Abstract
Store-operated calcium (Ca2+) entry (SOCE) is the principal Ca2+ entry route in non-excitable cells, including cancer cells. We previously demonstrated that Orai1 and STIM1, the molecular components of SOCE, are involved in tumorigenesis of clear cell renal cell carcinoma (CCRCC). However, a clinical relevance of Orai1 and STIM1 expression in CCRCC has been ill-defined. Here, we investigated the expression of Orai1 and STIM1 in CCRCC, and compared their expression with clinico-pathological parameters of CCRCC and the patients’ outcome. Immunohistochemical staining for Orai1 and STIM1 was performed on 126 formalin fixed paraffin embedded tissue of CCRCC and western blot analysis for Orai1 was performed on the available fresh tissue. The results were compared with generally well-established clinicopathologic prognostic factors in CCRCC and patient survival. Membrane protein Orai1 is expressed in the nuclei in CCRCC, whereas STIM1 shows the cytosolic expression pattern in immunohistochemical staining. Orai1 expression level is inversely correlated with CCRCC tumor grade, whereas STIM1 expression level is not associated with tumor grade. The higher Orai1 expression is significantly associated with lower Fuhrman nuclear grade, pathologic T stage, and TNM stage and with favorable prognosis. The expression level of STIM1 is not correlated with CCRCC grade and clinical outcomes. Orai1 expression in CCRCC is associated with tumor progression and with favorable prognostic factors. These results suggest that Orai1 is an attractive prognostic marker and therapeutic target for CCRCC.
Keywords: Orai1; STIM1; Carcinoma, Renal Cell; Kidney; Carcinogenesis; Prognosis
Graphical Abstract

INTRODUCTION
Renal cell carcinoma (RCC) is most common kidney tumor in adults, which is originated in the lining epithelium of proximal convoluted tubules (1). The incidence of RCC is steadily increasing; and kidney cancer statistics shows about 25,000 patients are living with the cancer in Korea (2). Clear cell RCC (CCRCC) is the most common subtype (87.7%) of RCC (3). Initial treatment is mostly either partial or complete nephrectomy that remains the mainstay of curative treatment. It is relatively resistant to radiation therapy or chemotherapy, although some cases respond to targeted therapies which have shown improvement in the overall survival of advanced RCC patients, reaching over 2 years (4). Recently, improved understanding of the molecular pathogenesis of RCC has led to the development of specific targeted therapies for treating the RCC.
Intracellular Ca2+ homeostasis has been known to be essential for cellular processes involving exocytosis, enzyme activation, gene transcription, cell growth, cell proliferation, and apoptosis (5). Intracellular Ca2+ is increased two ways: release from intracellular stores, the endoplasmic reticulum (ER) or Ca2+ influx from extracellular (6). The most common mechanism of intracellular Ca2+ mobilization results from the activation of phospholipase C (PLC)-linked cell surface receptors, which produce intracellular second messengers, inositol 1,4,5-trisphosphate (IP3) leading depletion of ER Ca2+ (7,8,9). As a result, ER Ca2+ depletion evokes Ca2+ influx via plasma membrane, which is known as capacitive or store operated Ca2+ entry (SOCE) (6,10). The molecular components of SOCE are the stromal interaction molecules (STIM1 and 2), an ER Ca2+ sensor, and the Orais (Orai1-3), a pore forming subunits. The best characterized SOCE current is Ca2+-release-activated Ca2+ (CRAC) current (11). ER Ca2+ depletion initiates STIM1 oligomerizaton and translocation to plasma membrane junction leading binding to Orai1, thereby triggering sustained Ca2+ influx (12,13).
The Orai1/STIM1 signaling is predominant Ca2+ influx route in non-excitable cells including tumors that has been implicated in cancer hallmarks such as migration and cell cycle progression in human cancers including breast, prostate, cervix and glioblastoma (14,15,16,17,18). We previously reported that expression of Orai1 was much higher in CCRCC tumor tissues compared to that of adjacent non-tumor renal parenchymal tissue and Orai1/STIM1-mediated SOCE aggravated migration and proliferation of CCRCC cells suggesting that Orai1/STIM1 is crucial for tumorigenesis and progression in CCRCC (19). However, whether expression level of SOCE components is associated with CCRCC progression and with clinical relevance is unclear. Therefore, in this study, we examined whether expression of Orai1 and STIM1 is correlated with clinico-pathological parameters of CCRCC and patients’ survival.
MATERIALS AND METHODS
Patients and tissue samples
This study includes 126 formalin fixed paraffin embedded (FFPE) tissue samples of CCRCC that were collected from patients who underwent radical nephrectomy at the Yonsei University Wonju Severance Christian Hospital from 2001 to 2011. In addition, 8 fresh tissue samples were also available among them. All slides and pathologic reports of these cases included in this study were reviewed and tumor stages and nuclear grades were reclassified according to TNM classification and Fuhrman nuclear grading, respectively (20,21).
Immunohistochemistry (IHC)
Tissue microarray (TMA) technique was used. 126 FFPE tissue cores from representative blocks (well-preserved area without necrosis, hemorrhage, and artifact site) were harvested using a 5 mm Quick-ray tip-punch (Unitma, Seoul, Korea), placed on a TMA mold with 20 pores (Unitma), and re-embedded with paraffin. The 4 µm sections of TMA blocks were cut, attached onto coated slides.
The sections were deparaffinized with xylene, rehydrated in graded alcohols, and subjected to pretreatment with CC1 (Roche Diagnostics, Basel, Switzerland). The primary antibodies, Orai1 (Abcam, Cambridge, MA, USA) and STIM1 (Abcam) were applied and incubated for 60 minutes (dilution titers: 1:100, respectively) using an automated IHC machine, Ventana Benchmark XT (Roche Diagnostics).
The antibodies were detected with the Ultra View Universal DAB kit (Roche Diagnostics) and counterstained with hematoxylin (Roche Diagnostics) by manufacturer’s recommendation. A positive and negative control stain test was also performed.
Modified Allred scoring system which calculated by the sum of staining intensity and distribution was used to evaluate the results of IHCs. The staining intensity was scored to be 0 points (negative), 1 point (weak), 2 points (intermediate), or 3 points (strong) and the distribution of positive-stained cells was calculated as 1 point (< 1%), 2 points (1%-10%), 3 points (11%-33%), 4 points (34%-67%), or 5 points (> 67%) (19,22). The total staining score from 0 to 2 points was interpreted as negative and a score from 3 to 8 points was interpreted as positive.
Western blot analysis
Western blot analysis was performed on 8 paired fresh tissue including CCRCC tissue and adjacent non-tumor renal tissue. Paired fresh tissues were lysed using 2 mL of PRO-PREP lysis buffer (iNtRon Biotechnology, Daejeon, Korea), and then ground for 15-20 seconds on ice using a homogenizer (ProScience, Woburn, MA, USA). Equal amount of protein lysates used for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to the immobilon-P membrane (Millipore, Billerica, MA, USA). The membranes were blocked with 5% skim milk and incubated with primary antibodies, including Orai1 (Abcam) and β-actin (Santa Cruz Biotechnology, Dallas, TX, USA) overnight at 4°C. The membranes were then incubated with secondary antibodies, including Anti-Rabbit IgG (HRP) (Santa Cruz Biotechnology) and Anti-mouse IgG (Santa Cruz Biotechnology) at room temperature for an hour. Immunoblotting bands were detected and quantified using Biospectrum Imaging System (UVP, Upland, CA, USA) and ImageJ software (available at http://rsb.info.nih.gov/ij/index.html).
Statistical analysis
Student’s t-test and χ2 test were used to compare the continuous and categorical variables. The period of overall survival was measured from the date of surgery to the date of death due to the tumor. Tumor recurrence was defined as the presence of clinically diagnosed or pathologically confirmed metastases. Survival analysis was performed using the Cox regression method after normalizing following parameters: sex, age, Fuhrman nuclear grade, and pathologic T stage. Statistical analysis was performed using PASW, version 20.0 (SPSS Inc., Chicago, IL, USA). A value of P < 0.05 was considered statistically significant.
Ethics statement
This study was conducted in accordance with the Declaration of Helsinki and was reviewed and approved by the institutional review board of Yonsei University Wonju College of Medicine (YWMR-12-0-014). Informed consent was exempted by the board.
RESULTS
Clinico-pathological characteristics of study population
The patients included in this study comprised of 94 male and 32 female, aged 16 to 79 years old (57.4 ± 10.5). The tumor size was ranged from 1.5 to 18 cm (5.3 ± 2.7 cm). Fuhrman nuclear grades in the tumors were as follows: grade 1, n = 15 (11.9%); grade 2, n = 57 (45.2%); grade 3, n = 42 (33.4%); grade 4, n = 12 (9.5%). Meanwhile, TNM stage of the tumors was follows: stage I, n = 87 (69%), stage II, n = 13 (10.3%), stage III, n = 22 (17.5%), stage IV, n = 4 (3.2%). Based on clinical data, 123 patients with CCRCC had available follow up data. Of the patients with follow up information, 9 (7.3%) had tumor recurrence. Until the last follow up, 12 patients (9.8%) had died due to the tumor. The clinico-pathological data of the patients are summarized in Table 1.
Table 1. Summary of clinical and pathological findings.
| Characteristics | No. (%) |
|---|---|
| Gender | |
| Male | 94 (74.6) |
| Female | 32 (25.4) |
| Age, yr | |
| Mean | 57.4 ± 10.5 |
| Range | 16-79 |
| Tumor size, cm | |
| Mean | 5.3 ± 2.7 |
| Range | 1.5-18 |
| Fuhrman nuclear grade | |
| 1 | 15 (11.9) |
| 2 | 57 (45.2) |
| 3 | 42 (33.4) |
| 4 | 12 (9.5) |
| TNM stage | |
| I | 87 (69.0) |
| II | 13 (10.3) |
| III | 22 (17.5) |
| IV | 4 (3.2) |
| Follow up data | |
| Tumor recurrence | 9 (7.3) |
| Death due to tumor | 12 (9.8) |
Orai1 expression is associated with favorable clinico-pathological parameters of CCRCC
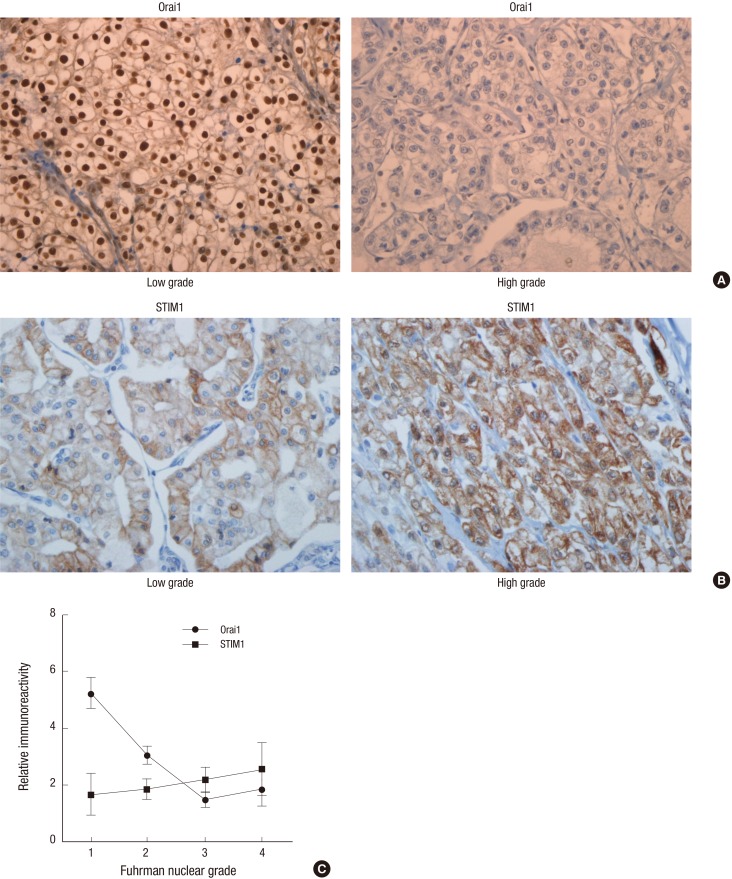
Orai1 was expressed in 66 (50%) cases of CCRCC, showing nuclear staining pattern whereas nuclear and cytoplasmic pattern in non-tumor renal tissue by IHC (Fig. 1A). Orai1 was positive in 65.3% of cases with a low (1 + 2) Fuhrman nuclear grade and 29.6% of cases with a high (3 + 4) Fuhrman nuclear grade (Fig. 1A and 1C, Table 2, P < 0.001). Consistent with IHC analysis, the protein level of Orai1 in fresh tissues from patient with low Fuhrman nuclear grade was elevated compared to that of high Fuhrman nuclear grade, in immunoblotting analysis (Fig. 2A and 2B). In the perirenal fat invasion, Orai1 positivity was seen in 53.6% of cases without perirenal fat invasion and 25% of cases with perirenal fat invasion (Table 2). For cystic change, Orai1 was positive in 67.6% of cases with cystic change and 43.5% of cases without cystic change (Table 2, P = 0.016). Orai1 was expressed in 56.7% of cases with pathologic T stage 1 and 33.3% of stage 2-4 cases (Table 2). Moreover, Orai1 positivity was seen in 57.5% of cases with TNM stage I and 33.3% of cases with TNM stage II-IV, which was again a statistically significant (P = 0.012). Although Orai1 expression seems to be higher in cases without sarcomatoid or rhabdoid feature, tumor necrosis, renal pelvis, renal sinus fat, and vascular invasions, these differences were not statistically significant (Table 2).
Fig. 1.
Immunohistochemistry (IHC) analysis of Orai1 and STIM1 in clear cell renal cell carcinoma (CCRCC). Expression of Orai1 (A) and STIM1 (B) in low and high grade tumors, respectively (×400). The mean staining score of IHC of Orai1 and STIM1 in Fuhrman nuclear grade 1-4 (C).
Table 2. Correlation of Orai1 and STIM1 expression and clinico-pathological parameters of clear cell renal cell carcinoma.
| Parameters | Orai1 expression | STIM1 expression | ||
|---|---|---|---|---|
| No.(%) of positive cases | P value | No.(%) of positive cases) | P value | |
| Perirenal fat invasion | ||||
| Absent | 59 (53.6) | 0.032 | 40 (36.7) | 0.507 |
| Present | 4 (25.0) | 6 (40.0) | ||
| Cystic change | ||||
| Present | 23 (67.6) | 0.016 | 38 (41.8) | 0.056 |
| Absent | 40 (43.5) | 8 (24.2) | ||
| Fuhrman nuclear grade | ||||
| Low grade (1 + 2) | 47 (65.3) | 0.000 | 24 (33.8) | 0.245 |
| High grade (3 + 4) | 16 (29.6) | 22 (41.5) | ||
| Pathologic T stage | ||||
| 1 | 51 (56.7) | 0.018 | 32 (36.0) | 0.413 |
| 2-4 | 12 (33.3) | 14 (40.0) | ||
| TNM stage | ||||
| I | 50 (57.5) | 0.012 | 31 (36.0) | 0.433 |
| II-IV | 13 (33.3) | 15 (39.5) | ||
Fig. 2.
Correlation of Orai1/STIM1 expression and tumor size. (A) Expression level of Orai1 in low and high grades, was analyzed with immunoblotting. (B) Relative (Rel.) expression of Orai1 in low and high grade tumor tissues. (C) Correlation between tumor size versus Orai1 negative and Orai1 positive groups. (D) Correlation between tumor size versus STIM1 negative and STIM1 positive groups; (NS) not significant.
*P = 0.033 low vs. high grade tumors; †P < 0.01 Orai1 negative versus positive tumors.
The mean staining score of IHC results was also compared in each group to overcome the limitation of quantification method, above used. The mean staining scores of Orai1 was statistically significant in clinico-pathological parameters including perirenal fat invasion, cystic change, Fuhrman nuclear grades, pathologic T stage, and TNM stages (Table 3). The mean staining score of IHC of Orai1 was high in Fuhrman nuclear grade 1 and was declined in higher Fuhrman nuclear grade (Fig. 1C). Importantly, the level of Orai1 expression was significantly associated with tumor size (Fig. 2C) an important indicator of CCRCC progression suggesting that Orai1 expression is associated with clinical outcomes.
Table 3. Difference of mean staining score of Orai1 and STIM1 expressions and clinico-pathological parameters of clear cell renal cell carcinoma.
| Parameters | Orai1 expression | STIM1 expression | ||
|---|---|---|---|---|
| Mean ± SD | P value | Mean ± SD | P value | |
| Perirenal fat invasion | ||||
| Absent | 2.83 ± 2.42 | 0.029 | 1.95 ± 2.71 | 0.759 |
| Present | 1.44 ± 1.90 | 2.20 ± 2.88 | ||
| Cystic change | ||||
| Present | 3.79 ± 2.60 | 0.001 | 1.18 ± 2.20 | 0.027 |
| Absent | 2.23 ± 2.18 | 2.27 ± 2.84 | ||
| Fuhrman nuclear grade | ||||
| Low grade (1 + 2) | 3.49 ± 2.45 | 0.000 | 1.79 ± 2.63 | 0.363 |
| High grade (3 + 4) | 1.54 ± 1.81 | 2.25 ± 2.84 | ||
| Pathologic T stage | ||||
| 1 | 2.98 ± 2.45 | 0.015 | 1.98 ± 2.79 | 0.966 |
| 2-4 | 1.83 ± 2.08 | 2.00 ± 2.57 | ||
| TNM stage | ||||
| I | 3.02 ± 2.46 | 0.009 | 1.99 ± 2.80 | 0.977 |
| II-IV | 1.82 ± 2.02 | 1.97 ± 2.56 | ||
SD, standard deviation.
Survival analysis for Orai1 was performed after normalizing following parameters: sex, age, Fuhrman nuclear grade, and pathologic T stage. There was no significant difference between Orai1 positive group and Orai1 negative group in both survival and recurrence rate.
STIM1 expression in CCRCC and its correlation with clinico-pathological parameters of CCRCC
Expression of ER Ca2+ sensor STIM1 an essential component of SOCE was detected in 46 (37.1%) cases of CCRCC. STIM1 was detected in the cytoplasm and/or membrane of tumor and non-tumor tissue (Fig. 1B). STIM1 was positive in 33.8% of cases with a low (1 + 2) Fuhrman nuclear grade and 41.5% of cases with a high (3 + 4) Fuhrman nuclear grade (Table 2). STIM1 expression level in CCRCC was not correlated with tumor grades such as Fuhrman nuclear grade, TNM and pathologic T stages (Fig. 1B and 1C). STIM1 was positive in 41.8% of cases without cystic change and 24.2% of cases with cystic change. Furthermore, STIM1 expression seems to be higher in cases without sarcomatoid or rhabdoid feature, tumor necrosis, perirenal fat, renal pelvis, renal sinus fat, and vascular invasion. In addition, STIM1 expression seemed to be higher in low pathologic T stage and TNM stage tumors as compared to high pathologic T stage and TNM stage tumors, respectively. These findings are summarized in Table 2.
The mean staining score of STIM1 was not significant in clinico-pathological parameters except cystic changes (Table 3, P = 0.027). In addition, there was no significant difference between STIM1 positive group and STIM1 negative group in tumor size (Fig. 2D) and both survival and recurrence rate (not shown).
DISCUSSION
We previously suggested that Orai1/STIM1-mediated store operated Ca2+ entry (SOCE) is primary Ca2+ entry mechanism which plays a critical role in tumorigenesis of CCRCC. In addition to our previous study, here, tumor expression level of Orai1 is associated with clinical outcomes of CCRCC. Interestingly, Orai1, not STIM, was expressed in nuclei of CCRCC indicating that plasma membrane protein Orai1 translocates to the nucleus of human CCRCC. This mechanism is reminiscent of type 1 insulin-like growth factor receptor (IGF-1R) translocation in CCRCC (23). In this report, nuclear IGF-1R was associated with poor prognosis. In contrast, high nuclear Orai1 expression was closely related with favorable prognostic factors of CCRCC in the present study. Underlying mechanism and pathological role of nuclear translocation of Orai1 in CCRCC awaits future investigation.
Several studies have demonstrated that increased expression of Orai1 protein level was associated with unfavorable prognosis in breast and lung cancers (14,24). However, high Orai1 expression was significantly related to absence of perirenal fat invasion, presence of cystic change, lower Fuhrman nuclear grade, lower pathologic T stage, and lower TNM stage suggesting that high Orai1 expression may be associated with favorable prognostic factors in CCRCC in the current study. Sustained depletion of ER Ca2+ which is crucial for ER chaperone protein function may cause ER stress leading apoptosis. Thus, Orai1-mediated Ca2+ influx is vital for refilling of ER Ca2+ protecting apoptosis. Higher expression of Orai1 at least in part, may involve in protecting tumor progression via preventing ER stress. In addition, Orai1-mediated SOCE activity and its function could be variable due to different organ, present tumor diversity, specified source of patients, and less number of specimens.
We expected the difference in survival of patients since the IHC and western blot results show significant difference between them. However, there was no difference between Orai1 positive and Orai1 negative patients’ survival. It may be due to the characteristics of our study population that mainly included low stage tumors, not advanced cases, since high stage patients usually do not tend to get surgery. As well as, the patients with low grade tumor have relatively good prognosis, so small number of patients who deceased in our study.
STIM1, an ER Ca2+ sensor, functions as a molecular component of SOCE channel in CCRCC and it is critical for cell migration and proliferation in CCRCC cell lines (19). STIM1 expression was detected in 46 (36.5%) of CCRCC with cytoplasm or membrane staining pattern in tumor tissues. The expression shows a trend that aggressive CCRCC reveal higher STIM1 positivity. However, the result of mean value comparison shows that STIM1 expression was significantly higher when the cystic change in CCRCC was absent (P = 0.027); Cystic change is known as favorable prognostic factor, as previously described. It has been argued whether STIM1 is associated with adverse or favorable prognosis of cancer. While STIM1 silencing in cervical cancer cells leads to decrease in proliferation by arresting the cell cycle at the S and G2/M phases (18). STIM1 knockdown had no significant impact on glioblastoma cell proliferation, as long as Orai1 knockdown induced significant inhibition of the tumor cell growth (17).
Wilms’ tumor suppressor 1 (WT1) and early growth response 1 (EGR1) regulate STIM1 expression in various kinds of cancer (25,26). STIM1 expression was reciprocally regulated by either overexpression of WT1 or knockdown of EGR1. Therefore, we examined WT1 expression status in CCRCC. All 126 cases of CCRCC did not expressed WT1 by immunohistochemistry (data are not shown). WT1 is expressed in differentiating glomerular epithelium but not in mesenchymal derived epithelial structures such as proximal and distal tubules in fetal kidney (27). WT1 staining was observed in human fetal kidney and high grade CCRCC, but do not react with normal tubules of the kidney (28), whereas common WT1 mutations are not involved in CCRCC (29). According to the results of our study, we may predict STIM1 expression might not be related with WT1 expression in the developing pathway of CCRCC, a tumor whose cell of origin is believed to be from the proximal tubule.
Development of the systemic target therapy for RCC has been greatly improved patients’ outcome. Predominant treatment strategy of metastatic RCC is cytokine-based treatment such as sorafenib, sunitinib bevacizumab, temisirolimus, and everolimus (1,4). However, the treatment of metastatic RCC remains a challenge and a major health problem due to lack of specific targets. This study provides a new perspective on the renal carcinogenesis, and offers clues for a prognosis and therapeutic strategies for CCRCC.
ACKNOWLEDGMENT
We would like to thank Mr. Joong Seob Kim and Mr. Tae-Young Kang (Department of Pathology, Yonsei University Wonju Severance Christian Hospital, Wonju, Republic of Korea) for their technical support.
Footnotes
Funding: This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2012R1A1A1004233 to M.E.) and the Ministry of Education (NRF-2010-0024789 to S.-K.C), and a research grant from Yonsei University Wonju College of Medicine (YUWCM-2010-27 to M.E.).
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Study design and literature review: Eom M, Cha S. Data collection and management: Lkhagvadorj S, Kim JH, Jung JH, Chung HC. Statistical analysis: Oh SS. Analysis and interpretation of findings: Lkhagvadorj S, Kim JH, Lee M, Eom M, Cha S. Preparation of manuscript: Lkhagvadorj S, Kim JH, Eom M, Cha S. Final approval: all authors.
References
- 1.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119–1132. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 2.Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013;45:1–14. doi: 10.4143/crt.2013.45.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patard JJ, Leray E, Rioux-Leclercq N, Cindolo L, Ficarra V, Zisman A, De La Taille A, Tostain J, Artibani W, Abbou CC, et al. Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol. 2005;23:2763–2771. doi: 10.1200/JCO.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 4.Cho IC, Chung J. Current status of targeted therapy for advanced renal cell carcinoma. Korean J Urol. 2012;53:217–228. doi: 10.4111/kju.2012.53.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 6.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 7.Smyth JT, Hwang SY, Tomita T, DeHaven WI, Mercer JC, Putney JW. Activation and regulation of store-operated calcium entry. J Cell Mol Med. 2010;14:2337–2349. doi: 10.1111/j.1582-4934.2010.01168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capiod T. The need for calcium channels in cell proliferation. Recent Patents Anticancer Drug Discov. 2013;8:4–17. doi: 10.2174/15748928130102. [DOI] [PubMed] [Google Scholar]
- 9.Berridge MJ. Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta. 2009;1793:933–940. doi: 10.1016/j.bbamcr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 11.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 12.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 14.Yang S, Zhang JJ, Huang XY. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell. 2009;15:124–134. doi: 10.1016/j.ccr.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Vanden Abeele F, Shuba Y, Roudbaraki M, Lemonnier L, Vanoverberghe K, Mariot P, Skryma R, Prevarskaya N. Store-operated Ca2+ channels in prostate cancer epithelial cells: function, regulation, and role in carcinogenesis. Cell Calcium. 2003;33:357–373. doi: 10.1016/s0143-4160(03)00049-6. [DOI] [PubMed] [Google Scholar]
- 16.Liu H, Hughes JD, Rollins S, Chen B, Perkins E. Calcium entry via ORAI1 regulates glioblastoma cell proliferation and apoptosis. Exp Mol Pathol. 2011;91:753–760. doi: 10.1016/j.yexmp.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Motiani RK, Hyzinski-García MC, Zhang X, Henkel MM, Abdullaev IF, Kuo YH, Matrougui K, Mongin AA, Trebak M. STIM1 and Orai1 mediate CRAC channel activity and are essential for human glioblastoma invasion. Pflugers Arch. 2013;465:1249–1260. doi: 10.1007/s00424-013-1254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen YF, Chiu WT, Chen YT, Lin PY, Huang HJ, Chou CY, Chang HC, Tang MJ, Shen MR. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc Natl Acad Sci USA. 2011;108:15225–15230. doi: 10.1073/pnas.1103315108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JH, Lkhagvadorj S, Lee MR, Hwang KH, Chung HC, Jung JH, Cha SK, Eom M. Orai1 and STIM1 are critical for cell migration and proliferation of clear cell renal cell carcinoma. Biochem Biophys Res Commun. 2014;448:76–82. doi: 10.1016/j.bbrc.2014.04.064. [DOI] [PubMed] [Google Scholar]
- 20.Edge SB. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010. pp. 323–328. [Google Scholar]
- 21.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–663. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 23.Aleksic T, Chitnis MM, Perestenko OV, Gao S, Thomas PH, Turner GD, Protheroe AS, Howarth M, Macaulay VM. Type 1 insulin-like growth factor receptor translocates to the nucleus of human tumor cells. Cancer Res. 2010;70:6412–6419. doi: 10.1158/0008-5472.CAN-10-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhan ZY, Zhong LX, Feng M, Wang JF, Liu DB, Xiong JP. Over-expression of Orai1 mediates cell proliferation and associates with poor prognosis in human non-small cell lung carcinoma. Int J Clin Exp Pathol. 2015;8:5080–5088. [PMC free article] [PubMed] [Google Scholar]
- 25.Ritchie MF, Zhou Y, Soboloff J. WT1/EGR1-mediated control of STIM1 expression and function in cancer cells. Front Biosci (Landmark Ed) 2011;16:2402–2415. doi: 10.2741/3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ritchie MF, Yue C, Zhou Y, Houghton PJ, Soboloff J. Wilms tumor suppressor 1 (WT1) and early growth response 1 (EGR1) are regulators of STIM1 expression. J Biol Chem. 2010;285:10591–10596. doi: 10.1074/jbc.M109.083493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rivera MN, Haber DA. Wilms’ tumour: connecting tumorigenesis and organ development in the kidney. Nat Rev Cancer. 2005;5:699–712. doi: 10.1038/nrc1696. [DOI] [PubMed] [Google Scholar]
- 28.Campbell CE, Kuriyan NP, Rackley RR, Caulfield MJ, Tubbs R, Finke J, Williams BR. Constitutive expression of the Wilms tumor suppressor gene (WT1) in renal cell carcinoma. Int J Cancer. 1998;78:182–188. doi: 10.1002/(sici)1097-0215(19981005)78:2<182::aid-ijc11>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Wang S, Sitaram RT, Andersson C, Ljungberg B, Li A. Single nucleotide polymorphisms in the Wilms’ tumour gene 1 in clear cell renal cell carcinoma. PLoS One. 2013;8:e58396. doi: 10.1371/journal.pone.0058396. [DOI] [PMC free article] [PubMed] [Google Scholar]