Abstract
Background:
Deep venous thrombosis (DVT) and pulmonary embolism (PE) are recognized as major causes of morbidity and mortality in orthopaedic trauma patients. Despite the high incidence of these complications following orthopaedic trauma, there is a paucity of literature investigating the clinical risk factors for DVT in this specific population. As our healthcare system increasingly emphasizes quality measures, it is critical for orthopaedic surgeons to understand the clinical factors that increase the risk of DVT following orthopaedic trauma.
Objectives:
Utilizing the ACS-NSQIP database, we sought to determine the incidence and identify independent risk factors for DVT following orthopaedic trauma.
Patients and Methods:
Using current procedural terminology (CPT) codes for orthopaedic trauma procedures, we identified a prospective cohort of patients from the 2006 to 2013 ACS-NSQIP database. Using Wilcoxon-Mann-Whitney and chi-square tests where appropriate, patient demographics, comorbidities, and operative factors were compared between patients who developed a DVT within 30 days of surgery and those who did not. A multivariate logistic regression analysis was conducted to calculate odds ratios (ORs) and identify independent risk factors for DVT. Significance was set at P < 0.05.
Results:
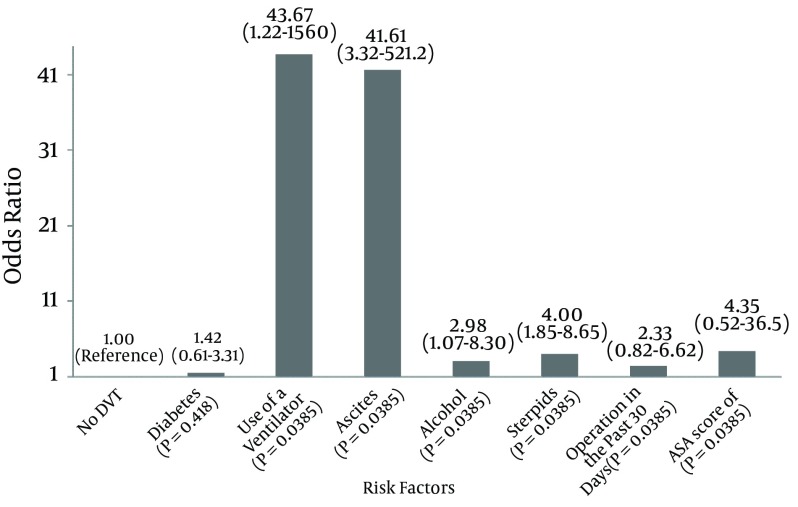
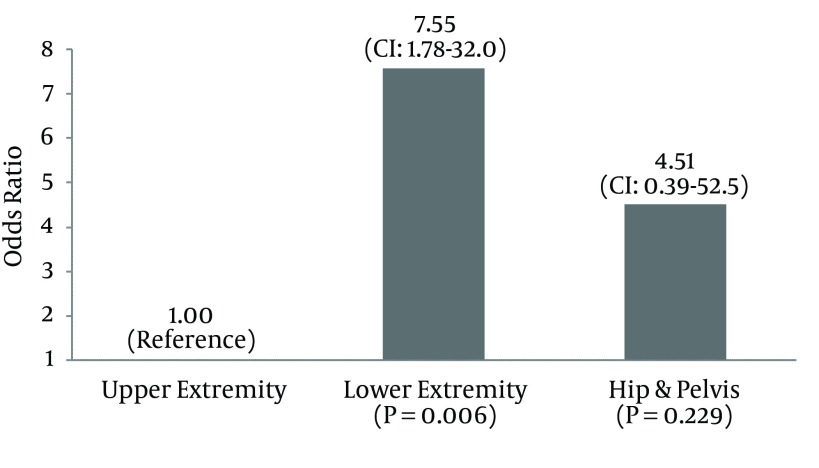
56,299 orthopaedic trauma patients were included in the analysis, of which 473 (0.84%) developed a DVT within 30 days. In univariate analysis, twenty-five variables were significantly associated with the development of a DVT, including age (P < 0.0001), BMI (P = 0.037), diabetes (P = 0.01), ASA score (P < 0.0001) and anatomic region injured (P < 0.0001). Multivariate analysis identified several independent risk factors for development of a DVT including use of a ventilator (OR = 43.67, P = 0.039), ascites (OR = 41.61, P = 0.0038), steroid use (OR = 4.00, P < 0.001), and alcohol use (OR = 2.98, P = 0.0370). Compared to patients with upper extremity trauma, those with lower extremity injuries had significantly increased odds of developing a DVT (OR = 7.55, P = 0.006). The trend toward increased odds of DVT among patients with injuries to the hip/pelvis did not reach statistical significance (OR = 4.51, P = 0.22). Smoking was not found to be an independent risk factor for developing a DVT (P = 0.1217).
Conclusions:
This is the largest study to date using the NSQIP database to identify risk factors for DVT in orthopaedic trauma patients. Although the incidence of DVT was low in our cohort, the presence of certain risk factors significantly increased the odds of developing a DVT following orthopaedic trauma. These findings will enable orthopaedic surgeons to target at-risk patients and implement post-operative care protocols aimed at reducing the morbidity and mortality associated with DVT in orthopaedic trauma patients.
Keywords: Pulmonary Embolism, Venous Thromboembolism, Orthopaedic Trauma, Thromboprophylaxis, Deep Venous Thrombosis
1. Background
Traumatic injury results in significant physiologic changes that place trauma patients at elevated risk for venous thromboembolism (VTE), a term that encompasses both deep venous thrombosis (DVT) and pulmonary embolism (PE). Serum levels of inflammatory cytokines (including interleukin-6 (IL-6), IL-8, and tumor necrosis factor-alpha), procoagulant microparticles, and thrombin are increased following trauma. This systemic inflammatory response results in a hypercoagulable state that increases the likelihood of developing VTE (1). Hypercoagulability, along with endothelial injury and venous stasis, comprise Virchow’s Triad, the set of conditions that contributes to venous thrombosis. Due to the frequent requirement for post-operative immobilization and protected weight bearing following orthopaedic trauma surgery, all three conditions of Virchow’s Triad are often present in these patients (2).
Prior to the implementation of routine thromboprophylaxis, reported rates of VTE following major trauma were extremely high. Geerts et al. reported a 58% incidence of lower extremity DVT and a 0.9% incidence of fatal PE in 349 patients admitted for major traumatic injuries who did not receive thromboprophylaxis (3). Despite its relatively low incidence compared to DVT, PE is still the third most common cause of in-hospital death among trauma patients (4). DVT rates also vary by anatomic region injured, ranging from 50% in patients with abdominal, thoracic, or facial injuries to 80% in patients with femur fractures (3). Numerous risk factors for DVT have been reported in the trauma literature, including age, injury severity, polytrauma, fracture of the pelvis, femur, or tibia, spinal cord injury, central vein cannulation, number of procedures, and medical comorbidities including diabetes and obesity (3, 5-8).
Both chemical and mechanical thromboprophylaxis have been shown to decrease rates of VTE in the setting of trauma (9, 10). Pharmacologic prophylaxis with low-molecular weight heparin (LMWH) was shown to significantly decrease the incidence of both DVT and PE in a large cohort of more than 2200 trauma patients (11). And mechanical prophylaxis with pneumatic sequential compression devices (SCDs) significantly decreased VTE incidence from 4% to 11% (P = 0.02) in a prospective randomized controlled trial of 300 orthopaedic trauma patients compared with no VTE prophylaxis (12). A growing understanding of the importance of thromboprophylaxis in trauma patients has led to the development of institutional protocols for VTE prophylaxis at trauma centers around the world. Additionally, several professional organizations have published clinical guidelines for thromboprophylaxis in trauma patients (9, 13, 14).
However, protocols differ widely, and even with appropriate prophylaxis, DVT and PE still occur following orthopaedic trauma. A prospective cohort study of more than 300 orthopaedic trauma patients reported a VTE rate of 11.5% despite thromboprophylaxis (10). In a study of 200,000 orthopaedic trauma patients receiving thromboprophylaxis, the overall incidence of PE was still 0.46%, and the in-hospital mortality rate among patients who developed a PE was 12% (5). Identifying those patients who are at greatest risk for the development of VTE following orthopaedic trauma may enable surgeons to implement targeted protocols to decrease rates of VTE among these high-risk patients, thereby decreasing the morbidity and mortality associated with DVT and PE.
Large-scale databases such as the national trauma data bank (NTDB) are useful resources for conducting large-scale analyses of outcomes. However Kardooni et al. recently reported on the potential hazards of utilizing the NTDB for this purpose (15). The national surgical quality improvement program (NSQIP) database is a high-quality, multicenter dataset that has been utilized extensively to study outcomes in surgical patients (16-21).
2. Objectives
Utilizing the NSQIP database, the purpose of our study was to determine the incidence of DVT following orthopaedic trauma surgery and to identify independent risk factors for the development of DVT in orthopaedic trauma patients.
3. Patients and Methods
3.1. Data Extraction
Institutional Review Board approval was obtained, and our study complies with the ethical guidelines of the 1975 Declaration of Helsinki. Access to the NSQIP dataset collected between 2005 and 2013 was granted by the American College of Surgeons. The 135 patient variables reported within this multi-center database include preoperative risk factors, intraoperative variables, and 30-day postoperative mortality and morbidity outcomes for patients undergoing major surgical procedures in both inpatient and outpatient settings. At each participating institution, two risk-assessment nurses trained as Surgical Clinical Reviewers (SCR) were appointed to collect data directly from patients’ medical records. Inter-rater reliability disagreement of < 5% per site was considered acceptable. Audit reports of NSQIP data collection have identified disagreement rates of < 1.8% (22).
3.2. Patient Selection
From the entire 2005-2013 NSQIP database, we included only patients who underwent an orthopaedic trauma procedure as defined by current procedural terminology (CPT) codes for orthopaedic trauma (n = 89). Patient demographics including age, gender, and race were recorded, along with preoperative comorbidities including body mass index (BMI), recent weight loss (greater than 10% in the last 6 months), insulin dependent diabetes mellitus, smoking status, alcohol use, functional status, dyspnea, history of chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), hypertension requiring medication, history of esophageal varices, disseminated cancer, steroid use, bleeding disorders, hemodialysis, chemotherapy within 30 days of surgery, and radiotherapy within 90 days of surgery. Operative factors including systemic inflammatory response syndrome (SIRS), sepsis, or septic shock at time of surgery, operative time, wound class, and American society of anesthesiologists (ASA) score were also recorded. Institutional thromboprophylaxis protocols were followed at each site.
3.3. Data Analysis
Using Wilcoxon-Mann-Whitney and chi-square tests where appropriate, patient demographics, comorbidities, and operative factors were compared between patients who developed a DVT within 30 days and those who did not. Using a multivariate logistic regression analysis, odds ratios (ORs) for DVT were calculated to determine independent risk factors for DVT.
Statistical analysis was performed using Stata 12 (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP) and SSPS Statistics (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp). Significance was set at P < 0.05.
4. Results
Application of our inclusion criteria to the NSQIP database yielded 56,299 orthopaedic trauma patients who were included in the analysis. Patient demographics are shown in Table 1. 473 patients developed a DVT within 30 days of surgery, representing an overall DVT incidence of 0.84%.
Table 1. Patient Demographics.
| DVT | No DVT | P Value | |
|---|---|---|---|
| Age (mean) | 79 | 72 | < 0.0001 |
| BMI (mean) | 25.4 | 25.0 | 0.037 |
| Frequency a | 473 (0.08) | 55826 (99.2) | NA |
| Location a | < 0.0001 | ||
| Upper extremity | 30 (6.34) | 11373 (20.4) | |
| Lower extremity | 433 (91.54) | 42819 (76.7) | |
| Hip/pelvis | 6 (1.3) | 762 (1.4) | |
| ASA Status a | < 0.0001 | ||
| 1 | 15 (3.17) | 5423 (9.7) | |
| 2 | 101 (21.4) | 17631 (31.6) | |
| 3 | 253 (53.5) | 26070 (46.7) | |
| 4 | 104 (22.0) | 6577 (11.8) |
Abbreviation: NA, not available.
aValues are presented as No (%).
Unadjusted univariate analysis using Wilcoxon-Mann-Whitney and chi square tests where appropriate identified twenty-five patient variables that were significantly associated with the development of a DVT, including age (P < 0.0001), BMI (P = 0.0377), diabetes (P = 0.01), ASA score (P < 0.0001) and anatomic region injured (P < 0.0001). The complete univariate analysis is included in Table A1.
Multivariate analysis identified seven independent risk factors for development of a DVT. As shown in Figure 1, the use of a ventilator increased the odds of DVT by 43.67 times (P = 0.039) while ascites increased the odds of DVT 41.61 times (P = 0.0038). Alcohol use (OR = 2.98, P = 0.037) and steroid use (OR = 4.00, P < 0.001) were also independent risk factors for DVT following orthopaedic trauma, as was the presence of sepsis (OR = 5.43, P = 0.001) and gangrene (OR = 5.14, P = 0.0175). Compared to patients with upper extremity trauma, those with lower extremity injuries had 7.55 times (CI: 1.78 - 32.04, P = 0.006) greater odds of developing a DVT within 30 days. As shown in Figure 2, the trend toward greater odds of DVT among patients with injuries to the hip/pelvis did not reach statistical significance (OR = 4.51, CI: 0.39 - 52.50, P = 0.229).
Figure 1. Multivariate Analysis Showing Odds Ratio DVT.
Figure 2. Multivariate Analysis of Odds Ratio DVT by Region Injured.
Smoking was not found to be an independent risk factor for developing a DVT (P = 0.122), nor was ASA score (P = 0.403), BMI (P = 0.163), or diabetes (P = 0.418). The complete multivariate analysis is included in Table A2
5. Discussion
Despite the abundance of literature relevant to VTE in the general trauma population, high-quality evidence specific to VTE prophylaxis and treatment in the orthopaedic trauma population is relatively limited. Stannard et al. reported a DVT rate of 11.5% in a prospective cohort of 312 patients who sustained high-energy skeletal trauma despite pharmacologic prophylaxis (23). Using the national trauma data bank (NTDB), Godzik et al. investigated the incidence of PE in 200,000 patients with pelvic and lower-extremity fractures who received thromboprophylaxis according to the protocols of each institution (5). The overall incidence of PE was 0.46%, and the in-hospital mortality rate among patients who developed a PE was 12%. These authors also identified independent risk factors for PE in this patient population including multiple fractures, history of warfarin use, morbid obesity, and emergency department disposition to an intensive care unit or to the operating room. These studies underscore the fact that both DVT and PE still occur despite routine VTE prophylaxis.
Table 2 summarizes the existing literature documenting VTE incidence and risk factors following orthopaedic trauma. Only three of the seven referenced studies are specific to an orthopaedic trauma population. The other four studies include major trauma patients, and although many of these patients also have orthopaedic injuries, the conclusions of these studies are not necessarily generalizable to an orthopaedic trauma population.
Table 2. Literature Review of Incidence and Risk Factors for Venous Thromboembolism in Orthopaedic Trauma.
| Study | Study Design, Patient Population | N | Prophylaxis used? | Incidence of VTE | Risk factors for VTE identified |
|---|---|---|---|---|---|
| Geerts et al. (3) | Single center prospective cohort, major trauma patients | 349 | No | 58% (1% fatal PE) | Age, blood transfusion, surgery, fracture of the femur or tibia, and spinal cord injury. |
| O'Malley et al. (6) | Single center retrospective cohort, major trauma patients | 1,316 | Yes | 2.3% (PE) | Age > 55 years, multi-system injury, cannulation of central veins, and pelvic fractures (but not long-bone fractures) |
| Paffrath et al. (7) | Multicenter retrospective cohort, major trauma patients | 7,937 | Yes | 1.8% (PE) | ISS score, Pelvic AIS score 2 or higher, # of operations, medical comorbidities (diabetes, renal failure, malignancy, coagulation disorders) |
| Tuttle-Newhall et al. (8) | State-wide trauma registry, major trauma patients | 318,554 | Yes | 0.3% (PE) | Age > 55 years, increasing ISS and AIS (extremity, soft tissue, chest regions), |
| Godzik et al. (5) | Retrospective Database Review, Ortho Trauma Patients | 199,952 | Yes | 0.46% (PE) | Multiple fractures, history of warfarin use, morbid obesity, ED disposition to ICU or OR |
| Fisher et al. (12) | Prospective RCT (SCDs vs. None), Ortho Trauma Patients | 304 | Yes/No | 11% vs. 4% | 11% incidence for control group, 4% incidence in experimental group |
| Stannard et al. (10) | Prospective Cohort Study, Ortho Trauma Patients | 312 | Yes | 11.5% | 11.5% incidence of VTE despite prophylaxis |
Large-scale databases offer significant advantages for determining population-based epidemiologic data. Utilizing such databases avoids the significant costs and inconvenience of conducting large multi-center studies. Although the NTDB has been used to investigate VTE incidence and risk factors in orthopaedic trauma, a recent article reported on the potential hazards of utilizing this particular database for this purpose due to the significant variability in practices for reporting complications among the participating institutions (15). Therefore, we decided to utilize the ACS-NSQIP database for our study, which has a standardized protocol for reporting complications. Inter-rater disagreement rates of < 1.8% have been reported among the surgical clinical reviewers who record outcomes data directly from patients’ charts.
Ours is the largest study to date utilizing the NSQIP database to identify risk factors for DVT in orthopaedic trauma patients. Although the incidence of DVT (0.84%) was low in our cohort, the presence of certain risk factors significantly increased the odds of developing a DVT following orthopaedic trauma. A ventilator requirement and the presence of sepsis, both markers of severity of illness, were identified as risk factors in our study. These findings are consistent with those of Paffrath and Tuttle-Newhall, who reported increased rates of DVT with increasing injury severity (7, 8). The presence of ascites, a marker of end-organ dysfunction, is also consistent with previous reports of renal failure as an independent risk factor for DVT (7). Our study is the first to identify steroid use and alcohol use as DVT risk factors. Furthermore, compared with upper extremity fractures, our study demonstrated significantly increased odds of DVT with lower extremity fractures as well as a nonsignificant trend toward increased odds with pelvic fractures. These findings substantiate previous reports of higher DVT rates in femur, tibia, and pelvic fractures (3, 6).
Our study does have some limitations. Although institutional protocols for DVT prophylaxis were followed for this cohort, the NSQIP database does not permit an assessment of the thromboprophylaxis status of each patient. Certain associated injuries or other medical conditions may represent a contra-indication to pharmacologic anticoagulation, and the impact of this variable on our results could not be assessed. In addition, smoking was not found to be an independent risk factor for DVT in our study even though tobacco use has been associated with increased DVT risk (24). This is likely due to the fact that our cohort had an extremely low overall incidence of DVT (0.84%) combined with a relatively low rate of smoking (less than 20% of the cohort). Finally, the NSQIP database does not permit an assessment of the specific thromboprophylactic agent(s) administered and the timing or duration of their administration, variables that have been shown to impact DVT rates in other studies (25-28).
These limitations notwithstanding, our study used a reliable large-scale database to identify several independent risk factors for DVT following orthopaedic trauma surgery, many of which had not previously been reported in the literature. In so doing, our study contributes meaningfully to the growing body of literature aimed at identifying factors that place orthopaedic trauma patients at increased risk for VTE. Such knowledge will enable surgeons to develop and implement effective post-operative VTE prophylaxis protocols aimed at reducing the morbidity and mortality associated with DVT following orthopaedic trauma.
Acknowledgments
The authors wish to acknowledge Ashley Dodd for her assistance with this study.
Appendices
Table A1. Univariate Analysis of Patient Variables Associated with DVT in Orthopaedic Trauma.
| Demographics | No DVT a | DVT a | P Value |
|---|---|---|---|
| Frequency | N = 55826 (%) | N = 473 (%) | NA |
| Gender | 0.1805 | ||
| Male | 19755 (35.39) | 320 (67.65) | |
| Female | 36071 (64.61) | 153 (32.35) | |
| Race | < 0.0001 b | ||
| White | 40570 (72.67) | 384 (81.18) | |
| Black | 2759 (4.94) | 28 (5.92) | |
| Other | 12497 (22.39) | 61 (12.90) | |
| Ethnicity | 0.4724 | ||
| Non-Hispanic | 42322 (75.81) | 407 (86.05) | |
| Hispanic | 2964 (5.31) | 24 (5.07) | |
| (Not specified) | 10540 (18.88) | 42 (8.88) | |
| Age c | 72 (54 - 85) | 79 (67 - 86) | <0.0001 b |
| BMI c | 25 (21.0 - 29.5) | 25.4 (21.9 - 29.6) | 0.0377 b |
| Diabetes | 0.01 b | ||
| No | 46894 (84.00) | 373 (78.86) | |
| Non-Insulin | 4889 (8.76) | 55 (11.63) | |
| Insulin | 4043 (7.24) | 45 (9.51) | |
| Smoker | 9736 (17.44) | 60 (12.68) | 0.0079 b |
| Alcohol | 964 (1.73) | 8 (1.69) | 1 |
| Dyspnea | 0.0191 b | ||
| No | 52208 (93.52) | 430 (90.91) | |
| Moderate Exertion | 3054 (5.47) | 33 (6.98) | |
| Rest | 564 (1.01) | 10 (2.11) | |
| Functional Status 1 | 0.5678 | ||
| Independent | 5922 (10.61) | 57 (12.05) | |
| Partially Dependent | 798 (1.43) | 10 (2.11) | |
| Totally Dependent | 118 (0.21) | 2 (0.42) | |
| Functional Status 2 | < 0.0001 b | ||
| Independent | 44335 (79.42) | 336 (71.04) | |
| Partially Dependent | 9142 (16.38) | 102 (21.56) | |
| Totally Dependent | 1844 (3.30) | 32 (6.77) | |
| Ventilator | 104 (0.19) | 7 (1.48) | < 0.0001 b |
| COPD | 4448 (7.97) | 41 (8.67) | 0.6349 |
| Pneumonia | 135 (0.24) | 3 (0.63) | 0.1941 |
| Ascites | 103 (0.18) | 6 (1.27) | < 0.0001 b |
| Esophageal Varices | 24 (0.04) | 0 | 1 |
| Congestive Heart Failure | 1223 (2.19) | 14 (2.96) | 0.3277 |
| Myocardial Infarction | 208 (0.37) | 3 (0.63) | 0.553 |
| Percutaneous Coronary Intervention | 1196 (2.14) | 12 (2.54) | 0.5965 |
| Cardiac Surgery | 1242 (2.22) | 13 (2.75) | 0.4724 |
| Angina | 178 (0.32) | 3 (0.63) | 0.3989 |
| Hypertension Medication | 29729 (53.25) | 320 (67.65) | < 0.0001 b |
| Peripheral Vascular Disease | 612 (1.10) | 4 (0.85) | 0.8055 |
| Gangrene | 334 (0.60) | 6 (1.27) | 0.1151 |
| Renal Failure | 301 (0.54) | 10 (2.11) | < 0.0001 b |
| Dialysis | 907 (1.62) | 13 (2.75) | 0.0823 |
| Impaired Sensorium | 347 (0.62) | 4 (0.85) | 0.7082 |
| Coma | 3 (0.01) | 0 | 1 |
| Hemiplegia | 335 (0.60) | 7 (1.48) | 0.0252 b |
| Transient Ischemic Attacks | 881 (1.58) | 5 (1.06) | 0.5044 |
| Cerebrovascular Accident with Deficit | 911 (1.63) | 13 (2.75) | 0.0655 |
| CVA without Deficit | 803 (1.44) | 11 (2.33) | 0.1283 |
| CNS Tumor | 51 (0.09) | 0 | 1 |
| Paraplegia | 130 (0.23) | 1 (0.21) | 1 |
| Quadriplegia | 29 (0.05) | 0 | 1 |
| Disseminated Cancer | 1253 (2.24) | 25 (5.29) | < 0.0001 b |
| Open wound or Infection | 3782 (6.77) | 45 (9.51) | 0.0235 b |
| Steroids | 2325 (4.16) | 37 (7.82) | 0.0001 b |
| Weight Loss | 551 (0.99) | 10 (2.11) | 0.0261 b |
| Bleeding Disorders | 5985 (10.72) | 72 (15.22) | 0.0021 b |
| Transfusion | 1964 (3.52) | 28 (5.92) | 0.0071 b |
| Chemotherapy | 227 (0.41) | 4 (0.85) | 0.2375 |
| Radiotherapy | 110 (0.20) | 4 (0.85) | 0.0075 b |
| Preoperative Sepsis | < 0.0001 b | ||
| Sepsis | 564 (1.01) | 12 (2.54) | |
| Septic Shock | 89 (0.16) | 5 (1.06) | |
| SIRS | 3752 (6.72) | 53 (11.21) | |
| Prior operation within 30 Days | 538 (0.96) | 15 (3.17) | < 0.0001 b |
| ASA classification | < 0.0001 b | ||
| 1 | 5431 (9.73) | 15 (3.17) | |
| 2 | 17656 (31.63) | 101 (21.35) | |
| 3 | 26107 (46.76) | 253 (53.49) | |
| 4 | 6586 (11.80) | 104 (21.99) | |
| 5 | 46 (0.08) | 0 | |
| Operation Time | 68 (47 - 99) | 74 (49 - 113) | 0.0015 |
| Injury Location | < 0.0001 b | ||
| Upper Extremity | 11554 (20.69) | 30 (6.34) | |
| Lower Extremity | 43498 (77.92) | 437 (92.39) | |
| Pelvis | 774 (1.39) | 6 (1.27) | |
| Pulmonary Embolism | 230 (0.41) | 42 (8.88) | <0.001 b |
Abbreviation: NA, not available.
a Values are presented as No (%).
b Significant P values are indicated with asterisks.
c Values are presented as mean (range).
Table A2. Multivariate Analysis Showing Odds Ratios (ORs) for DVT.
| Risk Factor | OR | Lower CI | Upper CI | P Value |
|---|---|---|---|---|
| Intercept | 0.00 | 0.00 | 0.00 | < 0.0001 |
| Male | 1.13 | 0.65 | 1.98 | .6607 |
| Black | 1.85 | 0.84 | 4.08 | .1282 |
| Other Race | 1.13 | 0.43 | 2.94 | .8065 |
| Hispanic | 1.01 | 0.37 | 2.73 | .9810 |
| Age | 1.02 | 1.00 | 1.04 | .1267 |
| BMI | 0.98 | 0.96 | 1.01 | .1683 |
| Non-Insulin Diabetes | 1.22 | 0.53 | 2.81 | .6483 |
| Diabetes with Insulin | 1.42 | 0.61 | 3.31 | .4183 |
| Smokes | 0.51 | 0.21 | 1.20 | .1217 |
| Alcohol Use | 2.98 | 1.07 | 8.30 | .0370 a |
| Dyspnea on Moderate Exertion | 0.73 | 0.27 | 2.01 | .5458 |
| Dyspnea at Rest | 2.34 | 0.54 | 10.06 | .2532 |
| FNSTATUS1=Partially Dependent | 0.75 | 0.34 | 1.67 | .4813 |
| FNSTATUS1=Totally Dependent | 0.83 | 0.14 | 4.89 | .8359 |
| FNSTATUS2=Partially Dependent | 1.15 | 0.63 | 2.09 | .6543 |
| FNSTATUS2=Totally Dependent | 1.16 | 0.39 | 3.41 | .7928 |
| Ventilator | 43.67 | 1.22 | 1,560.73 | .0385 a |
| COPD | 0.64 | 0.22 | 1.82 | .4013 |
| Pneumonia | 0.31 | 0.01 | 8.93 | .4912 |
| Ascites | 41.61 | 3.32 | 521.16 | .0038 a |
| Esophageal Varices | 0.00 | 0.00 | 1.42558E + 68 | .9234 |
| Congestive Heart Failure | 1.54 | 0.40 | 5.90 | .5315 |
| Myocardial Infarction | 3.12 | 0.59 | 16.52 | .1811 |
| Percutaneous Coronary Intervention | 0.49 | 0.13 | 1.79 | .2804 |
| Previous Cardiac Surgery | 0.86 | 0.28 | 2.59 | .7830 |
| Angina | 0.00 | 0.00 | 6.00798E + 35 | .8625 |
| Hyperlipidemia Medication | 0.78 | 0.44 | 1.40 | .4084 |
| Peripheral Vascular Disease | 0.14 | 0.02 | 1.31 | .0854 |
| Gangrene | 5.14 | 1.33 | 19.82 | .0175 a |
| Renal Failure | 2.18 | 0.41 | 11.63 | .3626 |
| Dialysis | 2.31 | 0.58 | 9.12 | .2326 |
| Impaired Sensorium | 0.47 | 0.06 | 3.81 | .4753 |
| Coma | 0.00 | 0.00 | Inf | .9884 |
| Hemiplegia | 1.13 | 0.20 | 6.54 | .8878 |
| Transient Ischemic Attack | 0.60 | 0.14 | 2.65 | .5024 |
| Cerebrovascular Accident with Deficit | 1.34 | 0.41 | 4.31 | .6286 |
| Cerebrovascular Accident without Deficit | 1.96 | 0.72 | 5.29 | .1871 |
| CNS Tumor | 0.00 | 0.00 | 3.50296E + 62 | .9144 |
| Paraplegia | 0.00 | 0.00 | 1.52209E + 43 | .8718 |
| Quadriplegia | 0.00 | 0.00 | 1.6768E + 155 | .9702 |
| Disseminated Cancer | 1.41 | 0.32 | 6.14 | .6500 |
| Open Wound or Infection | 0.98 | 0.42 | 2.30 | .9700 |
| Steroid Use | 4.00 | 1.85 | 8.65 | .0004 a |
| Weight Loss | 2.59 | 0.58 | 11.64 | .2149 |
| Bleeding Disorder | 0.67 | 0.29 | 1.58 | .3636 |
| Transfusion | 3.72 | 0.39 | 35.39 | .2528 |
| Chemotherapy | 1.22 | 0.18 | 8.13 | .8406 |
| Radiotherapy | 2.10 | 0.30 | 14.71 | .4553 |
| Sepsis | 5.43 | 1.98 | 14.89 | .0010 a |
| Septic Shock | 0.00 | 0.00 | 6.13541E + 38 | .8506 |
| SIRS | 1.76 | 0.84 | 3.68 | .1316 |
| Operation in Past 30 Days | 2.33 | 0.82 | 6.62 | .1108 |
| ASA 2 | 3.49 | 0.44 | 27.76 | .2369 |
| ASA 3 | 4.35 | 0.52 | 36.46 | .1757 |
| ASA 4 | 2.65 | 0.27 | 26.03 | .4025 |
| ASA 5 | 0.00 | 0.00 | 8.29E + 299 | .9808 |
| Operation Time | 1.01 | 1.00 | 1.01 | .0028 |
| Lower Extremity Surgery | 7.55 | 1.78 | 32.04 | .0061 a |
| Pelvis Surgery | 4.51 | 0.39 | 52.50 | .2293 |
a Significant P values are indicated with asterisks.
Footnotes
Authors’ Contribution:Study concept and design: Manish K. Sethi, William T. Obremskey, Paul S. Whiting; analysis and interpretation of data: Sarah E. Greenberg, Jacob P. VanHouten, Paul S. Whiting; drafting of the manuscript: Paul S. Whiting, Gabrielle A. White-Dzuro, Sarah E. Greenberg, Frank R. Avilucea; critical revision of the manuscript for important intellectual content: Manish K. Sethi, William T. Obremskey, Frank R. Avilucea; statistical analysis: Sarah E. Greenberg, Jacob P. Van Houten.
References
- 1.Holley AD, Reade MC. The ‘procoagulopathy’ of trauma. Curr Opin Crit Care. 2013;19(6):578–86. doi: 10.1097/mcc.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 2.Scolaro JA, Taylor RM, Wigner NA. Venous thromboembolism in orthopaedic trauma. J Am Acad Orthop Surg. 2015;23(1):1–6. doi: 10.5435/JAAOS-23-01-1. [DOI] [PubMed] [Google Scholar]
- 3.Geerts WH, Code KI, Jay RM, Chen E, Szalai JP. A prospective study of venous thromboembolism after major trauma. N Engl J Med. 1994;331(24):1601–6. doi: 10.1056/NEJM199412153312401. [DOI] [PubMed] [Google Scholar]
- 4.Shackford SR, Davis JW, Hollingsworth-Fridlund P, Brewer NS, Hoyt DB, Mackersie RC. Venous thromboembolism in patients with major trauma. Am J Surg. 1990;159(4):365–9. doi: 10.1016/s0002-9610(05)81272-3. [DOI] [PubMed] [Google Scholar]
- 5.Godzik J, McAndrew CM, Morshed S, Kandemir U, Kelly MP. Multiple lower-extremity and pelvic fractures increase pulmonary embolus risk. Orthopedics. 2014;37(6):e517–24. doi: 10.3928/01477447-20140528-50. [DOI] [PubMed] [Google Scholar]
- 6.O'Malley KF, Ross SE. Pulmonary embolism in major trauma patients. J Trauma. 1990;30(6):748–50. doi: 10.1097/00005373-199006000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Paffrath T, Wafaisade A, Lefering R, Simanski C, Bouillon B, Spanholtz T, et al. Venous thromboembolism after severe trauma: incidence, risk factors and outcome. Injury. 2010;41(1):97–101. doi: 10.1016/j.injury.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Tuttle-Newhall JE, Rutledge R, Hultman CS, Fakhry SM. Statewide, population-based, time-series analysis of the frequency and outcome of pulmonary embolus in 318,554 trauma patients. J Trauma. 1997;42(1):90–9. doi: 10.1097/00005373-199701000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Barrera LM, Perel P, Ker K, Cirocchi R, Farinella E, Morales Uribe CH, et al. Thromboprophylaxis for trauma patients. 2013;3:CD008303. doi: 10.1002/14651858.CD008303.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stannard JP, Lopez-Ben RR, Volgas DA, Anderson ER, Busbee M, Karr DK, et al. Prophylaxis against deep-vein thrombosis following trauma: a prospective, randomized comparison of mechanical and pharmacologic prophylaxis. J Bone Joint Surg Am. 2006;88(2):261–6. doi: 10.2106/JBJS.D.02932. [DOI] [PubMed] [Google Scholar]
- 11.Gritsiouk Y, Hegsted DA, Schlesinger P, Gardiner SK, Gubler KD. A retrospective analysis of the effectiveness of low molecular weight heparin for venous thromboembolism prophylaxis in trauma patients. Am J Surg. 2014;207(5):648–51. doi: 10.1016/j.amjsurg.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Fisher CG, Blachut PA, Salvian AJ, Meek RN, O'Brien PJ. Effectiveness of pneumatic leg compression devices for the prevention of thromboembolic disease in orthopaedic trauma patients: a prospective, randomized study of compression alone versus no prophylaxis. J Orthop Trauma. 1995;9(1):1–7. doi: 10.1097/00005131-199502000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest J. 2012;141(2_suppl):e278S–325S. doi: 10.1378/chest.11-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogers FB, Cipolle MD, Velmahos G, Rozycki G, Luchette FA. Practice management guidelines for the prevention of venous thromboembolism in trauma patients: the EAST practice management guidelines work group. J Trauma. 2002;53(1):142–64. doi: 10.1097/00005373-200207000-00032. [DOI] [PubMed] [Google Scholar]
- 15.Kardooni S, Haut ER, Chang DC, Pierce CA, Efron DT, Haider AH, et al. Hazards of benchmarking complications with the National Trauma Data Bank: numerators in search of denominators. J Trauma. 2008;64(2):273–7. doi: 10.1097/TA.0b013e31816335ae. [DOI] [PubMed] [Google Scholar]
- 16.Belmont PJ, Davey S, Orr JD, Ochoa LM, Bader JO, Schoenfeld AJ. Risk factors for 30-day postoperative complications and mortality after below-knee amputation: a study of 2,911 patients from the national surgical quality improvement program. J Am Coll Surg. 2011;213(3):370–8. doi: 10.1016/j.jamcollsurg.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Martin CT, Pugely AJ, Gao Y, Wolf BR. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: a review of 12,271 patients from the national surgical quality improvement program database. J Bone Joint Surg Am. 2013;95(14):1–10. doi: 10.2106/JBJS.L.01440. [DOI] [PubMed] [Google Scholar]
- 18.Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193–9. doi: 10.2106/JBJS.K.01682. [DOI] [PubMed] [Google Scholar]
- 19.Radcliff TA, Henderson WG, Stoner TJ, Khuri SF, Dohm M, Hutt E. Patient risk factors, operative care, and outcomes among older community-dwelling male veterans with hip fracture. J Bone Joint Surg Am. 2008;90(1):34–42. doi: 10.2106/JBJS.G.00065. [DOI] [PubMed] [Google Scholar]
- 20.Schoenfeld AJ, Ochoa LM, Bader JO, Belmont PJ. Risk factors for immediate postoperative complications and mortality following spine surgery: a study of 3475 patients from the National Surgical Quality Improvement Program. J Bone Joint Surg Am. 2011;93(17):1577–82. doi: 10.2106/JBJS.J.01048. [DOI] [PubMed] [Google Scholar]
- 21.Suleiman LI, Ortega G, Ong'uti SK, Gonzalez DO, Tran DD, Onyike A, et al. Does BMI affect perioperative complications following total knee and hip arthroplasty? J Surg Res. 2012;174(1):7–11. doi: 10.1016/j.jss.2011.05.057. [DOI] [PubMed] [Google Scholar]
- 22.American College of Surgeons National Surgical Quality Improvement Program . User Guide for the 2013 Participant Use Data File. ACS NSQIP; 2012. [Google Scholar]
- 23.Stannard JP, Singhania AK, Lopez-Ben RR, Anderson ER, Farris RC, Volgas DA, et al. Deep-vein thrombosis in high-energy skeletal trauma despite thromboprophylaxis. J Bone Joint Surg Br. 2005;87(7):965–8. doi: 10.1302/0301-620X.87B7.15989. [DOI] [PubMed] [Google Scholar]
- 24.Platzer P, Thalhammer G, Jaindl M, Obradovic A, Benesch T, Vecsei V, et al. Thromboembolic complications after spinal surgery in trauma patients. Acta Orthop. 2006;77(5):755–60. doi: 10.1080/17453670610012944. [DOI] [PubMed] [Google Scholar]
- 25.Geerts WH, Jay RM, Code KI, Chen E, Szalai JP, Saibil EA, et al. A comparison of low-dose heparin with low-molecular-weight heparin as prophylaxis against venous thromboembolism after major trauma. N Engl J Med. 1996;335(10):701–7. doi: 10.1056/NEJM199609053351003. [DOI] [PubMed] [Google Scholar]
- 26.Nathens AB, McMurray MK, Cuschieri J, Durr EA, Moore EE, Bankey PE, et al. The practice of venous thromboembolism prophylaxis in the major trauma patient. J Trauma. 2007;62(3):557–62. doi: 10.1097/TA.0b013e318031b5f5. [DOI] [PubMed] [Google Scholar]
- 27.Rasmussen MS, Jørgensen LN, Wille-Jørgensen P, Rasmussen MS. Prolonged thromboprophylaxis with Low Molecular Weight heparin for abdominal or pelvic surgery. 2009;1:CD004318. doi: 10.1002/14651858.CD004318.pub2. [DOI] [PubMed] [Google Scholar]
- 28.Agency for Healthcare Research and Quality. Venous Thromboembolism Prophylaxis in Orthopedic Surgery. 2012. [PubMed]