SYNOPSIS
Patient reported outcome (PRO) measures are an important component to assessing disease impact and therapy response in patients with psoriatic arthritis (PsA). Overall there are few PsA-specific PROs. Most PROs used in PsA are borrowed from other diseases (e.g. rheumatoid arthritis and ankylosing spondylitis) or general population PROs. PROs are used in PsA clinical trials and in the clinical management of PsA. In this review, we discuss the most commonly used PRO in PsA including their inclusion in composite measures. Future studies may be helpful to determine the best performing PROs in patients with PsA.
Keywords: psoriatic arthritis, patient reported outcome, outcome measure, composite measures
INTRODUCTION
Psoriatic arthritis (PsA) is a chronic inflammatory arthritis associated with psoriasis. It affects people heterogeneously with a range of clinical manifestations (e.g., inflammatory arthritis, dactylitis, enthesitis, spondylitis, skin psoriasis, nail disease). The disease has a significant impact on patients’ physical function, energy level, social participation, mood, and quality of life (1). Physician-based outcome measures do not capture the patient’s experience of the disease. Patient input in assessing disease status and the effectiveness of their treatments is an important aspect of the management of PsA. Patient reported outcomes (PROs) give us the ability to integrate patient input in a way that is complementary to physician assessments and laboratory measures. PROs are measures of self- reported health status used to evaluate the patient’s perception of symptoms, function and other aspects of their life potentially impacted by disease.
In PsA, PROs are used in clinical trials and clinical practice. PROs are key components of efficacy end-points in clinical trials and are incorporated with physician-based measures in composite disease activity indices, including the primary outcome in PsA randomized controlled trials (RCTs), the American College of Rheumatology 20% improvement response criteria (ACR20). As a part of the OMERACT PsA Core Domain Set (2), PROs representing patient global assessment, pain, physical function, and health related quality of life are expected to be measured in all PsA RCTs in addition to physician assessments of joints and skin. Beyond these domains, PROs are used to capture work productivity, fatigue, psychological endpoints and other symptoms. A wide range of PROs exist and few have been developed specifically for PsA. Most measures used in PsA have been developed for other diseases (e.g. Health Assessment Questionnaire Disability index for rheumatoid arthritis, Functional Assessment of Chronic Illness Therapy-Fatigue for cancer-related anemia) or are generic and meant to assess population health (e.g., Medical Outcomes Study Short Form-36, European Quality of Life Index-5 Dimensions). Furthermore, even fewer PROs have been developed with input from patients with PsA. Patient input into PsA outcome measures has previously been reviewed and for a majority of measures there has been no patient input (3). For a few measures, patient input has been incorporated by developing items from qualitative research among patients with PsA (Psoriatic Arthritis Quality of Life index, Psoriasis Symptom Inventory, Worst Itch-Numerical Rating Scale) or using patient research partner opinions of the relative importance of domains (Psoriatic Arthritis Impact of Disease) (4). Measures of PsA have been reviewed previously (5).
In this review, we discuss PROs used in observational and interventional studies of psoriatic arthritis. We have organized the PROs into categories based on the domains they address.
METHODS
We performed a systematic literature search on July 22, 2015 in PubMed. We included the following search terms for psoriatic arthritis: (“Arthritis, Psoriatic”[Mesh] OR “Psoriatic arthritis” OR “psoriatic arthropathy” OR “arthritis psoriatica” OR “arthropathic psoriasis” OR “psoriasis arthropathica” OR “psoriatic arthropathy” OR “psoriatic polyarthritis” OR “psoriatic rheumatism”) and the Oxford Patient Reported Outcome Measurement filter (source: Oxford Department of Public Health PROM Group). We obtained 1422 entries which were reviewed by title and abstract for inclusion. We excluded duplicates and studies specifically for children. After this review, 247 articles were retained. We performed additional searches for individual outcome measures. For each measure we synthesized the available data on the use of the outcome measure in PsA.
PATIENT REPORT OUTCOMES IN PSORIATIC ARTHRITIS STUDIES
PROs may be disease specific or generic and may address one or more health dimensions or domains. Domains assessed by PROs used in PsA are shown in Table 1 and studied measurement characteristics of PROs in PsA are abstracted in Table 2. The most frequently used PROs in PsA are discussed below.
Table 1.
Domains and Patient Reported Outcomes in Psoriatic Arthritis Studies
| Domain | Patient Reported Outcome |
|---|---|
| Pain | Pain VAS |
| Patient Global | Patient
global Skin Joints Skin and Joints |
| Health Related Quality of Life | Medical Outcomes Study Short
Form-36 Euro-Qol 5 Dimensions PsA Quality of Life Index Dermatologic Life Quality Index Ankylosing Spondylitis Quality of Life Index |
| Impact of Disease | Arthritis Impact Measurement
Scales Psoriatic Arthritis Impact of Disease |
| Disease Activity | Routine Assessment of Patient Index
Data Rheumatoid Arthritis Disease Activity Index Bath Ankylosing Spondylitis Disease Activity Index |
| Disability and Physical Function | Health Assessment Questionnaire Disability
Index Bath Ankylosing Spondylitis Functional Index Disabilities of arm, shoulder and hand questionnaire |
| Skin | Psoriasis Symptom Inventory Worst Itch Numerical Rating Scale |
| Fatigue | Functional Assessment Chronic Illness
Therapy-Fatigue Fatigue Visual Analog Scale/Numerical Rating Scale |
| Productivity | Work Productivity Survey (arthritis specific) |
Table 2.
Studied Measurement Characteristics of Patient Reported Outcomes in Psoriatic Arthritis
| Patient Reported Outcome |
Populatio n |
Reliabilit y Internal consiste ncy |
Reliabil ity e.g. test- retest |
Measurem ent error |
Conten t validity |
Construct validity |
Criterio n validity |
Responsiven ess |
Interpretabi lity (existence of cutoffs) |
|---|---|---|---|---|---|---|---|---|---|
| Medical Outcomes Study Short Form-36 (SF-36) | general | Cronbach’s alpha >0.8 for all 8 scales (25) | NR | NR | NR | Hypothesis testing based on
convergent/divergent validity (25) Structural validity of PCS and MCS dimensions with confirmatory factor analysis (76) |
NR | Area under Receiver Operator Curve (77) | PsA MID calculated (13) |
| Euro-Qol 5 Dimensions (EQ-5D) | general | NR | NR | NR | NR | NR | NR | NR | NR |
| PsA Quality of Life Index (PsAQoL) | psoriatic arthritis | Internal consistency 0.91 Rasch analysis: person separation index 0.93 (27) |
Test retest reliability 0.89 (27) | NR | qualitative research | Hypothesis testing convergent validity (27) | NR | NR | NR |
| Dermatologic Life Quality Index (DLQI) | dermatological conditions | NR | NR | NR | NR | NR | NR | NR | NR |
| Psoriatic Arthritis Impact of Disease (9 and 12 item) | psoriatic arthritis | Cronbach’s alpha=0.93–0.94 (4) | Test-retest reliability at 2–10 days ICC 0.95 and 0.94 (4) | NR | Patient prioritized domains (4) | Hypothesis testing convergent validity (4) | NR | Provided SRM (4) | Preliminary
values PASS=4 MCII=3 (4) |
| Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) | ankylosing spondylitis | NR | NR | NR | NR | Hypothesis testing correlation w disease activity (45, 46) | NR | Area under Receiver Operator Curve calculated for predicting high disease activity/change in treatment (46) | NR |
| Health Assessment Questionnaire Disability Index (HAQ-DI) | rheumatoid arthritis | NR | NR | NR | NR | NR | NR | Area under Receiver Operator Curve calculated (77) | PsA MID 0.131 (78) PsA MCII 0.35 (51) |
| Disabilities of arm, shoulder and hand questionnaire (DASH) | rheumatoid arthritis | NR | NR | NR | NR | Hypothesis testing, correlations with disease activity measures (55) | NR | NR | NR |
| Psoriasis Symptom Inventory (PSI) | psoriasis | Cronbach’s alpha =0.95–0.97 (63) | Test re-test (0–2 week and 2–4 week) ICC=0.7 and 0.87 (63) | (psoriasis) Limits of agreement (62) | Qualitative research | Structural validity using confirmatory factor
analysis and Rasch analysis:
uni-dimensionality Hypothesis testing correlations w BSA, SF-36 (63) |
NR | Comparison of PSI change scores w change in patient global (63) | NR |
| Functional Assessment Chronic Illness Therapy-Fatigue (FACIT-F) | cancer | Cronbach’s alpha =0.96 (66) | Test – retest ICC=0.95 (66) | NR | NR | Hypothesis testing (66) | Correlation with Fatigue Severity Scale =−0.79 | NR | No PsA MID RA MID is 4 |
| Work Productivity Survey (WPS) | rheumatoid arthritis | NR | NR | NR | Literature review | Hypothesis testing (72) | NR | Report SRM (72) | NR |
Pain
Pain is a prevalent and debilitating symptom in arthritis. Pain assessment is part of the Outcome Measures in Rheumatology Clinical Trials core domain set and one of the three PROs in the ACR response indices. It is an outcome measure that is uniformly collected in PsA RCTs and longitudinal studies. Pain is generally measured using a 100 mm visual analog scale (VAS) or an 11 point numerical rating scale (NRS) (range 0–10) with anchors “no pain” (left, 0) to “pain as bad as it could be” (right, 100 or 10 respectively) and a recall period of seven days.
PsA Global Assessment Scales
As noted above, global assessment scales are a part of the 2006 OMERACT PsA Core Domain Set and are captured in most clinical trials and as part of many composite measures. Global assessment scales are meant to measure the impact of a patient’s disease on his/her life. These questions may be phrased in slightly different ways and generally specify a time period over which to rate the effect of their disease. The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) has advocated for measuring three distinct global assessments which include separate skin and joint global assessments and a dual skin and joint global assessment (6). The skin and joint global item is formulated “In all the ways in which your PSORIASIS and ARTHRITIS, as a whole, affects you, how would you rate the way you felt over the past week?” and responses are recorded on a 100mm visual analog scale with anchors “Excellent” (left) and “Poor” (right). VAS are most often used in measuring a global assessment although some have used NRS or Likert-type scales, such as the MultiDimensional Health Assessment Questionnaire (MDHAQ) (7).
Health Related Quality of Life (HRQL)
While the term “health-related quality of life” (HRQL) (8) has not been precisely defined, measures of HRQL are generally felt to measure the impact of chronic disease or therapeutic interventions on a patient’s quality of life. Self –rated health has long been shown to predict short and long-term mortality in the elderly after adjustment for physician assessment, co-morbidities, health-service utilization, demographics, income and life satisfaction (9). HRQL represents a broad concept and draws from different domains of health (such as fatigue, physical function, emotional function, etc.) to derive a final score. The most commonly used HRQL outcome measures are generic (e.g., SF36 and EQ5D) although some HRQL measures have been developed specifically for PsA (PsAQoL). HRQL measures are often secondary efficacy end points in RCTs and can be incorporated into composite measures assessing the cost effectiveness of interventions in PsA. Below we discuss those measures most frequently used in PsA. Other measures that less commonly used in PsA include the Arthritis Impact Measurement Scales (AIMS and AIMS2), Ankylosing Spondylitis Quality of Life index (ASQoL), Routine Assessment of Patient Index Data (RAPID3), and the Rheumatoid Arthritis Disease Activity Index (RADAI).
The Medical Outcomes Study Short Form-36 (SF-36) was developed for use in the general population for clinical care, economic evaluations and health surveys (10, 11). It can be administered as a PRO but has also been administered by trained individuals via telephone. While a free version of the questionnaire (RAND-36) is available, (12) the SF36 is proprietary and the scoring is complex. The SF-36 questionnaire assesses the following eight health domains on a scale of 0–100, 100 being the best score: 1) limitations in physical activities (due to health problems); 2) limitations in social activities (due to physical or emotional problems); 3) limitations in usual role activities (due to physical health problems); 4) bodily pain; 5) general mental health (psychological distress and well-being); 6) limitations in usual role activities (due to emotional problems); 7) vitality (energy and fatigue); and 8) general health perceptions. Scores can also be summarized on 2 components using a population normed T score metric with a mean of 50 and standard deviation of 10. These sub-scores are termed the Physical Component Score (PCS) and Mental Component Score (MCS). Minimal important differences for improvement in PsA were examined using Rasch analysis in one (13) small study (20 patients with PsA starting biologic) and are estimated at 3.74 for PCS and 1.7 for MCS. Corresponding changes in an RA population are 4.4 for PCS and 3.1 for MCS (14). The SF-36 is widely used in PsA RCTs as the preferred measure for HRQL due to its responsiveness with treatment (13, 15, 16).
The EuroQol 5-Dimensions (EQ-5D)
The EQ-5D is a commonly used generic quality of life instrument developed in Europe. It assesses mobility, self-care, usual activities, pain and anxiety/depression. The final score is calculated using a derived formula. The score ranges from −0.594 to 1 where zero is equivalent to death (and therefore patients can rate their health status as being worse than death). EQ-5D has been measured in many PsA RCTs, particularly those conducted in Europe. However, it is widely used among many different diseases and has been validated in a variety of disease status. It is frequently used in economic analyses. An advantage and disadvantage of the EQ5D is it’s brevity – it is easily and rapidly completed however, there are only three possible answers for each question which contributes to a ceiling effect (18, 19).
Psoriatic Arthritis Quality of Life index (PsAQoL)
PsAQoL is a PsA disease specific measure of quality of life composed of 20 yes/no questions, making this a relatively easy and rapid questionnaire for completion. The items address domains including social participation, fatigue, mood, and daily activities. This instrument was developed using results from focus groups conducted among patients with PsA and subsequent item surveys with patients. The PsAQoL had excellent test-retest reliability and two studies have demonstrated correlation of PsAQoL with other instruments, suggesting construct validity (27, 28). Additionally, the PsAQoL is sensitive to change. The PsAQoL has been adapted in additional languages for Sweden and Netherlands (29, 30). Similar to the SF-36, PsAQoL is proprietary. While not frequently used in clinical trials, the PsAQoL was used in the recent Tight Control in Psoriatic Arthritis (TiCOPA) trial and it is part of several candidate PsA disease activity indices (see Table 3) (24–26).
Table 3.
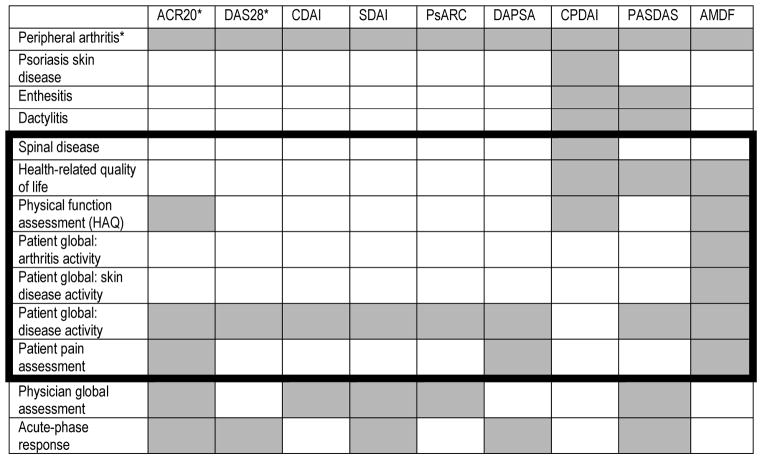
Composite Outcome Measures in Psoriatic Arthritis
Patient reported outcomes are shown within the dark lines.
Peripheral arthritis is captured through the number of swollen and tender joints. The ACR20 is defined as 20% improvement in tender and swollen joint counts as well as 20% improvement in 3 of the other 5 measures. The ACR20 and DAS28 assess the PIPs, MCPs, wrists, elbows, shoulders, and knees. DAPSA, CPDAI, PASDAS, and AMDF use the 66/68 joint counts. The DAS66/68 adds the hips, DIPs, sternoclavicular, temporomandibular, acromioclavicular, talotibial, midtarsal, metatarsophalangeal and interphalageal joints of the toes.
Abbreviations: ACR20 = American College of Rheumatology 20% Response; DAS28 = Disease Activity Score 28 Joints; PsAJAI = Psoriatic arthritis Joint Activity Index; DAPSA = Disease Activity in Psoriatic Arthritis; CDAI = Clinical Disease Activity Index; SDAI = Simplified Disease Activity Index; CPDAI= Composite Psoriatic Disease Activity Index; PASDAS= Psoriatic Arthritis Disease Activity Score; AMDF= Arithmetic Mean of Desirability Functions.
Adapted with permission from Coates LC, FitzGerald O, Mease PJ, DD. Gladman, et al. Development of a disease activity and responder index for psoriatic arthritis--report of the Psoriatic Arthritis Module at OMERACT 11. The Journal of rheumatology. 2014;41(4):782–91; with permission.
Psoriatic Arthritis Impact of Disease (PsAID)
The PsAID is a measure developed by the European League Against Rheumatism (EULAR) and is composed of domains selected by an international group of patients with PsA. The PSAID is not specifically a HRQL PRO. It is instead intended for use as a patient-reported measure of disease impact on life in general. The PSAID has two versions, one with nine domains for RCTs and one with 12 domains for clinical care. PSAID domains include: 1) pain (pain in joints, spine and skin), 2) skin problems (including itching), 3) fatigue (being physically tired, but also mental fatigue, lack of energy), 4) ability to work/leisure, 5) functional capacity, 6) feeling of discomfort, 7) sleep disturbance, 8) anxiety, fear and uncertainty (about the future, treatments, fear of loneliness), 9) coping (adjustment to the disease, managing, being in charge, making do with the disease), 10) embarrassment and/or shame due to appearance, 11) social participation, 12) depression. (Numbers 10–12 are added to the 9-item questionnaire). The questionnaire uses a weighted scoring system (weights were derived by patient impression of importance) and has a range of 0–10 (higher scores are worse) with 4 being considered a patient acceptable symptom state (4). The proposed Minimal Clinically Important Difference (MCID) is 3. Given that this is a relatively new measure, few studies have included the PSAID but studies are underway to determine sensitivity to change and convergent validity.
Patient Reported Disease Activity, Disability and Physical Function
Patient reported disease activity measures have been developed for RA (e.g., RAPID3) and ankylosing spondylitis (AS) (e.g., BASDAI) and these measures have been extended to other rheumatologic diseases including PsA. One issue with patient reported disease activity measures is the lack of correlation between self-reported joint counts with physician assessments in PsA (33). In one study, there was weak correlation for tender joints; no correlation for swollen joints; and weak to moderate correlation for damaged joints. A study in the same cohort showed discrepancies between patient and physician global assessments (34); these patient-physician discrepancies were significantly associated in a multivariable regression model with scores for fatigue, pain, tender and swollen joints and HRQL. Nevertheless, these measures may provide different and complimentary information to physician-reported measures. Below we discuss the most commonly used patient-reported disease activity measures in PsA trials and in the clinical management of PsA. Additionally, we discuss PRO measures for disability and physical function, which have different meanings than disease activity. While physical function may correlate with disease activity, disability may not (35).
Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) has been developed in patients with ankylosing spondylitis and exclusively axial disease (43). BASDAI is a questionnaire consisting of six VAS items assessing fatigue, axial joint pain, peripheral joint pan, soft tissue tenderness to touch, severity and duration of morning stiffness consisting BASDAI. Responses are recorded on unlabeled 10 cm VAS with left and right anchor “None” and “Very severe” except for the morning stiffness duration item, from “0” to “2 or more hours” with additional labels for ½, 1 and 1½ hours. Score range is 0–10 calculated as the mean of the six items. BASDAI has been used in PsA with and without axial disease and scores were generally higher in the axial versus peripheral PsA phenotype (44, 45). In axial PsA (grade 2 or more unilateral sacroiliitis, inflammatory back pain and stiffness) BASDAI did not differentiate between levels of disease activity defined by change in treatment in either axial or peripheral PsA (45). In the Toronto PsA cohort, BASDAI showed good discriminative ability for high and low disease activity in axial PsA (similarly defined as grade 2 or more sacroiliitis, inflammatory back pain or spinal mobility limitation). Three definitions were used for high disease activity: patient global>6; physician global>6; and change in treatment. BASDAI discriminative ability for high disease activity using the three definitions was calculated as Area Under the Curve (AUC) (95% CI): 0.92 (0.88–0.95); 0.78 (0.67–0.88); and 0.69 (0.63–0.76) respectively (46).
Health Assessment Questionnaire Disability Index (HAQ-DI) is a widely used outcome measurement instrument for disability, developed in patients with rheumatoid arthritis (47). HAQ-DI scores have been shown to predict future function, survival and resource utilization in RA (48), correlate with radiographic scores in RA.(49) Total HAQ-DI score range is 0–3 and normal scores are 0.5 or lower. Minimal clinically important improvement (MCII) in RA has been determined to be decrease of 0.375 in the total score (50) (equivalent to 3 points improvement in the raw score), and very similar, a decrease of 0.35 in PsA (51). The HAQ-DI has been measured in every PsA RCT as it is part of the ACR responder indices and is usually also reported as a separate endpoint. HAQ-DI has been shown to be limited by floor effect much more in PsA (30%) than RA (8%) (52, 53) a fact supported by a comparative review of RA versus PsA RCTs where mean HAQ-DI scores are systematically higher in RA vs PsA (17). While this may be interpreted as a lower level of disability, it may in fact be a reflection of common oligoarticular involvement with PsA.
Disabilities of arm, shoulder and hand questionnaire (DASH) was studied in one longitudinal PsA cohort. Correlations with clinical measures of disease/joint activity were lower for the total joint core compared to upper extremity score as expected since DASH measures upper limb function (55). Due to common lower extremity involvement in PsA the measure is not sufficient for assessing the construct of disability in a majority of PsA patients.
Skin symptoms and related impact
While a complete review of the quality of life and disease activity indices for skin are beyond the scope of this review, we will briefly discuss those commonly included in clinical trials of PsA.
Dermatology Life Quality Index (DLQI)
The DLQI is a quality of life index designed for patients with skin disease. This 10-item questionnaire (with one additional item that branches) which ascertains the impact of skin disease on work and leisure activities, social participation/relationships, symptoms related to skin disease like itch and pain (56, 57). The DLQI is widely used in clinical trials for psoriasis and PsA and correlates well with the Psoriasis Area and Severity Index.(58). This questionnaire is easy to complete, sensitive to change and has been validated in multiple populations, in particular psoriasis (59).
Psoriasis Symptom Inventory (PSI)
The PSI is a recently developed PRO assessing psoriasis symptoms that can be administered on paper or electronically (60). Rather than a HRQL index, this can be used more as a disease activity index. PSI was developed in people with psoriasis in the US who participated in focus groups and interviews to generate and subsequently clarify concepts and patient preferred terms. PSI has eight items assessing the severity of each of these symptoms: 1) itch; 2) redness; 3) scaling; 4) burning; 5) stinging; 6) cracking; 7) flaking; and 8) pain due to psoriasis (61). Item response options are a 5-point Likert type (score 0: not at all severe; 1: mild; 2: moderate; 3: severe; 4: very severe) and two versions exist, differing only in the recall period (24 hours and seven days). The PSI score range is 0–32 with higher score representing worse symptoms. PSI items test-retest reliability has been studies in 139 patients with psoriasis and it was good (individual items ICCs>0.7) as well as correlations with DLQI and SF-36 items. As expected, the highest correlations were observed with corresponding skin symptom items (62). PSI was tested in 154 people with PsA showing good test-retest reliability (total score ICC 0.7), moderate correlation with body surface area and patient global (−0.5 and 0.4 respectively) and low correlations with SF-36 concepts and physician global (63).
Worst Itch Numerical Rating Scale (WI-NRS)
The WI-NRS was developed in patients with psoriasis (n=22) and PsA (n=12) and consists of a single NRS (0–10, “no itch” to “worst imaginable itch”) for itch with an assessment over the past 24 hours. The item was developed using qualitative research with patients with psoriasis and psoriatic arthritis followed by item cognitive debriefing (64).
Fatigue
Functional Assessment Chronic Illness Therapy-Fatigue (FACIT-F)
FACIT-F is a 13-item PRO initially developed in patients with cancer as the Functional Assessment of Cancer Therapy (65) and adapted for use in other chronic conditions. Score range is 0–52 with higher scores reflecting less fatigue. FACIT-F reliability and validity was examined in a longitudinal PsA study (66). Minimal important change in RA is 4 points (67) and it has not been specifically studied in PsA.
Fatigue VAS use is common especially in clinical care (including an 11-point NRS scale as a part of the MDHAQ) and longitudinal studies. Fatigue VAS have been criticized for lack of standardization because this causes great difficulty with comparisons across studies (68).
Work Productivity
Work productivity and work disability are related concepts. PsA is associated with increased work disability (69) which can be reversed with treatment (70).
The arthritis-specific Work Productivity Survey (WPS) has been developed in rheumatoid arthritis on the basis of a literature review (71) and has data supporting its validity in PsA from one RCT (72). WPS is an interviewer-administered ten item questionnaire assessing employment status, missed workdays, productivity and arthritis interference both in the work place and at home, with a recall period of one month. Two of the items are visual analog scales with anchors “no interference” (left) and “complete interference” (right) and additional items are reports of numbers of days missed or not as productive.
Sleep Disturbance
There is evidence that PsA is associated with sleep disturbance (73) and patients prioritized this impact in the EULAR PsAID measure, yet sleep is rarely assessed in PsA research or clinical care. The Medical Outcomes Study Sleep Scale has been used in a study of psoriasis and fibromyalgia but not specifically in PsA (74, 75). The PsAID questionnaire is the only PsA specific PRO assessing sleep disturbance as one of its domains. Further studies are needed to address optimal PROs for sleep in PsA.
INCLUSION OF PATIENT REPORTED OUTCOMES IN COMPOSITE MEASURES FOR PSORIATIC ARTHRITIS
Composite outcome measures have been developed to attempt to integrate patient and clinician measures (Table 3) and to address several domains in a single measure. In composite indices, patient and physician measures are aggregated into a single score. There are two types of composite measures: those that are response indices and have a dichotomous cutoff (e.g. ACR20) and those that calculate a score for disease activity (e.g. DAPSA) that is sensitive to change and a cutoff is derived to serve as a threshold for “response.” This second type of measure can be a static or dynamic measure of disease activity and often has defined categories of disease activity (e.g., remission, low, moderate and high disease activity). Patient measures most often included are the HAQ (or a functional assessment), pain and global assessments. A few of the composite measures include quality of life (via the SF36, PsAQoL, DLQI and/or ASQoL). Below we address the PROs in each composite index and how these were selected. The domains assessed by each composite measure are shown in Table 3. The Psoriatic Arthritis Joint Activity Index (PsAJAI) was previous developed but has not yet been used in additional studies since development and thus is not included below.(79)
American College of Rheumatology 20%, 50%, and 70% Response Criteria (ACR20, ACR50 and ACR70)
The ACR20 is the most commonly used response index and is the primary outcome for trials in PsA. The ACR criteria define response as a binary outcome. These criteria were initially developed for rheumatoid arthritis and utilize a 28 joint count. This has been modified in PsA to include a 66/68 joint count. The ACR criteria were developed using physician surveys and analysis of RA clinical trial data. These criteria define response at the 20%, 50%, and 70% thresholds based on the reduction in tender and swollen joint counts, physician’s global assessment, acute phase reactant, and three PROs, HAQ-DI, patient pain assessment and patient global assessment (80).
Disease Activity Score (DAS) of 28 or 66/68 joints, the Clinical Disease Activity Index (CDAI), and the Simplified Disease Activity Index (SDAI)
Similar to the ACR outcomes, these measures were developed initially in RA. The DAS has been modified for PsA to include the 66/68 joint counts. These disease activity measures include only one PRO: the patient global assessment of health in the case of DAS, and the patient global assessment of arthritis for CDAI and SDAI. In addition, these measures include the swollen and tender joint counts and one or both of the C-reactive protein or sedimentation rate and physician (or evaluator) global assessment (81).
Disease Activity index for Psoriatic Arthritis (DAPSA)
Development of the DAPSA was based on a principal component analysis that revealed three significant components: two PROs (patient pain and global assessments), joint involvement (66/68 swollen joint counts), and acute phase response (C-Reactive Protein) (82) The Disease Activity Index for Reactive Arthritis (DAREA) had previously been derived for reactive arthritis and contained these same elements. It was thus tested in PsA and found to have good discrimination (AUC 0.74–0.80) (83). However, subsequent studies have suggested that other composite indices may have larger effect sizes (84). Recently DAPSA cutoffs for disease activity states and treatment response have been derived using patient level data from three PsA RCTs (85) therefore this index is now usable and interpretable.
Composite Psoriatic Disease Activity Index (CPDAI)
The domains of the CPDAI were derived from consensus among GRAPPA members and include joint disease, skin involvement, enthesitis, dactylitis and spinal disease. Instruments to measure each domain were similarly chosen by consensus. For each domain, activity is defined as none, mild, moderate or severe and these categories can be defined by more than one instrument. Each domain is assigned a point value depending on the severity (0–3 respectively) and these individual scores are then summed to a final score (range 0–15). PROs included in the CPDAI include the HAQ for peripheral arthritis, DLQI for skin disease, and BASDAI or ASQoL for spine disease (86).
The Psoriatic Arthritis Disease Activity Score (PASDAS) and the Arithmetic Mean of Desirability Functions (AMDF)
Both the PASDAS and AMDF were developed as a part of the GRACE project and were derived (although in different ways) from the same datasets. In this dataset (the GRAppa Composite Exercise or GRACE study), PROs included patient global assessments (overall global, skin, and joints), the DLQI, ASQoL index, PsAQoL index, SF36 and the individual components and the HAQ. PASDAS was derived using a principal component analysis and AMDF was derived using desirability functions (desirability was derived using physician surveys). Both have somewhat complex formulas. The PASDAS includes the physician and patient global assessments, the SF36 physical component scale, the tender and swollen joint counts, the Leeds enthesitis count, tender dactylitis count, and the C-reactive protein. The AMDF includes the same elements (different formula) but adds the mental component scale of the SF36 (87). These measures have not yet been used in PsA clinical trials but have shown large effect sizes in a clinical trial dataset.(88)
Minimal Disease Activity (MDA)
MDA are a set of criteria that define the “state of disease activity deemed a useful target of treatment by both the patient and physician, given current treatment possibilities and limitations.” Each domain is assessed as active or not active based on suggested thresholds. The OMERACT PsA Core Domains, agreed upon in 2006, were used to define MDA. However, HRQL was excluded because of lack of correlation between HRQL and other measures of disease activity to be included. Rheumatologists and dermatologists were then asked to decide whether patient profiles were in a state of MDA. Thresholds for each of the domains were maintained when >70% consensus was achieved. The final version of the MDA includes the following components (threshold) patient pain and global VAS assessments (less than or equal to 15 and 20 respectively), the HAQ (less than or equal to 0.5), tender and swollen joint counts (less than or equal to 1), enthesitis count (less than or equal to 1), and psoriasis severity characterized by either Psoriasis Area and Severity Index (PASI) or Body Surface Area (BSA) (less than or equal to 1, and 3% respectively) (89). MDA state is defined as achieving the threshold for five out of the seven components.
Psoriatic Arthritis Response Criteria (PsARC)
The PsARC is a composite responder index that includes tender (68) and swollen (66) joint counts and physician and patient global assessments (measured on 5-point likert scales)(90). It was the first composite measure derived specifically for PsA. Similar to the ACR response criteria, this is a binary score where a patient can meet the definition of response if they have either a 30% reduction in tender joints or swollen joints or a 1-point improvement in either the physician or patient global assessment scale and the other items must not worsen (91). PsARC is generally not used as the primary outcome measure in RCTs but rather as a secondary outcome (92, 93).
SUMMARY
Psoriatic arthritis is a complex disease. Patients with PsA have highly varied manifestations of PsA (e.g. peripheral arthritis, spondylitis, enthesitis, dactylitis) and are likewise varied in terms of how they experience their disease and the level of impact it has on their lives. From these perspectives, PsA can be difficult to measure. The patients’ perspective of their illness and their response to therapy can be captured using PROs. While numerous PROs exist in general, only a few addressing each domain have been validated in PsA and even fewer have been developed specifically for PsA. However, some existing PROs do perform relatively well in PsA RCTs. Additional studies are needed to understand what patients think is important in defining the activity of their disease. With such knowledge we can more precisely define the unidimensional concepts that need to be assessed in PsA such that a set of PROs with optimized measurement properties for PsA can be selected and standardized for PsA assessment in clinical trials and clinical practice.
KEY POINTS.
Psoriatic arthritis is a chronic and heterogeneous inflammatory arthritis associated with psoriasis.
Patient reported outcomes are essential in assessing health status and treatment effects in psoriatic arthritis
Additional studies are needed to understand what patients think is important in defining the activity of their disease.
Acknowledgments
Dr. Orbai’s work is supported by the Rheumatology Research Foundation Scientist Development Award and by the Johns Hopkins Arthritis Discovery Fund. Dr. Ogdie is supported by K23 AR063764.
Footnotes
DISCLOSURE STATEMENT
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ana-Maria Orbai, Assistant Professor of Medicine, Division of Rheumatology, Johns Hopkins University, Baltimore, MD, USA.
Alexis Ogdie, Assistant Professor of Medicine and Epidemiology, Division of Rheumatology, Perelman School of Medicine, University of Pennsylvania, Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania, Philadelphia, PA, USA.
References
- 1.Taylor WJ, Mease PJ, Adebajo A, Nash PJ, Feletar M, Gladman DD. Effect of psoriatic arthritis according to the affected categories of the international classification of functioning, disability and health. J Rheumatol. 2010;37(9):1885–91. doi: 10.3899/jrheum.091315. [DOI] [PubMed] [Google Scholar]
- 2.Gladman DD, Mease PJ, Strand V, Healy P, Helliwell PS, Fitzgerald O, et al. Consensus on a core set of domains for psoriatic arthritis. J Rheumatol. 2007;34(5):1167–70. [PubMed] [Google Scholar]
- 3.Tillett W, Adebajo A, Brooke M, Campbell W, Coates LC, FitzGerald O, et al. Patient involvement in outcome measures for psoriatic arthritis. Curr Rheumatol Rep. 2014;16(5):418. doi: 10.1007/s11926-014-0418-7. [DOI] [PubMed] [Google Scholar]
- 4.Gossec L, de Wit M, Kiltz U, Braun J, Kalyoncu U, Scrivo R, et al. A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: elaboration and preliminary validation of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire, a 13-country EULAR initiative. Ann Rheum Dis. 2014;73(6):1012–9. doi: 10.1136/annrheumdis-2014-205207. [DOI] [PubMed] [Google Scholar]
- 5.Mease PJ. Measures of psoriatic arthritis: Tender and Swollen Joint Assessment, Psoriasis Area and Severity Index (PASI), Nail Psoriasis Severity Index (NAPSI), Modified Nail Psoriasis Severity Index (mNAPSI), Mander/Newcastle Enthesitis Index (MEI), Leeds Enthesitis Index (LEI), Spondyloarthritis Research Consortium of Canada (SPARCC), Maastricht Ankylosing Spondylitis Enthesis Score (MASES), Leeds Dactylitis Index (LDI), Patient Global for Psoriatic Arthritis, Dermatology Life Quality Index (DLQI), Psoriatic Arthritis Quality of Life (PsAQOL), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Psoriatic Arthritis Response Criteria (PsARC), Psoriatic Arthritis Joint Activity Index (PsAJAI), Disease Activity in Psoriatic Arthritis (DAPSA), and Composite Psoriatic Disease Activity Index (CPDAI) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S64–85. doi: 10.1002/acr.20577. [DOI] [PubMed] [Google Scholar]
- 6.Cauli A, Gladman DD, Mathieu A, Olivieri I, Porru G, Tak PP, et al. Patient global assessment in psoriatic arthritis: a multicenter GRAPPA and OMERACT study. J Rheumatol. 2011;38(5):898–903. doi: 10.3899/jrheum.100857. [DOI] [PubMed] [Google Scholar]
- 7.Pincus T, Skummer PT, Grisanti MT, Castrejon I, Yazici Y. MDHAQ/RAPID3 can provide a roadmap or agenda for all rheumatology visits when the entire MDHAQ is completed at all patient visits and reviewed by the doctor before the encounter. Bulletin of the NYU hospital for joint diseases. 2012;70(3):177–86. [PubMed] [Google Scholar]
- 8.Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Annals of internal medicine. 1993;118(8):622–9. doi: 10.7326/0003-4819-118-8-199304150-00009. [DOI] [PubMed] [Google Scholar]
- 9.Mossey JM, Shapiro E. Self-rated health: a predictor of mortality among the elderly. American journal of public health. 1982;72(8):800–8. doi: 10.2105/ajph.72.8.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical care. 1993;31(3):247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 12.Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Annals of medicine. 2001;33(5):350–7. doi: 10.3109/07853890109002089. [DOI] [PubMed] [Google Scholar]
- 13.Leung YY, Zhu TY, Tam LS, Kun EW, Li EK. Minimal important difference and responsiveness to change of the SF-36 in patients with psoriatic arthritis receiving tumor necrosis factor-alpha blockers. J Rheumatol. 2011;38(9):2077–9. doi: 10.3899/jrheum.101256. [DOI] [PubMed] [Google Scholar]
- 14.Kosinski M, Zhao SZ, Dedhiya S, Osterhaus JT, Ware JE., Jr Determining minimally important changes in generic and disease-specific health-related quality of life questionnaires in clinical trials of rheumatoid arthritis. Arthritis and rheumatism. 2000;43(7):1478–87. doi: 10.1002/1529-0131(200007)43:7<1478::AID-ANR10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 15.Saad AA, Ashcroft DM, Watson KD, Symmons DP, Noyce PR, Hyrich KL. Improvements in quality of life and functional status in patients with psoriatic arthritis receiving anti-tumor necrosis factor therapies. Arthritis Care Res (Hoboken) 2010;62(3):345–53. doi: 10.1002/acr.20104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strand V, Sharp V, Koenig AS, Park G, Shi Y, Wang B, et al. Comparison of health-related quality of life in rheumatoid arthritis, psoriatic arthritis and psoriasis and effects of etanercept treatment. Ann Rheum Dis. 2012;71(7):1143–50. doi: 10.1136/annrheumdis-2011-200387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee S, Mendelsohn A, Sarnes E. The burden of psoriatic arthritis: a literature review from a global health systems perspective. P T. 2010;35(12):680–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmad MA, Xypnitos FN, Giannoudis PV. Measuring hip outcomes: common scales and checklists. Injury. 2011;42(3):259–64. doi: 10.1016/j.injury.2010.11.052. [DOI] [PubMed] [Google Scholar]
- 19.Leung YY, Png ME, Wee HL, Thumboo J. Comparison of EuroQol-5D and short form-6D utility scores in multiethnic Asian patients with psoriatic arthritis: a cross-sectional study. J Rheumatol. 2013;40(6):859–65. doi: 10.3899/jrheum.120782. [DOI] [PubMed] [Google Scholar]
- 20.Gignac MA, Cao X, McAlpine J, Badley EM. Measures of disability: Arthritis Impact Measurement Scales 2 (AIMS2), Arthritis Impact Measurement Scales 2-Short Form (AIMS2-SF), The Organization for Economic Cooperation and Development (OECD) Long-Term Disability (LTD) Questionnaire, EQ-5D, World Health Organization Disability Assessment Schedule II (WHODASII), Late-Life Function and Disability Instrument (LLFDI), and Late-Life Function and Disability Instrument-Abbreviated Version (LLFDI-Abbreviated) Arthritis care & research. 2011;63(Suppl 11):S308–24. doi: 10.1002/acr.20640. [DOI] [PubMed] [Google Scholar]
- 21.Husted J, Gladman DD, Farewell VT, Long JA. Validation of the revised and expanded version of the Arthritis Impact Measurement Scales for patients with psoriatic Arthritis. J Rheumatol. 1996;23(6):1015–9. [PubMed] [Google Scholar]
- 22.Husted J, Gladman DD, Long JA, Farewell VT. Relationship of the Arthritis Impact Measurement Scales to changes in articular status and functional performance in patients with psoriatic arthritis. J Rheumatol. 1996;23(11):1932–7. [PubMed] [Google Scholar]
- 23.Long JA, Husted JA, Gladman DD, Farewell VT. The relationship between patient satisfaction with health and clinical measures of function and disease status in patients with psoriatic arthritis. J Rheumatol. 2000;27(4):958–66. [PubMed] [Google Scholar]
- 24.Husted JA, Gladman DD, Cook RJ, Farewell VT. Responsiveness of health status instruments to changes in articular status and perceived health in patients with psoriatic arthritis. The Journal of rheumatology. 1998;25(11):2146–55. [PubMed] [Google Scholar]
- 25.Husted JA, Gladman DD, Farewell VT, Long JA, Cook RJ. Validating the SF-36 health survey questionnaire in patients with psoriatic arthritis. The Journal of rheumatology. 1997;24(3):511–7. [PubMed] [Google Scholar]
- 26.Taal E, Rasker JJ, Riemsma RP. Sensitivity to change of AIMS2 and AIMS2-SF components in comparison to M-HAQ and VAS-pain. Annals of the rheumatic diseases. 2004;63(12):1655–8. doi: 10.1136/ard.2003.014522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKenna SP, Doward LC, Whalley D, Tennant A, Emery P, Veale DJ. Development of the PsAQoL: a quality of life instrument specific to psoriatic arthritis. Annals of the rheumatic diseases. 2004;63(2):162–9. doi: 10.1136/ard.2003.006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brodszky V, Pentek M, Balint PV, Geher P, Hajdu O, Hodinka L, et al. Comparison of the Psoriatic Arthritis Quality of Life (PsAQoL) questionnaire, the functional status (HAQ) and utility (EQ-5D) measures in psoriatic arthritis: results from a cross-sectional survey. Scand J Rheumatol. 2010;39(4):303–9. doi: 10.3109/03009740903468982. [DOI] [PubMed] [Google Scholar]
- 29.Billing E, McKenna SP, Staun M, Lindqvist U. Adaptation of the Psoriatic Arthritis Quality of Life (PsAQoL) instrument for Sweden. Scand J Rheumatol. 2010;39(3):223–8. doi: 10.3109/03009740903347975. [DOI] [PubMed] [Google Scholar]
- 30.Wink F, Arends S, McKenna SP, Houtman PM, Brouwer E, Spoorenberg A. Validity and reliability of the Dutch adaptation of the Psoriatic Arthritis Quality of Life (PsAQoL) Questionnaire. PLoS One. 2013;8(2):e55912. doi: 10.1371/journal.pone.0055912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doward LC, Spoorenberg A, Cook SA, Whalley D, Helliwell PS, Kay LJ, et al. Development of the ASQoL: a quality of life instrument specific to ankylosing spondylitis. Annals of the rheumatic diseases. 2003;62(1):20–6. doi: 10.1136/ard.62.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leung YY, Tam LS, Kun EW, Ho KW, Li EK. Comparison of 4 functional indexes in psoriatic arthritis with axial or peripheral disease subgroups using Rasch analyses. J Rheumatol. 2008;35(8):1613–21. [PubMed] [Google Scholar]
- 33.Chaudhry SR, Thavaneswaran A, Chandran V, Gladman DD. Physician scores vs patient self-report of joint and skin manifestations in psoriatic arthritis. Rheumatology (Oxford) 2013;52(4):705–11. doi: 10.1093/rheumatology/kes355. [DOI] [PubMed] [Google Scholar]
- 34.Eder L, Thavaneswaran A, Chandran V, Cook R, Gladman DD. Factors explaining the discrepancy between physician and patient global assessment of joint and skin disease activity in psoriatic arthritis patients. Arthritis Care Res (Hoboken) 2015;67(2):264–72. doi: 10.1002/acr.22401. [DOI] [PubMed] [Google Scholar]
- 35.Husted JA, Tom BD, Farewell VT, Schentag CT, Gladman DD. A longitudinal study of the effect of disease activity and clinical damage on physical function over the course of psoriatic arthritis: Does the effect change over time? Arthritis Rheum. 2007;56(3):840–9. doi: 10.1002/art.22443. [DOI] [PubMed] [Google Scholar]
- 36.Castrejon I, Bergman MJ, Pincus T. MDHAQ/RAPID3 to recognize improvement over 2 months in usual care of patients with osteoarthritis, systemic lupus erythematosus, spondyloarthropathy, and gout, as well as rheumatoid arthritis. Journal of clinical rheumatology: practical reports on rheumatic & musculoskeletal diseases. 2013;19(4):169–74. doi: 10.1097/RHU.0b013e3182936b98. [DOI] [PubMed] [Google Scholar]
- 37.Pincus T, Swearingen CJ, Bergman M, Yazici Y. RAPID3 (Routine Assessment of Patient Index Data 3), a rheumatoid arthritis index without formal joint counts for routine care: proposed severity categories compared to disease activity score and clinical disease activity index categories. The Journal of rheumatology. 2008;35(11):2136–47. doi: 10.3899/jrheum.080182. [DOI] [PubMed] [Google Scholar]
- 38.Pincus T, Furer V, Keystone E, Yazici Y, Bergman MJ, Luijtens K. RAPID3 (Routine Assessment of Patient Index Data 3) severity categories and response criteria: Similar results to DAS28 (Disease Activity Score) and CDAI (Clinical Disease Activity Index) in the RAPID 1 (Rheumatoid Arthritis Prevention of Structural Damage) clinical trial of certolizumab pegol. Arthritis care & research. 2011;63(8):1142–9. doi: 10.1002/acr.20481. [DOI] [PubMed] [Google Scholar]
- 39.Castrejon I, Dougados M, Combe B, Guillemin F, Fautrel B, Pincus T. Can remission in rheumatoid arthritis be assessed without laboratory tests or a formal joint count? possible remission criteria based on a self-report RAPID3 score and careful joint examination in the ESPOIR cohort. The Journal of rheumatology. 2013;40(4):386–93. doi: 10.3899/jrheum.121059. [DOI] [PubMed] [Google Scholar]
- 40.Danve A, Reddy A, Vakil-Gilani K, Garg N, Dinno A, Deodhar A. Routine Assessment of Patient Index Data 3 score (RAPID3) correlates well with Bath Ankylosing Spondylitis Disease Activity index (BASDAI) in the assessment of disease activity and monitoring progression of axial spondyloarthritis. Clinical rheumatology. 2015;34(1):117–24. doi: 10.1007/s10067-014-2827-4. [DOI] [PubMed] [Google Scholar]
- 41.Castrejon I, Yazici Y, Pincus T. Patient self-report RADAI (Rheumatoid Arthritis Disease Activity Index) joint counts on an MDHAQ (Multidimensional Health Assessment Questionnaire) in usual care of consecutive patients with rheumatic diseases other than rheumatoid arthritis. Arthritis care & research. 2013;65(2):288–93. doi: 10.1002/acr.21793. [DOI] [PubMed] [Google Scholar]
- 42.Leeb BF, Haindl PM, Brezinschek HP, Mai HT, Deutsch C, Rintelen B. Patient-centered psoriatic arthritis (PsA) activity assessment by Stockerau Activity Score for Psoriatic Arthritis (SASPA) BMC Musculoskelet Disord. 2015;16:73. doi: 10.1186/s12891-015-0512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. The Journal of rheumatology. 1994;21(12):2286–91. [PubMed] [Google Scholar]
- 44.Taylor WJ, Harrison AA. Could the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) be a valid measure of disease activity in patients with psoriatic arthritis? Arthritis and rheumatism. 2004;51(3):311–5. doi: 10.1002/art.20421. [DOI] [PubMed] [Google Scholar]
- 45.Fernandez-Sueiro JL, Willisch A, Pertega-Diaz S, Tasende JA, Fernandez-Lopez JC, Villar NO, et al. Validity of the bath ankylosing spondylitis disease activity index for the evaluation of disease activity in axial psoriatic arthritis. Arthritis Care Res (Hoboken) 2010;62(1):78–85. doi: 10.1002/acr.20017. [DOI] [PubMed] [Google Scholar]
- 46.Eder L, Chandran V, Shen H, Cook RJ, Gladman DD. Is ASDAS better than BASDAI as a measure of disease activity in axial psoriatic arthritis? Ann Rheum Dis. 2010;69(12):2160–4. doi: 10.1136/ard.2010.129726. [DOI] [PubMed] [Google Scholar]
- 47.Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis and rheumatism. 1980;23(2):137–45. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 48.Wolfe F, Kleinheksel SM, Cathey MA, Hawley DJ, Spitz PW, Fries JF. The clinical value of the Stanford Health Assessment Questionnaire Functional Disability Index in patients with rheumatoid arthritis. The Journal of rheumatology. 1988;15(10):1480–8. [PubMed] [Google Scholar]
- 49.Navarro-Compan V, Landewe R, Provan SA, Odegard S, Uhlig T, Kvien TK, et al. Relationship between types of radiographic damage and disability in patients with rheumatoid arthritis in the EURIDISS cohort: a longitudinal study. Rheumatology (Oxford) 2015;54(1):83–90. doi: 10.1093/rheumatology/keu284. [DOI] [PubMed] [Google Scholar]
- 50.Ward MM, Guthrie LC, Alba MI. Clinically important changes in individual and composite measures of rheumatoid arthritis activity: thresholds applicable in clinical trials. Annals of the rheumatic diseases. 2014 doi: 10.1136/annrheumdis-2013-205079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mease PJ, Woolley JM, Bitman B, Wang BC, Globe DR, Singh A. Minimally important difference of Health Assessment Questionnaire in psoriatic arthritis: relating thresholds of improvement in functional ability to patient-rated importance and satisfaction. J Rheumatol. 2011;38(11):2461–5. doi: 10.3899/jrheum.110546. [DOI] [PubMed] [Google Scholar]
- 52.Taylor WJ, McPherson KM. Using Rasch analysis to compare the psychometric properties of the Short Form 36 physical function score and the Health Assessment Questionnaire disability index in patients with psoriatic arthritis and rheumatoid arthritis. Arthritis Rheum. 2007;57(5):723–9. doi: 10.1002/art.22770. [DOI] [PubMed] [Google Scholar]
- 53.Fries JF, Bruce B, Rose M. Comparison of the health assessment questionnaire disability index and the short form 36 physical functioning subscale using Rasch analysis: comment on the article by Taylor and McPherson. Arthritis Rheum. 2008;59(4):598–9. doi: 10.1002/art.23520. author reply 9. [DOI] [PubMed] [Google Scholar]
- 54.Calin A, Garrett S, Whitelock H, Kennedy LG, O’Hea J, Mallorie P, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. The Journal of rheumatology. 1994;21(12):2281–5. [PubMed] [Google Scholar]
- 55.Navsarikar A, Gladman DD, Husted JA, Cook RJ. Validity assessment of the disabilities of arm, shoulder, and hand questionnaire (DASH) for patients with psoriatic arthritis. J Rheumatol. 1999;26(10):2191–4. [PubMed] [Google Scholar]
- 56.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)--a simple practical measure for routine clinical use. Clinical and experimental dermatology. 1994;19(3):210–6. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 57.Nichol MB, Margolies JE, Lippa E, Rowe M, Quell J. The application of multiple quality-of-life instruments in individuals with mild-to-moderate psoriasis. Pharmaco Economics. 1996;10(6):644–53. doi: 10.2165/00019053-199610060-00010. [DOI] [PubMed] [Google Scholar]
- 58.Mattei PL, Corey KC, Kimball AB. Psoriasis Area Severity Index (PASI) and the Dermatology Life Quality Index (DLQI): the correlation between disease severity and psychological burden in patients treated with biological therapies. Journal of the European Academy of Dermatology and Venereology: JEADV. 2014;28(3):333–7. doi: 10.1111/jdv.12106. [DOI] [PubMed] [Google Scholar]
- 59.Lundberg L, Johannesson M, Silverdahl M, Hermansson C, Lindberg M. Health-related quality of life in patients with psoriasis and atopic dermatitis measured with SF-36, DLQI and a subjective measure of disease activity. Acta Derm Venereol. 2000;80(6):430–4. doi: 10.1080/000155500300012873. [DOI] [PubMed] [Google Scholar]
- 60.Bushnell DM, Martin ML, Scanlon M, Chen T, Chau D, Viswanathan HN. Equivalence and measurement properties of an electronic version of the Psoriasis Symptom Inventory. Quality of life research: an international journal of quality of life aspects of treatment, care and rehabilitation. 2014;23(3):897–906. doi: 10.1007/s11136-013-0527-1. [DOI] [PubMed] [Google Scholar]
- 61.Martin ML, McCarrier KP, Chiou CF, Gordon K, Kimball AB, Van Voorhees AS, et al. Early development and qualitative evidence of content validity for the Psoriasis Symptom Inventory (PSI), a patient-reported outcome measure of psoriasis symptom severity. The Journal of dermatological treatment. 2013;24(4):255–60. doi: 10.3109/09546634.2012.759639. [DOI] [PubMed] [Google Scholar]
- 62.Bushnell DM, Martin ML, McCarrier K, Gordon K, Chiou CF, Huang X, et al. Validation of the Psoriasis Symptom Inventory (PSI), a patient-reported outcome measure to assess psoriasis symptom severity. The Journal of dermatological treatment. 2013;24(5):356–60. doi: 10.3109/09546634.2012.742950. [DOI] [PubMed] [Google Scholar]
- 63.Wilson HD, Mutebi A, Revicki DA, Mease PJ, Genovese MC, Erondu N, et al. Reliability and validity of the psoriasis symptom inventory in patients with psoriatic arthritis. Arthritis Care Res (Hoboken) 2015 doi: 10.1002/acr.22653. [DOI] [PubMed] [Google Scholar]
- 64.Naegeli AN, Flood E, Tucker J, Devlen J, Edson-Heredia E. The Worst Itch Numeric Rating Scale for patients with moderate to severe plaque psoriasis or psoriatic arthritis. Int J Dermatol. 2015;54(6):715–22. doi: 10.1111/ijd.12645. [DOI] [PubMed] [Google Scholar]
- 65.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. Journal of pain and symptom management. 1997;13(2):63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 66.Chandran V, Bhella S, Schentag C, Gladman DD. Functional assessment of chronic illness therapy-fatigue scale is valid in patients with psoriatic arthritis. Ann Rheum Dis. 2007;66(7):936–9. doi: 10.1136/ard.2006.065763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cella D, Yount S, Sorensen M, Chartash E, Sengupta N, Grober J. Validation of the Functional Assessment of Chronic Illness Therapy Fatigue Scale relative to other instrumentation in patients with rheumatoid arthritis. The Journal of rheumatology. 2005;32(5):811–9. [PubMed] [Google Scholar]
- 68.Hewlett S, Dures E, Almeida C. Measures of fatigue: Bristol Rheumatoid Arthritis Fatigue Multi-Dimensional Questionnaire (BRAF MDQ), Bristol Rheumatoid Arthritis Fatigue Numerical Rating Scales (BRAF NRS) for severity, effect, and coping, Chalder Fatigue Questionnaire (CFQ), Checklist Individual Strength (CIS20R and CIS8R), Fatigue Severity Scale (FSS), Functional Assessment Chronic Illness Therapy (Fatigue) (FACIT-F), Multi-Dimensional Assessment of Fatigue (MAF), Multi-Dimensional Fatigue Inventory (MFI), Pediatric Quality Of Life (PedsQL) Multi-Dimensional Fatigue Scale, Profile of Fatigue (ProF), Short Form 36 Vitality Subscale (SF-36 VT), and Visual Analog Scales (VAS) Arthritis care & research. 2011;63(Suppl 11):S263–86. doi: 10.1002/acr.20579. [DOI] [PubMed] [Google Scholar]
- 69.Tillett W, de-Vries C, McHugh NJ. Work disability in psoriatic arthritis: a systematic review. Rheumatology (Oxford) 2012;51(2):275–83. doi: 10.1093/rheumatology/ker216. [DOI] [PubMed] [Google Scholar]
- 70.Kristensen LE, Englund M, Neovius M, Askling J, Jacobsson LT, Petersson IF. Long-term work disability in patients with psoriatic arthritis treated with anti-tumour necrosis factor: a population-based regional Swedish cohort study. Ann Rheum Dis. 2013;72(10):1675–9. doi: 10.1136/annrheumdis-2012-202229. [DOI] [PubMed] [Google Scholar]
- 71.Osterhaus JT, Purcaru O, Richard L. Discriminant validity, responsiveness and reliability of the rheumatoid arthritis-specific Work Productivity Survey (WPS-RA) Arthritis research & therapy. 2009;11(3):R73. doi: 10.1186/ar2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Osterhaus JT, Purcaru O. Discriminant validity, responsiveness and reliability of the arthritis-specific Work Productivity Survey assessing workplace and household productivity in patients with psoriatic arthritis. Arthritis Res Ther. 2014;16(4):R140. doi: 10.1186/ar4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Callis Duffin K, Wong B, Horn EJ, Krueger GG. Psoriatic arthritis is a strong predictor of sleep interference in patients with psoriasis. J Am Acad Dermatol. 2009;60(4):604–8. doi: 10.1016/j.jaad.2008.10.059. [DOI] [PubMed] [Google Scholar]
- 74.Strober BE, Sobell JM, Duffin KC, Bao Y, Guerin A, Yang H, et al. Sleep quality and other patient-reported outcomes improve after patients with psoriasis with suboptimal response to other systemic therapies are switched to adalimumab: results from PROGRESS, an open-label Phase IIIB trial. The British journal of dermatology. 2012;167(6):1374–81. doi: 10.1111/bjd.12000. [DOI] [PubMed] [Google Scholar]
- 75.Williams DA, Arnold LM. Measures of fibromyalgia: Fibromyalgia Impact Questionnaire (FIQ), Brief Pain Inventory (BPI), Multidimensional Fatigue Inventory (MFI-20), Medical Outcomes Study (MOS) Sleep Scale, and Multiple Ability Self-Report Questionnaire (MASQ) Arthritis care & research. 2011;63(Suppl 11):S86–97. doi: 10.1002/acr.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leung YY, Ho KW, Zhu TY, Tam LS, Kun EW, Li EK. Testing scaling assumptions, reliability and validity of medical outcomes study short-form 36 health survey in psoriatic arthritis. Rheumatology (Oxford) 2010;49(8):1495–501. doi: 10.1093/rheumatology/keq112. [DOI] [PubMed] [Google Scholar]
- 77.Husted JA, Cook RJ, Farewell VT, Gladman DD. Methods for assessing responsiveness: a critical review and recommendations. J Clin Epidemiol. 2000;53(5):459–68. doi: 10.1016/s0895-4356(99)00206-1. [DOI] [PubMed] [Google Scholar]
- 78.Kwok T, Pope JE. Minimally important difference for patient-reported outcomes in psoriatic arthritis: Health Assessment Questionnaire and pain, fatigue, and global visual analog scales. J Rheumatol. 2010;37(5):1024–8. doi: 10.3899/jrheum.090832. [DOI] [PubMed] [Google Scholar]
- 79.Gladman DD, Tom BD, Mease PJ, Farewell VT. Informing response criteria for psoriatic arthritis (PsA). II: Further considerations and a proposal--the PsA joint activity index. The Journal of rheumatology. 2010;37(12):2559–65. doi: 10.3899/jrheum.100479. [DOI] [PubMed] [Google Scholar]
- 80.Felson DTAJ, Boers M, et al. American College of Rheumatology Preliminary Definition of Improvement in Rheumatoid Arthritis. Arthritis and rheumatism. 1995;38(6):727–35. doi: 10.1002/art.1780380602. [DOI] [PubMed] [Google Scholar]
- 81.Prevoo ML, van’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis and rheumatism. 1995;38(1):44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 82.Nell-Duxneuner VP, Stamm TA, Machold KP, Pflugbeil S, Aletaha D, Smolen JS. Evaluation of the appropriateness of composite disease activity measures for assessment of psoriatic arthritis. Annals of the rheumatic diseases. 2010;69(3):546–9. doi: 10.1136/ard.2009.117945. [DOI] [PubMed] [Google Scholar]
- 83.Schoels M, Aletaha D, Funovits J, Kavanaugh A, Baker D, Smolen JS. Application of the DAREA/DAPSA score for assessment of disease activity in psoriatic arthritis. Annals of the rheumatic diseases. 2010;69(8):1441–7. doi: 10.1136/ard.2009.122259. [DOI] [PubMed] [Google Scholar]
- 84.Helliwell PS, FitzGerald O, Fransen J. Composite disease activity and responder indices for psoriatic arthritis: a report from the GRAPPA 2013 meeting on development of cutoffs for both disease activity states and response. The Journal of rheumatology. 2014;41(6):1212–7. doi: 10.3899/jrheum.140172. [DOI] [PubMed] [Google Scholar]
- 85.Schoels MM, Aletaha D, Alasti F, sMOlen J. Disease activity in psoriatic artthritis (PsA): defining remission and treatment success using teh DAPSA score. Ann Rheum Dis. 2015 doi: 10.1136/annrheumdis-2015-207507. [DOI] [PubMed] [Google Scholar]
- 86.Mumtaz A, Gallagher P, Kirby B, Waxman R, Coates LC, Veale JD, et al. Development of a preliminary composite disease activity index in psoriatic arthritis. Annals of the rheumatic diseases. 2011;70(2):272–7. doi: 10.1136/ard.2010.129379. [DOI] [PubMed] [Google Scholar]
- 87.Helliwell PS, FitzGerald O, Fransen J, Gladman DD, Kreuger GG, Callis-Duffin K, et al. The development of candidate composite disease activity and responder indices for psoriatic arthritis (GRACE project) Ann Rheum Dis. 2013;72(6):986–91. doi: 10.1136/annrheumdis-2012-201341. [DOI] [PubMed] [Google Scholar]
- 88.Helliwell PS, Kavanaugh A. Comparison of composite measures of disease activity in psoriatic arthritis using data from an interventional study with golimumab. Arthritis care & research. 2014;66(5):749–56. doi: 10.1002/acr.22204. [DOI] [PubMed] [Google Scholar]
- 89.Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Annals of the rheumatic diseases. 2010;69(1):48–53. doi: 10.1136/ard.2008.102053. [DOI] [PubMed] [Google Scholar]
- 90.Clegg DO, Reda DJ, Mejias E, Cannon GW, Weisman MH, Taylor T, et al. Comparison of sulfasalazine and placebo in the treatment of psoriatic arthritis. A Department of Veterans Affairs Cooperative Study. Arthritis and rheumatism. 1996;39(12):2013–20. doi: 10.1002/art.1780391210. [DOI] [PubMed] [Google Scholar]
- 91.Fransen J, Antoni C, Mease PJ, Uter W, Kavanaugh A, Kalden JR, et al. Performance of response criteria for assessing peripheral arthritis in patients with psoriatic arthritis: analysis of data from randomised controlled trials of two tumour necrosis factor inhibitors. Annals of the rheumatic diseases. 2006;65(10):1373–8. doi: 10.1136/ard.2006.051706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mease PJ, Fleischmann R, Deodhar AA, Wollenhaupt J, Khraishi M, Kielar D, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA) Annals of the rheumatic diseases. 2014;73(1):48–55. doi: 10.1136/annrheumdis-2013-203696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mease PJ, Goffe BS, Metz J, VanderStoep A, Finck B, Burge DJ. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet (London, England) 2000;356(9227):385–90. doi: 10.1016/S0140-6736(00)02530-7. [DOI] [PubMed] [Google Scholar]
- 94.Gladman DD, Tom BD, Mease PJ, Farewell VT. Informing response criteria for psoriatic arthritis. I: discrimination models based on data from 3 anti-tumor necrosis factor randomized studies. The Journal of rheumatology. 2010;37(9):1892–7. doi: 10.3899/jrheum.091172. [DOI] [PubMed] [Google Scholar]
- 95.Gladman DPF, Illouz O, Sampalis JS. Evaluation of response using the psoriatic arthritis joint activity index scoring tool in patients treated with adalimumab: post hoc analysis of the AC-CLAIM study. The Journal of rheumatology. 2009;38:2571. [Google Scholar]
- 96.Coates LC, FitzGerald O, Mease PJ, Gladman DD, et al. Development of a disease activity and responder index for psoriatic arthritis--report of the Psoriatic Arthritis Module at OMERACT 11. The Journal of rheumatology. 2014;41(4):782–91. doi: 10.3899/jrheum.131250. [DOI] [PubMed] [Google Scholar]