Abstract
Background
Chronic obstructive pulmonary disease (COPD) is one of the most common lung diseases. It is a chronic inflammatory process characterised by airway obstruction and progressive lung inflammation, associated with difficulty breathing and insensitivity to corticosteroid therapy. Although there is some preliminary evidence to suggest a beneficial effect of acupuncture on COPD, its mechanism of action has not been investigated. Our aim was to examine the anti-inflammatory effects of acupuncture in a rat model of COPD induced by exposure to cigarette smoke (CS).
Methods
Sixty Sprague–Dawley rats were exposed to the smoke of 15 cigarettes for 1 h/day, 6 days/week for 3 months to induce COPD and treated with acupuncture at BL13 (Feishu), BL23 (Shenshu) and Dingchuan (COPD+Acupuncture, n=15), sham acupuncture (COPD+Sham, n=15) or left untreated (n=15). Exposed rats were compared with controls not exposed to CS (control, n=15). Pulmonary function was measured, and tumour necrosis factor-α (TNF-α) and interleukin-8 (IL-8) levels were determined in bronchoalveolar lavage fluid by ELISA. Histone deacetylase 2 (HDAC2) protein and mRNA expression were examined in lung tissue and in bronchus.
Results
Acupuncture treatment appeared to protect pulmonary function and reduce the COPD-induced inflammatory response by decreasing cell inflammation and the production of TNF-α and IL-8. Acupuncture also enhanced HDAC2 mRNA and protein expression, suggesting a possible direct effect on protein structure through post-translational modifications.
Conclusions
Our results suggest that acupuncture regulates inflammatory cytokines and contributes to lung protection in a rat model of smoke-induced COPD by modulating HDAC2.
Keywords: ACUPUNCTURE
Introduction
Chronic obstructive pulmonary disease (COPD) is caused by chronic inflammation of the peripheral airways and lung parenchyma. It is a common and debilitating chronic inflammatory disease characterised by the progressive development of poorly reversible airflow obstruction. Its prevalence is increasing and safe, effective anti-inflammatory therapies are lacking.1 Moreover, the discovery of new treatments is hindered by a fundamental lack of knowledge of the cellular, molecular and genetic mechanisms of the disease. COPD is frequently complicated by remodelling of the airways, which often involves the development of emphysema. It is also characterised by episodes of acute worsening of symptoms, which negatively affect progression of the disease and survival.
Genetic predisposition plays an important role in the development of COPD; however, the most critical risk factors for COPD include cigarette smoking2 and exposure to dust, fumes, polluted air particles and biomass fuels. Corticosteroids are the most widely used anti-inflammatory therapy in COPD but have limited clinical benefit, particularly in more severe disease. These drugs only partially suppress cytokine production by COPD alveolar macrophages. Moreover, a subset of subjects with COPD are relatively insensitive to this treatment.
In recent years, acupuncture therapy has been widely accepted as a useful therapeutic technique with negligible risks for the clinical prevention of inflammatory diseases, including asthma, rhinitis, inflammatory bowel disease, rheumatoid arthritis, epicondylitis, complex regional pain syndrome type 1 and vasculitis. Clinical and experimental studies have demonstrated the therapeutic effects of electroacupuncture (EA) on COPD.3 For example, patients with COPD receiving traditional acupuncture experienced improvement in dyspnoea on exertion and 6-min walking distance, indicating better exercise tolerance.4 5 However, the mechanisms underlying the anti-inflammatory action of acupuncture are still unclear. In particular, epigenetic mechanisms such as histone modification have not been extensively investigated to date. Alterations in histone acetyltransferase and histone deacetylase (HDAC) mRNA expression and protein activity have direct consequences on the transcription of genes that may be involved in inflammatory lung diseases. Gene expression is regulated by acetylation of core histones, which open up the chromatin allowing transcription factors and RNA polymerase to bind to DNA and initiate gene transcription.6 HDACs play a critical role in the suppression of gene expression by reversing the hyperacetylation of core histones. For instance, a significant reduction in total HDAC activity was observed in the lung tissue of patients with COPD compared with healthy controls.7 In addition, a recent study observed a reduction in HDAC2 in patients with COPD compared with healthy controls only during exacerbations,8 suggesting that lung environment may have a determinant effect. HDAC2 activity and expression are also reduced in peripheral lung and alveolar macrophages, resulting in amplification of the inflammatory response to COPD. Recent research has shown that suppression of HDAC2 by either a pharmacological inhibitor (trichostatin A, TSA) or a small interfering RNA (siRNA) targeting HDAC2 does not block the inhibitory effects of budesonide in fibroblasts, which contrasts with HDAC-dependent inhibition in human bronchial epithelial cells and monocytic cells. The observation that the anti-inflammatory effects of budesonide in lung fibroblasts are HDAC-independent suggests that budesonide has the potential to modulate fibroblast-mediated tissue remodelling following airway inflammation in COPD and provides a mechanism for the therapeutic benefits of glucocorticoids in COPD. Nevertheless, treatments that restore HDAC2 may be clinically feasible in reducing cigarette smoke (CS)-induced inflammatory responses.9
On this background, the present study was conducted to investigate the effects of acupuncture therapy on HDAC2 levels and inflammation in a CS-induced rat model of COPD.
Methods
Animals and COPD model
This study was approved by the Animal Research Committee of Hubei Province. All protocols were based on the Guidelines for the Care and Use of Laboratory Animals published by the National Institutes of Health (USA) and efforts were made to minimise the number of animals and any discomfort to them. Sixty male Sprague–Dawley rats were kept in an animal house maintained at 21±2°C with a 12 h/12 h light/dark cycle and were given free access to food and water. Rats weighing 300–350 g were randomly divided into four groups (n=15 each), namely, control, COPD model (COPD), COPD with (real) acupuncture (COPD+Acupuncture) and COPD with sham acupuncture (COPD+Sham). The latter three groups were exposed to smoke from 15 cigarettes for 1 h per day (in the morning) for 6 days/week for a total of 3 months, as previously described.10
Acupuncture treatment
Manual acupuncture was applied to verum and sham groups after CS exposure and was performed by a single acupuncturist with 15 years experience (Jia Li). All rats (including the control and model groups) were mildly anaesthetised with 6% chloral hydrate at 4 mL/kg (a reduced dose to enable rats to crawl when given appropriate stimulation). All rats completely recovered from the anaesthesia within about 30 min. Stainless-steel acupuncture needles (15 mm long, 0.3 mm diameter from Suzhou Medical Appliance Factory, China) were inserted into the back of the rats at acupuncture points BL13 (Feishu), BL23 (Shenshu) and Dingchuan to a depth of 5 mm. The needles were evenly twisted through 360° at 2 Hz frequency for 1 min every 5 min, as previously described.11 This pattern was repeated six times (30 min) during the session, following which the needle was withdrawn. BL13, BL23 and Dingchuan were located at the depressions lateral to the lower border of the spinous processes of the third thoracic, second lumbar and seventh cervical vertebrae, respectively, approximately 0.8 cm from the midline of the adult rat (approximately 1/20 of the thorax circumference). The COPD+Sham group was stimulated unilaterally at tissues located 5 mm away from the above acupuncture points on the left side. Acupuncture treatments were carried out once a day for 6 days/week for 3 months after modelling.
Pulmonary function
Ten rats per group were anesthetised by intraperitoneal injection with 7 mL/kg 6% chloral hydrate. Basal lung function was measured in unrestrained rats using a whole-body flow plethysmography system (Buxco Electronics, North Carolina, USA). This method involved the continuous monitoring of a number of ventilatory parameters including inspiratory capacity (IC), peak expiratory flow (PEF) and minute volume (MV).
Bronchoalveolar lavage fluid
Twelve rats per group were anesthetised intraperitoneally with pentobarbital (40 mg/kg) and the lungs were lavaged through a tracheal cannula by instillation of 25 mL/kg aliquots of 4°C sterile phosphate-buffered saline solution. Bronchoalveolar lavage fluid (BALF) was retrieved by gentle aspiration and the lavage procedure was repeated three times. All BALF samples from the same rat were pooled and immediately centrifuged at 1200 rpm for 10 min at 4°C. The cell-free supernatant was obtained and stored at −20°C for analysis of tumour necrosis factor-α (TNF-α) and interleukin-8 (IL-8) levels with an ELISA method using commercially available kits (reference nos. PRTA00 and FAB2164P-100, respectively; R&D Systems, Minneapolis, Minnesota, USA).
Immunostaining
Localisation of HDAC2 was assessed by immunohistochemical staining in 10 rats per group. At the end of the treatment period, rats were euthanised with 400 mg/kg chloral hydrate and lung tissues were sampled, snap frozen and stored at −85°C. Tissue sections of 5 μm thickness were mounted on glass slides coated with aminosilane, deparaffinised with xylene, and hydrated in decreasing concentrations of ethanol. After fixing with 4% paraformaldehyde and permeating with 0.2% Triton, endogenous peroxidase activity was blocked with 2% bovine serum albumin (BSA) in 0.1 M phosphate-buffered saline with Tween (PBST) for 30 min at room temperature. After a brief rinse in 0.1 M PBST, the sections were transferred and incubated with polyclonal rabbit anti-acetyl-histone H2 antibody (1:500; Cell Signalling) in 2% BSA/PBST overnight at 4°C on a shaker. After incubation, sections were rinsed in 0.1 M PBST for 5 min and reacted with biotinylated secondary antibodies (1:2000, rabbit IgG; Vector Laboratories, California, USA) for 1 h at room temperature. Lastly, the sections were mounted on 1% gelatinised slides, air-dried, dehydrated serially in alcohol and covered with Permount mounting medium. Image J software (National Institutes of Health, Maryland, USA) was used for OD step wedge calibration and subsequent image analysis. A control immunostaining experiment was also conducted, which was identical to the above immunohistochemical procedure but without the primary antibody.
Immunoblot analysis
HDAC2 activity was measured by Western blot analysis in 10 rats per group. Briefly, small quick-frozen bronchi were individually homogenised on dry ice and boiled in 50 μl of sodium dodecyl sulfate (SDS) sample buffer (1.5% dithiothreitol, 2% SDS, 80 mM Tris-HCl (pH 6.8), 10% glycerol and 0.01% bromophenol blue) for 5 min and then separated by SDS polyacrylamide gel electrophoresis. Antibody against HDAC2 was purchased from Santa Cruz Biotechnology (Texas, USA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody was purchased from Fitzgerald Industries International (Massachusetts, USA). The levels of proteins in the immunoblots were quantified by scanning densitometry (Fuji Multigauge V.3.0 Software). The luminescent signals from all immunoblots were within the linear range.
Real-time PCR for HDAC2
Gene expression of HDAC2 was measured in the bronchial tissues of all rats (n=15 per group) using quantitative real-time (RT) PCR. Total RNA was isolated using Trizol reagent according to the manufacturer's instructions. RNA (1 µg) was extracted and reverse transcribed into cDNA using the PrimeScript RT Reagent Kit (Takara, Japan). Following RT-PCR reactions, mRNA expression of HDAC2 was expressed relative to the sample's own GAPDH content. Primer sequences for HDAC2 and GAPDH were designed as follows: HDAC2, FW: 5′-GCTGCTTCAACCTAACTG-3′; RV: 5′-CTCATACGTCCAACATCG-3′; GAPDH, FW: 5′-CCTGGAGAAACCTGCCAAG-3′; RV: 5′-CACAGGAGACAACCTGGTCC-3′. After PCR reaction, the products were analysed by electrophoresis on 1.2% agarose gel and visualised by ethidium bromide staining.
Statistical analyses
All statistical analyses were performed using Prism 4 software (GraphPad Software, California, USA). Statistical differences were evaluated using one-way ANOVA followed by Tukey's multiple comparison test at the p<0.05 level of significance. Data are presented as the mean±SEM.
Results
Figure 1 shows the effect of acupuncture on pulmonary function after CS-induced COPD in rats. Compared to controls, both the COPD and COPD+Sham groups demonstrated significant decreases in IC, PEF and MV. However, the above parameters increased for rats in the COPD+Acupuncture group compared with COPD rats. No significant difference was observed between the COPD and COPD+Sham groups.
Figure 1.
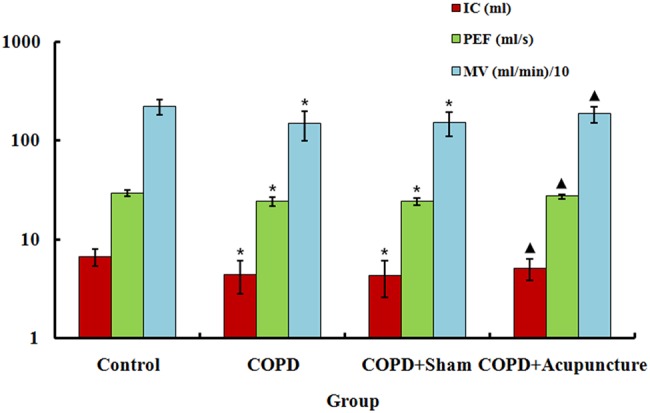
Basal lung function measured in 60 unrestrained rats assigned to a control group (n=15) or exposed to cigarette smoke for 3 months to induce chronic obstructive pulmonary disease (COPD) and treated with verum acupuncture (COPD+Acupuncture, n=15), sham acupuncture (COPD+Sham, n=15) or left untreated (COPD, n=15). IC, inspiratory capacity; MV, minute volume; PEF, peak expiratory flow. *p<0.01, compared to control group; ▴p<0.05, compared to COPD group. Data are presented as the mean±SEM.
As shown in figure 2, TNF-α and IL-8 levels in BALF markedly increased in the COPD group compared with the control group. By contrast, levels were decreased in the COPD+Acupuncture group compared with the COPD group but remained higher than in the Control group. No significant difference was observed between the COPD and COPD+Sham groups.
Figure 2.
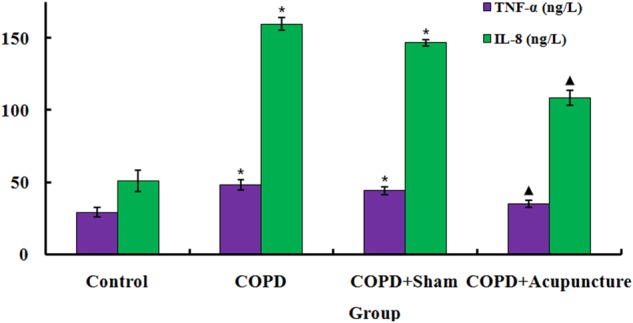
Tumour necrosis factor-α (TNF-α) and interleukin (IL-8) levels measured in bronchoalveolar lavage fluid obtained via tracheal cannulation of 60 unrestrained rats assigned to a control group (n=12) or exposed to cigarette smoke for 3 months to induce chronic obstructive pulmonary disease (COPD) and treated with verum acupuncture (COPD+Acupuncture, n=12), sham acupuncture (COPD+Sham, n=12) or left untreated (COPD, n=12). *p<0.01, compared to control group; ▴p<0.05, compared to COPD group. Data are presented as the mean±SEM.
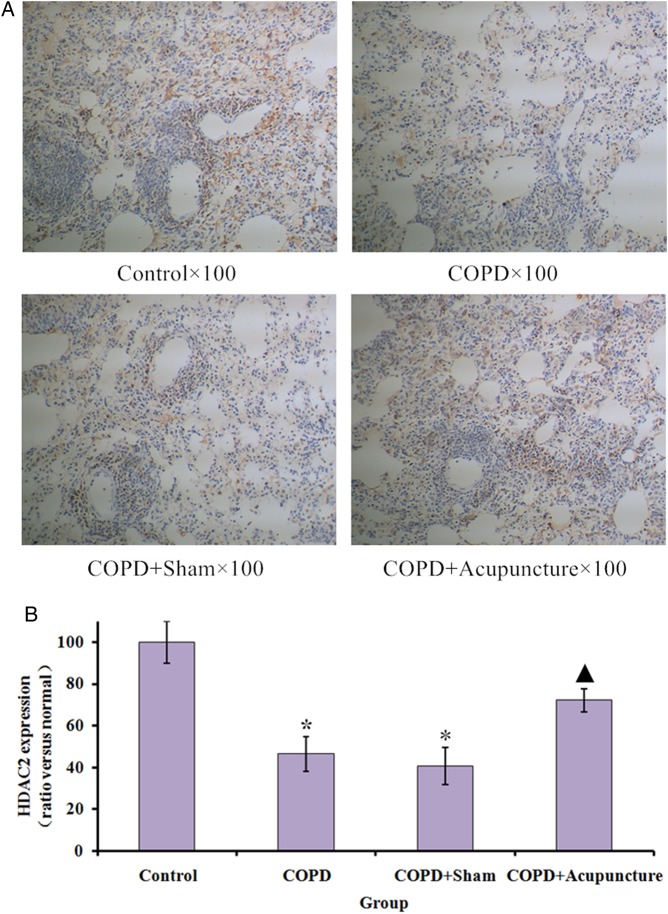
HDAC2 immunostaining revealed expression in the COPD group was significantly lower than in the Control group, while acupuncture treatment significantly enhanced HDAC2 protein expression (figure 3A, B). No significant difference was observed between the COPD and COPD+Sham groups. As demonstrated in figure 4A, B, HDAC2 protein expression was also significantly lower in the COPD group compared with the Control group, and the acupuncture group demonstrated higher HDAC2 expression compared with the COPD group. Similarly in figure 5, mRNA expression of HDAC2 was decreased in the COPD versus Control groups but relatively increased in the COPD+Acupuncture group. However, no significant difference was observed between the COPD and COPD+Sham groups in either protein or mRNA expression of HDAC2.
Figure 3.
Histone deacetylase 2 (HDAC2) immunostaining in lung tissues (×100 magnification) of 60 unrestrained rats assigned to a control group (n=10) or exposed to cigarette smoke for 3 months to induce chronic obstructive pulmonary disease (COPD) and treated with verum acupuncture (COPD+Acupuncture, n=10), sham acupuncture (COPD+Sham, n=10) or left untreated (COPD, n=10). *p<0.01, compared to control group; ▴p<0.05, compared to COPD group. Data are presented as the mean±SEM.
Figure 4.
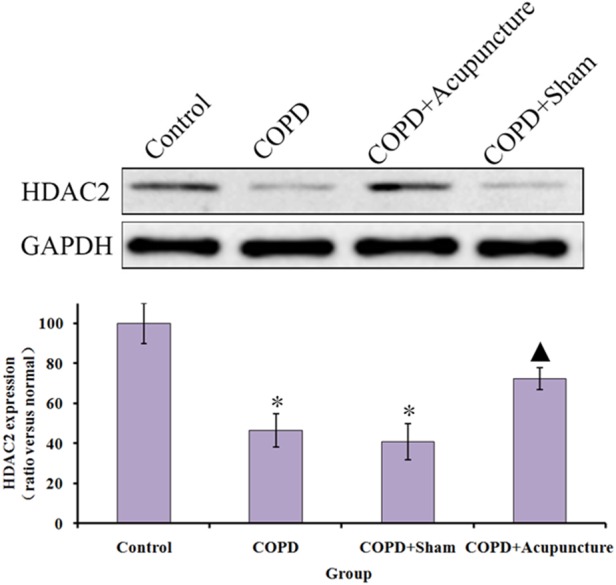
Protein expression of histone deacetylase 2 (HDAC2; measured by Western blot analysis) in lung tissue of 60 unrestrained rats assigned to a control group (n=10) or exposed to cigarette smoke for 3 months to induce chronic obstructive pulmonary disease (COPD) and treated with verum acupuncture (COPD+Acupuncture, n=10), sham acupuncture (COPD+Sham, n=10) or left untreated (COPD, n=10). *p<0.01, compared to control group; ▴p<0.05, compared to COPD group. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. Data are presented as the mean±SEM.
Figure 5.
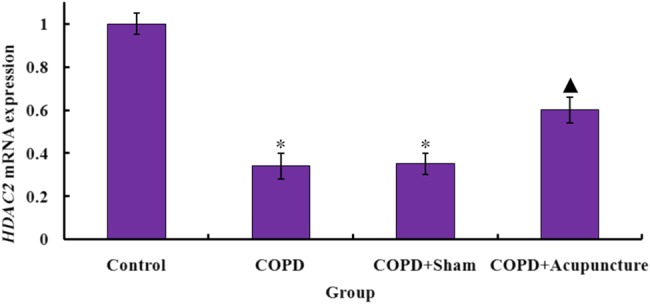
Histone deacetylase 2 (HDAC2) mRNA expression (measured by real-time-PCR in triplicate and expressed relative to glyceraldehyde 3-phosphate dehydrogenase) in the bronchus of 60 unrestrained rats assigned to a control group (n=15) or exposed to cigarette smoke for 3 months to induce chronic obstructive pulmonary disease (COPD) and treated with verum acupuncture (COPD+Acupuncture, n=15), sham acupuncture (COPD+Sham, n=15) or left untreated (COPD, n=15). *p<0.01, compared to control group; ▴p<0.05, compared to COPD group. Data are presented as the mean±SEM.
Discussion
Cigarette smoking is by far the most important risk factor for COPD and is strongly linked to inflammation in the airways and lung parenchyma. Consequently, the severity of airflow obstruction is correlated with the degree of pulmonary inflammation. Many researchers favour the CS-induced rat model as it features the same primary insult as seen in the clinical condition and produces emphysema, airway remodelling and physiological alterations similar to those in humans. Cigarettes contain over 4000 chemical compounds and 400 or more toxic substances including nicotine, tar, acrolein, carbon monoxide, hydrogen cyanide, ammonia, aromatic compounds, arsenic, mercury and chromium. The pulmonary vascular structure in smokers has been extensively investigated, and it is known that CS induces remodelling of pulmonary vessels.12 Based on previous studies, 3 months of CS exposure in the CS-induced rat model is enough to induce alveolar wall destruction and airspace enlargement.10 In the present experiment, reductions in PEF, IC and MV in rats with CS-induced COPD were normalised after acupuncture treatment, supporting a potential role of acupuncture in reversing airway obstruction and protecting pulmonary function. Acupuncture also decreased levels of IL-8 and TNF-α in BALF and improved tissue levels of HDAC2 protein expression. Furthermore, no significant differences were observed between the COPD and COPD+Sham groups, which suggests point or regional specificity of the acupuncture protocol.
COPD affects the airways and lung parenchyma resulting in progressive and mostly irreversible airflow obstruction, and is associated with persistent inflammation and oxidative stress in susceptible individuals. It is increasingly acknowledged as a systemic disease because of its clinically significant extra-pulmonary consequences, although the lung is the primary diseased organ.13 Inflammatory processes in COPD are complex. Compared with normal subjects, levels of neutrophil chemotactic mediators (such as IL-8 and leukotriene B) and pro-inflammatory cytokines (such as TNF- α) are increased in the sputum of patients with COPD. COPD inflammation is accompanied by neutrophilia that persists many years after smoking cessation. IL-8 is a neutrophil attractant that is over-expressed in COPD and is considered to be a major driver of neutrophilia.14 The findings of the present study suggest that acupuncture treatment for COPD affects IL-8 and TNF-α.
Histone acetylation is reversed by HDACs. There are 11 HDAC isoenzymes that deacetylate histones and other proteins within the nucleus. Specific HDACs may regulate different groups of genes. In contrast, HDAC enzymes including the class I HDACs (HDAC1, HDAC2 and HDAC3) condense chromatin, forming a more compact, transcriptionally repressive conformation. HDAC2 appears to be critically important for the regulation of inflammatory genes.15 A concurrent reduction in mRNA expression of HDACs 2, 5, 8 and protein expression of HDAC216 suggests a possible deficiency of certain types in COPD. HDAC is a key molecule in the repression of pro-inflammatory cytokine production in alveolar macrophages. Thus, patients with COPD have a progressive reduction in total HDAC activity that reflects the severity of the disease.16 In particular, HDAC2 is essential for corticosteroid-dependent anti-inflammatory processes, and is associated with pro-inflammatory transcription factors bound to promoter regions of pro-inflammatory genes together with activation of the glucocorticoid receptor (GR).17 It has been shown previously that HDAC2 is subject to oxidative modification that possibly alters its activity. For example, hyperphosphorylation disrupts HDAC2 interaction with other co-repressors.18 An important mechanism of corticosteroid resistance in COPD, which is also linked to amplification of the inflammatory process, is a reduction in the critical nuclear HDAC2 enzyme.19 Therefore, restoration or attenuation of HDAC2 loss enhances glucocorticoid sensitivity by deacetylating GR.
A number of recent basic science and clinical studies have suggested that acupuncture may reduce lung injury associated with COPD, possibly through downregulation of inflammatory cytokines. Anti-inflammatory and antioxidant effects have been implicated in the clinical benefit of EA20 and it has been shown that acupuncture is superior to placebo needling in improving dyspnoea in COPD patients receiving standard medication.4 Several Chinese studies have suggested that acupuncture significantly improves patients' lung function and quality of life. Regarding point selection, BL1321 and Dingchuan22 have been used classically in COPD and share the same segmental innervation as the lung (T1–T5). BL23 is also commonly used in clinical practice, particularly for renal and pulmonary conditions.23
In the present study, IL-8 and TNF-α expression were increased in peripheral lung tissues from rats with COPD, and HDAC2 activity was decreased. The reduction in HDAC2 activity may in part account for the increased inflammatory response in the respiratory tract of patients with COPD. Moreover, HDAC2 was increased in rats treated with verum acupuncture but not sham acupuncture.
Limitations
Although the CS-induced rat model of COPD successfully mirrors human COPD caused by cigarette smoking, some COPD cases develop in the absence of CS, and so these findings may not be applicable to all patients with COPD. Ultimately further studies are needed to determine the efficacy of acupuncture in clinical practice. As rats received acupuncture treatment under mild anaesthesia, a confounding influence of the resultant narcosis on the effect of acupuncture cannot be excluded; however, the use of anaesthesia in all experimental groups controlled for this aspect when groups were compared in the present study.
Conclusion
This study found that acupuncture treatment attenuated the CS-induced inflammatory response in a rat model of COPD by inhibiting the production of IL-8 and TNF-α, which complex with HDAC2. This study provides insights into the potential applications of acupuncture in the treatment of COPD, and the development of new and effective anti-inflammatory therapies.
Footnotes
Contributors: Jia Li designed the research; Song Wu and Hua Wang performed the research; Hongtu Tang analysed the data; Wei Huang, Lushan Wang, Huanjiao Zhou and Miao Zhou helped with data interpretation and discussion; Jing Li wrote the paper.
Funding: This work was supported by a project grant from NSFC (Natural Science Foundation of China, nos. 81403485, 81403460, 81473787 and 81170049). The funding agency provided relevant information and helped in the design of the study, data collection and analysis, decision to publish, and preparation of the manuscript.
Competing interests: None declared.
Ethics approval: This study was prospectively approved by the Animal Research Committee of Hubei Province and was conducted in accordance with guidelines for animal welfare equivalent to the National Research Council's ‘Guide for the Care and Use of Laboratory Animals’.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347–65. 10.1164/rccm.201204-0596PP [DOI] [PubMed] [Google Scholar]
- 2.Kim V, Rogers TJ, Criner GJ. New concepts in the pathobiology of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008;5:478–85. 10.1513/pats.200802-014ET [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coyle ME, Shergis JL, Huang ET, et al. Acupuncture therapies for chronic obstructive pulmonary disease: a systematic review of randomized, controlled trials. Altern Ther Health Med 2014;20:10–23. [PubMed] [Google Scholar]
- 4.Suzuki M, Muro S, Ando Y, et al. A randomized, placebo-controlled trial of acupuncture in patients with chronic obstructive pulmonary disease (COPD): the COPD-acupuncture trial (CAT). Arch Intern Med 2012;172:878–86. [DOI] [PubMed] [Google Scholar]
- 5.Jobst K, Chen JH, McPherson K, et al. Controlled trial of acupuncture for disabling breathlessness. Lancet 1986;2:1416–19. 10.1016/S0140-6736(86)92732-7 [DOI] [PubMed] [Google Scholar]
- 6.Szyf M. Epigenetics, DNA methylation, and chromatin modifying drugs. Annu Rev Pharmacol Toxicol 2009;49:243–63. 10.1146/annurev-pharmtox-061008-103102 [DOI] [PubMed] [Google Scholar]
- 7.Lakshmi SP, Reddy AT, Zhang Y, et al. Down-regulated peroxisome proliferator-activated receptor γ (PPARγ) in lung epithelial cells promotes a PPARγ agonist-reversible proinflammatory phenotype in chronic obstructive pulmonary disease (COPD). J Biol Chem 2014;289:6383–93. 10.1074/jbc.M113.536805 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Tamimi A, Serdarevic D, Hanania NA. The effects of cigarette smoke on airway inflammation in asthma and COPD: therapeutic implications. Respir Med 2012;106:319–28. 10.1016/j.rmed.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Nelson A, Weiler ZM, et al. Anti-inflammatory effects of budesonide in human lung fibroblast are independent of histone deacetylase 2. J Inflamm Res 2013;6:109–19. 10.2147/JIR.S43736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L, Bao H, Wu J, et al. Baicalin is anti-inflammatory in cigarette smoke-induced inflammatory models in vivo and in vitro: a possible role for HDAC2 activity. Int Immunopharmacol 2012;13:15–22. 10.1016/j.intimp.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 11.Liu H, Shen X, Tang H, et al. Using MicroPET imaging in quantitative verification of the acupuncture effect in ischemia stroke treatment. Sci Rep 2013;3:1070 10.1038/srep01070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright JL, Levy RD, Churg A. Pulmonary hypertension in chronic obstructive pulmonary disease: current theories of pathogenesis and their implications for treatment. Thorax 2005;60:605–9. 10.1136/thx.2005.042994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans RA, Morgan MD. The systemic nature of chronic lung disease. Clin Chest Med 2014;35:283–93. 10.1016/j.ccm.2014.02.009 [DOI] [PubMed] [Google Scholar]
- 14.Postma DS, Reddel HK, ten Hacken NH, et al. Asthma and chronic obstructive pulmonary disease: similarities and differences. Clin Chest Med 2014;35:143–56. 10.1016/j.ccm.2013.09.010 [DOI] [PubMed] [Google Scholar]
- 15.Ito K, Yamamura S, Essilfie-Quaye S, et al. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J Exp Med 2006;203:7–13. 10.1084/jem.20050466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito K, Ito M, Elliott WM, et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med 2005;352:1967–76. 10.1056/NEJMoa041892 [DOI] [PubMed] [Google Scholar]
- 17.Barnes PJ. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol 2013;131:636–45. 10.1016/j.jaci.2012.12.1564 [DOI] [PubMed] [Google Scholar]
- 18.Galasinski SC, Resing KA, Goodrich JA, et al. Phosphatase inhibition leads to histone deacetylases 1 and 2 phosphorylation and disruption of corepressor interactions. J Biol Chem 2002;277:19618–26. 10.1074/jbc.M201174200 [DOI] [PubMed] [Google Scholar]
- 19.Barnes PJ. Reduced histone deacetylase in COPD: clinical implications. Chest 2006;129:151–5. 10.1378/chest.129.1.151 [DOI] [PubMed] [Google Scholar]
- 20.Geng WY, Liu ZB, Song NN, et al. Effects of electroacupuncture at Zusanli (ST36) on inflammatory cytokines in a rat model of smoke-induced chronic obstructive pulmonary disease. J Integr Med 2013;11:213–19. 10.3736/jintegrmed2013024 [DOI] [PubMed] [Google Scholar]
- 21.Zhang XF, Zhu J, Geng WY, et al. Electroacupuncture at Feishu (BL13) and Zusanli (ST36) down-regulates the expression of orexins and their receptors in rats with chronic obstructive pulmonary disease. J Integr Med 2014;12:417–24. 10.1016/S2095-4964(14)60040-6 [DOI] [PubMed] [Google Scholar]
- 22.Ngai SP, Jones AY, Hui-Chan CW, et al. An adjunct intervention for management of acute exacerbation of chronic obstructive pulmonary disease (AECOPD). J Altern Complement Med 2013;19:178–81. 10.1089/acm.2011.0222 [DOI] [PubMed] [Google Scholar]
- 23.Hu J. Clinical observation on 25 cases of hormone dependent bronchial asthma treated by acupuncture. J Tradit Chin Med 1998;18:27–30. [PubMed] [Google Scholar]