Abstract
The aim of this study was to evaluate the bone mineral density and the prevalence of osteoporosis in postmenopausal Korean women with low-energy distal radius fractures and compared with those of aged-matched normal Korean women. Two hundred and six patients with distal radius fractures between March 2006 and March 2010 were included in this study. Patients were divided into three groups by age; group 1 (50-59 years), group 2 (60-69 years), and group 3 (70-79 years). Controls were age-matched normal Korean women. The bone mineral density values at all measured sites, except for the spine, were significantly lower in group 1 than those of control. While the bone mineral density values in group 2 and 3 were lower than those of controls, these differences were not statistically significant. All groups had significantly higher prevalence of osteoporosis at the Ward’s triangle; however, at the spine, femoral neck and trochanteric area it was not significantly different from those of age-matched controls. Although the prevalence of osteoporosis of the postmenopausal women with low-energy distal radius fractures may not be higher than that of the control, osteoporosis should be evaluated especially in younger postmenopausal patients to prevent other osteoporotic hip and/or spine fractures.
Keywords: Osteoporosis, Distal Radius Fracture, Post-Menopausal Women, Prevalence
Graphical Abstract
INTRODUCTION
Distal radius fractures of the upper extremities are relatively common accounting for approximately 17% of all fractures in patients visiting the emergency room (1). In recent years, osteoporotic fractures have become a major health issue; and many studies have been done on relationship between distal radius fractures and osteoporosis. However, there have been still controversies whether this relationship is statistically significant or not (2,3,4). Also, the prevalence of low bone mineral density (BMD) and osteoporosis in patients with low-energy distal radial fractures compared with that of normal Korean population without such fractures is not well known. In previous study, Lee et al. (5) assessed age- and site-related BMD and the prevalence of osteoporosis in Korean women with a distal radius fracture, and compared these with reference data derived from a study done in a large population-based cohort in Korea (6). For the study, they recruited only small number of 54 consecutive Korean women, 50 to 79 years of age, with a distal radius fracture. However, in this study we recruited large number of 206 consecutive Korean women patients. This study has shown that BMD is lower in patients with a distal radius fracture in women younger than 60 years of age or over 70 years of age than in normal controls. In addition, low-energy distal radius fractures are predictive of future 15 years prior to osteoporotic hip and spine fractures (7,8,9). Considering these findings, it is important to analyze the relationship between BMD and distal radius fractures in postmenopausal women for the prevention of secondary fractures (10). The aim of this study was to compare the BMD and prevalence of osteoporosis of postmenopausal Korean women with low-energy distal radius fractures with those of aged-matched normal Korean women.
MATERIALS AND METHODS
We retrospectively reviewed 206 patients who visited our hospital between March 2006 and March 2010 because of distal radius fractures. Inclusion criteria of this study were low-energy distal radius fractures caused by out-stretched injury of the wrist and postmenopausal women older than 50 years of age. Patients previously diagnosed systemic diseases affecting bone metabolism (e.g., metabolic bone disease, renal osteodystrophy) or those who had been taken steroid for extended period were excluded from this study. We also excluded patients with previous distal radius fractures, those with high-energy injuries caused by a vehicle accident or fall, those with pathologic fractures, and those who had previously diagnosed osteoporosis.
The BMD was measured at the spine and proximal femur using a Lunar Prodigy Advance™ dual energy X-ray absorptiometry (GE Healthcare, Madison, WI, USA). At the spine, BMD values were obtained from the first to the fourth lumbar vertebral body. At the proximal femur, BMD values were obtained from the femoral neck, Ward’s triangle, and the intertrochanteric area separately. For each area, the T-score and Z-score, which represent differences in BMD from the maximum bone density of young and same aged people, respectively, were calculated. According to the diagnostic criteria of the World Health Organization (WHO), osteoporosis was defined as any T-score lower than -2.5, osteopenia as any T-scores between -2.5 and -1.0, and normal bone density as any T-scores equal to or greater than -1.0 (11).
Patients were divided into three groups by age; group 1 (50-59 years), group 2 (60-69 years), and group 3 (70-79 years). The BMD values and prevalence of osteoporosis in each age group were compared with those of age-matched control. The BMD values and prevalence of osteoporosis of the control were based on the reference data from healthy community dwelling Korean population (6). These reference data were obtained from a study that used a large cohort of community dwelling Korean women, 20 to 79 years of age. This study included the entire female age group, including women with osteoporosis, and had 599 women 50 to 59 years of age, 894 women 60 to 69 years of age, and 313 women 70 to 79 years of age, and it provided age- and site-specific BMD reference values. This study measured the lumbar (L1–4) and hip locations. The equipment used in this study was DPX Bravo (GE Healthcare, Madison, WI, USA), which is small and measures only hip and spine BMD.
Statistical analysis
Results are reported as means with standard deviations or frequencies with 95% confidence intervals (CI). When appropriate, ranges are supplied. Continuous variables between patients and controls were compared with use of independent-sample t tests. The prevalence of osteoporosis in each group was compared by chi-square test. All statistical analyses were performed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA), and P-values < 0.05 were considered statistically significant.
Ethics statement
This study protocol was reviewed and approved by the institutional review board of Asan Medical Center (IRB No. AMC-IRB 2010-0530). Informed consent was waived for this study since it is an observational study with minimal risk.
RESULTS
The mean height, weight, and body mass index (BMI) of the total 206 patients were 155.7 ± 5.2 cm, 55.7 ± 5.6 kg, and 23.1 ± 2.8 kg/m2, respectively. The differences of the mean height, weight, and BMI in each group were not statistically significant compared to control group (Table 1).
Table 1. The mean height, weight, and body mass index (BMI) of the patients and control groups.
| Groups | Height, cm | Weight, kg | BMI, kg/m2 | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Group 1 | ||||||
| Patients (n = 71) |
153.8 | 5.5 | 59.2 | 7.1 | 24.9 | 3.5 |
| Controls (n = 599) |
153.1 | 4.9 | 58.9 | 8.1 | 25.4 | 2.9 |
| P = 0.125 | P = 0.735 | P = 0.101 | ||||
| Group 2 | ||||||
| Patients (n = 93) |
153.2 | 5.2 | 54.5 | 5.4 | 23.3 | 2.8 |
| Controls (n = 894) |
150.8 | 5.1 | 55.7 | 8.2 | 24.5 | 3.2 |
| P = 0.592 | P = 0.470 | P = 0.100 | ||||
| Group 3 | ||||||
| Patients (n = 42) |
151.8 | 3.2 | 52.3 | 6.7 | 24.1 | 3.5 |
| Controls (n = 313) |
147.6 | 5.8 | 51.8 | 8.7 | 23.8 | 3.4 |
| P = 0.733 | P = 0.154 | P = 0.190 | ||||
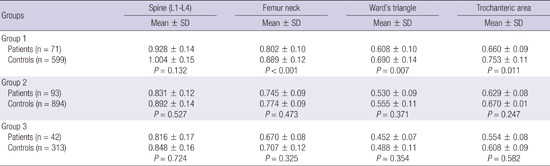
In group 1, except for the spine, all BMD values of the patients were significantly lower than those of age-matched control. While the BMD values in groups 2 and 3 were lower than those of age-matched controls, those differences were not statistically significant (Table 2).
Table 2. Comparison of bone mineral density (g/cm2) at measured sites according to age groups.
| Groups | Spine (L1-L4) | Femur neck | Ward's triangle | Trochanteric |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Group 1 | ||||
| Patients (n = 71) |
0.928 ± 0.14 | 0.802 ± 0.10 | 0.608 ± 0.10 | 0.660 ± 0.09 |
| Controls (n = 599) |
1.004 ± 0.15 | 0.889 ± 0.12 | 0.690 ± 0.14 | 0.753 ± 0.11 |
| P = 0.132 | P < 0.001 | P = 0.007 | P = 0.011 | |
| Group 2 | ||||
| Patients (n = 93) |
0.831 ± 0.12 | 0.745 ± 0.09 | 0.530 ± 0.09 | 0.629 ± 0.08 |
| Controls (n = 894) |
0.892 ± 0.14 | 0.774 ± 0.09 | 0.555 ± 0.11 | 0.670 ± 0.01 |
| P = 0.527 | P = 0.473 | P = 0.371 | P = 0.247 | |
| Group 3 | ||||
| Patients (n = 42) |
0.816 ± 0.17 | 0.670 ± 0.08 | 0.452 ± 0.07 | 0.554 ± 0.08 |
| Controls (n = 313) |
0.848 ± 0.16 | 0.707 ± 0.12 | 0.488 ± 0.11 | 0.608 ± 0.09 |
| P = 0.724 | P = 0.325 | P = 0.354 | P = 0.582 |
L1-L4, from the first lumbar vertebrae to the fourth vertebrae; SD, standard deviation.
One hundred and six (51.5%) of the 206 patients were newly diagnosed with osteoporosis. Osteoporosis was diagnosed most frequently at the Ward’s triangle (101 of 206 patients; 49.0%), followed by the spine (93 of 206 patients; 45.1%), the femoral neck (14 of 206 patients; 6.8%), and the trochanteric area (9 of 206 patients; 4.4%). Compared with age-matched controls, all groups had significantly higher prevalence rates of osteoporosis at the Ward’s triangle; however, the prevalence of osteoporosis at the spine, femoral neck and trochanteric area were not significantly different from those of age-matched controls (Table 3).
Table 3. Comparison of prevalence of osteoporosis (%) at measured sites according to age groups.
| Groups | Spine | Femur neck | Ward's triangle | Trochanter area |
|---|---|---|---|---|
| Group 1 | ||||
| Patients (n = 71) |
29.6 | 1.4 | 25.4 | 2.8 |
| Controls (n = 599) |
21.5 | 1.7 | 5.8 | 0.5 |
| P = 0.513 | -* | P < 0.001 | P = 0.117 | |
| Group 2 | ||||
| Patients (n = 93) |
54.8 | 4.3 | 50.5 | 4.3 |
| Controls (n = 894) |
51.3 | 11.4 | 36.4 | 4.3 |
| P = 0.724 | -* | P = 0.036 | -* | |
| Group 3 | ||||
| Patients (n = 42) |
50.0 | 23.8 | 85.7 | 7.1 |
| Controls (n = 313) |
60.2 | 36.7 | 62.8 | 13.1 |
| -* | -* | P = 0.031 | -* | |
| Total | ||||
| Patients (n = 206) |
45.1 | 6.8 | 49.0 | 4.4 |
| Controls (n = 1,806) |
40.1 | 12.4 | 28.4 | 4.4 |
| P = 0.831 | -* | P = 0.042 | -* |
*The prevalence of osteoporosis of control is higher than patients.
DISCUSSION
We analyzed the BMD and prevalence of osteoporosis in postmenopausal Korean women with low-energy distal radius fractures. The patients were divided into three groups by age, and results were compared with age-matched normal Korean women without fractures. This study showed that prevalence of osteoporosis at all measurement sites in the patients was not significantly lower than those of age-matched controls except at the Ward’s triangle. The Ward’s triangle, however, is not considered by International Society for Clinical Densitometry to be a suitable site for bone density measurement, because the rate of osteoporotic prevalence at this site can be overestimated (12,13). In this regard, our results seems to be against the previous studies, which showed the prevalence of osteoporosis in patients with distal radius fractures to be higher than that of the control (3,14,15,16). In this study, however, 106 new cases of 206 patients (51.5%) of distal radius fractures were diagnosed as osteoporosis. The rates of newly diagnosed osteoporosis were 21 of the 71 patients (29.6%) aged 50-59 years, 51 of the 93 patients (54.8%) aged 60-69 years, and 39 of the 42 patients (92.8%) aged 70-79 years. Although the prevalence of osteoporosis of patients in our study was comparable with those of age-matched controls at all measured sites, except the Ward’s triangle, our results indicate that osteoporosis should be evaluated and managed by screening BMD in patients with distal radius fractures.
Our study results showed that the BMD values were lower in all measured area in all patients’ groups; however, it was interesting that only the BMD in the femur area in group 1 was significantly lower than age-matched control. There has been still inconsistency in correlation between low BMD and age. In one study, patients younger than 65 years of age with distal radius fractures had significantly low BMD values, whereas low BMD values associate with older patients in another study (2,17). Our results are partly consistent with those of a previous study, which showed younger postmenopausal women with distal radius fractures to have low BMD values. The rate of bone loss in lumbar vertebrae decreases after menopause and stabilizes in women at 60-69 years of age, whereas in the femur it continues or accelerates with age (18,19). Therefore, postmenopausal women at 50-59 years of age with low-energy distal radius fractures should be evaluated for osteoporosis to prevent other osteoporotic fractures such as those of the hip and spine.
The BMD of women peaks in their 30s and declines thereafter with age, greatly decreasing during the perimenopausal period (20). While healthy Korean women have peak BMD values, bone loss is more rapid after 50 years of age compared to Caucasian and Lebanese women (6). Early detection of osteoporosis in younger postmenopausal women with distal radius fractures may help to prevent future osteoporotic fractures. Low BMD values associate with the severity of osteoporotic distal radius fractures (21). In contrast with distal radius fractures, hip and spine fractures were associated with an increased mortality (22). Our results indicate that an osteoporotic hip fracture can occur after a distal radius fracture in younger postmenopausal women with distal radius fractures.
The major limitation of this study was that normative data were based on the reference data from Korean community dwelling population (6). BMD values obtained by different densitometers cannot be compared because technical differences in the devices exist. Nevertheless, we were able to compare BMD values with reference data because the densitometer and measurement sites were identical.
Footnotes
Funding: This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea. (Grant number: HI13C1634)
DISCLOSURE: Each author certifies that he or she, or a member of his or her immediate family, has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with this article.
AUTHOR CONTRIBUTION: Conception and coordination of the study: Jeon IH, Yoon JO. Design of ethical issues: Jung HJ, Kim JS. Data acquisition: Jung HJ, Park HY. Data analysis and interpretation: Jung HJ, Park HY. Statistical analysis: Jung HJ, Park HY. Drafting of the manuscript: Jung HJ, Park HY, Kim JS, Yoon JO. Critical revision of the manuscript: Jung HJ, Jeon IH. Receiving grant: Jeon IH. Approval of final manuscript: all authors.
References
- 1.Chung KC, Shauver MJ, Birkmeyer JD. Trends in the United States in the treatment of distal radial fractures in the elderly. J Bone Joint Surg Am. 2009;91:1868–1873. doi: 10.2106/JBJS.H.01297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanterewicz E, Yañez A, Pérez-Pons A, Codony I, Del Rio L, Díez-Pérez A. Association between Colles’ fracture and low bone mass: age-based differences in postmenopausal women. Osteoporos Int. 2002;13:824–828. doi: 10.1007/s001980200114. [DOI] [PubMed] [Google Scholar]
- 3.Oyen J, Brudvik C, Gjesdal CG, Tell GS, Lie SA, Hove LM. Osteoporosis as a risk factor for distal radial fractures: a case-control study. J Bone Joint Surg Am. 2011;93:348–356. doi: 10.2106/JBJS.J.00303. [DOI] [PubMed] [Google Scholar]
- 4.Sosa M, Saavedra P, Gómez-Alonso C, Mosquera J, Torrijos A, Muñoz-Torres M, Valero Díaz de la Madrid C, Díaz Curiel M, Martínez Díaz Guerra G, Pérez-Cano R, et al. Postmenopausal women with Colles’ fracture have bone mineral density values similar to those of controls when measured with calcaneus quantitative ultrasound. Eur J Intern Med. 2005;16:561–566. doi: 10.1016/j.ejim.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Lee JO, Chung MS, Baek GH, Oh JH, Lee YH, Gong HS. Age- and site-related bone mineral densities in Korean women with a distal radius fracture compared with the reference Korean female population. J Hand Surg Am. 2010;35:1435–1441. doi: 10.1016/j.jhsa.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Cui LH, Choi JS, Shin MH, Kweon SS, Park KS, Lee YH, Nam HS, Jeong SK, Im JS. Prevalence of osteoporosis and reference data for lumbar spine and hip bone mineral density in a Korean population. J Bone Miner Metab. 2008;26:609–617. doi: 10.1007/s00774-007-0847-8. [DOI] [PubMed] [Google Scholar]
- 7.Kanis JA, Johnell O, De Laet C, Johansson H, Oden A, Delmas P, Eisman J, Fujiwara S, Garnero P, Kroger H, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35:375–382. doi: 10.1016/j.bone.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 8.Mallmin H, Ljunghall S, Persson I, Naessén T, Krusemo UB, Bergström R. Fracture of the distal forearm as a forecaster of subsequent hip fracture: a population-based cohort study with 24 years of follow-up. Calcif Tissue Int. 1993;52:269–272. doi: 10.1007/BF00296650. [DOI] [PubMed] [Google Scholar]
- 9.Earnshaw SA, Cawte SA, Worley A, Hosking DJ. Colles’ fracture of the wrist as an indicator of underlying osteoporosis in postmenopausal women: a prospective study of bone mineral density and bone turnover rate. Osteoporos Int. 1998;8:53–60. doi: 10.1007/s001980050048. [DOI] [PubMed] [Google Scholar]
- 10.Rozental TD, Makhni EC, Day CS, Bouxsein ML. Improving evaluation and treatment for osteoporosis following distal radial fractures. A prospective randomized intervention. J Bone Joint Surg Am. 2008;90:953–961. doi: 10.2106/JBJS.G.01121. [DOI] [PubMed] [Google Scholar]
- 11.Kanis JA, Melton LJ, 3rd, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–1141. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 12.Lewiecki EM, Kendler DL, Kiebzak GM, Schmeer P, Prince RL, El-Hajj Fuleihan G, Hans D. Special report on the official positions of the International Society for Clinical Densitometry. Osteoporos Int. 2004;15:779–784. doi: 10.1007/s00198-004-1677-3. [DOI] [PubMed] [Google Scholar]
- 13.Kanis JA, Glüer CC. An update on the diagnosis and assessment of osteoporosis with densitometry. Committee of Scientific Advisors, International Osteoporosis Foundation. Osteoporos Int. 2000;11:192–202. doi: 10.1007/s001980050281. [DOI] [PubMed] [Google Scholar]
- 14.Itoh S, Ohta T, Samejima H, Shinomiya K. Bone mineral density in the distal radius in a healthy Japanese population and in relation to fractures of the distal radius. J Hand Surg [Br] 1999;24:334–337. doi: 10.1054/jhsb.1999.0073. [DOI] [PubMed] [Google Scholar]
- 15.Gong HS, Oh WS, Chung MS, Oh JH, Lee YH, Baek GH. Patients with wrist fractures are less likely to be evaluated and managed for osteoporosis. J Bone Joint Surg Am. 2009;91:2376–2380. doi: 10.2106/JBJS.H.01871. [DOI] [PubMed] [Google Scholar]
- 16.Löfman O, Hallberg I, Berglund K, Wahlström O, Kartous L, Rosenqvist AM, Larsson L, Toss G. Women with low-energy fracture should be investigated for osteoporosis. Acta Orthop. 2007;78:813–821. doi: 10.1080/17453670710014608. [DOI] [PubMed] [Google Scholar]
- 17.Lashin H, Davie MW. DXA scanning in women over 50 years with distal forearm fracture shows osteoporosis is infrequent until age 65 years. Int J Clin Pract. 2008;62:388–393. doi: 10.1111/j.1742-1241.2007.01395.x. [DOI] [PubMed] [Google Scholar]
- 18.Arlot ME, Sornay-Rendu E, Garnero P, Vey-Marty B, Delmas PD. Apparent pre- and postmenopausal bone loss evaluated by DXA at different skeletal sites in women: the OFELY cohort. J Bone Miner Res. 1997;12:683–690. doi: 10.1359/jbmr.1997.12.4.683. [DOI] [PubMed] [Google Scholar]
- 19.Ensrud KE, Palermo L, Black DM, Cauley J, Jergas M, Orwoll ES, Nevitt MC, Fox KM, Cummings SR. Hip and calcaneal bone loss increase with advancing age: longitudinal results from the study of osteoporotic fractures. J Bone Miner Res. 1995;10:1778–1787. doi: 10.1002/jbmr.5650101122. [DOI] [PubMed] [Google Scholar]
- 20.Sorenson JA, Cameron JR. A reliable in vivo measurement of bone-mineral content. J Bone Joint Surg Am. 1967;49:481–497. [PubMed] [Google Scholar]
- 21.Clayton RA, Gaston MS, Ralston SH, Court-Brown CM, McQueen MM. Association between decreased bone mineral density and severity of distal radial fractures. J Bone Joint Surg Am. 2009;91:613–619. doi: 10.2106/JBJS.H.00486. [DOI] [PubMed] [Google Scholar]
- 22.Johnell O, Kanis JA, Odén A, Sernbo I, Redlund-Johnell I, Petterson C, De Laet C, Jönsson B. Mortality after osteoporotic fractures. Osteoporos Int. 2004;15:38–42. doi: 10.1007/s00198-003-1490-4. [DOI] [PubMed] [Google Scholar]


