself. The consequences of such a model could be dire with patients scattered among competing hospitals with variable standards of care and inconsistent quality metrics. Additionally, the comparatively lower prevalence of ischemic stroke will result in multiple centers carrying out a small volume of procedures instead of a regional high-volume center to which all such patients could be efficiently transferred and treated.
Limitations
The process at our institution was developed based on the manpower and resources within our system—a tertiary level academic medical center with in-house resident teams. We realize that our specific methodology may not be applicable to other systems with different resources.
Conclusion
Time-critical interventions such as endovascular stroke therapy can benefit from a systemically implemented protocol-driven approach. While the methodology can be different in different places based on available resources, its key elements include clear definition of roles and parallel processing of tasks. It also requires constant monitoring and process improvements for consistent performance. As the field grows it is important to regionalize systems of care based on population densities, facility capabilities, and health system networks.
Footnotes
Contributors: ATR: study design, data analysis, manuscript preparation. MSS, SHB, ART, JSC: manuscript preparation. GRH:data analysis.
Competing interests: None declared.
Ethics approval: Institutional review board.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Powers WJ, Derdeyn CP, Biller J, et al. . 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015;46:3020–35. 10.1161/STR.0000000000000074 [DOI] [PubMed] [Google Scholar]
- 2.Fiorella DJ, Fargen KM, Mocco J, et al. . Thrombectomy for acute ischemic stroke: an evidence-based treatment. J Neurointerv Surg 2015;7:314–15. 10.1136/neurintsurg-2015-011707 [DOI] [PubMed] [Google Scholar]
- 3.Kotwal RS, Howard JT, Orman JA, et al. . The effect of a golden hour policy on the morbidity and mortality of combat casualties. JAMA Surg Published Online First: 30 Sep 2015. doi:10.1001/jamasurg.2015.3104 10.1001/jamasurg.2015.3104 [DOI] [PubMed] [Google Scholar]
- 4.Ashmeade TL, Haubner L, Collins S, et al. . Outcomes of a neonatal golden hour implementation project. Am J Med Qual Published Online First: 5 Sep 2014. pii: 1062860614548888. 10.1177/1062860614548888 [DOI] [PubMed] [Google Scholar]
- 5.Mazighi M, Chaudhry SA, Ribo M, et al. . Impact of onset-to-reperfusion time on stroke mortality: a collaborative pooled analysis. Circulation 2013;127:1980–5. 10.1161/CIRCULATIONAHA.112.000311 [DOI] [PubMed] [Google Scholar]
- 6.Berkhemer OA, Fransen PS, Beumer D, et al. . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20. 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 7.Kelleher DC, Carter EA, Waterhouse LJ, et al. . Effect of a checklist on advanced trauma life support task performance during pediatric trauma resuscitation. Acad Emerg Med 2014;21:1129–34. 10.1111/acem.12487 [DOI] [PubMed] [Google Scholar]
- 8.Broderick JP, Palesch YY, Demchuk AM, et al. . Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med 2013;368:893–903. 10.1056/NEJMoa1214300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kidwell CS, Jahan R, Gornbein J, et al. . A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med 2013;368: 914–23. 10.1056/NEJMoa1212793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frankel HL, Crede WB, Topal JE, et al. . Use of corporate Six Sigma performance-improvement strategies to reduce incidence of catheter-related bloodstream infections in a surgical ICU. J Am Coll Surg 2005;201:349–58. 10.1016/j.jamcollsurg.2005.04.027 [DOI] [PubMed] [Google Scholar]
- 11.Kothari RU, Pancioli A, Liu T, et al. . Cincinnati prehospital stroke scale: reproducibility and validity. Ann Emerg Med 1999; 33:373–8. 10.1016/S0196-0644(99)70299-4 [DOI] [PubMed] [Google Scholar]
- 12.Kohn LT, Corrigan J, Donaldson MS. To err is human: building a safer health system. Washington DC: National Academy Press, 2000. [PubMed] [Google Scholar]
- 13.Kles CL, Murrah CP, Smith K, et al. . Achieving and sustaining zero: preventing surgical site infections after isolated coronary artery bypass with saphenous vein harvest site through implementation of a staff-driven quality improvement process. Dimens Crit Care Nurs 2015;34:265–72. 10.1097/DCC.0000000000000131 [DOI] [PubMed] [Google Scholar]
- 14.Sanders JH, Karr T. Improving ED specimen TAT using lean Six Sigma. Int J Health Care Qual Assur 2015;28:428–40. 10.1108/IJHCQA-10-2013-0117 [DOI] [PubMed] [Google Scholar]
- 15.Mason SE, Nicolay CR, Darzi A. The use of lean and Six Sigma methodologies in surgery: a systematic review. Surgeon 2015;13:91–100. 10.1016/j.surge.2014.08.002 [DOI] [PubMed] [Google Scholar]
- 16.Lucas AG, Primus K, Kovach JV, et al. . Rethinking behavioral health processes by using design for Six Sigma. Psychiatr Serv 2015;66:112–14. 10.1176/appi.ps.201400384 [DOI] [PubMed] [Google Scholar]
- 17.Pyzdek T, Keller PA. The Six Sigma handbook. New York: McGraw-Hill Education, 2014. [Google Scholar]
- 18.Kwak YH, Anbari FT. Benefits, obstacles, and future of Six Sigma approach. Technovation 2006;26:708–15. 10.1016/j.technovation.2004.10.003 [DOI] [Google Scholar]
- 19.Rai AT, Evans K. Hospital-based financial analysis of endovascular therapy and intravenous thrombolysis for large vessel acute ischemic strokes: the ‘bottom line’. J Neurointerv Surg 2015;7:150–6. 10.1136/neurintsurg-2013-011085 [DOI] [PubMed] [Google Scholar]
- 20.Ahmed B, Lischke S, Straight F, et al. . Consistent door-to-balloon times of less than 90 minutes for STEMI patients transferred for primary PCI. J Invasive Cardiol 2009;21:429–33. [PubMed] [Google Scholar]
- 21.Rai AT. Red pill, blue pill: reflections on the emerging large vessel stroke ‘market’. J Neurointerv Surg 2015;7:623–5. 10.1136/neurintsurg-2015-011971 [DOI] [PubMed] [Google Scholar]
- 22.Mozaffarian D, Benjamin EJ, Go AS, et al. . Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015;131:e29–322. 10.1161/CIR.0000000000000152 [DOI] [PubMed] [Google Scholar]