Abstract
Objective
Acupuncture has been shown to be effective for the treatment of chemotherapy-related nausea and vomiting. The aim of this study was to explore the mechanisms of action underlying the anti-emetic effect of electroacupuncture (EA).
Design
Forty-eight rats received saline (n=12) or 6 mg/kg cisplatin (n=36) to establish a chemotherapy-induced nausea and vomiting model. EA was performed at CV12 (n=12), bilateral PC6 (n=12), or sham points (n=12) 3 days before and 1–2 days after cisplatin administration (4–5 times in total), at 0.5–1 mA intensity and 2/15 Hz frequency for 10 min. Kaolin intake, food intake and bodyweight change were evaluated as markers of nausea and vomiting severity. Concentrations of serotonin (5-hydroxytryptamine, 5-HT) in the duodenum and c-Fos expression in the nucleus of the solitary tract (NTS) were measured using high performance liquid chromatography and immunohistochemistry, respectively.
Results
Cisplatin administration led to increased kaolin intake and reduced food intake and bodyweight over the following 2 days. EA at CV12 significantly reversed the cisplatin-induced change in kaolin intake (on days 1 and 2) and food intake and bodyweight (on day 1). EA at CV12 also attenuated the cisplatin-induced increase in 5-HT in the duodenum and suppressed c-Fos expression in the NTS. EA at PC6 influenced kaolin intake (on day 1 only) and c-Fos expression, but had no statistically significant effect on food intake, bodyweight or 5-HT expression.
Conclusions
This study demonstrated beneficial effects of EA on chemotherapy-induced nausea and vomiting in a rat model. The anti-emetic effect of EA may be mediated through inhibition of 5-HT secretion in the duodenum and activity of the NTS.
Keywords: ONCOLOGY, GASTROENTEROLOGY)
Introduction
Nausea and vomiting are major side effects of chemotherapy and can severely impair quality of life. They are also a key reason for non-compliance with cancer treatment. Cisplatin has been widely used as an antineoplastic drug in the treatment of solid tumours for more than 30 years.1 It is classified into the highest emetic risk group according to the American Society of Clinical Oncology guidelines,2 which often limits its therapeutic use. Despite the availability of antiemetic medications, including serotonin (5-HT3A) receptor antagonists, glucocorticosteroids, cannabinoids, and neurokinin (NK)-1 antagonist, a considerable number of patients still suffer from nausea and vomiting.3 Moreover, these drugs frequently cause additional side effects such as headaches, constipation, diarrhoea, asthenia, and somnolence.4
Acupuncture can treat and prevent various diseases through stimulation of the body surface with needles, heat or pressure. A large systematic review of randomised controlled trials has shown that acupuncture is effective in preventing or attenuating post-chemotherapy nausea and vomiting.5 However, the mechanism underlying the antiemetic effect of acupuncture remains unclear.
Serotonin (5-hydroxytryptamine, 5-HT) is an important neurotransmitter, which is implicated in cisplatin-induced emesis.1 6 7 5-HT is released from the secretory granules of the enterochromaffin (EC) cells, which are located mostly in the duodenum. After the administration of chemotherapy, free radicals are generated, leading to release of 5-HT from the EC cells of the stomach and intestine. 5-HT activates vagal afferent nerves, which are connected to the brain stem structures associated with nausea and vomiting. The nucleus of the solitary tract (nucleus tractus solitarii, NTS) is the main target for incoming fibres from the vagal nerve and plays an important role in the emetic response.8 9
The aim of this study was to examine the effects of electroacupuncture (EA) at two widely used acupuncture points for the treatment of nausea and vomiting, namely CV12 (Zhongwan) and PC6 (Neiguan),5 on cisplatin-induced anorexic behaviour in rats, including changes in food intake, bodyweight and kaolin intake (reflecting ‘pica’ behaviour, which has been validated as an index of nausea and vomiting in rats).10–12 We also aimed to measure the 5-HT concentration in the duodenum and the expression of c-Fos (a commonly used marker of neuronal activity) in the NTS to explore the underlying mechanism.
Methods
Animals
Male Wistar rats (aged 6 weeks and weighing 180–250 g, from the Academy of Military Medical Sciences, Beijing, China) were used for this study. The rats were housed in individual cages at a temperature of 22±2°C and humidity of 50–60%, and maintained under a 12 h light/dark schedule (lights on at 07:00). Water and food were provided ad libitum. The experiment was approved by the Capital Medical University Animal Experiments and Experimental Animals Management Committee, Beijing (AEEI-2015-075). All experiments were performed according to the National Guideline for the Care and Use of Laboratory Animals, Amendment 2 (State Council of China, 2013). The study commenced 7 days after the animals arrived at the laboratory to allow for acclimatisation. To minimise suffering, the rats were treated with great care throughout the experiment.
Measurement of kaolin and food intake
Kaolin (H2Al2Si2O8·H2O, Sigma-Aldrich) was mixed with 1% gum Arabic (Kanto Chemical Co, Tokyo, Japan) in distilled water to form a thick paste. The mixture was extruded from a 5 mL syringe, which was cut into a column of the same size as normal food pellets and dried thoroughly.13 Kaolin (30 g) and normal food (70 g) were placed into two separate containers in the cage 3 days before the experiment (day 6 to day 4) to allow the animals to adapt to the presence of both containers (figure 1A). The consumption of kaolin and food during each 24 h period was measured by collecting, drying and weighing the remaining kaolin and food (both in the container and spilled in the cage) on a daily basis. Rats with kaolin intake <3.0 g/day on the last 3 days of adaptation (n=48) were chosen for use in the experiment. The number of rats was restricted to the minimum necessary and based on the power calculation of a previous study.14
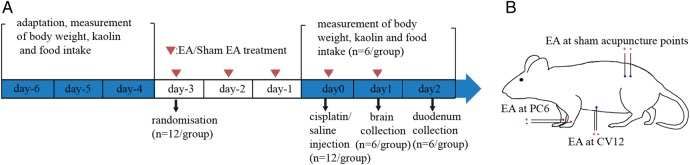
Figure 1.
Timeline of experiment (A) and location of classical and sham acupuncture points (B). EA, electroacupuncture.
Experimental procedure
After adaptation (day 6 to day 4), rats were randomised into four groups (n=12 each): saline plus EA at sham points; cisplatin plus EA at sham points; cisplatin plus EA at CV12; and cisplatin plus EA at PC6. SPSS software was used for assignment, using a completely randomised block design including bodyweight as a blocking factor for allocation. Animals were injected intraperitoneally (ip) with either saline or 6 mg/kg cisplatin (cis-diamineplatinum (II) dichloride, crystalline, dissolved in saline before use, Sigma, St Louis, Missouri, USA) at 10:00 on day 0, as previously described.15 16
Half of the rats (n=6 per group) received EA or sham-EA three times before and once after cisplatin injection at 15:00 each day (day 3 to day 0, figure 1A). Twenty-four hours after cisplatin injection (day 1), these rats were euthanased with 2% pentobarbital sodium (50 mg/kg ip) and their brains were removed for quantification of c-Fos expression (figure 1A). A 24 h time point was chosen based on previous data demonstrating near maximal c-Fos expression at this stage post-chemotherapy in the rat and musk shrew.9 17
All other rats (n=6 per group) received EA or sham-EA three times before and twice after cisplatin injection at 15:00 each day (day 3 to day 1, figure 1A). Kaolin and food intake and bodyweight change per 24 h period were measured at 24 and 48 h after cisplatin injection (day 1 and day 2), as described above. After these measurements, the rats were euthanased and the duodenums removed for measurement of 5-HT using high performance liquid chromatography (HPLC).
EA and sham treatment
During treatment, each animal was fixed in a controller, with the central part of the abdomen and the four limbs exposed. For EA, stainless steel disposable acupuncture needles (0.25×25 mm, Zhong Yan Tai He Medical Instrument Co, Ltd, Beijing) were inserted at sham acupuncture points or classical points (CV12 or bilateral PC6), which were located according to a standard atlas of rat acupuncture.18 In the PC6 group, the point was located on the flexor aspect of the forearm, between the radius and ulna, 3 mm proximal to the wrist joint, and needled perpendicularly to a depth of 1 mm. The left and right sides were paired for EA. In the CV12 group, the point was located in the midline of the abdomen, 2 cm below the sternocostal angle and half of the distance to the umbilicus, and needled perpendicularly to a depth of 3 mm. A second acupuncture needle was inserted perpendicularly to a depth of 1 mm at a non-acupuncture point located 1 mm lateral to CV12 to form a pair for EA. In the sham groups, a pair of non-acupuncture points on the lower back, 4 cm lateral to the spine, 2–3 cm above the left hip joint (figure 1B) were needled perpendicularly to a depth of 1 mm. In all groups, EA was performed using an EA device (HANS-100A) at an intensity of 0.5–1 mA (according to the reaction of each animal) and frequency of 2/15 Hz for a total of 10 min/day. Each rat received 4–5 treatments in total, depending on its subgroup allocation.
Measurement of duodenal 5-HT
Duodenal 5-HT was measured using HPLC with electrochemical detection. One milligram of duodenal tissue was dissolved in 0.1 mol/L of analytical grade perchloric acid (HCLO4,), treated with an ultrasonic cell crusher, and centrifuged at a speed of 16992 g for 10 min at 4°C. Twenty microlitres of supernatant was obtained and injected into an HPLC system (2695, Waters Alliance, USA) fitted with an Atlantis C18 column (2.1×150 mm, 3 μm, Waters Alliance) and electrochemical detector (2465, Waters Alliance). The mobile phase consisted of 50 mM citric acid (C6H8O7, analytical grade), sodium acetate (C2H3O2Na, analytical grade), buffer solution (pH 3.5, 1.8 mM dibutylamine, 0.3 mM Na2EDTA, Sigma), and methanol (CH3OH, HPLC grade) (96:4, v/v). The column temperature was set to 35°C, detection voltage +0.75V, and flow rate 0.35 mL/min. The HPLC method met requirements with elution of 5-HT after 19.3 min. Resolution far exceeded 1.5, confirming that 5-HT could be measured precisely without interference from other endogenous substances.
c-Fos immunohistochemistry
Brain samples were fixed in 10% buffered formalin at 4°C for 24–48 h and sectioned (6 μm thickness) using a microtome (Leica RM2235) after paraffin embedding. The NTS area of the brainstem was identified according to a rat brain atlas19 and transferred onto gelatin-coated slides. Standard immunohistochemical procedures were used to visualise c-Fos.
Sections were incubated overnight at 4°C with a primary antibody directed against c-Fos (Santa Cruz Biotechnology Inc USA; sc-52; 1:1000), then incubated for 1 h with goat anti-rabbit secondary antibody (Dako REAL EnVision). After DAB reaction for 3 min, the sections were counterstained with haematoxylin and eosin and viewed under a microscope (Olympus BX51). Images were captured (Nikon DS-U3) and quantified with Image Pro Plus 5.1 software by two independent investigators blinded to experimental conditions. Counts for three coronal brain sections per animal were averaged.
Statistical analysis
Data are expressed as mean±SEM. Groups were compared by one-way analysis of variance (ANOVA) followed by post-hoc test of least significant difference using SPSS V.17.0 software. A probability level of p<0.05 was set as the threshold of statistical significance.
Results
Baseline characteristics
Before the experiment (at day 3, immediately after randomisation), there were no statistically significant baseline differences between the study groups (p>0.05, table 1).
Table 1.
Baseline data (n=6 per group)
| Group | Kaolin intake (over 24 h, g) | Normal food intake (over 24 h, g) | Bodyweight (g) |
|---|---|---|---|
| Saline+sham | 1.12±0.38 | 31.04±3.1 | 269.86±11.8 |
| Cisplatin+sham | 0.91±0.39 | 30.64±3.12 | 271.00±16.11 |
| Cisplatin+CV12 | 1.37±0.40 | 29.25±2.49 | 279.14±16.58 |
| Cisplatin+PC6 | 1.39±0.42 | 30.84±3.07 | 277.29±15.49 |
Data are expressed as mean±SEM. No statistically significant differences were found between the groups.
Kaolin intake
On days 1 and 2 after cisplatin treatment, sham-treated rats showed a significant increase in kaolin intake (cisplatin+sham vs saline+sham: 6.43±0.64 g vs 1.43±0.37 g, p<0.01; and 5.25±0.71 g vs 1.16±0.4 g, p<0.01, respectively; figure 2A). On days 1 and 2 after cisplatin treatment, kaolin intake was significantly lower in rats treated with EA at CV12 (cisplatin+CV12 vs cisplatin+sham: 3.2±0.39 g vs 6.43±0.64 g, p<0.01; and 3.03±0.53 g vs 5.25±0.71 g, p=0.02, respectively). On day 1 after cisplatin treatment, kaolin intake was significantly lower in rats treated with EA on PC6 (cisplatin+PC6 vs cisplatin+sham: 4.53±0.6 g vs 6.43±0.64 g, p=0.02), although this effect was not significant on day 2 (cisplatin+PC6 vs cisplatin+sham: 4.4±0.79 g vs 5.25±0.71 g, p=0.36).
Figure 2.
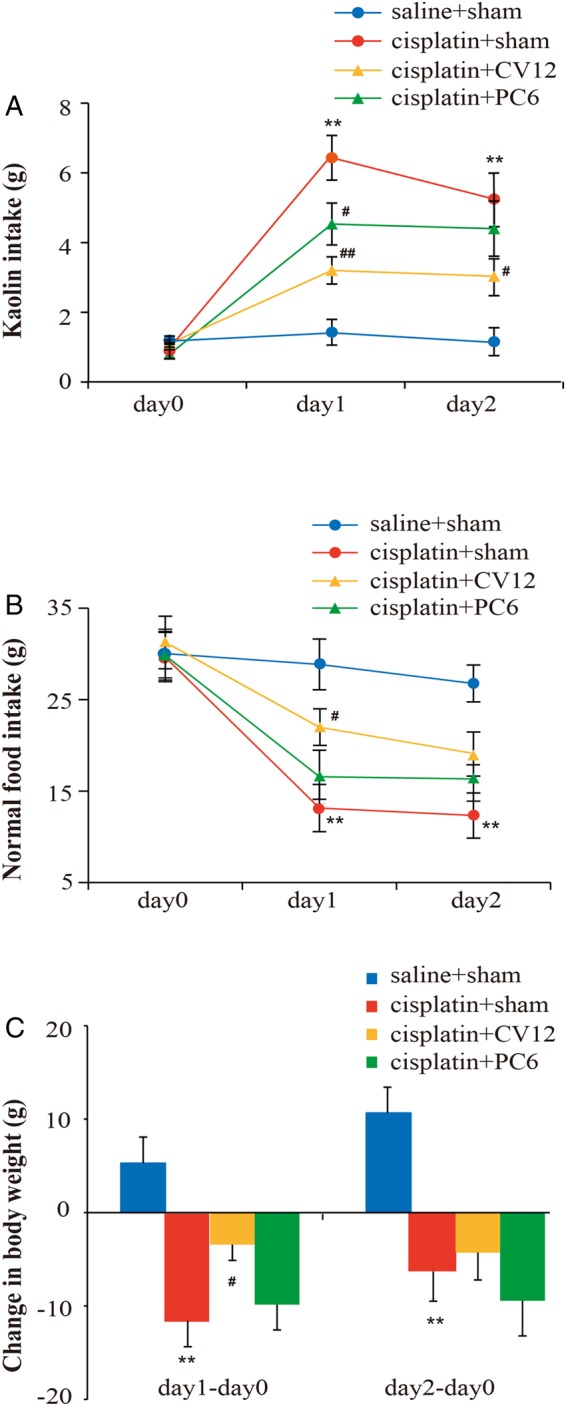
Changes in kaolin intake (A), normal food intake (B), and bodyweight (C) over a 48 h time period following intraperitoneal injection of saline (n=12) or 6 mg/kg cisplatin (n=36) in male Wistar rats receiving a total of 4–5 electroacupuncture (EA) treatments at sham points (saline+sham and cisplatin+sham groups, n=12 each), CV12 (cisplatin+CV12 group, n=12) or PC6 (cisplatin+PC6 group, n=12). Data are mean±SEM. *p<0.05, **p<0.01, cisplatin+sham versus saline+sham. #p<0.05, ##p<0.01, cisplatin+CV12/PC6 versus cisplatin+sham.
Normal food intake
On days 1 and 2 after cisplatin treatment, sham-treated rats displayed a significant decrease in normal food intake (cisplatin+sham vs saline+sham: 13.14±2.59 g vs 28.86±2.78 g, p<0.01; and 12.33±2.47 g vs 26.77±2.02 g, p<0.01, respectively; figure 2B). Relative to the sham-treated group, normal food intake was significantly greater following EA at CV12 on day 1 (cisplatin+CV12 vs cisplatin+sham: 22±2 g vs 13.14±2.59 g, p=0.03) but not on day 2 (cisplatin+CV12 vs cisplatin+sham: 19.05±2.41 g vs 12.33±2.47 g, p=0.06). EA at PC6 had no statistically significant effect on the cisplatin-induced reduction in normal food intake in rats on either day 1 or day 2 (cisplatin+PC6 vs cisplatin+sham: 16.57±2.9 g vs 13.14±2.59 g, p=0.36; and 16.33±2.43 g vs 12.33±2.47 g, p=0.24, respectively).
Bodyweight
Bodyweight of the rats increased after saline treatment, but decreased after cisplatin injection. There were significant differences in bodyweight changes between rats treated with EA at sham points following saline and cisplatin on day 1 and day 2 (cisplatin+sham vs saline+sham: −11.57±2.79 g vs 5.29±2.79 g, p<0.01; and −6±3.18 g vs 10.57±2.75 g, p=0.01, respectively; figure 2C). EA at CV12 had a statistically significant effect on cisplatin-induced bodyweight loss on day 1 (cisplatin+CV12 vs cisplatin+sham: −3.43±1.68 g vs −11.57±2.79 g, p=0.03), but not on day 2 (cisplatin+CV12 vs cisplatin+sham: −4.29±2.92 g vs −6±3.18 g, p=0.71). EA at PC6 had no statistically significant effect on cisplatin-induced bodyweight loss on either day 1 or day 2 (cisplatin+PC6 vs cisplatin+sham: −9.86±2.71 g vs −11.57±2.79 g, p=0.64; and −9.43±3.77 g vs −6±3.18 g, p=0.45, respectively).
Duodenal 5-HT concentration
Cisplatin (6 mg/kg ip) significantly increased 5-HT concentration in the duodenum in rats compared to saline (cisplatin+sham vs saline+sham: 0.68±0.08 μg/mL vs 0.11±0.03 μg/mL, p<0.01; figure 3). The cisplatin-induced increase in 5-HT in the duodenum was notably lower following EA at CV12 (cisplatin+CV12 vs cisplatin+sham: 0.21±0.06 μg/mL vs 0.68±0.08 μg/mL, p<0.01) but not at PC6 (cisplatin+PC6 vs cisplatin+sham: 0.59±0.14 μg/mL vs 0.68±0.08 μg/mL, p=0.48).
Figure 3.
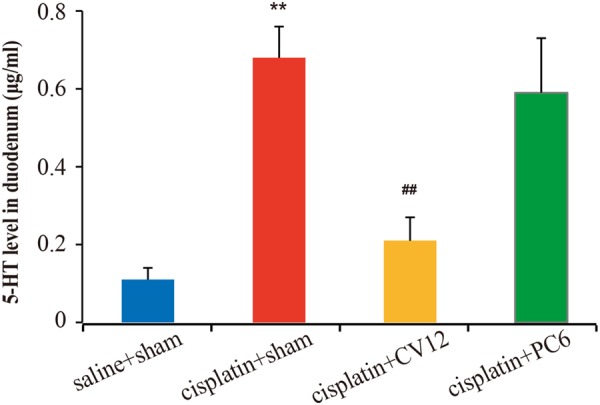
Concentrations of serotonin (5-hydroxytryptamine, 5-HT), measured by high performance liquid chromatography, in the duodenum 48 h following intraperitoneal injection of saline (n=6) or 6 mg/kg cisplatin (n=18) in male Wistar rats receiving five electroacupuncture (EA) treatments at sham points (saline+sham and cisplatin+sham groups, n=6 each), CV12 (cisplatin+CV12 group, n=6) or PC6 (cisplatin+PC6 group, n=6). Data are mean±SEM. **p<0.01, cisplatin+sham versus saline+sham. ##p<0.01, cisplatin+CV12/PC6 versus cisplatin+sham.
c-Fos expression in NTS
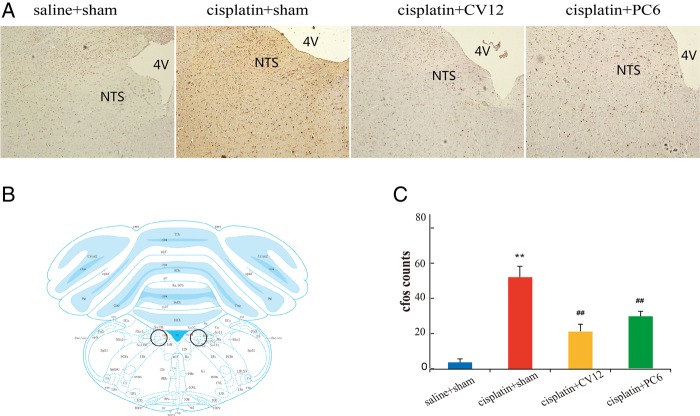
As is apparent in figure 4A, rats treated with saline and receiving EA at sham points exhibited few c-Fos expressing neurons in the NTS, the neuroanatomical location of which is annotated in figure 4B. Cisplatin (6 mg/kg ip) produced greater c-Fos-like immunoreactivity than saline in the NTS (cisplatin+sham vs saline+sham: 67±6 vs 4±2, p<0.01; figure 4C). The cisplatin-induced increase in NTS c-Fos cell counts in the rats was significantly lower following EA at both CV12 and PC6 (cisplatin+CV12 vs cisplatin+sham: 23±4 vs 67±6, p<0.01; and cisplatin+PC6 vs cisplatin+sham: 36±4 vs 67±6, p<0.01, respectively).
Figure 4.
Representative immunohistochemistry images taken at ×100 magnification (A) and c-Fos positive cell counts (C) in the brainstem nucleus of the solitary tract (NTS) located according to a rat brain atlas (B).19 24 h following intraperitoneal injection of saline (n=6) or 6 mg/kg cisplatin (n=18) in male Wistar rats receiving four electroacupuncture (EA) treatments at sham points (saline+sham and cisplatin+sham groups, n=6 each), CV12 (cisplatin+CV12 group, n=6) or PC6 (cisplatin+PC6 group, n=6). Data are mean±SEM. **p<0.01, cisplatin+sham versus saline+sham. ##p<0.01, cisplatin+CV12/PC6 versus cisplatin+sham. 4V, fourth ventricle.
Discussion
The clinical efficacy of acupuncture in treating chemotherapy-induced nausea and vomiting has been reported in many studies.20–23 The present study used an animal model to attempt to explain the underlying mechanism using behavioural and in vitro assessment. We observed nausea-like behaviours, increased peripheral 5-HT secretion, and excessive neuronal activation in the NTS in cisplatin-treated rats. Furthermore, EA appeared to partially suppress these changes.
Rats do not possess the emetic reflex. Instead, the ‘pica’ behaviour of consuming non-food substances (such as kaolin) is thought to be a manifestation of gastrointestinal discomfort and to reflect emetogenic potential.10 24 Kaolin ingestion has been validated as an index of nausea and vomiting, and it has been reported that anti-cancer drugs trigger an increased kaolin intake.25–27 In accordance with previous studies,15 25 we found an increase in kaolin intake and a decrease in food intake and bodyweight 2 days after cisplatin injection. This ‘pica’ behaviour appeared to be alleviated via pre- and post-chemotherapy EA treatment at CV12 during the acute phase (24 h after cisplatin treatment). The lack of any significant effect of EA in the delayed phase (24–48 h after cisplatin treatment) may reflect the possibility that nausea in the rats was already decreasing by the second day of our study, and/or that EA at a single point was unable to sustain a long term effect in treating chemotherapy-induced nausea and vomiting.
5-HT is thought to be the most important neurotransmitter in chemotherapy-induced nausea and vomiting, and recognition of its critical role is perhaps one of the most important advances in research on chemotherapy-induced nausea and vomiting in the past 20 years. 5-HT interacts with its receptor on vagal afferent nerves and transmits the sensation of nausea to the brain.6 Thus, selective antagonists of the 5-HT3 receptor (such as ondansetron, granisetron, dolasetron and palonosetron) are currently the most effective class of antiemetics in preventing acute chemotherapy-induced nausea and vomiting.28–30 The effect of acupuncture at CV12 on 5-HT transmission found in the present study is in line with previous evidence that suggests that acupuncture may exert some of its effects through the regulation of certain neurotransmitters.31 32 Nonetheless, further (more specific) studies on the 5-HT receptors and the dose–response relationship are needed.
We also demonstrated a relationship between EA and neuronal activation in the central nervous system via measurement of c-Fos immunoreactivity. c-Fos is a gene that demonstrates early expression following repeated depolarisation of neurons and is regarded as a metabolic marker of neuronal activation.33 Regions of the central nervous system that are related to nausea and vomiting include the area postrema, NTS, vestibular system, amygdala, and insular cortex.34 Among them, the NTS in the lower brainstem is the primary target site of vagal afferents and plays an important integrative role in the emetic response. Induction of c-Fos within the NTS has previously been observed in cisplatin-induced nausea and vomiting in animals in accordance with the present findings.9 17 34 Our study indicates that EA at both CV12 and PC6 may suppress the emetic response through inhibition of NTS activation.
Point specificity remains a topic of major debate in acupuncture research. Whether the effect of EA is due to general mechanical stimulation or is point-specific is unclear. In this study, significant differences were observed between EA at classical acupuncture points and EA at distant non-acupuncture points. This suggests that point location may be important; however, to what extent these represent regional as opposed to point-specific differences could not be determined using the present experimental design. Acupuncture point selection clearly remains an important consideration when providing acupuncture treatment clinically. The most commonly used points for nausea and vomiting include PC6, CV12, ST36, and LI4,5 35 and a recently published study used non-invasive electro-stimulation at Kl1.36 Point selection may be guided by traditional Chinese medical theory. Classically PC6 is used for cardiovascular disease, while CV12 is used for gastrointestinal disease, although both points are classically indicated for nausea and vomiting. Few studies have been conducted to compare the effects of different points in treating chemotherapy-induced nausea and vomiting. In the present study, EA at both CV12 and PC6 influenced neuronal activation in the NTS, while EA at CV12 appeared to be more effective than PC6 in regulating ‘local’ gastrointestinal function, reflected by the changes in kaolin intake, normal food intake, and duodenal 5-HT values. The results suggest that EA at CV12 and PC6 may exert their effects through different neurobiological pathways. Further study is needed to explore the influence of variable degrees of stimulation at PC6 and evaluate its effects.
There are several limitations to the present study. Firstly, given the lack of emesis in the rat, it is impossible to replicate fully the human condition. Secondly, administration of chemotherapy in this rodent model induced both acute (24 h) and delayed (24–120 h) nausea and vomiting, which is more difficult to treat using anti-emetic drugs (5-HT3 antagonists). The longer term effects of EA treatment for >2 days should therefore be considered in future studies. Thirdly, the study of EA at a single point is unlikely to explain fully the mechanism of action, since acupuncture points are mostly needled in combination to treat diseases in a clinical setting. Future research should include evaluation of the effects and underlying mechanism of different acupuncture point combinations.
In conclusion, our study showed that EA could alleviate cisplatin-induced nausea and vomiting by reducing secretion of 5-HT in the duodenum and suppressing the activation of the NTS in the brainstem. Further studies are needed to elucidate the precise mechanisms underlying the anti-emetic effect of acupuncture.
Footnotes
Contributors: YC and LW conceived and designed the study. YC performed the experiments. GS, LL and PP performed the data analyses. All authors wrote, read and approved the final manuscript.
Funding: The study was supported by national 973 project of China (2014CB543203) and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201412).
Competing interests: None declared.
Ethics approval: This study received prospective ethical approval from the Capital Medical University Animal Experiments and Experimental Animals Management Committee, Beijing (reference number AEEI-2015-075). All experiments were performed according to the National Guideline for the Care and Use of Laboratory Animals, Amendment 2 (State Council of China, 2013).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Percie DSN, Rudd JA, Apfel CC, et al. Cisplatin-induced emesis: systematic review and meta-analysis of the ferret model and the effects of 5-HT(3) receptor antagonists. Cancer Chemother Pharmacol 2011;67:667–86. 10.1007/s00280-010-1339-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kris MG, Hesketh PJ, Somerfield MR, et al. American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol 2006;24:2932–47. 10.1200/JCO.2006.06.9591 [DOI] [PubMed] [Google Scholar]
- 3.Navari RM. Pharmacological management of chemotherapy-induced nausea and vomiting: focus on recent developments. Drugs 2009;69:515–33. 10.2165/00003495-200969050-00002 [DOI] [PubMed] [Google Scholar]
- 4.Haus U, Spath M, Farber L. Spectrum of use and tolerability of 5-HT3 receptor antagonists. Scand J Rheumatol Suppl 2004;119:12–18. 10.1080/03009740410006961 [DOI] [PubMed] [Google Scholar]
- 5.Ezzo J, Vickers A, Richardson MA, et al. Acupuncture-point stimulation for chemotherapy-induced nausea and vomiting. J Clin Oncol 2005;23:7188–98. 10.1200/JCO.2005.06.028 [DOI] [PubMed] [Google Scholar]
- 6.Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med 2008;358:2482–94. 10.1056/NEJMra0706547 [DOI] [PubMed] [Google Scholar]
- 7.Hattori T, Yakabi K, Takeda H. Cisplatin-induced anorexia and ghrelin. Vitam Horm 2013;92:301–17. 10.1016/B978-0-12-410473-0.00012-X [DOI] [PubMed] [Google Scholar]
- 8.Hagbom M, Istrate C, Engblom D, et al. Rotavirus stimulates release of serotonin (5-HT) from human enterochromaffin cells and activates brain structures involved in nausea and vomiting. PLoS Pathog 2011;7:e1002115 10.1371/journal.ppat.1002115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Jonghe BC, Horn CC. Chemotherapy agent cisplatin induces 48-h Fos expression in the brain of a vomiting species, the house musk shrew (Suncus murinus). Am J Physiol Regul Integr Comp Physiol 2009;296:R902–11. 10.1152/ajpregu.90952.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horn CC, Kimball BA, Wang H, et al. Why can't rodents vomit? A comparative behavioral, anatomical, and physiological study. PLoS ONE 2013;8:e60537 10.1371/journal.pone.0060537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horn CC. The medical implications of gastrointestinal vagal afferent pathways in nausea and vomiting. Curr Pharm Des 2014;20:2703–12. 10.2174/13816128113199990568 [DOI] [PubMed] [Google Scholar]
- 12.Schwartz GJ, Zeltser LM. Functional organization of neuronal and humoral signals regulating feeding behavior. Annu Rev Nutr 2013;33:1–21. 10.1146/annurev-nutr-071812-161125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saito R, Takano Y. [Easy method for emesis using rats]. Nihon Yakurigaku Zasshi 2006;127:461–6. 10.1254/fpj.127.461 [DOI] [PubMed] [Google Scholar]
- 14.Qian Q, Chen W, Guo C, et al. Xiao-Ban-Xia-Tang inhibits cisplatin-induced pica by down regulating obestatin in rats. J Ethnopharmacol 2011;135:186–93. 10.1016/j.jep.2011.03.008 [DOI] [PubMed] [Google Scholar]
- 15.Sathyanath R, Hanumantha RB, Kim HG, et al. Saponin and non-saponin fractions of red ginseng ameliorate cisplatin-induced pica in rats. Pharm Biol 2013;51: 1052–60. 10.3109/13880209.2013.775660 [DOI] [PubMed] [Google Scholar]
- 16.Liu YL, Malik N, Sanger GJ, et al. Pica—a model of nausea? Species differences in response to cisplatin. Physiol Behav 2005;85:271–7. 10.1016/j.physbeh.2005.04.009 [DOI] [PubMed] [Google Scholar]
- 17.Horn CC, Ciucci M, Chaudhury A. Brain Fos expression during 48 h after cisplatin treatment: neural pathways for acute and delayed visceral sickness. Auton Neurosci 2007;132:44–51. 10.1016/j.autneu.2006.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li ZR. Experimental acupuncture science. China Press of Traditional Chinese Medicine, 2003. [Google Scholar]
- 19.Paxinos G, Charles W. The rat brain. 6th edn Academic Press, Elsevier Inc., 2007. [Google Scholar]
- 20.Streitberger K, Ezzo J, Schneider A. Acupuncture for nausea and vomiting: an update of clinical and experimental studies. Auton Neurosci 2006;129:107–17. 10.1016/j.autneu.2006.07.015 [DOI] [PubMed] [Google Scholar]
- 21.Bao T. Use of acupuncture in the control of chemotherapy-induced nausea and vomiting. J Natl Compr Canc Netw 2009;7:606–12. [DOI] [PubMed] [Google Scholar]
- 22.Konno R. Cochrane review summary for cancer nursing: acupuncture-point stimulation for chemotherapy-induced nausea or vomiting. Cancer Nurs 2010;33:479–80. 10.1097/NCC.0b013e3181f104bc [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Jin HF, Fan YH, et al. Effects and mechanisms of transcutaneous electroacupuncture on chemotherapy-induced nausea and vomiting. Evid Based Complement Alternat Med 2014;2014:860631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamoto K, Nakai M, Nohara K, et al. The anti-cancer drug-induced pica in rats is related to their clinical emetogenic potential. Eur J Pharmacol 2007;554:34–9. 10.1016/j.ejphar.2006.09.058 [DOI] [PubMed] [Google Scholar]
- 25.Tatsushima Y, Egashira N, Matsushita N, et al. Pemirolast reduces cisplatin-induced kaolin intake in rats. Eur J Pharmacol 2011;661:57–62. 10.1016/j.ejphar.2011.04.026 [DOI] [PubMed] [Google Scholar]
- 26.Raghavendran HR, Rekha S, Shin JW, et al. Effects of Korean ginseng root extract on cisplatin-induced emesis in a rat-pica model. Food Chem Toxicol 2011;49:215–21. 10.1016/j.fct.2010.10.019 [DOI] [PubMed] [Google Scholar]
- 27.Horn CC, De Jonghe BC, Matyas K, et al. Chemotherapy-induced kaolin intake is increased by lesion of the lateral parabrachial nucleus of the rat. Am J Physiol Regul Integr Comp Physiol 2009;297:R1375–82. 10.1152/ajpregu.00284.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin SJ, Hatoum HT, Buchner D, et al. Impact of 5-HT3 receptor antagonists on chemotherapy-induced nausea and vomiting: a retrospective cohort study. BMC Health Serv Res 2012;12:215 10.1186/1472-6963-12-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamura H, Yokoyama H, Takayanagi R, et al. Theoretical evaluation of antiemetic effects of 5-HT receptor antagonists for prevention of vomiting induced by cisplatin. Eur J Drug Metab Pharmacokinet 2015;40:39–44. [DOI] [PubMed] [Google Scholar]
- 30.Mori-Vogt S, Blazer M. Palonosetron for the prevention of chemotherapy-induced nausea and vomiting. Expert Rev Anticancer Ther 2013;13:919–36. 10.1586/14737140.2013.814412 [DOI] [PubMed] [Google Scholar]
- 31.Takahashi T. Mechanism of acupuncture on neuromodulation in the gut—a review. Neuromodulation 2011;14:8–12; discussion 12 10.1111/j.1525-1403.2010.00295.x [DOI] [PubMed] [Google Scholar]
- 32.Soligo M, Nori SL, Protto V, et al. Acupuncture and neurotrophin modulation. Int Rev Neurobiol 2013;111:91–124. 10.1016/B978-0-12-411545-3.00005-5 [DOI] [PubMed] [Google Scholar]
- 33.Yuan SY, Vilimas PI, Zagorodnyuk VP, et al. Novel spinal pathways identified by neuronal c-Fos expression after urethrogenital reflex activation in female guinea pigs. Neuroscience 2014;288C:37–50. [DOI] [PubMed] [Google Scholar]
- 34.Holland RA, Leonard JJ, Kensey NA, et al. Cisplatin induces neuronal activation and increases central AMPA and NMDA receptor subunit gene expression in mice. Physiol Behav 2014;136:79–85. 10.1016/j.physbeh.2014.02.038 [DOI] [PubMed] [Google Scholar]
- 35.Gottschling S, Reindl TK, Meyer S, et al. Acupuncture to alleviate chemotherapy-induced nausea and vomiting in pediatric oncology—a randomized multicenter crossover pilot trial. Klin Padiatr 2008;220:365–70. 10.1055/s-0028-1086039 [DOI] [PubMed] [Google Scholar]
- 36.Shen Y, Liu L, Chiang JS, et al. Randomized, placebo-controlled trial of K1 acupoint acustimulation to prevent cisplatin-induced or oxaliplatin-induced nausea. Cancer 2015;121:84–92. 10.1002/cncr.28973 [DOI] [PMC free article] [PubMed] [Google Scholar]