Abstract
Clinicopathological correlates of prostate cancer associated with a pseudocapsule at T2-weighted MR imaging are presented in a retrospective series of 15 patients. Fourteen of 15 tumors involved the peripheral zone. Extracapsular extension was seen in 14 cases. Tumor Gleason score was 8 or above in 12 of 15 cases, and ductal type adenocarcinoma was identified in 4. Step section histopathological correlation (n = 5) demonstrated that the pseudocapsule corresponded with dense compressive or reactive peritumoral fibrosis. A pseudocapsule around prostate cancer at T2-weighted MR imaging is a rare finding that appears to be associated with high grade and stage disease.
Keywords: MR imaging, prostate cancer, pseudocapsule, pathology
1.1 Introduction
MR imaging is increasingly used for localization and staging of prostate cancer [1, 2]. On T2-weighted imaging, prostate cancer is typically seen as focal, non-encapsulated, low signal intensity that has an ellipsoid or crescentic subcapsular shape in the peripheral zone or an infiltrative “erased charcoal” appearance in the central gland. Such foci are especially suspicious when accompanied by additional abnormalities on multiparametric imaging, such as restricted diffusion, early intense enhancement, rapid washout, or a malignant spectral signature [3, 4]. Peripheral zone and central gland cancers typically do not have a tumor capsule, and encapsulation is generally considered indicative of nodules derived from benign prostatic hyperplasia [4]. There is only one case report describing the finding of a pseudocapsule surrounding a prostate cancer [5]. However, we have encountered several cases of prostate cancer accompanied by the finding of a pseudocapsule Therefore, we undertook this study to report the clinicopathological correlates of prostate cancer associated with a pseudocapsule at T2-weighted MR imaging.
1.2 Materials and methods
1.2.1 Subjects
This was a retrospective study conducted at two institutions and approved by both Institutional Review Boards, with waiver of the requirement for informed consent. The study was compliant with the requirements of the Health Insurance Portability and Accountability Act. Based on the records of our multidisciplinary urology tumor boards and the senior author (---), we retrospectively identified 15 patients with prostate cancer associated with a pseudocapsule at T2-weighted MR imaging seen at two institutions between 2000 and 2015. In all cases, the diagnosis of prostate cancer was established by transrectal ultrasound guided biopsy. All available clinical, imaging, and histopathological records were reviewed by the principal investigator (---), with attention to demographics, clinical presentation, imaging findings, histopathological results, management, and outcome. In patients who proceeded to radical prostatectomy at our institutions, we reviewed the histopathological correlate of the pseudocapsule in conjunction with two attending genitourinary pathologists (---, ---).
1.2.2 MRI technique
The detailed scan protocols used at the two institutions are shown in Table 1. At institution A, studies were performed on a 3T whole body MRI scanner (Ingenia; Philips Healthcare, Netherlands). At institution B, studies were performed on 3T scanner (General Electric Healthcare Technologies, Waukesha, WI). At both institutions, a body coil was used for excitation and an inflatable endorectal coil (Medrad; Pittsburgh, PA) in conjunction with a pelvic phase array coil was used for signal reception.
Table 1.
Details of the diagnostic endorectal multiparametric MRI protocols used at the two institutions in the study.
| Parameter | T1 | T2 | |
|---|---|---|---|
| Institution A | Sequence | Axial fast spin echo | Axial & coronal fast spin echo |
| Anatomic coverage | Pelvis | Prostate | |
| TR/TE (msec/msec) | 400-600/10 | Minimum/120 | |
| Echo train length | 4 | 26 | |
| PI acceleration factor | 1.6 | 1.3 | |
| Matrix | 352 × 352 | 560 × 560 | |
| Field of view (cm) | 30 | 18 | |
| Number of excitations | 2 | 3 | |
| Slice thickness/gap (mm) | 5/0.5 | 3/0 | |
| Specific to sequence | Transverse frequency direction | Anteroposterior frequency direction for axials | |
| Fat suppression | NA | NA | |
| Institution B | Sequence | Axial 3D spoiled gradient echo | Oblique axial & coronal fast spin echo |
| Anatomic coverage | Pelvis | Prostate | |
| TR/TE (msec/msec) | In Phase | 6000/102 | |
| Echo train length | NA | 16 | |
| PI acceleration factor | NA | NA | |
| Matrix | 192 × 128 | 384 × 384 | |
| Field of view (cm) | 24 | 18 (Coronal = 14) | |
| Number of excitations | 2 | 2 | |
| Slice thickness/gap (mm) | 4.2/2 | 3/0 | |
| Specific to sequence | Frequency direction A/P | Frequency direction A/P Coronal: Frequency direction S/I |
|
| Fat suppression | NA | NA |
1.2.3 MRI interpretation
All MR images of the 15 retrospectively identified patients with a pseudocapsule cancer were reviewed by two attending radiologists (---, ---) on a picture archiving and communication system workstation (Impax; Agfa, Mortsel, Belgium) and, by consensus, the two readers identified dominant tumor site and size, presence of tumor pseudocapsule, and staging findings. Tumor size was measured as the maximum axial diameter. Using an amalgam of previously described methodologies [3, 6, 7], prostate cancer was defined as focal low T2 signal intensity with an ellipsoid or crescentic subcapsular morphology in the peripheral zone or infiltrative “erased charcoal” non-encapsulated appearance in the central gland accompanied by focal reduction in apparent diffusion coefficient or accompanied by focal early intense enhancement or rapid washout at perfusion imaging. A pseudocapsule was defined as a visible rim of low T2 signal intensity around all or part of the tumor margin that was not abutting the true capsule of the prostate gland or the pseudocapsule of the central gland. The radial distance of extracapsular extension, if present, was measured, using a previously described methodology [8].
1.3 Results
The clinical, pathological, and radiological findings in the study population are summarized in Table 2. Mean patient age was 67 years (range, 52 to 87). Mean baseline serum prostate specific antigen level was 26.2 ng/mL (range, 0.2 to 76). Mean tumor diameter at MR imaging was 3.4 cm (range, 1.0 to 7.2). Fourteen of 15 tumors involved the peripheral zone. MR findings of extracapsular extension, seminal vesicle invasion, pelvic adenopathy, and pelvic bone metastases were seen in 14, 7, 2 and 2 cases, respectively. The mean radial distance of extracapsular extension was 11 mm (range, 1 to 24). Eleven of 14 patients had extracapsular extension over 5 mm. Tumor Gleason score was 8 or above in 12 of 15 cases, and ductal type adenocarcinoma was identified in 4 of 15. Patients with known treatment (n = 11) were managed primarily by radical prostatectomy (n = 6), radiation (n = 3), or androgen deprivation therapy alone (n = 2). After a median follow up of 18 months (range, 3 to 44) in these 11 patients, 6 are alive with disease, 4 are in remission, and one has died of progressive disease. Step section histopathological correlation in the patients who underwent radical prostatectomy at our institutions (n = 5) demonstrated that the pseudocapsule corresponded with dense compressive or reactive peritumoral fibrosis (Figures 1 and 2).
Table 2.
Clinical and imaging characteristics of the 15 patients in the study group with pseudoencapsulated prostate cancer at T2-weighted MRI.
| Age | Baseline serum PSA* (ng/mL) |
Baseline clinical stage |
Gleason score |
Dominant tumor location |
Size (cm) |
Radial diameter of extracapsular extension (mm) |
Seminal vesicle invasion |
Enlarged nodes |
Bone metastasis |
Follow up (month) |
Treatment and outcome | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 69 | 29.9 | T3 | G5+4 (ductal) |
Left peripheral zone |
4.6 | 24 | No | No | No | 6 | Androgen deprivation therapy prior to prostatectomy; PSA currently undetectable. |
| 2 | 66 | 37.0 | T1 | G4+4 (ductal) |
Left peripheral zone |
4.0 | 20 | Yes | No | No | N/A | Lost to follow-up. |
| 3 | 58 | 34.0 | Unknown | G4+4 | Right peripheral zone |
3.0 | 12 | No | No | Yes | 42 | Androgen deprivation therapy prior to prostatectomy. Radiation to single bone metastasis. Stable PSA < 0.5. |
| 4 | 58 | 2.2 | T2 | 4+3 | Left apical peripheral zone |
1.0 | 4 | No | No | No | 35 | Prostatectomy. PSA remains undetectable. |
| 5 | 52 | 28.0 | Unknown | 4+4 (ductal) |
Left peripheral zone |
2.0 | 1 | No | Yes | No | N/A | Lost to follow-up. |
| 6 | 72 | 70.0 | T1 | 4+4 | Predominantly exophytic and superior to the bladder |
6.9 | 12 | Yes | No | No | N/A | Lost to follow-up. |
| 7 | 57 | 0.2** | T2 | 4+4 | Entire gland involved |
4.6 | 24 | Yes | No | No | 44 | Androgen deprivation therapy and radiation. Alive without recent PSA assay. |
| 8 | 60 | 3.9 | T2 | 4+3 | Right peripheral zone and central gland |
3.5 | 10 | No | No | No | 5 | Prostatectomy. Biochemical recurrence with current PSA of 6.5. |
| 9 | 81 | 9.9 | T1 | 4+4 (ductal) |
Left peripheral zone |
3.3 | 12 | No | No | No | 26 | Androgen deprivation therapy and radiation. CurrentPSA < 0.1. |
| 10 | 87 | 76.0 | T3 | 4+5 | Left peripheral zone |
4.9 | 13 | Yes | No | No | 12 | Androgen deprivation therapy. Current PSA < 0.5 and stable. |
| 11 | 79 | 6.2 | Unknown | 5+5 | Bilateral peripheral zone |
7.2 | 10 | Yes | No | No | N/A | Lost to follow-up. |
| 12 | 61 | 46.0 | Unknown | 3+4 | Left peripheral zone |
2.6 | 6 | Yes | Yes | Yes | 16 | Androgen deprivation therapy. Died of disease 16 months after diagnosis. |
| 13 | 60 | 6.9 | T1 | 5+4 | Right-base peripheral zone |
1.1 | 6 | Yes | No | No | 3 | Prostatectomy. |
| 14 | 65 | 33 | T1 | 3+4 | Right-apex central gland |
1.6 | 0 | No | No | No | 4 | Prostatectomy. |
| 15 | 78 | 9.7 | T2 | 4+4 | Left mid gland peripheral zone |
1.4 | 2 | No | No | No | 4 | Radiation and adjuvant androgen deprivation therapy, with falling PSA. |
Prostatic specific antigen.
Tumor was poorly differentiated at pathological examination, likely explaining low PSA level.
Figure 1.
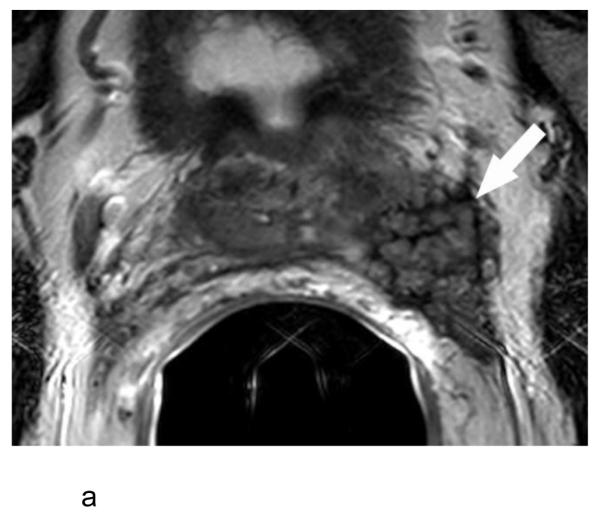
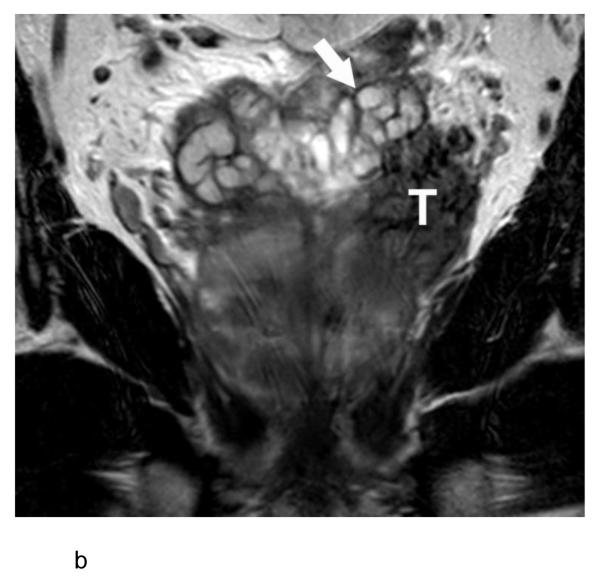
a:- Axial T2-weighted MR image in a 69 year old man with a serum PSA of 29.9 ng/ml and Gleason score 5+4 prostate cancer in 13 of 13 cores on systematic biopsy (patient #1 in Table 1) showing a large mass (arrow) arising from the left side of the prostate with extensive extracapsular extension. The mass is surrounded by a thin rim of low T2 signal intensity, which also interdigitates within the mass, resulting in a vermiform appearance.
b:- Coronal T2-weighted MR image shows the tumor mass (T) distinct from the left seminal vesicle (arrow), indicating the vermiform appearance in Figure 1a does not represent seminal vesicle invasion.
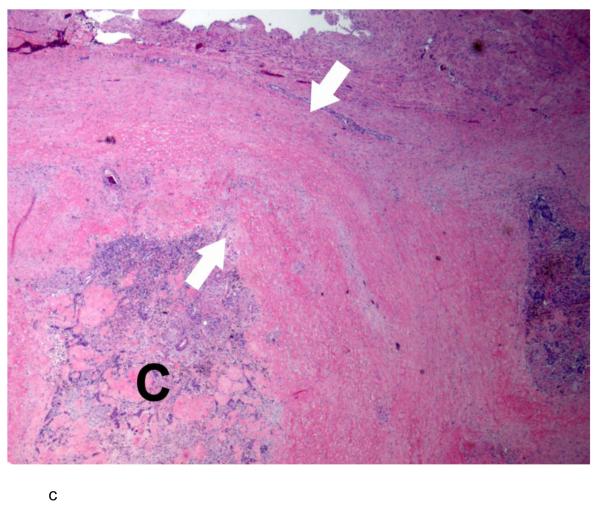
c:- Photomicrograph of a hematoxylin and eosin-stained slide of the extracapsular tumor mass shows a nodule of prostate cancer (C) surrounded by a thick band of stromal desmoplastic fibrosis (between arrows). The cancer demonstrates involution as a consequence of pre-operative androgen deprivation therapy.
Figure 2.
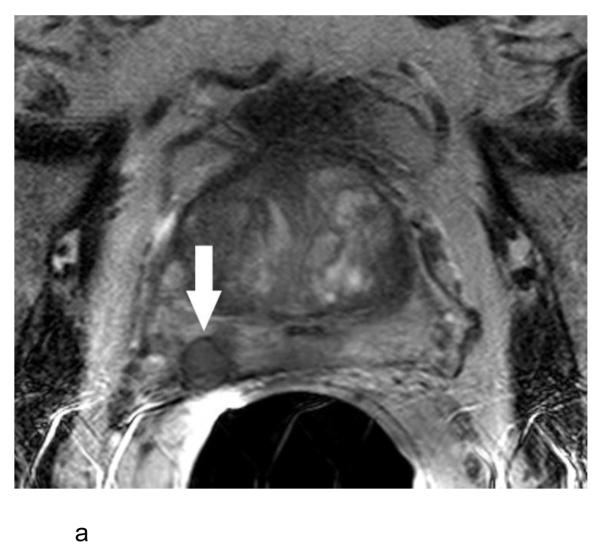
a:- Axial T2-weighted MR image in a 60 year old man with a serum PSA of 9.5 ng/ml and Gleason score 5+4 prostate cancer in 5 of 12 cores on systematic biopsy (patient #13 in Table 1) showing a circular nodule (arrow) of low T2 signal intensity in the peripheral zone of the right base surrounded by a thin rim of low T2 signal intensity.
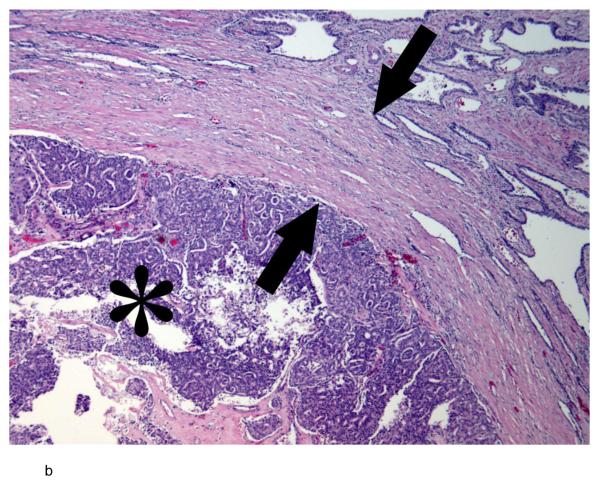
b:- Photomicrograph of a hematoxylin and eosin-stained slide of the tumormargin shows an expansile nodule of high grade prostate cancer (asterisk) surrounded by a thick band of fibrosis (between arrows) that appears to represent compressed adjacent prostatic parenchyma.
1.4 Discussion
This study describes a series of patients with prostate cancer that demonstrated a pseudocapsule at T2-weighted MR imaging. Our results suggest this represents dense compressive or reactive peritumoral fibrosis around the tumor, and that this finding is associated with high grade and stage disease. These observations have a number of practical implications. First, a pseudocapsule of low T2 signal in the prostate is generally considered a feature of benign prostate hyperplasia, and absence of a capsule is generally considered a feature of prostate cancer [4]. Our study adds a note of caution to this approach. For prostate lesions with a pseudocapsule that are large, in the peripheral zone, or appear associated with extension beyond the prostate capsule or seminal vesicle invasion, malignancy should be included in the differential. However, review of the cases in our study suggests that experienced readers of prostate MR imaging should have little difficulty in distinguishing a pseudocapsule prostate cancers from benign prostate hyperplasia; most of the tumors in this study were in the peripheral zone or associated with locally advanced disease. Second, recognition that a pseudocapsule around prostate cancer at T2-weighted MR imaging portends an aggressive tumor of high grade may be of clinical utility, highlighting the need for more intensive treatment, such as extended androgen deprivation therapy. This could be useful in cases where standard systematic biopsy under-graded the primary tumor, a well-recognized phenomenon [8]. In such a scenario, radiology could play a key role in guiding management. Our finding that 11 of 15 patients had extracapsular extension exceeding 5 mm in radial distance further reinforces the prognostic importance of tumor encapsulation. In a study of patients evaluated by MRI prior to radiation therapy, extracapsular extension greater than 5 mm in radial distance was associated with early metastatic recurrence in 3 of 5 patients [8]. Of course, tumor encapsulation may not be an independent negative prognostic factor, but recognizing the association with worse prognosis could still be clinically helpful.
To our knowledge, only one prior report has described a pseudocapsule around prostate cancer at MR imaging, in which a 72 year old man was found to have a surgically confirmed Gleason score 3+4 cancer extending exophytically from the prostate with a T2 hypointense rim or capsule [5]. This is in accordance with the finding that all 15 patients in our series had high or intermediate grade Gleason scores. Interestingly, the implication that a pseudocapsule of prostate cancer is associated with high grade disease and presumably a poorer prognosis contrasts with the published literature on radiologically identified pseudocapsule around other malignancies. For example, a pseudocapsule around renal cell carcinoma and hepatocellular carcinoma appears to be associated with a more favorable prognosis [10-14].
Our study has several limitations. This was a retrospective study with a small sample size. As the patients were identified based on the records of tumor boards and the senior author, it is possible that there may have been selection bias in case identification. Specifically, nodules with a pseudocapsule at MR imaging may be more likely to be diagnosed as malignant if they are large, advanced, or in the peripheral zone, and so our study might have been skewed towards inclusion of such cases. However, if smaller nodules with a pseudocapsule in the central gland were malignant with any frequency, it seems likely that this would be a recognized clinical phenomenon, given that such cases would presumably return for re-imaging due to progressive rises in prostatic specific antigen. We are unaware of any studies describing such observations. Because the cases were collected over a long time period from two institutions, patients were not studied with a standard multiparametric protocol incorporating diffusion and perfusion imaging. As a result, we do not know how diffusion and perfusion findings might help in the distinction of encapsulated prostate cancer from encapsulated nodules of benign prostatic hyperplasia. Larger and more comprehensive studies will be required to address these limitations, incorporating contemporary multiparametric MR sequences across all patients, comparison with benign encapsulated nodules, and systematic comparative analyses. The lack of histopathological correlation in many cases is a further limitation, but reflects the advanced nature of these tumors, such that many patients were managed without surgery.
1.5 Conclusions
In conclusion, the finding of a pseudocapsule around prostate cancer at T2-weighted MR imaging is a rare finding that corresponds with compressive or reactive peritumoral fibrosis at histopathology and appears to be associated with high grade and stage disease.
Acknowledgments
DP supported by NIH grant 1R25EB016671
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
1.6 References
- 1.Yacoub JH, Oto A, Miller FH. MR imaging of the prostate. Radiol Clin North Am. 2014;52:811–37. doi: 10.1016/j.rcl.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Porten SP, Smith A, Odisho AY, et al. Updated trends in imaging use in men diagnosed with prostate cancer. Prostate Cancer Prostatic Dis. 2014;17:246–51. doi: 10.1038/pcan.2014.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jung AJ, Westphalen AC, Kurhanewicz J, et al. Clinical utility of endorectal MRI-guided prostate biopsy: preliminary experience. J Magn Reson Imaging. 2014;40:314–23. doi: 10.1002/jmri.24383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.PIRADS [accessed February 6, 2015];Acr.org. (v2). http://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Resources/PIRADS/PIRADS%20V2.pdf.
- 5.Satoa Y, Oguroa T, Kumagaia S, et al. Prostatic adenocarcinoma with pseudocapsule. World J Nephrol Urol. 2013;2:18–20. [Google Scholar]
- 6.Siddiqui MM, Rais-Bahrami S, Truong H, et al. Magnetic Resonance Imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol. 2013;64:713–719. doi: 10.1016/j.eururo.2013.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pokorny MR, de Rooij M, Duncan E, et al. Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasound-guided biopsy versus magnetic resonance (MR) imaging with subsequent MR-guided biopsy in men without previous prostate biopsies. Eur Urol. 2014;66:22–9. doi: 10.1016/j.eururo.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 8.McKenna DA, Coakley FV, Westphalen AC, et al. Prostate cancer: role of pretreatment MR in predicting outcome after external-beam radiation therapy--initial experience. Radiology. 2008;247:141–6. doi: 10.1148/radiol.2471061982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grossfeld GD, Chang JJ, Broering JM, et al. Under staging and under grading in a contemporary series of patients undergoing radical prostatectomy: results from the Cancer of the Prostate Strategic Urologic Research Endeavor database. J Urol. 2001;165:851–6. [PubMed] [Google Scholar]
- 10.Lu DS, Siripongsakun S, Kyong Lee J, et al. Complete tumor encapsulation on magnetic resonance imaging: a potentially useful imaging biomarker for better survival in solitary large hepatocellular carcinoma. Liver Transpl. 2013;19:283–91. doi: 10.1002/lt.23597. [DOI] [PubMed] [Google Scholar]
- 11.Chu KK, Chan SC, Fan ST, et al. Radiological prognosticators of hepatocellular carcinoma treated by hepatectomy. Hepatobiliary Pancreat Dis Int. 2012;11:612–7. doi: 10.1016/s1499-3872(12)60232-x. [DOI] [PubMed] [Google Scholar]
- 12.Ishigami K, Yoshimitsu K, Nishihara Y, et al. Hepatocellular carcinoma with a pseudocapsule on gadolinium-enhanced MR images: correlation with histopathologic findings. Radiology. 2009;250:435–43. doi: 10.1148/radiol.2501071702. [DOI] [PubMed] [Google Scholar]
- 13.Roy C, Sr, El Ghali S, Buy X, et al. Significance of the pseudocapsule on MRI of renal neoplasms and its potential application for local staging: a retrospective study. AJR Am J Roentgenol. 2005;184:113–20. doi: 10.2214/ajr.184.1.01840113. [DOI] [PubMed] [Google Scholar]
- 14.Yamashita Y, Honda S, Nishiharu T, et al. Detection of pseudocapsule of renal cell carcinoma with MR imaging and CT. AJR Am J Roentgenol. 1996;166:1151–5. doi: 10.2214/ajr.166.5.8615260. [DOI] [PubMed] [Google Scholar]