Abstract
This study aimed to evaluate the association between body mass index (BMI) and progression in triple-negative breast cancer (TNBC). We retrospectively reviewed the medical records of 50 patients with TNBC who underwent breast-conserving surgery or mastectomy between 2007 and 2014. All patients were classified according to BMI (median 23.5 kg/m2, range 17.2–31.6 kg/m2): 31 patients (62%) were classified as being overweight or obese (BMI ≥ 23 kg/m2) and 19 patients (38%) were classified as having a normal body weight (BMI < 23 kg/m2). The median follow-up for patients was 31.1 months (range, 6.7–101.9 months). Progression occurred in 7 patients (14%), including 5 ipsilateral breast tumor recurrences, 2 regional lymph node metastases, and 5 distant metastases. Progression was significantly correlated with overweight or obese patients (P = 0.035), while none of the normal weight patients showed progression. The 3-year disease-free survival (DFS) and overall survival (OS) rates were 85.0% and 87.7%, respectively. DFS was significantly reduced in overweight or obese patients compared to that in normal weight patients (P = 0.035). However, OS was not significantly compromised by being overweight or obese (P = 0.134). In conclusion, being overweight or obese negatively affects DFS in TNBC patients.
Keywords: Triple-Negative Breast Cancer, Overweight, Obesity, Body Mass Index, Prognosis
Graphical Abstract
INTRODUCTION
Breast cancer is not a homogeneous disease, but is composed of several different molecular subtypes that are in turn classified based on the impact of treatment choice and their positivity for 3 receptors: the estrogen hormone receptor, progesterone hormone receptor, and human epidermal growth factor receptor-2 (HER-2) (1). There are 4 molecular subtypes of breast cancer: luminal A, luminal B, HER-2 positive, and triple-negative breast cancer (TNBC). Among those subgroups, TNBC, which refers to the negative expression of all 3 receptors, is known to have a poor prognosis (2). TNBC patients also present with earlier relapse and death compared to those with other subtypes (2). Moreover, the need for effective new treatments for TNBC is unmet, as patients in this subgroup do not respond to endocrine or targeted therapy (3).
According to recent studies, cancer progression is related to metabolic changes in the body (4,5), and the notion of breast cancer being a metabolic disease has been receiving more attention recently (6). According to Turkoz et al. (7), being overweight or obese significantly increases the risk of TNBC; moreover, other recent studies showed that the prognosis of TNBC is closely related to being overweight or obese (8,9,10). In a large-scale, multicenter study, Hao et al. (11) recently reported that the prognosis of TNBC, especially survival, is closely associated with being overweight in Chinese patients.
The aim of this study was to investigate the association between body weight and prognosis of patients with TNBC. We evaluated the relationship between being overweight and the progression of TNBC in Korean patients.
MATERIALS AND METHODS
We retrospectively reviewed the records of 304 patients who underwent curative surgery for breast cancer between February 2007 and October 2014 in Busan Paik Hospital. All the patients were women pathologically diagnosed with invasive ductal carcinoma. Of these patients, those with distant metastases and/or bilateral breast cancer were excluded from the study. Only patients who had not previously undergone cancer-related breast surgery were enrolled. After categorizing patients according to the molecular subtype of their diseases, 50 were classified as TNBC patients and evaluated in this study.
Intrinsic molecular subtypes were classified based on the St. Gallen consensus of 2013 (1). For pathologic studies with immunohistochemistry, TNBC was defined according to the expression of estrogen receptor, progesterone receptor, and HER-2. The primary antibodies used for this study were as follows: estrogen receptor (1:90; Novocastra Laboratories Ltd., Newcastle upon Tyne, UK), progesterone receptor (1:170; Novocastra Laboratories Ltd.), HER-2 (Ventana Medical Systems Inc., Tucson, AZ, USA), Ki-67 (1:280; Dako, Glostrup, Denmark), and p53 (1:150; Dako). For estrogen and progesterone receptor status, activity less than 10% was considered negative. HER-2 expression was graded from 0 to 3+ according to the American Society of Clinical Oncology guidelines (12); HER-2 negativity was defined as a score of 2 or less (13). Positivity for p53 (14) and the Ki-67 labeling index were also determined on the basis of pathologic examination (15).
Menopausal status indicates changes in estrogen and/or progesterone hormonal levels and is an important factor in breast cancer prognosis (11,16). However, in the current study, it was difficult to directly identify patients’ menopausal statuses from their medical records. Therefore, we divided patients into 2 subgroups based on age as a substitute for grouping by menopausal status in order to estimate the difference in the prognostic effect of being overweight between premenopausal and postmenopausal women. The median age of Korean women who experienced menopause was estimated at 49.4 years according to a report from the Korean Occupational Safety and Health Research Institute (2010) (17). Therefore, patients in this study aged < 50 years were considered pre-menopausal and patients aged ≥ 50 years were considered postmenopausal.
The body mass index (BMI) was calculated as the body weight divided by the square of the height. Based on the Asian BMI classification (18), a BMI < 23 kg/m2 indicated normal weight while a BMI ≥ 23 kg/m2 indicated overweight or obese. We attempted to determine the cut-off BMI value at which disease-free survival (DFS) would be affected.
DFS was defined as the time interval from the date of surgery to overall progression (including ipsilateral breast tumor recurrence, regional lymph nodal failure, and distant metastases). Overall survival (OS) was defined as the time interval from the date of surgery to the date of death or the last follow-up.
For statistical analysis, SPSS version 18.0 (SPSS, Chicago, IL, USA) was used. The Fisher exact test was used to determine the clinical factors related to treatment failure. DFS and OS rates were estimated by using the Kaplan-Meier method. The log-rank test was used to compare clinical variables. Statistical significance was defined as P < 0.05 (two-sided test). The maximal chi-square method was used to determine which BMI value could best segregate patients into the poor- and good-prognosis subgroups according to DFS, with the log-rank test used to measure the strength of the grouping. MaxStat, a maximal chi-square method in R 2.13.0 (R Development Core Team, Vienna, Austria, http://www.R-project.org) was used to identify the optimal cut-off point.
Ethics statement
The study was reviewed and approved by the institutional review board of Inje University Busan Paik Hospital (IRB Approval No. 15-0235). The requirement for informed consent was waived owing to the retrospective nature of the study.
RESULTS
Patients and treatment
Table 1 summarizes patient characteristics and cancer treatments. The median age of the patients was 54 years (range, 30–77 years), with 31 patients (62%) aged ≥ 50 years (assumed to be postmenopausal) and 19 patients (38%) aged < 50 years (assumed to be premenopausal). The median BMI of the patients was 23.5 kg/m2 (range, 17.2–31.6 kg/m2), with 19 patients (38%) classified as having a normal weight (BMI < 23 kg/m2) and 31 patients (62%) classified as being overweight or obese (BMI ≥ 23 kg/m2). Among the 31 patients determined to be above normal weight, 18 were classified as obese according to Asian BMI classification (BMI ≥ 25 kg/m2) (18). Twenty-six patients (52%) had cancer in the right breast while 24 patients (48%) had cancer in the left breast. According to pathologic reports, 28 patients (56%) showed p53 positivity and 37 patients (74%) showed a Ki-67 labeling index of ≥ 20%. Curative surgery used in this study included breast-conserving surgery or modified radical mastectomy. Systemic chemotherapy (CT) was administered to most patients (n = 41, 82%). The majority of patients (n = 38, 76%) underwent CT in an adjuvant setting, except for 3 patients who were treated with neoadjuvant CT. Several CT regimens based on anthracycline, taxane, and platinum were used. For adjuvant radiotherapy (RT), most patients who underwent breast-conserving surgery underwent postoperative RT (30/36, 83.3%). Conversely, only patients with a high risks of relapse (especially those who had multiple metastatic regional lymph nodes) underwent adjuvant RT after modified radical mastectomy (4/14, 28.6%). The median total RT dose was 60.4 Gy (range, 50–66 Gy). The most commonly applied RT scheme (n = 17) was whole breast irradiation with tangential photon beam RT (total 50.4 Gy with 1.8 Gy per fraction) followed by single electron beam boost (total 10 Gy with 2 Gy per fraction).
Table 1. Patient characteristics and cancer treatments.
| Characteristics | No. (%) of patients |
|---|---|
| Age, yr | |
| < 50 | 19 (38.0) |
| ≥ 50 | 31 (62.0) |
| BMI, kg/m2 | |
| < 23 | 19 (38.0) |
| ≥ 23 | 31 (62.0) |
| p53 | |
| Positive | 28 (56.0) |
| Negative | 22 (44.0) |
| Ki-67 LI (%) | |
| < 20 | 13 (26.0) |
| ≥ 20 | 37 (74.0) |
| Pathologic stage | |
| Stage I | 20 (40.0) |
| Stage II | 26 (52.0) |
| Stage III | 4 (8.0) |
| Tumor multiplicity | |
| Yes | 6 (12.0) |
| No | 44 (88.0) |
| Types of surgery | |
| BCS | 36 (72.0) |
| MRM | 14 (28.0) |
| Radiotherapy | |
| Yes | 34 (68.0) |
| No | 16 (32.0) |
| Chemotherapy | |
| Yes | 41 (82.0) |
| No | 9 (18.0) |
BMI, body mass index; BCS, breast-conserving surgery; MRM, modified-radical mastectomy.
Table 2 shows the comparison between patients with normal weight (BMI < 23 kg/m2) and patients who were overweight or obese (BMI ≥ 23 kg/m2). None of the clinical factors showed a difference in distribution according to BMI.
Table 2. Comparison of patients according to overweight or obesity.
| Characteristics | BMI < 23 (kg/m2) No. |
BMI ≥ 23 (kg/m2) No. |
P value |
|---|---|---|---|
| Age, yr | 0.766 | ||
| < 50 | 8 | 11 | |
| ≥ 50 | 11 | 20 | |
| p53 | 1 | ||
| Positive | 11 | 17 | |
| Negative | 8 | 14 | |
| Ki-67 LI, % | 0.742 | ||
| < 20 | 4 | 9 | |
| ≥ 20 | 15 | 22 | |
| Pathologic stage | 0.073 | ||
| Stage I | 11 | 9 | |
| Stage II–III | 8 | 22 | |
| Tumor multiplicity | 1 | ||
| Yes | 2 | 4 | |
| No | 17 | 27 | |
| Types of surgery | 0.522 | ||
| BCS | 15 | 21 | |
| MRM | 4 | 10 | |
| Radiotherapy | 0.349 | ||
| Yes | 8 | 8 | |
| No | 11 | 23 | |
| Chemotherapy | 1 | ||
| Yes | 3 | 6 | |
| No | 16 | 25 |
BMI, body mass index; BCS, breast-conserving surgery; MRM, modified-radical mastectomy.
Recurrence
The median follow-up for patients was 31.1 months (range, 6.7–101.9 months). During the follow-up period, recurrence occurred in 7 patients (14%), including 5 cases of ipsilateral breast tumor recurrence, 2 of regional lymph node metastasis, and 5 of distant metastasis (i.e., some of the patients had multiple types of progression). The sites of the observed distant metastases were the lungs, brain, and bone.
Overweight or obesity was found to be significantly related to disease progression (P = 0.035). All observed progression occurred in overweight or obese patients, while normal weight patients did not experience progression. No patient (0%) with normal weight developed ipsilateral breast tumor recurrence, regional lymph nodal recurrence, or distant metastasis.
Survival
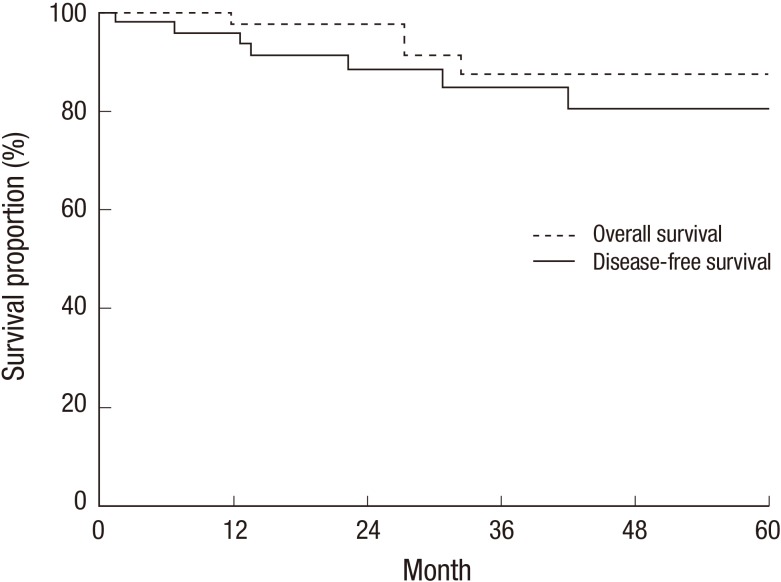
During the follow-up period, 4 breast cancer-related deaths were noted. The 3-year DFS and OS rates were 85.0% and 87.7%, respectively (Fig. 1).
Fig. 1.
Disease-free survival and overall survival.
Prognostic factors for survival
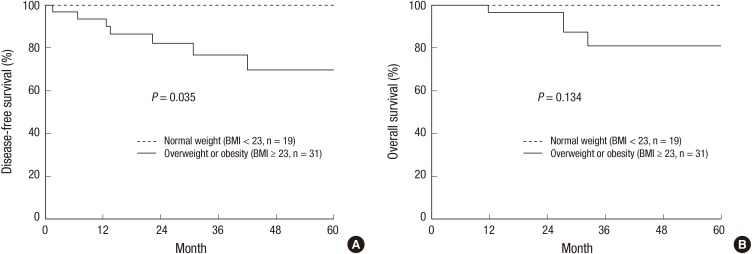
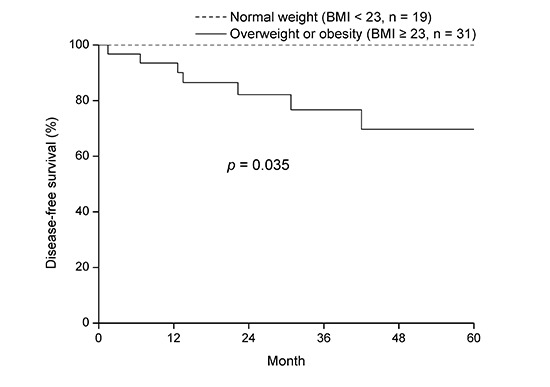
Table 3 shows the prognostic factors for DFS. Being overweight or obese was significantly correlated with DFS (P = 0.035). A reduced 3-year DFS was observed in overweight or obese patients compared to patients of normal weight (P = 0.035, Fig. 2A). Moreover, younger patients tended to have poor DFS (P = 0.067, Table 3). However, OS was not significantly compromised by being overweight or obese (P = 0.134, Fig. 2B).
Table 3. Prognostic factors for disease-free survival.
| Characteristics | No. of patients | 3-year PFS | P value |
|---|---|---|---|
| Age, yr | 0.067 | ||
| < 50 | 19 | 73.2 | |
| ≥ 50 | 31 | 92.9 | |
| BMI, kg/m2 | 0.035 | ||
| < 23 | 19 | 100 | |
| ≥ 23 | 31 | 76.7 | |
| p53 | 0.138 | ||
| Positive | 28 | 85.3 | |
| Negative | 22 | 73.7 | |
| Ki-67 LI, % | 0.366 | ||
| < 20 | 13 | 92.3 | |
| ≥ 20 | 37 | 81.5 | |
| Pathologic stage | 0.491 | ||
| Stage I | 20 | 84.4 | |
| Stage IIIII | 30 | 85.4 | |
| Tumor multiplicity | 0.347 | ||
| Yes | 6 | 100 | |
| No | 44 | 83 | |
| Types of surgery | 0.343 | ||
| BCS | 36 | 88.1 | |
| MRM | 14 | 76.6 | |
| Radiotherapy | 0.398 | ||
| Yes | 34 | 85.4 | |
| No | 16 | 85.6 | |
| Chemotherapy | 0.258 | ||
| Yes | 41 | 82.5 | |
| No | 9 | 100 |
BMI, body mass index; BCS, breast-conserving surgery; MRM, modified-radical mastectomy.
Fig. 2.
Disease-free survival (A) and overall survival (B) according to overweight and obesity.
Prognostic value of BMI for DFS
The cut-off value of BMI for classifying patients according to the DFS was 23 kg/m2 (by using the maximal χ 2 method, M = 2.2675, P = 0.299).
Effects of age and being overweight on prognosis
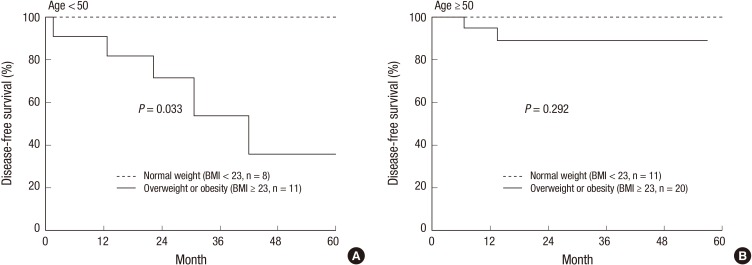
The detrimental effect of being overweight or obese was clearly indicated in the DFS rate of younger women (age < 50 years, P = 0.033, Fig. 3A). In contrast, older women (age ≥ 50 years, P = 0.292, Fig. 3B) did not show a significant difference in DFS according to BMI.
Fig. 3.
Disease-free survival by overweight or obesity in (A) young women (age < 50) and (B) old women (age ≥ 50).
DISCUSSION
Our study revealed that being obese or overweight is closely related to disease progression in TNBC. DFS was also significantly affected by being obese or overweight. The estimated BMI cut-off value that best segregated DFS was 23 kg/m2 according to our study results. BMI ≥ 23 kg/m2 is also the cut-off value that defines being “overweight” in Asians. The harmful effect on prognosis of being overweight or obese was more clearly apparent in younger patients (age < 50 years) than in older patients (age ≥ 50 years).
Obesity or overweight is a potentially modifiable risk factor of breast cancer (19). Lifestyle changes (including exercise) and weight control can be attempted to improve treatment outcomes in TNBC patients. Explaining the risk of cancer progression related to overweight and obesity to TNBC patients in clinics may be a helpful motivating factor for lifestyles changes.
The mechanism between the progression of breast cancer and metabolic changes has not yet been fully explored. Leptin and insulin-like growth factor-1 have been mentioned as playing roles in the poor prognostic effects of being overweight or obese in breast cancer patients (8). Leptin, which is secreted by adipocytes, acts as a growth factor (8), and the insulin-leptin-adiponectin axis is suggested to be a link between breast cancer and metabolic syndrome (20). Additionally, Liu et al. (21) reported that lipids activate growth promoting signals such as those involving lysophosphatidic acid, insulin, insulin-like growth factor-1, and endothelial growth factor to promote cancer cell growth. Poly (ADP-ribose) polymerase inhibitors are already used in TNBC treatment (3). However, further investigation is required to understand the specific association between being overweight and TNBC progression. Such research would be helpful towards discovering new cancer treatment strategies for TNBC.
Menopausal status and related changes in estrogen/progesterone hormone levels may affect the outcome of TNBC. Generally, young patients with breast cancer are known to have poorer prognosis than aged patients. According to a recent study, TNBC is categorized into 7 heterogeneous pathologic subgroups according to gene expression profiles (basal-like 1, basal-like 2, immunomodulatory, mesenchymal, mesenchymal stem–like, luminal androgen receptor, and unstable) (22). The predominantly poor prognostic effect of being overweight or obese on young patients in our study may be related to the tumor’s intrinsic clinicopathologic features. If young and old TNBC patients belong to different pathologic subgroups, the prognostic effect of being overweight or obese may potentially be attributed to pathology.
More attention is required for overweight and obese patients, who require closer follow-up. Short-interval, frequent follow-up sessions may be helpful for early detection of relapse. Intensified adjuvant treatment for overweight patients, including combination CT (23,24) and dose-escalated RT (25), can be attempted as treatment options to improve outcomes. In cases with clinically suspected recurrence, active and immediate diagnostic evaluation and treatment should be performed.
Because this study dealt with only 50 patients for evaluation, there are limitations to our results. The finding that being obese or overweight showed no effect on OS may be associated with the relatively small number of patients and short-term follow-up in this study. Large-scale and long-term follow-up studies are warranted to clarify the prognostic significance of being overweight in TNBC. Selection bias should be also taken into consideration because of the retrospective design of this study. Our results should be interpreted while taking into account that overweight or obese patients might have received relatively lower doses of CT owing to their larger body weights.
In conclusion, being overweight or obese is associated with worse TNBC prognosis. Furthermore, progression after surgery in TNBC patients is related to their BMI. Further efforts are required to determine the mechanisms underlying how overweight induces poor prognosis in TNBC. We expect that controlling metabolism will provide a path towards new treatments for TNBC.
Footnotes
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Research conception & design: Choi Y, Lee YH. Performing the experiments: Choi Y. Data acquisition: Choi Y, Kim T, Yoon HK, Park SK, Ahn KJ, Cho H. Data analysis and interpretation: Choi Y. Statistical analysis: Choi Y. Drafting of the manuscript: Choi Y. Critical revision of the manuscript: Kim T, Yoon HK. Approval of final manuscript: all authors.
References
- 1.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn HJ, Albain KS, Andre F, Bergh J, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 3.Hudis CA, Gianni L. Triple-negative breast cancer: an unmet medical need. Oncologist. 2011;16(Suppl 1):1–11. doi: 10.1634/theoncologist.2011-S1-01. [DOI] [PubMed] [Google Scholar]
- 4.Seyfried TN, Flores RE, Poff AM, D’Agostino DP. Cancer as a metabolic disease: implications for novel therapeutics. Carcinogenesis. 2014;35:515–527. doi: 10.1093/carcin/bgt480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boeing H. Obesity and cancer--the update 2013. Best Pract Res Clin Endocrinol Metab. 2013;27:219–227. doi: 10.1016/j.beem.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Yazici O, Aksoy S, Sendur MA, Babacan T, Ozdemir N, Ozisik Y, Zengin N, Altundag K. The effect of obesity on recurrence pattern in early breast cancer patients. J BUON. 2015;20:954–962. [PubMed] [Google Scholar]
- 7.Turkoz FP, Solak M, Petekkaya I, Keskin O, Kertmen N, Sarici F, Arik Z, Babacan T, Ozisik Y, Altundag K. Association between common risk factors and molecular subtypes in breast cancer patients. Breast. 2013;22:344–350. doi: 10.1016/j.breast.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Dean SJ, Rhodes A. Triple negative breast cancer: the role of metabolic pathways. Malays J Pathol. 2014;36:155–162. [PubMed] [Google Scholar]
- 9.Hauner D, Hauner H. Metabolic syndrome and breast cancer: is there a link? Breast Care (Basel) 2014;9:277–281. doi: 10.1159/000365951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vona-Davis L, Rose DP, Hazard H, Howard-McNatt M, Adkins F, Partin J, Hobbs G. Triple-negative breast cancer and obesity in a rural Appalachian population. Cancer Epidemiol Biomarkers Prev. 2008;17:3319–3324. doi: 10.1158/1055-9965.EPI-08-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao S, Liu Y, Yu KD, Chen S, Yang WT, Shao ZM. Overweight as a prognostic factor for triple-negative breast cancers in Chinese women. PLoS One. 2015;10:e0129741. doi: 10.1371/journal.pone.0129741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 13.Ahn HJ, Jung SJ, Kim TH, Oh MK, Yoon HK. Differences in clinical outcomes between Luminal A and B type breast cancers according to the St. Gallen Consensus 2013. J Breast Cancer. 2015;18:149–159. doi: 10.4048/jbc.2015.18.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller LD, Smeds J, George J, Vega VB, Vergara L, Ploner A, Pawitan Y, Hall P, Klaar S, Liu ET, et al. An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc Natl Acad Sci USA. 2005;102:13550–13555. doi: 10.1073/pnas.0506230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luporsi E, André F, Spyratos F, Martin PM, Jacquemier J, Penault-Llorca F, Tubiana-Mathieu N, Sigal-Zafrani B, Arnould L, Gompel A, et al. Ki-67: level of evidence and methodological considerations for its role in the clinical management of breast cancer: analytical and critical review. Breast Cancer Res Treat. 2012;132:895–915. doi: 10.1007/s10549-011-1837-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheridan W, Scott T, Caroline S, Yvonne Z, Vanessa B, David V, Karen G, Stephen C. Breast cancer in young women: have the prognostic implications of breast cancer subtypes changed over time? Breast Cancer Res Treat. 2014;147:617–629. doi: 10.1007/s10549-014-3125-1. [DOI] [PubMed] [Google Scholar]
- 17.Kim IK, Choi HM, Kim MH. Menopausal knowledge and management in peri-menopausal women. J Korean Soc Menopause. 2012;18:124–131. [Google Scholar]
- 18.Expert Consultation WH. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 19.Ballard-Barbash R, Hunsberger S, Alciati MH, Blair SN, Goodwin PJ, McTiernan A, Wing R, Schatzkin A. Physical activity, weight control, and breast cancer risk and survival: clinical trial rationale and design considerations. J Natl Cancer Inst. 2009;101:630–643. doi: 10.1093/jnci/djp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis AA, Kaklamani VG. Metabolic syndrome and triple-negative breast cancer: a new paradigm. Int J Breast Cancer. 2012;2012:809291. doi: 10.1155/2012/809291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu R, Huang Y. Lipid signaling in tumorigenesis. Mol Cell Pharmacol. 2014;6:1–9. [PMC free article] [PubMed] [Google Scholar]
- 22.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erler JT, Linding R. Network medicine strikes a blow against breast cancer. Cell. 2012;149:731–733. doi: 10.1016/j.cell.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Kim HA, Seong MK, Kim EK, Kang E, Park S, Hur MH, Song BJ, Noh WC, Korea Breast Cancer Society Evaluation of the survival benefit of different chemotherapy regimens in patients with T1-2N0 triple-negative breast cancer. J Breast Cancer. 2015;18:271–278. doi: 10.4048/jbc.2015.18.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sioshansi S, Huber KE, Wazer DE. The implications of breast cancer molecular phenotype for radiation oncology. Front Oncol. 2011;1:12. doi: 10.3389/fonc.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]