Abstract
Given the rapid growth of the population of cancer survivors, increased attention has been paid to their health problems. Although gastric cancer is one of the most common cancers, empirical evidence of survivorship care is limited. The objectives of this study were to describe the health care status of gastric cancer survivors and to report the experience of using the shared-care model during a one-year experience at the cancer survivorship clinic in Seoul National University Hospital. This is a descriptive, single-center study of 250 long-term gastric cancer survivors who were referred to the survivorship clinic. The status of their health behaviors, comorbid conditions, secondary cancer screenings, and survivorship care status were investigated through questionnaires and examining the medical records. Among the survivors, 7.2% were current smokers, 8.8% were at-risk drinkers, and 32.4% were physically inactive. Among the patients who did not know their bone density status, the majority were in the osteopenic (37.1%) or osteoporotic range (24.1%). Screening among the eligible population within the recommended time intervals were 76.3% for colorectal cancer, but only 13.6% for lung cancer. All of the survivors were provided with counseling and medical management at the survivorship clinic, as appropriate. In conclusion, Long-term gastric cancer survivors have various unmet needs. Shared-care through survivorship clinics can be an effective solution for providing comprehensive care to cancer survivors.
Keywords: Cancer, Gastric Cancer, Cancer Survivorship Care, Shared-Care Model, Korea
Graphical Abstract
INTRODUCTION
With the improvements in cancer survival, the number of cancer survivors continues to rise worldwide. In Korea, currently the number is estimated to be 1.1 million, which means that one in 45 persons is living after cancer treatment (1).
Gastric cancer is the fifth most common type of cancer worldwide in 2012 (2). Although the incidence of gastric cancer is decreasing in developed countries, it is still the second most common cancer in Asian countries, including China, Japan, and Korea. While its survival rate generally is poor (2), in Korea, however, due to the existence of national screening programs and opportunistic screening for gastric cancer, the proportion of early gastric cancer has increased from 25% to 50% over the past two decades. As a result, 5-year survival rates of all gastric cancers now are as high as 70% (1). Accordingly, the number of gastric cancer survivors in Korea currently is approximately 200,000, which accounts for about one-sixth of all cancer survivors in Korea.
Given the rapid growth of cancer survivors, increasing attention has been paid to their health problems and the management of them. Cancer survivors are vulnerable to second primary cancers and late complications. Most of them are elderly, so many of them also suffer from comorbid chronic conditions. In addition, many of the survivors show health behaviors which indicate the need for professional education (3). However, a cancer diagnosis may shift the attention away from non-cancer problems, and cancer survivors often do not receive optimal treatment for comorbidities or preventive services.
So far, some models of comprehensive care for cancer survivors have been suggested. Among them is the shared-care model used by both oncologists and primary care physicians (PCPs), which has been highlighted the most (4). In this model, patients are back-transferred from the oncologist to the PCP after completion of cancer therapy. While oncologists do the oncology follow-up of the primary cancer, the PCP assumes responsibility for ensuring that the physical and emotional needs of the survivors are met (4). When the survivors reach long-term survival, the oncologists generally finish their follow-up, and the patients are followed up exclusively by their PCP. Shared-care has been found to enhance the quality of non-cancer care (5), and to have a positive influence on patients’ attitudes towards the healthcare system (6).
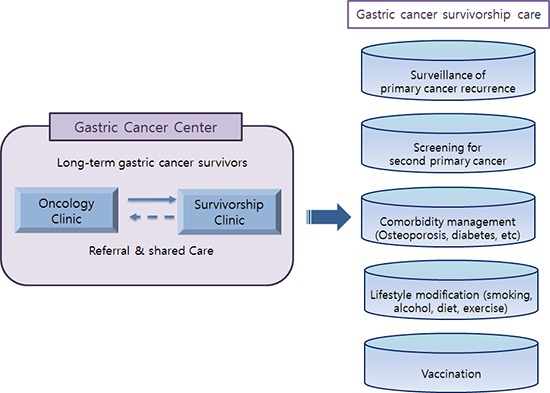
In Korea, an institution-based shared-care model, involving PCPs within a cancer center, was suggested as a potential solution, given the current oncology practices in Korea (7). It was considered the preferred model of care for the following reasons: easy information sharing and communication between physicians, and patients’ preferences for being cared for at the same institution where they undergo cancer treatment. Seoul National University Hospital is a national referral hospital, and the Gastric Cancer Center is renowned for its high quality cancer care. A cancer survivorship clinic run by the Family Medicine Department was established in 2011, and in October 2013, a dedicated survivorship clinic was established within the Gastric Cancer Center. The surgical oncologists of the center refer their patients to PCPs in the survivorship clinic, usually 3-5 years after surgery, for preparation for the completion of follow-up by the oncologists.
Until now, there are only few studies on health behavior and long-term problems of gastric cancer survivors and how these can be managed through shared care. The objectives of this study were to describe the health care status of long-term gastric cancer survivors, and to present what problems could be detected and solved at a survivorship clinic with shared-care, based on a one-year experience at a survivorship clinic in a tertiary cancer center.
MATERIALS AND METHODS
Study population and data collection procedure
The study participants were patients who had been transferred from the Gastric Cancer Center to the survivorship clinic from October 2013 to September 2014. During the period, 384 patients were referred to the clinic and 326 visited it. Among the patients who visited the clinic, we excluded those who had been referred less than 3 years since their surgery date and gastrointestinal stromal tumor patients. As a result, 250 patients were included in the analysis.
Measures
All of the patients who visited the clinic completed a questionnaire about their health behaviors. Current status of smoking and drinking alcohol was assessed, and in cases of former smokers and drinkers, they were asked whether they quit it before or after their cancer diagnosis. To assess physical activity, patients were asked whether they did regular exercise or not, and the frequency and duration of their exercise. Patients were asked whether they had been diagnosed with hypertension, diabetes mellitus, or hypercholesterolemia. Questions about the results of former bone density examinations were also asked to assess the patients’ perceptions of their bone density status. For secondary cancer screening, patients were asked whether and when they had their last screening for colorectal, breast, cervical, and lung cancer. In the case of vaccinations, patients were asked when they received the influenza and pneumococcal vaccinations. During the next part of the consultation, the doctors asked the patients questions about their medical history based on their responses to the questionnaire. All patients referred had blood tests for the routine follow-up in the oncology clinic. Some tests, such as the DEXA bone densitometry were ordered as appropriate after the consultation. The education and prescriptions provided during the consultation were reviewed in the patients’ medical records for this study.
Statistical analyses
Descriptive statistics, including, measures of central tendency (mean), measures of spread (standard deviation), and rates (percentages) were calculated. At-risk drinking was defined by the criteria of the National Institute on Alcohol Abuse and Alcoholism (8). Adequate physical activity was defined by guidelines for adult physical activity from the World Health Organization (WHO) (9). The criteria to judge the adequacy of second cancer screening and upper age limit were developed based on the Korean National Cancer Center screening guidelines. For cervical cancer screening, it was based on the Korean Society of Gynecologic Oncology recommendations, because of the lack of recommendations for upper age limit from Korean National Cancer Center guidelines. A yearly influenza vaccination and lifetime pneumococcal vaccination were considered appropriate. Fasting blood sugar (FBS) levels were categorized as normal, prediabetic, and diabetic, according to the American Diabetes Association criteria (10), but the recommendation for repeated testing to confirm a diagnosis of diabetes mellitus was not considered. A total cholesterol level ³ 240 mg/dL was considered to be hypercholesterolemia, based on the Adult Treatment Panel III guideline (11). Bone density was categorized as normal, osteopenia (-2.5 < T-score ≤ -1.0), and osteoporotic (T score ≤ 2.5) using the WHO criteria (12). STATA software (ver. 13.0; STATA Corp., Houston, TX, USA) was used for all statistical analyses.
Ethics statement
The study protocol was reviewed and approved by the institutional review board of Seoul National University Hospital (IRB No. 1503-056-655). Informed consent was waived by the board considering the retrospective study design and very low potential risk for the subjects.
RESULTS
Study population characteristics
The median age of the 250 patients included in the study sample was 62 (36-85) years old. A total of 103 patients were transferred to the center 3-5 years after surgery, and 147 (58.8%) patients were referred more than 5 years after surgery. Regarding the types of surgery, most of the patients had a distal gastrectomy and Billroth I reconstruction (n = 143, 57.2%) (Table 1).
Table 1. Characteristics of the long-term cancer survivors.
| Characteristics | Patients (n = 250) | |
|---|---|---|
| No. | % | |
| Age, median (SD), yr | 62.00 | (9.8) |
| Sex | ||
| Male | 150 | 60.0 |
| Female | 100 | 40.0 |
| Time between surgery and consultation | ||
| 3 yr < 5 yr | 103 | 41.2 |
| 5 yr < | 147 | 58.8 |
| Types of surgery | ||
| Distal gastrectomy | ||
| Billroth I | 143 | 57.2 |
| Billroth II | 25 | 10.0 |
| Roux en Y | 1 | 0.4 |
| Proximal gastrectomy | 11 | 4.4 |
| Pylorus-preserving gastrectomy | 28 | 11.2 |
| Total gastrectomy | 42 | 16.8 |
Health behavior
As for smoking, 42.4% of the patients were former smokers. Among the former smokers, 91.5% reported quitting after they received the cancer diagnosis (data not shown). Current smokers were 7.2% of the patients. All of them received consultation at the survivorship clinic, and 38.9% were prescribed smoking cessation medication (e.g., varenicline for most cases). Some of the other patients chose to obtain prescriptions from local clinics because of the distance from their residence to the cancer center.
As for drinking alcohol, 21.6% of the patients were former drinkers, and among them, 87.0% stated that they quit drinking after receiving the cancer diagnosis (data not shown in Tables). Current drinkers comprised 36.4% of the sample, and 8.8% of them were at-risk drinkers. All of the at-risk drinkers received consultation about their drinking problem, but only one person was prescribed a medication (naltrexone) for it.
Regarding physical activity, 32.4% of the patients were inactive, 21.6% reported that they engaged in regular exercise, but did so less than 150 minutes per week. Only 46.0% of the patients exercised more than 150 minutes per week, as recommended by WHO (Table 2).
Table 2. Health behaviors and prevalence of chronic metabolic diseases and management by the clinic.
| Behaviors or other diseases | No. (%) of patients | Management or newly diagnosis No. (%) |
Patients No. (%) |
|---|---|---|---|
| Health behaviors | (Management at survivorship clinic) | ||
| Smoking | |||
| Never | 126 (50.4) | ||
| Former | 106 (42.4) | ||
| Current | 18 (7.2) | Medication + Consultation | 7 (38.89) |
| Consultation only* | 11 (61.11) | ||
| Drinking | |||
| Never | 105 (42.0) | ||
| Former | 54 (21.6) | ||
| Current | 91 (36.4) | ||
| At - risk drinker | 22 (8.8) | Medication + Consultation | 1 (4.55) |
| Consultation only* | 21 (95.45) | ||
| Exercise | |||
| Inactive | 81 (32.4) | ||
| Active | |||
| < 150 min per wk | 54 (21.6) | ||
| > 150 min per wk | 115 (46.0) | ||
| Chronic metabolic diseases | (Newly diagnosis) | ||
| Hypertension | |||
| Have | 70 (28.0) | ||
| Do not have | 176 (70.4) | Blood pressure was not routinely checked | |
| Do not know | 4 (1.6) | ||
| Diabetes mellitus | |||
| Have | 29 (11.6) | ||
| Do not have | 217 (86.8) | FBS ≥ 126†,∥ | 11 (4.98)‡ |
| Do not know | 4 (1.6) | 100 ≤ FBS ≤ 125†,∥ | 79 (35.75)‡ |
| Hypercholesterolemia | |||
| Have | 41 (16.4) | ||
| Do not have | 205 (82.0) | Total Cholesterol ≥ 240∥ | 6 (2.87)§ |
| Do not know | 4 (1.6) |
*Consultation only includes treatment at a nearby hospital for logistic reasons (e.g., residence in distant areas); †FBS, fasting blood sugar; ‡Among the patients who did not know whether he/she had diabetes mellitus or the patients who stated that he/she did not have diabetes mellitus; §Among the patients who did not know whether he/she had hypercholesterolemia or the patients who stated that he/she did not have hypercholesterolemia; ∥The unit of fasting blood glucose and total cholesterol was mg/dL.
Comorbid cardiovascular conditions
Regarding comorbid cardiovascular conditions, 28.0%, 11.6%, and 16.4% of the patients reported that they had hypertension, diabetes mellitus, and hypercholesterolemia, respectively. Among the patients (88.4%) who did not know whether they had diabetes and those who stated they did not have the disease, 35.8% were suspected of having prediabetes (100 mg/dL ≤ FBS ≤ 125 mg/dL) and 4.98% had blood sugar within the diabetic range (FBS ≥ 126 mg/dL). Among the patients (83.6%) who said that they did not have, or did not know whether they had hypercholesterolemia, 2.9% of them were newly diagnosed with hypercholesterolemia (total cholesterol ≥ 240 mg/dL) at the survivorship clinic (Table 2).
Bone density
Among the 88.4% of the patients who reported they did not know their bone density status, over half were prescribed a DEXA bone densitometry; 37.1% were found to have osteopenia, and another 24.1% were found to have osteoporosis. Most of the patients with osteopenia were prescribed calcium plus a vitamin D supplement, and the patients with osteoporosis were prescribed bisphosphonate in most cases or raloxifen in some cases. Among the patients who perceived their recent bone density tests as normal, the results of 33.3% of them actually were in the osteopenic range (Table 3).
Table 3. Diagnosis of osteoporosis/osteopenia by the cancer survivorship clinic.
| Perceived state of bone density | Patients No. (%) | Examination not ordered or planned for future visit* No. |
Results of examination ordered by survivorship clinic No. (%) |
Proportion of newly diagnosed Osteoporosis/osteopenia No. (%) |
||
|---|---|---|---|---|---|---|
| Normal | Osteopenia | Osteoporosis | ||||
| Normal | 10 (4.0) | 4 | 4 (66.7) | 2 (33.3) | 0 (0.0) | 2 (33.3) |
| Osteopenia | 18 (7.2) | 10 | 0 (0.0) | 8 (100.0) | 0 (0.0) | 0 (0.0) |
| Osteoporosis | 1 (0.4) | 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Do not know | 221 (88.4) | 105 | 45 (38.8) | 43 (37.1) | 28 (24.1) | 71 (61.2) |
*DEXA bone densitometry was not ordered when patients reported a recent examination, had plans to be examined in other centers, or refused the examination. Some patients planned to be examined at the next visit, which was after the data collection period.
Second primary cancer screening
Among the eligible patients for screenings, 76.3% had a colonoscopy within the generally recommended interval (≤ 5 years). Among the eligible female patients, 71.6% and 34.2% had breast and cervical cancer screening within the recommended intervals, respectively. Of all of the patients, 23.6% were eligible for lung cancer screening, but only 13.6% of the eligible patients had appropriate screening (Table 4).
Table 4. Secondary cancer screening and vaccination status among the cancer survivors.
| Regimens | Eligible patients No. (%)** |
Done within recommended interval No. (%)†† |
Done beyond recommended interval No. (%)†† |
Never done/Do not know No. (%)†† |
Unmet needs No. (%)†† |
|---|---|---|---|---|---|
| Secondary cancer screening | |||||
| Colorectal cancer* | 249 (99.6) | 190 (76.3) | 21 (8.4) | 38 (15.3) | 59 (23.69) |
| Breast cancer† | 81 (32.4) | 58 (71.6) | 16 (19.8) | 7 (8.6) | 23 (28.39) |
| Cervical cancer‡ | 82 (32.8) | 28 (34.2) | 41 (50.0) | 13 (15.9) | 54 (65.85) |
| Lung cancer§ | 59 (23.6) | 8 (13.6) | 9 (15.3) | 42 (71.2) | 51 (86.44) |
| Vaccination | |||||
| Influenza∥ | 250 (100) | 149 (59.6) | NA | 101 (40.4) | 101 (40.40) |
| Pneumococcus¶ | 250 (100) | 99 (39.6) | NA | 151 (60.4) | 151 (60.40) |
*Colorectal cancer, ≤ 80 years old; colonoscopy ≤ 5 years was considered a screening within the recommended interval; †Breast cancer: females only, ≤ 69 years old; mammography or breast sonography ≤ 2 years was considered a screening within the recommended interval; ‡Cervical cancer, females only, ≤ 70 years old; Papanicolaou test ≤ 1 year was considered a screening within the recommended interval; §Lung cancer, more than 30 packs/year smoking history, currently smoke or have quit within the past 15 years, ≤ 74 years old; Low dose chest CT ≤ 1 year was considered a screening within the recommended interval; ∥Influenza vaccination within 1 year was considered a vaccination received within the recommended interval; ¶Pneumococcal vaccination during the patient’s lifetime was considered a vaccination received as recommended; **The proportions of eligible patients for each examination were calculated using the overall study population; ††Each proportion was calculated for each examination.
Vaccinations
Regarding vaccinations, 59.6% of the patients had an influenza vaccination within the previous one year, and 39.6% of the patients stated that they had received the pneumococcal vaccination (Table 4).
DISCUSSION
This is a descriptive, single-center study based on a one-year experience in a gastric cancer survivorship clinic. To our knowledge, this is the first study to investigate the health status and health behaviors of long-term gastric cancer survivors, their unmet needs, and the feasibility of clinical approaches to shared-care.
Although our study did not make a direct comparison, the results suggest that gastric cancer survivors generally engage in healthier behaviors than the general population. In the general population, the rates of current smokers are estimated to be 22.8% in Korean adults (13). However, only 7.2% of the patients in this study reported that they were current smokers. As for drinking alcohol, 11.9% of adults in the general population are considered at-risk drinkers, but in this study, only 8.8% were categorized as at-risk drinkers. This finding might be due to health behavior changes that occur after receiving a cancer diagnosis which has been reported in previous studies (14). Indeed, among the former smokers and drinkers in the study, most of them reported that they quit smoking and drinking after the cancer diagnosis. However, on the other hand, a significant minority of patients were engaged in smoking or at-risk drinking. Although some patients resisted the acceptance of treatment, a significant portion benefited from it, especially the treatment for smoking cessation.
The prevalence of hypertension, diabetes, and hypercholesterolemia seemed to be lower than that in the general population. For example, in case of diabetes, the prevalence in the general population was estimated to be 13.0% and 20.9% in adults in their 50s and 60s (15), but it was only 11.60% in this population, with mean age of 61.56. Hypercholesterolemia prevalence also was lower in this study than that of general population in same age group (16.4% vs. 53.2%-55.7%) (16). Gastrectomy often results in weight loss and improves metabolic profiles similar to bariatric surgery (17), and previous studies showed improvement in blood pressure, blood glucose, and cholesterol after the surgery (18). At the same time, some patients were newly diagnosed with diabetes (5.0%) or hypercholesterolemia (2.9%) at the survivorship clinic. As these conditions may require medical attention, including medication, the early identification of such conditions and timely treatment through shared-care might be beneficial for the patients.
Among the various health problems of cancer survivors, osteoporosis is especially prevalent in gastric cancer survivors. The exact pathogenesis is unknown, but it is thought that the poor absorption of vitamin D and calcium results in secondary hyperparathyroidism, which increases rates of bone loss. In addition, low vitamin D levels or hypocalcemia can lead to osteoporosis (19). Nevertheless, osteoporosis in gastric cancer patients often is overlooked. In this study, 88.4% did not know their bone density status. After a referral to the survivorship clinic, up to 61.2% of them were newly diagnosed with osteopenia or osteoporosis. While there are effective treatments for such conditions, including bisphosphonate and calcium and vitamin D supplements, it is not certain whether those drugs can be absorbed effectively by gastrectomy patients. Further investigations of the prevalence and pathophysiology of osteoporosis in gastric cancer survivors and optimal methods of treatment are warranted in the future.
Second primary cancer is one of the most important health issues for cancer survivors. Cancer survivors are at greater risk for developing new cancers than general population. Approximately 8% of cancer survivors are affected by a second primary cancer, and 10% of all new cancers are diagnosed in cancer survivors (20). The data are limited for gastric cancer survivors. However, data from Osaka cancer registry of Japan showed the increased standardized incidence ratio (SIR) of 1.28 (95% CI, 1.24-1.33) for all second cancers in gastric cancer survivors (21). For specific types of second primary cancers, increased risks were found for colorectal (SIR, 1.40), breast (SIR, 1.63), and lung cancer (SIR, 1.26). Therefore, adequate screening for second primary cancers should be an integral component of cancer survivorship care.
In our study, the colorectal cancer screening rate was quite high, with the rate of 76.3%. However, this is not a generalizable finding in Korea, and it is due to the fact that gastric cancer surgeons in our center frequently perform colonoscopy when they perform gastroscopy. On the other hand, other cancers, such as breast, cervical, and lung cancers, generally were not screened appropriately. Previous studies revealed that many cancer survivors had limited knowledge about a second primary cancer and were unable to distinguish ‘cancer screening’ from ‘routine surveillance tests’ after cancer treatment (22). Moreover, many the oncologists did not consider second primary cancer screening to be their responsibility and did not cover it in routine care (23). In addition, oncologists may feel burden to discuss second primary cancer screening with patients because of limited time and additional costs for the screening. Therefore, shared-care from a survivorship clinic could be helpful to address this important unmet need. Indeed, during the study period, two cases of lung cancer were detected through proactive screening for lung cancer at the resectable stage (data not shown).
Another important health issue for cancer survivors is vaccination. Cancer survivors are susceptible to severe complications from influenza (24). Also, the risk of invasive pneumococcal disease is higher in adults with cancer than in generally healthy adults. Thus, influenza and pneumococcal vaccinations are recommended for all cancer survivors, regardless of age (25). Yet, the level of vaccination utilization has not been ideal among survivors (24), as our study found that only 59.6% and 39.4% of the patients received influenza and pneumococcal vaccinations, respectively. As most survivors were receptive to the need for vaccination after their consultation, visits to a survivorship clinic could provide an opportunity to educate the survivors of their needs.
Current study provides some evidence of the feasibility and potential effectiveness of institution-based shared care model. Around 85% of gastric cancer survivors visited survivorship clinic after referral: recommendation by trusted surgeons, same day referral option might have improved the acceptance by the survivors. We also showed non-cancer problems of survivors which could be detected and managed in survivorship clinic, implying that our model could be effective in improving the comprehensiveness of care in this population. In regard of gastric cancer survivorship care, we could detect many of unknown osteopenia and osteoporosis and provided appropriate management for them. Also, second primary cancers – colorectal, breast and lung cancers which are known to be prevalent among gastric cancer survivors were actively screened after referral to survivorship clinic.
Among the patients who were referred to the survivorship clinic, a significant portion of them (15.1%) did not visit it (data not shown). The follow-up loss rates differed by the intervals between the date that the surgical oncologist made the referral and the date that the patient was scheduled to visit the survivorship clinic. When the surgical oncologists scheduled the patient’s visit to the survivorship clinic on the very same date that the patient was seen and referred, most of the patients visited the clinic (96.0%, data not shown). However, as the interval between the referral and the planned visit lengthened, the follow-up loss rate increased (e.g. 22.5% when scheduled to visit the clinic after 6 months, data not shown). The patients’ convenience might have affected those rates. To increase the visit rate to the survivorship clinic, a system enabling same-day referrals is recommended.
This study has some limitations. First, it was based on an experience at one center, so it cannot be representative of other centers with different circumstances. Second, information collected through self-report questionnaires might be subject to recall bias. Third, we could not compare the effect of shared-care model to care not using that model, because of lack of literature on gastric cancer survivors. Further studies on direct comparison with care not using the model would be helpful. Fourth, economic status and insurance coverage might affect preventive behaviors such as second primary cancer screening, however we could not include that information. Future study including such information would help analyze the impact of economic status. Finally, as a descriptive study, we focused on the short-term process of care, and could not determine whether such care led to clinically meaningful improvements in outcomes or to survivors’ satisfaction. Further investigations of the long-term outcomes of the shared-care model are warranted in the future.
In conclusion, we found that the long-term gastric cancer survivors had various unmet needs, such as overlooked osteoporosis and second primary cancer screening, and through the shared-care program in the dedicated survivorship clinic, we could detect such needs and adequately address them. As oncologists alone might not deal with various primary or preventive care needs effectively, shared-care provided by PCPs in a survivorship clinic might be a potential solution for comprehensive survivorship care. The development of a standard protocol for the referral, screening, and subsequent management of non-cancer conditions would be necessary, as well as an increased awareness of the need for cancer survivorship care and the purpose of shared-care.
Footnotes
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conception and design of study: Lee JE, Shin DW, Son KY, Kong SH, Lee HJ, Cho B, Yang HK. Acquisition and cleansing of data: Lee JE, Kim JS. Data review: Shin DW, Lee H, Suh YS. Statistical analysis: Lee JE, Kim JS, Lee H. Manuscript preparation: Lee JE, Shin DW, Yang HK. Manuscript approval: all authors.
References
- 1.Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH, Lee JS. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2011. Cancer Res Treat. 2014;46:109–123. doi: 10.4143/crt.2014.46.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Shin DW, Cho B, Kim SY, Jung JH, Park JH. Management of cancer survivors in clinical and public health perspectives: current status and future challenges in Korea. J Korean Med Sci. 2013;28:651–657. doi: 10.3346/jkms.2013.28.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oeffinger KC, McCabe MS. Models for delivering survivorship care. J Clin Oncol. 2006;24:5117–5124. doi: 10.1200/JCO.2006.07.0474. [DOI] [PubMed] [Google Scholar]
- 5.Earle CC, Burstein HJ, Winer EP, Weeks JC. Quality of non-breast cancer health maintenance among elderly breast cancer survivors. J Clin Oncol. 2003;21:1447–1451. doi: 10.1200/JCO.2003.03.060. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen JD, Palshof T, Mainz J, Jensen AB, Olesen F. Randomised controlled trial of a shared care programme for newly referred cancer patients: bridging the gap between general practice and hospital. Qual Saf Health Care. 2003;12:263–272. doi: 10.1136/qhc.12.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin DW, Park JH. Long-term survivorship clinics led by primary care physicians within the cancer center may be a good option for coordinated survivorship care. Cancer. 2014;120:3752–3753. doi: 10.1002/cncr.28939. [DOI] [PubMed] [Google Scholar]
- 8.National Institute on Alcohol Abuse and Alcoholism. The Physicians' Guide to Helping Patients with Alcohol Problems. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism; 1995. [Google Scholar]
- 9.World Health Organization (CH) Global Recommendations on Physical Activity for Health. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 10.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36:S67–74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 12.World Health Organization (CH) Guidelines for Preclinical Evaluation and Clinical Trials in Osteoporosis. Geneva: World Health Organization; 1998. [Google Scholar]
- 13.Ministry of Health and Welfare; Korea Centers for Disease Control and Prevention. Korea Health Statistics 2013: Korea National Health and Nutrition Examination Survey (KNHANES VI-1) Cheongju: Korea Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 14.Humpel N, Magee C, Jones SC. The impact of a cancer diagnosis on the health behaviors of cancer survivors and their family and friends. Support Care Cancer. 2007;15:621–630. doi: 10.1007/s00520-006-0207-6. [DOI] [PubMed] [Google Scholar]
- 15.Kim DJ. The epidemiology of diabetes in Korea. Diabetes Metab J. 2011;35:303–308. doi: 10.4093/dmj.2011.35.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee MH, Kim HC, Ahn SV, Hur NW, Choi DP, Park CG, Suh I. Prevalence of dyslipidemia among Korean adults: Korea National Health and Nutrition Survey 1998-2005. Diabetes Metab J. 2012;36:43–55. doi: 10.4093/dmj.2012.36.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee YH, Han SJ, Kim HC, Hyung WJ, Lim JS, Lee K, Lee HJ, Lee EY, Kang ES, Ahn CW, et al. Gastrectomy for early gastric cancer is associated with decreased cardiovascular mortality in association with postsurgical metabolic changes. Ann Surg Oncol. 2013;20:1250–1257. doi: 10.1245/s10434-012-2688-5. [DOI] [PubMed] [Google Scholar]
- 18.Yang J, Li C, Liu H, Gu H, Chen P, Liu B. Effects of subtotal gastrectomy and Roux-en-Y gastrojejunostomy on the clinical outcome of type 2 diabetes mellitus. J Surg Res. 2010;164:e67–71. doi: 10.1016/j.jss.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Lim JS, Lee JI. Prevalence, pathophysiology, screening and management of osteoporosis in gastric cancer patients. J Gastric Cancer. 2011;11:7–15. doi: 10.5230/jgc.2011.11.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mariotto AB, Rowland JH, Ries LA, Scoppa S, Feuer EJ. Multiple cancer prevalence: a growing challenge in long-term survivorship. Cancer Epidemiol Biomarkers Prev. 2007;16:566–571. doi: 10.1158/1055-9965.EPI-06-0782. [DOI] [PubMed] [Google Scholar]
- 21.Tabuchi T, Ito Y, Ioka A, Miyashiro I, Tsukuma H. Incidence of metachronous second primary cancers in Osaka, Japan: update of analyses using population-based cancer registry data. Cancer Sci. 2012;103:1111–1120. doi: 10.1111/j.1349-7006.2012.02254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin DW, Baik YJ, Kim YW, Oh JH, Chung KW, Kim SW, Lee WC, Yun YH, Cho J. Knowledge, attitudes, and practice on second primary cancer screening among cancer survivors: a qualitative study. Patient Educ Couns. 2011;85:74–78. doi: 10.1016/j.pec.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Shin DW, Kim Y, Baek YJ, Mo HN, Choi JY, Cho J. Oncologists experience with second primary cancer screening: current practices and barriers and potential solutions. Asian Pac J Cancer Prev. 2012;13:671–676. doi: 10.7314/apjcp.2012.13.2.671. [DOI] [PubMed] [Google Scholar]
- 24.Shih YC, Pan IW. Influenza vaccination among individuals with cancer and their family members. Am J Prev Med. 2010;38:61–69. doi: 10.1016/j.amepre.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 25.Yoo S. Recent update in adult immunization. Korean J Fam Med. 2010;31:345–354. [Google Scholar]


