Abstract
Mixed carcinoma shows a mixture of glandular and signet ring/poorly cohesive cellular histological components and the prognostic significance of each component is not fully understood. This study aimed to investigate the significance of the poorly cohesive cellular histological component as a risk factor for lymph node metastasis and to examine the diagnostic reliability of endoscopic biopsy. Clinicopathologic characteristics of 202 patients who underwent submucosal invasive gastric carcinoma resection with lymph node dissection in 2005–2012 were reviewed. Mixed carcinoma accounted for 27.2% (56/202) of cases. The overall prevalence of lymph node metastasis was 17.3% (35/202). Lymphatic invasion (P < 0.001), family history of carcinoma (P = 0.025), tumor size (P = 0.004), Lauren classification (P = 0.042), and presence of any poorly cohesive cellular histological component (P = 0.021) positively correlated with the lymph node metastasis rate on univariate analysis. Multivariate analyses revealed lymphatic invasion, family history of any carcinoma, and the presence of any poorly cohesive cellular histological component to be significant and independent factors related to lymph node metastasis. Review of preoperative biopsy slides showed that preoperative biopsy demonstrated a sensitivity of 63.6% and a specificity of 100% in detecting the presence of the poorly cohesive cellular histological component, compared with gastrectomy specimens. The presence of any poorly cohesive cellular histological component was an independent risk factor associated with lymph node metastasis in submucosal invasive gastric carcinoma. Endoscopic biopsy had limited value in predicting the presence and proportion of the poorly cohesive cellular histologic component due to the heterogeneity of mixed carcinoma.
Keywords: Early Gastric Cancer, Lymph Node Metastasis, Biopsy, Mixed Carcinoma, Gastrointestinal Endoscopy
Graphical Abstract
INTRODUCTION
Traditionally, surgical resection with lymph node (LN) dissection was considered the only curative therapeutic option for early gastric carcinoma (EGC). However, surgical resection is associated with its own mortality and morbidity (1). In addition, even after successful surgery, a patient’s quality of life could be significantly reduced due to surgical complications (2).
Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) were introduced for organ-sparing, minimally invasive endoscopic removal of benign and early malignant lesions in the gastrointestinal tract (3). These endoscopic resections have little effect on the quality of life. However, as these procedures do not include LN dissection, the indication for endoscopic resection is limited to EGC with an ignorable risk of LN metastasis. Predicting the possibility of LN metastasis is important to determine the appropriateness of endoscopic resection for EGC (4,5).
Initially, the indication for endoscopic resection was limited to small, intestinal type intramucosal lesions without ulceration (6,7). With the accumulation of statistical data and advances in the technique, numerous attempts have been made to extend the indications for endoscopic resection, and attention has been focused on the clinicopathologic risk factors of LN metastasis.
Mixed carcinoma is a newly defined histologic type of gastric carcinoma in the 2010 World Health Organization (WHO) classification. This type of carcinoma shows a mixture of morphologically discrete glandular and signet ring/poorly cohesive cellular (PCC) histological components (8). The prognostic relevance of LN metastasis and of the proportion of each component in mixed carcinoma has not been fully understood. In addition, previous reports related to the risk of LN metastasis in histologically heterogeneous gastric carcinoma have concentrated on mixed carcinoma, consisting of differentiated and undifferentiated components based on the Japanese classification.
The aim of this study was to investigate the significance of the proportion of the PCC histological component as a risk factor for LN metastasis in submucosal invasive gastric carcinoma and to investigate the diagnostic reliability of endoscopic biopsy.
MATERIALS AND METHODS
Patients
A total of 202 patients who had undergone gastrectomy with LN dissection for submucosal invasive EGC at Konkuk University Medical Center between 2005 and 2012 were studied. Clinicopathological parameters including age, sex, family history of cancer, past medical history, follow-up data, and gross tumor type were retrieved from hospital databases. The preoperative biopsy slides were available for only 165 patients. The remaining 37 patients were diagnosed and referred from another hospital without proper biopsy slides. All available slides from preoperative biopsy and resection were reviewed, and the tumors were classified according to the 2010 WHO classification (8). We counted and recorded the numbers of the preoperative biopsied pieces. If any PCC histological components were identified, the proportions of PCC histological components were also evaluated. Additionally, depth of invasion, presence of lymphovascular and perineural invasion, and LN metastasis were also re-evaluated in the resection specimens.
Depth of invasion was recorded in three different ways. First, we measured the maximum depth of tumor invasion from the muscularis mucosa. Second, we recorded it according to the Japanese Classification of Gastric Carcinoma (JCGC) (9). The JCGC subclassifies submucosal invasion as SM1 (tumor invasion is less than 500 μm from the muscularis mucosa) or SM2 (tumor invasion is 500 μm or deeper from the muscularis mucosa). Third, we classified it into three groups (sm1, sm2, and sm3) based on the maximum depth of tumor invasion into the upper third (sm1), middle third (sm2), and lower third (sm3) of the submucosa when possible.
Statistical analysis
Continuous variables were tested for normality of distribution using the Kolmogorov-Smirnov test. Continuous variables showing normal distribution are described as the mean and standard deviation (SD), and were compared using the unpaired t-test. Continuous variables that did not show normal distribution are described as the median and interquartile range (IQR), and were compared using the Mann-Whitney U test. Categorical variables are described as the n (%) and were compared using the chi-square test or Fisher’s exact test. Multivariate analysis was performed using multiple logistic regression testing. Analysis of receiver operating characteristic (ROC) curves, logistic regression test analysis, intraclass correlation, and the Bland-Altman plot were applied to evaluate diagnostic accuracy. A P value of < 0.05 (two-tailed) was considered to indicate statistical significance. All statistical analyses were conducted using SPSS v. 19.0 (SPSS Inc., Chicago, IL, USA) and dBSTAT v. 5 (dBSTAT Co., Chuncheon, Korea).
Ethics statement
This study was approved by the institutional review board of Konkuk University Medical Center (KUH1210041). Informed consents were waived by the board.
RESULTS
Clinicopathologic features in this study
The mean age of patients was 61.4 years (range: 30–85). One hundred thirty-one (64.9%) patients were male, and 71 (35.1%) were female. The median tumor size was 3.0 (2.08–4.5) cm and the incidence of LN metastasis was 17.3% (35/202). The histologic types of submucosal invasive carcinomas according to the 2010 WHO classification were tubular adenocarcinomas in 119 cases (58.9%), mixed carcinomas in 56 cases (27.7%), poorly cohesive carcinomas in 18 cases (8.9%), medullary carcinomas in 7 cases (3.5%), and mucinous carcinomas in 2 cases (1.0%). The proportion of the PCC histological component in mixed carcinomas ranged from 2% to 95%. A family history of cancer was identified in 20.4% (40/202) of the patients: 24 gastric carcinomas, 2 urothelial carcinomas of the bladder, 2 non-small cell carcinomas of the lung, 2 hepatocellular carcinomas, 1 cholangiocarcinoma, 1 thyroid papillary carcinoma, 2 colorectal adenocarcinomas, 1 nasopharyngeal carcinoma, 1 laryngeal carcinoma, 3 pancreatic carcinomas, 3 ductal carcinomas of the breast, and 1 non-Hodgkin lymphoma. Eight patients had more than one cancer patient in their family.
The risk factors for LN metastasis
The relationship between each clinicopathologic parameter and the risk of LN metastasis is summarized in Table 1.
Table 1. Relationship between clinicopathologic parameters and lymph node metastasis in 202 submucosal invasive gastric carcinomas: results of univariate analysis.
| Parameters | Total | Status of LN metastasis | P value | ||
|---|---|---|---|---|---|
| Negative | Positive | ||||
| Total | 202 | 167 | 35 | ||
| Age | mean ± SD | 61.4 ± 11.6 | 61.7 ± 11.4 | 59.7 ± 13.0 | 0.352 |
| range | 30-85 | 30-85 | 32-77 | - | |
| Sex | M | 131 (64.9%) | 111 (66.5%) | 20 (57.1%) | - |
| F | 71 (35.1%) | 56 (33.5%) | 15 (42.9%) | 0.293 | |
| Family history of cancer | no | 156 (79.6%) | 133 (82.6%) | 23 (65.7%) | - |
| yes | 40 (20.4%) | 28 (17.4%) | 12 (34.3%) | 0.025 | |
| gastric cancer | 24 (12.2%) | 17 (10.6%) | 7 (20.0%) | 0.152 | |
| N/A | 6 | 6 | 0 | - | |
| Tumor size | median (IQR) | 3.0 (2.08-4.5) | 3.0 (2.0-4.0) | 3.6 (2.8-6.0) | 0.004 |
| > 3 cm | 98/202 (48.51%) | 74/167 (44.31%) | 24/35 (68.57%) | 0.015 | |
| 2010 WHO Classification | tubular (all) | 119 (58.9%) | 104 (62.3%) | 15 (42.9%) | - |
| tubular (p/d) | 16 (7.9%) | 14 (8.4%) | 2 (5.7%) | - | |
| mixed | 56 (27.7%) | 41 (24.6%) | 15 (42.9%) | - | |
| pcc | 18 (8.9%) | 15 (9.0%) | 3 (8.6%) | - | |
| mucinous | 2 (1.0%) | 1 (0.6%) | 1 (2.9%) | - | |
| medullary | 7 (3.5%) | 6 (3.6%) | 1 (2.9%) | 0.142 | |
| Presence of PCC component | 75 (37.1%) | 56 (33.5%) | 19 (54.3%) | 0.021 | |
| Lauren classification | intestinal | 116 (60.9%) | 108 (64.7%) | 15 (42.9%) | - |
| diffuse | 62 (30.7%) | 48 (28.7%) | 14 (40.0%) | - | |
| mixed | 17 (8.4%) | 11 (6.6%) | 6 (17.1%) | 0.026 | |
| Depth of invasion | median (IQR) | 1,298 (656-2,220) | 1,200 (555-2,035) | 1,850 (1,050-2,500) | 0.059 |
| > 500 µm | 160 (79.2%) | 132 (79.0%) | 28 (80.0%) | 0.899 | |
| sm1 | 57 (29.7%) | 48 (30.2%) | 9 (27.3%) | - | |
| sm2 | 54 (28.1%) | 50 (31.4%) | 4 (12.1%) | - | |
| sm3 | 81 (42.2%) | 61 (38.4%) | 20 (60.6%) | - | |
| NA | 10 | 8 | 2 | 0.074 | |
| Lymphatic invasion | present | 61 (30.2%) | 33 (19.8%) | 28 (80.0%) | < 0.001 |
LN, lymph node; SD, standard deviation; N/A, not available; IQR, interquartile range; p/d, poorly differentiated; pcc, poorly cohesive carcinoma; PCC component, signet ring/poorly cohesive cellular histological component.
Univariate analysis revealed that the presence of LN metastasis had a statistically significant correlation with tumor size (P = 0.004), family history of cancer (P = 0.025), Lauren classification (P = 0.026), lymphatic invasion (P < 0.001), and presence of any PCC histological components (P = 0.021). Age (P = 0.352), sex (P = 0.293), venous invasion (P = 0.873), and perineural invasion (P = 0.464) did not show statistical significance. The depth of invasion was deeper in the group with LN metastasis, but the difference was not sufficient to show statistical significance (P = 0.059). The rate of LN metastasis was higher in the mixed carcinoma group (26.8%) than in the pure poorly cohesive carcinoma group (16. 7%) or the poorly differentiated tubular carcinoma group (12.5%), but the differences were insufficient to show statistical significance (P = 0.533 and P = 0.327). Because the presence of any PCC histologic component showed statistical significance, we tried to find the optimal cutoff point of the percentage of the PCC histologic components using ROC curve analysis. However, we failed to find a statistically significant and clinically important cutoff point.
Multivariate analysis revealed that family history of cancer (P = 0.034), lymphatic invasion (P < 0.001), and presence of any PCC histological components (P = 0.015) are significant and independent factors associated with the presence of LN metastasis (Table 2). Tumor size showed no statistical significance. Lauren classification was excluded from multivariate analysis due to a strong correlation (P < 0.001) with the presence of any PCC histological components.
Table 2. Multivariate analysis of independent clinicopathologic factors associated with lymph node metastasis and their significance.
| Variables | Odds ratio | 95% CI | P value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Lymphatic invasion | 32.257 | 10.181 | 102.201 | < 0.001 |
| Presence of PCC component | 3.615 | 1.290 | 10.128 | 0.015 |
| Family history of cancer | 2.866 | 1.080 | 7.604 | 0.034 |
CI, confidence interval; PCC component, signet ring/poorly cohesive cellular histological component.
The diagnostic value of endoscopic biopsy
Preoperative biopsy specimens were available in 81.7% (165/202) of the patients. In detecting the PCC component, preoperative biopsy showed a sensitivity of 63.6%, a specificity of 100%, a false positive rate of 0%, and a false negative rate of 36.3%. The median of the number of biopsied pieces was 4 (IQR: 3–5, range: 1–10). The number of biopsied pieces showed no statistically significant correlation with the detection of PCC components (P = 0.573).
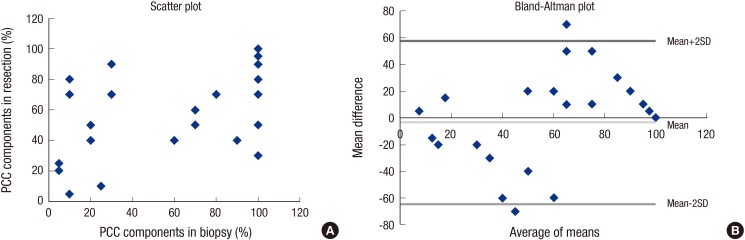
In predicting the exact percentage of the PPC components, preoperative biopsy showed an intraclass correlation coefficient of 0.586, which indicates a poor correlation. The relationship between the percentages in the biopsy and resection specimens is demonstrated in a scatter plot and a Bland-Altman plot (Fig. 1).
Fig. 1.
Relationship between the proportions of the poorly cohesive cellular histological component in biopsy and resection specimens.
DISCUSSION
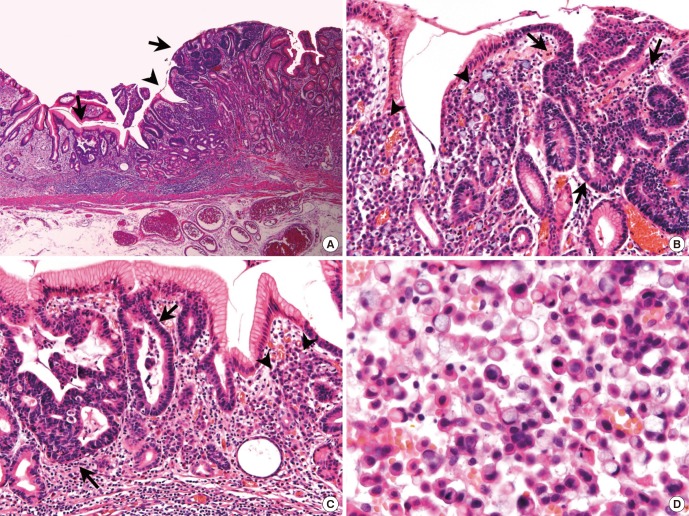
Mixed carcinoma is a newly described histologic type of gastric carcinoma in the 2010 WHO classification (8) (Fig. 2). In the 2000 WHO classification, the histologic type of gastric carcinoma is determined based on the predominant histologic pattern (10). In the 2010 WHO classification, carcinomas showing a mixture of morphologically discrete glandular and signet ring/PCC histological components are defined as mixed carcinoma (8). In this study, mixed carcinoma accounted for 27.2% (56/202) of all patients. This result suggests that the mixed carcinoma is not a rare type of gastric carcinoma, but a relatively common and clinically important type of gastric carcinoma.
Fig. 2.
A representative case of mixed carcinoma. (A-C) Between gland forming moderately differentiated tubular adenocarcinoma components (arrows), signet ring/poorly cohesive cellular histological components are present in lamina propria (arrow heads). (D) The signet ring/poorly cohesive cellular histological components show characteristic intracytoplasmic mucin vacuole, which pushes the nucleus to the cell periphery. Magnification: (A) × 40; (B-C) × 200; (D) × 600.
In the present study, we found that tumor size, Lauren classification, and lymphatic invasion have a relationship with LN metastasis. These results are in close agreement with those of numerous authors (11,12,13,14). On the other hand, the depth of invasion has been reported as an independent risk factor for LN metastasis by many previous studies, in contrast to the present study, which failed to find a correlation. In a review of the previous studies, we found that most studies were based on the JCGC. The JCGC divides tumors according to the depth of submucosal invasion into two groups: the SM1 group includes tumors which invade less than 500 μm from the muscularis mucosa and the SM2 group includes tumors which invade 500 μm or deeper from the muscularis mucosa (9). According to the JCGC, 79.2% (160/202) of patients were classified as the SM2 group in the present study. Perhaps the proportion in the SM2 group was too high to show a statistical difference.
Interestingly, family history of cancer was found to be a significant and independent risk factor associated with LN metastasis. This is the first study that statistically evaluated the relationship between family history of cancer and the risk of LN metastasis in gastric carcinoma, but similar findings have been reported in gastrointestinal and other carcinoma entities. Kupelian et al. (15) reported that prostate cancer patients with a family history of prostate carcinoma show worse clinical outcomes. Hata et al. (16) reported that a family history of gastric cancer is a risk factor for colorectal neoplasia. Minami et al. (17) reported that a family history of gastric cancer increases mortality in gastric cancer patients under 60 years old. Finally, Wong et al. (18) reported that esophageal cancer patients with a family history of esophageal cancer show a higher tendency toward LN metastasis. Yu et al. (19) reported that family history of cancer was not statistically correlated with sex, age, or the histologic type of gastric carcinoma. However, they did not evaluate the relationship between family history of cancer and the risk of LN metastasis. Considering that LN metastasis is one of the most important prognostic factors, it would be not surprising if there is an association between family history of cancer and LN metastasis. Maybe there are still unknown genetic factors that can help the lymphatic invasion but this question was out of our reach in this study.
The presence of any PCC histological component appeared as an independent risk factor associated with LN metastasis in submucosal invasive gastric carcinoma. Furthermore, the proportion of the PCC histological component was not in correlation with the risk of LN metastasis. To our knowledge, there were no previous reports on this subject based on the histologic type of the 2010 WHO classification. There are several similar studies, but they are based on the histologic type of the JCGC reported similar results. The JCGC defined mixed carcinoma as a mixture of differentiated (well or moderately differentiated tubular adenocarcinoma and papillary adenocarcinoma) and undifferentiated (poorly differentiated adenocarcinoma, signet-ring cell carcinoma, and mucinous adenocarcinoma) histological components (9). Accordingly, the mixed carcinoma of the JCGC classification is different from the mixed carcinoma of the 2010 WHO classification, and only overlaps partly.
Many previous studies based on the JCGC reported that the presence of an undifferentiated histological component is a risk factor for LN metastasis (20,21,22,23). However, the importance of the proportion of the undifferentiated histologic component is still debated. One previous report suggested that there is no significant difference in LN metastasis between differentiated-predominant mixed carcinoma and undifferentiated-predominant mixed carcinoma (21). But another report suggested that undifferentiated-predominant mixed carcinoma has the highest risk of LN metastasis (22). In addition, some previous reports suggest that mixed carcinoma has an even higher risk of LN metastasis than purely undifferentiated carcinoma (22,24). In accordance with previous studies, the rate of LN metastasis of the mixed carcinoma was higher than that of the purely poorly cohesive carcinoma or the poorly differentiated tubular carcinoma in our study. However, the difference was insufficient to show statistical significance. Further study would be needed to address this question.
Maybe it is evident that the presence of an undifferentiated histological component is a risk factor for LN metastasis in JCGC. But in practice, diagnoses are made solely based on WHO classification in many countries including Korea. The question is how we can apply the result of previous studies to WHO classification. In WHO classification, the diagnosis is made based on the predominant histologic pattern of the tumor. If there is minor portion of poorly differentiated tubular adenocarcinoma in moderately differentiated tubular adenocarcinoma, we may be able to make the diagnosis as ‘tubular adenocarcinoma, moderately to poorly differentiated’. But if there is minor portion of signet-ring/PCC histological components in tubular adenocarcinoma, we only are able to make the diagnosis as ‘mixed carcinoma’. Therefore, we have to find out the significance of the PCC histologic components and the meaning of mixed carcinoma in predicting the risk factors and deciding the therapeutic options. We think our study have solved this problem.
Preoperative endoscopic forceps biopsy showed a sensitivity of only 63.6% in detecting the presence of the PCC histologic component, and the number of the biopsied pieces showed no significant correlation. Moreover, it showed a poor correlation in predicting the proportion of PCC histologic components.
It is not uncommon to find a heterogeneous histologic component in the resection specimen, which was not identified in the forceps biopsy specimen (25). Several previous studies have reported the incidence of histologic discrepancies between forceps biopsied specimens and resected specimens as 2.3%–11.9% (26). Tumor location at the mid-stomach and easy friability were reported as risk factors for histologic discrepancy (27).
There are several explanations for our findings. First, mixed carcinomas tend to be glandular in the intramucosal component and to grow diffusely in the deeper portion (28). Second, the distribution of the PCC histological components is heterogeneous and the presence of the PCC histological component in the biopsy specimen largely depends on the biopsy sites. However, in this study, the number of biopsied pieces showed no significant correlation. We supposed that maybe the problem is not the number of biopsied pieces, but the number of biopsied sites. Further prospective study would be needed to address this question. Third, a part of mixed carcinoma is derived from intestinal type carcinoma in the course of the tumorigenesis (29).
As we mentioned above, there are no previous studies on mixed carcinoma based on the 2010 WHO classification. It would be possible to speculate, however, from the studies based on the Japanese classification. Several previous studies have suggested that histologically heterogeneous carcinoma shows more aggressive features, including larger tumor size, deeper invasion, ulcer formation, lymphatic invasion, and LN metastasis (21,23,25,30). There was also a suggestion that complete and curative resection rates are significantly lower in this type of carcinoma, mainly due to positive resection margins (20). The long-term outcome of the histologically heterogeneous carcinoma remains controversial. Shimizu et al. reported that this type of carcinoma shows a poorer 5-year survival rate after curative gastrectomy and LN dissection (21). In contrast, another report suggested that there are no differences in the survival rate between purely differentiated type carcinoma and histologically heterogeneous carcinoma after curative resection (24,25).
In conclusion, we suggest that a family history of cancer and the presence of any PCC histological components are significant and independent risk factors for LN metastasis. We also suggest that endoscopic biopsy has limited value in predicting the presence and proportion of the PCC histological component. This result reminds us that endoscopic resection is not only a therapeutic option, but also a diagnostic option. We should consider the possibility of additional resection before endoscopic resection.
Footnotes
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Study design: Han HS. Data acquisition, analysis and interpretation: Park HK, Lee KY, Yoo MW. Drafting of the manuscript: Park HK, Han HS. Review of manuscript for important intellectual content: Lee KY, Yoo MW, Hwang TS, Han HS. Approval of the final manuscript and submission: all authors.
References
- 1.Yi HW, Kim SM, Kim SH, Shim JH, Choi MG, Lee JH, Noh JH, Sohn TS, Bae JM, Kim S. Complications leading reoperation after gastrectomy in patients with gastric cancer: frequency, type, and potential causes. J Gastric Cancer. 2013;13:242–246. doi: 10.5230/jgc.2013.13.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeon YW, Han SI, Jeon CE, Kim JJ, Park SM. Quality of life in patients with stomach cancer after operation. J Korean Gastric Cancer Assoc. 2004;4:27–31. [Google Scholar]
- 3.ASGE Technology Committee Endoscopic mucosal resection and endoscopic submucosal dissection. Gastrointest Endosc. 2008;68:11–18. doi: 10.1016/j.gie.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 4.Gotoda T. Endoscopic resection of early gastric cancer: the Japanese perspective. Curr Opin Gastroenterol. 2006;22:561–569. doi: 10.1097/01.mog.0000239873.06243.00. [DOI] [PubMed] [Google Scholar]
- 5.Lee JH, Kim JG, Jung HK, Kim JH, Jeong WK, Jeon TJ, Kim JM, Kim YI, Ryu KW, Kong SH, et al. Clinical practice guidelines for gastric cancer in Korea: an evidence-based approach. J Gastric Cancer. 2014;14:87–104. doi: 10.5230/jgc.2014.14.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiki Y, Shimao H, Mieno H, Sakakibara Y, Kobayashi N, Saigenji K. Modified treatment of early gastric cancer: evaluation of endoscopic treatment of early gastric cancers with respect to treatment indication groups. World J Surg. 1995;19:517–522. doi: 10.1007/BF00294712. [DOI] [PubMed] [Google Scholar]
- 7.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 8.Lauwers GY, Carneiro F, Graham DY, Curado MP, Franceschi S, Montgomery E. Tumours of the stomach. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon: IARC Press; 2010. pp. 48–58. [Google Scholar]
- 9.Japanese Gastric Cancer Association Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 10.Fenoglio-Preiser C, Carneiro F, Correa P, Guilford P, Lambert R, Megraud F, Munoz N, Powell SM, Rugge M, Sasako M, et al. Tumours of the stomach. In: Hamilton SR, Aaltonen LA, editors. Pathology and Genetics of Tumours of the Digestive System: WHO Classification of Tumours. 3rd ed. Lyon: IARC Press; 2000. pp. 37–68. [Google Scholar]
- 11.Gotoda T, Sasako M, Ono H, Katai H, Sano T, Shimoda T. Evaluation of the necessity for gastrectomy with lymph node dissection for patients with submucosal invasive gastric cancer. Br J Surg. 2001;88:444–449. doi: 10.1046/j.1365-2168.2001.01725.x. [DOI] [PubMed] [Google Scholar]
- 12.Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, Fujisaki J, Sano T, Yamaguchi T. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009;12:148–152. doi: 10.1007/s10120-009-0515-x. [DOI] [PubMed] [Google Scholar]
- 13.Kunisaki C, Takahashi M, Nagahori Y, Fukushima T, Makino H, Takagawa R, Kosaka T, Ono HA, Akiyama H, Moriwaki Y, et al. Risk factors for lymph node metastasis in histologically poorly differentiated type early gastric cancer. Endoscopy. 2009;41:498–503. doi: 10.1055/s-0029-1214758. [DOI] [PubMed] [Google Scholar]
- 14.Kurihara N, Kubota T, Otani Y, Ohgami M, Kumai K, Sugiura H, Kitajima M. Lymph node metastasis of early gastric cancer with submucosal invasion. Br J Surg. 1998;85:835–839. doi: 10.1046/j.1365-2168.1998.00743.x. [DOI] [PubMed] [Google Scholar]
- 15.Kupelian PA, Kupelian VA, Witte JS, Macklis R, Klein EA. Family history of prostate cancer in patients with localized prostate cancer: an independent predictor of treatment outcome. J Clin Oncol. 1997;15:1478–1480. doi: 10.1200/JCO.1997.15.4.1478. [DOI] [PubMed] [Google Scholar]
- 16.Hata K, Shinozaki M, Toyoshima O, Toyoshima A, Matsumoto S, Saisho T, Tsurita G. Impact of family history of gastric cancer on colorectal neoplasias in young Japanese. Colorectal Dis. 2013;15:42–46. doi: 10.1111/j.1463-1318.2012.03108.x. [DOI] [PubMed] [Google Scholar]
- 17.Minami Y, Kawai M, Fujiya T, Suzuki M, Noguchi T, Yamanami H, Kakugawa Y, Nishino Y. Family history, body mass index and survival in Japanese patients with stomach cancer: a prospective study. Int J Cancer. 2015;136:411–424. doi: 10.1002/ijc.29001. [DOI] [PubMed] [Google Scholar]
- 18.Wong VC, Ko JM, Qi RZ, Li PJ, Wang LD, Li JL, Chan YP, Chan KW, Stanbridge EJ, Lung ML. Abrogated expression of DEC1 during oesophageal squamous cell carcinoma progression is age- and family history-related and significantly associated with lymph node metastasis. Br J Cancer. 2011;104:841–849. doi: 10.1038/bjc.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu J, Fu B, Zhao Q. Family history of malignant neoplasm and its relation with clinicopathologic features of gastric cancer patients. World J Surg Oncol. 2013;11:201. doi: 10.1186/1477-7819-11-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shim CN, Chung H, Park JC, Lee H, Shin SK, Lee SK, Lee YC. Early gastric cancer with mixed histology predominantly of differentiated type is a distinct subtype with different therapeutic outcomes of endoscopic resection. Surg Endosc. 2015;29:1787–1794. doi: 10.1007/s00464-014-3861-7. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu H, Ichikawa D, Komatsu S, Okamoto K, Shiozaki A, Fujiwara H, Murayama Y, Kuriu Y, Ikoma H, Nakanishi M, et al. The decision criterion of histological mixed type in “T1/T2” gastric carcinoma--comparison between TNM classification and Japanese Classification of Gastric Cancer. J Surg Oncol. 2012;105:800–804. doi: 10.1002/jso.23010. [DOI] [PubMed] [Google Scholar]
- 22.Hanaoka N, Tanabe S, Mikami T, Okayasu I, Saigenji K. Mixed-histologic-type submucosal invasive gastric cancer as a risk factor for lymph node metastasis: feasibility of endoscopic submucosal dissection. Endoscopy. 2009;41:427–432. doi: 10.1055/s-0029-1214495. [DOI] [PubMed] [Google Scholar]
- 23.Lee JH, Choi IJ, Han HS, Kim YW, Ryu KW, Yoon HM, Eom BW, Kim CG, Lee JY, Cho SJ, et al. Risk of lymph node metastasis in differentiated type mucosal early gastric cancer mixed with minor undifferentiated type histology. Ann Surg Oncol. 2015;22:1813–1819. doi: 10.1245/s10434-014-4167-7. [DOI] [PubMed] [Google Scholar]
- 24.Kozuki T, Yao T, Nakamura S, Matsumoto T, Tsuneyoshi M. Differences in p53 and cadherin-catenin complex expression between histological subtypes in diffusely infiltrating gastric carcinoma. Histopathology. 2002;41:56–64. doi: 10.1046/j.1365-2559.2002.01407.x. [DOI] [PubMed] [Google Scholar]
- 25.Min BH, Kim KM, Park CK, Lee JH, Rhee PL, Rhee JC, Kim JJ. Outcomes of endoscopic submucosal dissection for differentiated-type early gastric cancer with histological heterogeneity. Gastric Cancer. 2015;18:618–626. doi: 10.1007/s10120-014-0378-7. [DOI] [PubMed] [Google Scholar]
- 26.Joo M, Kim KM. Histologic discrepancy between gastric biopsy and resection specimen in the era of endoscopic treatment for early gastric cancer. Korean J Gastroenterol. 2014;64:256–259. doi: 10.4166/kjg.2014.64.5.256. [DOI] [PubMed] [Google Scholar]
- 27.Shim CN, Kim H, Kim DW, Chung HS, Park JC, Lee H, Shin SK, Lee SK, Lee YC. Clinicopathologic factors and outcomes of histologic discrepancy between differentiated and undifferentiated types after endoscopic resection of early gastric cancer. Surg Endosc. 2014;28:2097–2105. doi: 10.1007/s00464-014-3441-x. [DOI] [PubMed] [Google Scholar]
- 28.Carneiro F, Machado JC, Nabais S, Santos CM, Sobrinho Simões M. Mixed carcinoma of the stomach: a clinicopathological entity. Histopathology. 2003;43:94–95. doi: 10.1046/j.1365-2559.2003.01617.x. [DOI] [PubMed] [Google Scholar]
- 29.Honda T, Tamura G, Endoh Y, Nishizuka S, Kawata S, Motoyama T. Expression of tumor suppressor and tumor-related proteins in differentiated carcinoma, undifferentiated carcinoma with tubular component and pure undifferentiated carcinoma of the stomach. Jpn J Clin Oncol. 2005;35:580–586. doi: 10.1093/jjco/hyi166. [DOI] [PubMed] [Google Scholar]
- 30.Zheng HC, Li XH, Hara T, Masuda S, Yang XH, Guan YF, Takano Y. Mixed-type gastric carcinomas exhibit more aggressive features and indicate the histogenesis of carcinomas. Virchows Arch. 2008;452:525–534. doi: 10.1007/s00428-007-0572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]