Abstract
Human immunodeficiency virus (HIV)-1 integrase (IN), which mediates integration of viral cDNA into the cellular chromosome, is a validated antiviral drug target. Three IN inhibitors, raltegravir, elvitegravir and dolutegravir, have been clinically approved since 2008. However, drug resistance have emerged in infected patients receiving treatment using these drugs which share the same mechanism of action and have a low genetic barrier for resistance. Therefore, there is an urgent need to develop drugs with novel mechanism. IN requires a precise and dynamic equilibrium between several oligomeric species for its activities. The modulation of the process which is termed as IN oligomerization, presents an interesting allosteric target for drug development. In this research, we developed a magnetic beads based approach to assay the IN dimerization. Then, using the assay we screened a library of 1000 Food and Drug Administration (FDA)-approved drugs for IN dimerization inhibitors and identified dexlansoprazole as a potential IN dimerization inhibitor. In conclusion, the assay presented here has been proven to be sensitive and specific for the detection of IN dimerization as well as for the identification of antiviral drugs targeting IN dimerization. Moreover, a FDA-approved proton-pump inhibitors, dexlansoprazole, was identified as a potential inhibitor for IN dimerization.
Retroviruses such as HIV-1 are characterized by integration of reverse-transcribed viral genome into the host cell chromosome1. Viral integration, which is catalyzed by HIV-1 integrase (IN), comprises two spatially and temporally distinct steps, 3′ processing and strand transfer2. As a critical enzyme in the viral life cycle, IN is currently targeted by three FDA-approved drugs: raltegravir (RAL), elvitegravir (EVG) and dolutegravir (DTG)3. All these drugs have the same mechanism of action: blocking the strand transfer activity of IN and are collectively termed as IN strand transfer inhibitors (INSTIs). However, significant cross-resistance has been observed within INSTIs in infected patients receiving treatment4,5,6,7. As a consequence, there is an urgent need to develop novel drugs with mechanism distinct from INSTIs to avoid existing and emerging multi-drug resistant HIV-1 strains.
IN is found as an equilibrium of monomers, dimers, tetramers, and even higher multimeric forms during integration, which is termed as IN oligomerization8. IN dimerization has been shown to be a plausible therapeutic target, for which several compounds and peptides have been found to display inhibitory activity9.
Recently, an AlphaScreen technology-based method for screening IN dimerization inhibitor was reported. However, this method has an obvious limitation: the requirement of expensive and sophisticated instruments which are not available to all laboratories. Moreover, a homogeneous time-resolved fluorescence based (HTRF) assay for detection of IN dimerization was reported and used to study the dynamics of IN dimerization11. However, to the best of our knowledge, this assay has not been validated for high-throughput screening (HTS) or used for the screening of inhibitors targeting IN.
Drug repositioning is the process of identifying new uses for drugs outside the scope of their original medical indication12. By exploiting existing knowledge of drugs, drug repositioning can offer a faster and cheaper approach than traditional drug discovery13. Drug repositioning has become an increasingly important part of the drug development landscape, with many pharmaceutical and biotech companies now having repositioning programs14. With lower costs, shorter development times and higher success rates, drug repositioning is also ideally suited for academia-based drug discovery14.
In this study, we developed a novel IN dimerization assay. Using the method, we undertook a drug repositioning screen to identify unknown IN dimerization inhibitory activity for known drugs. Besides, to provide confidence in our hits during screening, we implemented a counterscreen to eliminate molecules that interfere with the screening method itself.
Results and Discussion
Principle of the assay
The principle of the method is illustrated in Fig. 1A. In the assay, GST-tagged IN (yellow) is mixed with His6-tagged IN (green) at the desired concentrations. Incubation at room temperature allows the formation of GST-IN/His6-IN heterodimers as well as GST-IN and His6-IN homodimers. Then, heterodimers will be captured by Ni2+ -coated magnetic beads (red) through C-terminal His6-tag and detected by alkaline phosphatase conjugated anti-GST antibody (dark red) through its N-terminal GST-tag. Whereas, neither of two kind of homodimers will be captured by Ni2+ -coated magnetic beads and detected by alkaline phosphatase conjugated anti-GST antibody simultaneously. Hence, GST-IN/His6-IN heterodimers will be detected as IN dimerization by the assay. The presence of modulating compounds in the assay will change the population of heterodimers or block the formation of them, resulting in an altered output signal and can be picked up by the spectrophotometer.
Figure 1. Development of an in vitro screening method to identify IN dimerization inhibitors.
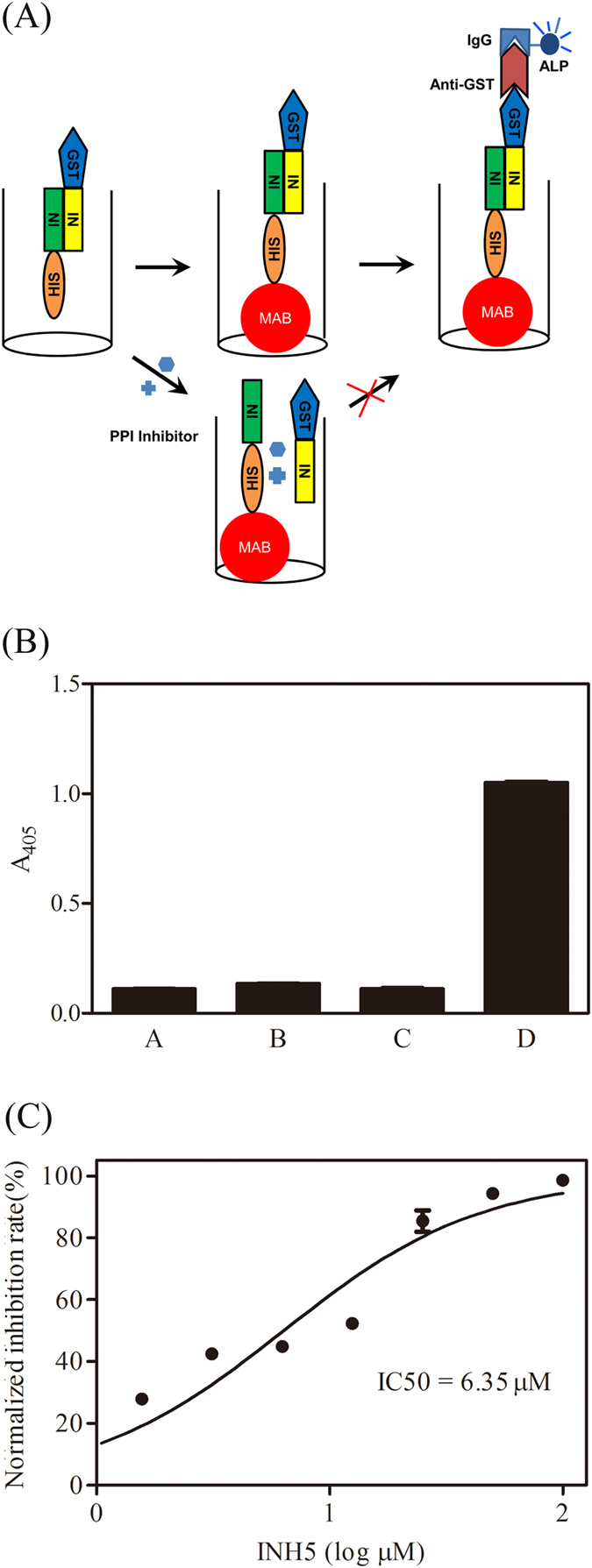
(A) The schemes depict the principle of the assay for IN dimerization. GST–tagged IN (yellow) is mixed with His6-tagged IN (green) at the desired concentrations. Incubation at room temperature allows the formation of GST-IN/His6-IN heterodimers as well as GST-IN and His6-IN homodimers. Then, heterodimers will be captured by Ni2+ -coated magnetic beads (red) through C-terminal His6-tag and detected by alkaline phosphatase conjugated anti-GST antibody (dark red) through its N-terminal GST-tag. Changes in the population of heterodimers due to the presence of modulating compounds will result in an altered output signal and can be picked up. (B) Assay performance under optimal reaction conditions. Bars (A–C) represent signals produced from negative controls in the absence of either GST-IN and His6-IN or GST-IN or His6-IN respectively. Bar D represent signals produced from IN dimerization. (C) Inhibition of IN dimerization by peptide INH5. Error bars represent SD from 3 replicate values.
Assay development, performance evaluation and validation
This study aimed to establish a high-throughput assay for screening inhibitors targeting IN dimerization. To optimize the assay, titration experiments were performed. The optimal concentration of GST-IN and His6-IN was determined to be 20 nM respectively. The primary antibody was prepared at a 1/4000 and secondary antibody at 1/5000. IN has the propensity to aggregate15, and theoretically IN tetramers and higher order oligomers containing both GST- and His6-tags may also be captured by magnetic beads and detected by alkaline phosphatase conjugated anti-GST antibody in the assay. Generally, we cannot fully exclude that only IN dimers contribute to signal generation. However, in previous studies, the dissociation constants of IN dimers and tetramer were determined to be 67.8 pM and 22 μM11,16. In their research, Tsiang et al. suggested that at the integrase concentrations used a maximum of 67.5 nM, the presence of integrase tetramer is negligible (0.068% of total integrase)11. Accordingly, in our study, both GST-IN and His6-IN were used at 20 nM, and the total concentration of IN was 40 nM which was less than 67.5 nM used by Tsiang et al. Therefore, in our assay, most of the IN existed in the form of dimer, the presence of IN tetramer and multimer should be omitted for simplicity.
Z′ factor has been defined to evaluate suitability of an assay for HTS and signal-to-noise ratio (S/N), signal-to-background ratio (S/B) are widely used to indicate the quality of an assay17,18,19. To test the suitability and quality of the assay, we measured the signal of all reactions under optimal reaction conditions. As shown in Fig. 1B, the negative controls in the absence of either GST-IN and His6-IN or GST-IN or His6-IN showed background readings measured as absorbance at 405 nm (A405), lower than 0.11. Wheras, the IN dimerization gave a signal of 1.05. Accordingly, we determined the S/N, S/B and z′ factor values of the assay and found that S/N, S/B and z′ factor values was 295, 10 and 0.98 respective, which all exceeded the minimum criteria (S/N > 10, S/B > 3 and z′ > 0.5). Taken together, these values of statistical parameters confirmed that the assay developed was high sensitivity, specificity, and was suitable to be developed for HTS.
Peptide INH5 (ATDQAEHLKTAVQMAVFIHNYKA-CONH2), which was previously reported as an inhibitor of IN dimerization, was used as a probe to test whether the HTS assay was effective for drug screening targeting IN dimerization20. As shown in Fig. 1C, INH5 inhibited the IN dimerization with an IC50 of 6.35 μM (95% confidence interval, 2.45 μM to 16.47 μM) which was are comparable to previous experiment data 4.50 μM, indicating that the assay can robustly detect decreases in signal and is therefore effective and suitable for a HTS application10.
Identifying IN dimerization inhibitory activities for existing drugs
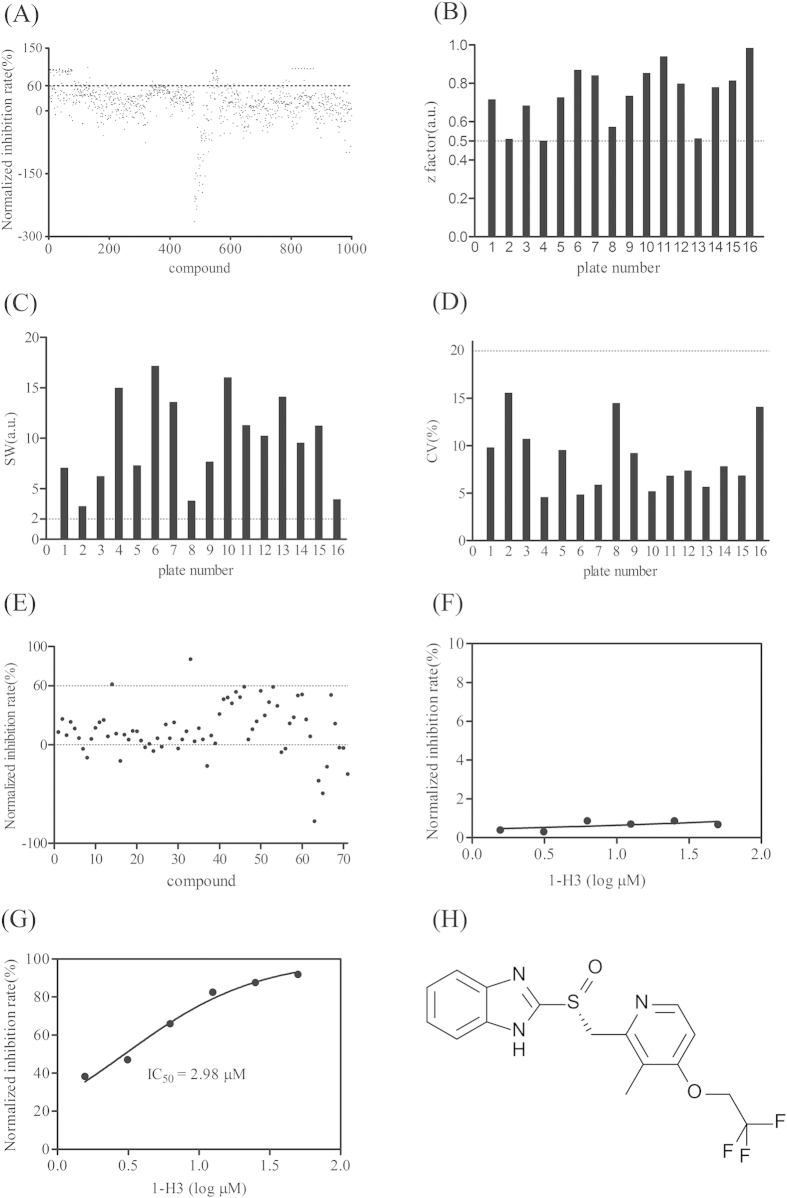
The optimized IN dimerization assay was used to screen a partial library of pharmacologically active compounds for inhibitors of IN dimerization. Compounds were screened as described in materials and methods section, with appropriate comparison controls included on each plate. Hits were scored as compounds that showed an inhibition ratio of the signal higher than 60% in the assays. The z′, signal window (SW), and coefficient of variation (CV) of each plate were calculated and then compared with the minimum pass criteria (z′ > 0.5, SW > 2.0, CV < 20%) to evaluate the quality of the HTS data. In a primary screening, at the concentration of 30 μM, 71 of the 1000 initial compounds were identified as hits, with a hit rate of 7.1% (Fig. 2A). All HTS campaigns in the primary screening exceeded the minimum criteria (z′ > 0.5, SW > 2, and CV < 20%) (Fig. 2B–D), indicating that the results of the screening were reliable and could be used for further investigated. Then, 71 compounds screened out from the primary screening were re-screened and only one compound, dexlansoprazole (Fig. 2H), showed an excellent inhibitory rate of 87.17% (Fig. 2E). All HTS campaigns in the confirmatory screening also exceeded the minimum criteria (z′ > 0.5, SW > 2, and CV < 20%), as such dexlansoprazole could be used for further study.
Figure 2. Results of the drug repositioning screen for IN dimerization inhibitors.
(A) Scatterplots of the primary screening using the developed assay. Dashed line shows the ‘hits’ selection criteria (>60% disruption of a PPI complex); z′ factor (B), SW (C)and CV (D) of each plate in the primary screening. Dashed lines show the minimum pass criteria (z′ > 0.5, S/N > 10, S/B > 3, SW > 2, CV < 20%). (E) Scatterplots of the confirmatory screening using the developed assay. Dashed line shows the ‘hits’ selection criteria (>60% disruption of a PPI complex). Z′ factor, SW and CV of the confirmatory screening were 0.99, 6.19 and 10.8% respectively. (F) Counterscreen using His6-GST protein was used to analyze the interference between dexlansoprazole and the assay system; (G) Dexlansoprazole identified in the screening exhibited a sigmoidal dose-dependent reduction of assay signal; (H) The chemical structure of dexlansoprazole.
Non-specificity counterscreen validation
In a previous study, Schorpp et al. suggested that in a drug screening system using His6-tag protein and nickel chelating beads, false-positive compounds could be identified21. This can be attributed to two possible reasons. One is hydrogen-bonded complexes formed between his-tagged protein and the compound would prevent immobilization of the protein on the beads surface. The other is the compound may act as nickel chelating agents and adsorb on the bead surface, thus preventing binding of the proteins to the beads surface. Thus, in this study, a nonspecific counterscreen was performed to confirm dexlansoprazole is a specific inhibitor for IN dimerization rather than a false positive interfering with the assay system. In the counterscreen assay, His6-GST protein was used, which could bind the Ni2+ coupling magnetic beads and be detected by GST antibody, generating an extremely strong signal. Any compound that interferes with beads or with the His6-tag of His6-GST protein will result in a decreased signal in the counterscreen assay. The results (Fig. 2F) showed that dexlansoprazole almost had no effect on the assay system, whereas displayed a dose-dependent effect on IN dimerization with an IC50 value of 2.98 μM (Fig. 2G). All these results indicate that dexlansoprazole is a specific small molecular inhibitor for IN dimerization.
Conclusion
Based on Ni-NTA magnetic agarose beads, we have developed a highly sensitive and effective screening method to identify inhibitors of IN dimerization, and have successfully identified a FDA-approved drug dexlansoprazole as a potential small molecular inhibitor of IN dimerization, which may therefore serve as a lead compound for further optimization.
Methods
Construction of protein expression plasmids
The full-length HIV-1 IN coding sequence was amplified by PCR from pET28a-IN with a His6 fusion tag on its N-terminus (conserved in our lab). Then, the amplified IN gene was subcloned into Nco I-Xho I sites of the vector pET28a to create the pET28a-IN for expression of recombinant C-terminal His6–tagged IN protein (IN-His6). In a similar manner, the amplified IN gene was subcloned into the BamH I-Sal I sites of the vector pGEX-4 T-1, creating pGEX-4T-IN for expression of recombinant N-terminal GST-tagged IN protein (GST-IN). The pGEX-4T-His6 plasmid for expression of recombinant C-terminal His6-tagged GST (His6-GST) used for counterscreen was constructed by inserting the His6-tag encoding sequences (CACCACCACCACCACCACTGA) to the BamH I-Sal I sites of the plasmid pGEX-4T-1.
Recombinant proteins expression and purification
IN-His6 was expressed and purified by affinity chromatography as previously described22. GST-IN and double-tagged GST-His6 protein were produced using standard conditions as previously described10. The concentration of the all protein was determined by the Bradford assay with bovine serum albumin (BSA) as a standard. 12% SDS-PAGE was performed to determine the purity of the proteins.
The IN dimerization assay
The IN dimerization assay was developed based on Ni-NTA magnetic agarose beads. The assay was performed in 96-well NBS microplate in a final volume of 100 μl per well. Briefly, the assay works as follows. Compounds and proteins were diluted to 10× and 5× working solutions in the 1× assay buffer (25 mM tris-HCl, pH 7.4, 150 mM NaCl, 1 mM MgCl2, 0.05% BSA[v/w]) respectively. First, 10 μl compound dilution, 50 μl 1× buffer, 20 μl each of protein dilutions were pipetted into the wells. The plate was sealed and left to incubate at 200 rpm for 3 h at room time (RT). Next, the 10 μl magnetic beads were added followed by incubating at 900 rpm for 30 min at RT. Then, the plate was placed on a magnetic concentrator, the supernatant was discarded, and the wells were washed 3 times with 200 μl wash buffer (50 mM sodium phosphate, pH 7.4, 150 mM NaCl, 0.005% Tween-20[v/v], 20 mM imidazole). The wells were incubated with 100 μl mouse anti-GST antibody diluted 1:4000 in antibody dilution buffer (50 mM sodium phosphate, pH 7.4, 150 mM NaCl, 0.005% Tween-20[v/v], 0.5%[w/v]BSA) at 900 rpm for 30 min at 37 °C, followed by washing three times as above. The wells were incubated with 100 μl rabbit anti-mouse IgG alkaline phosphatase conjugated antibody diluted 1:5000 in antibody dilution buffer at 900 rpm for 30 min at 37 °C, followed by washing three times as above. The color reaction was developed with 100 μl substrate buffer (10 mM diethanolamine, pH 9.8, 0.5 mM MgCl2, 8 mM p-NPP) for 30 min at 37 °C. The plate was read at 405 nm with an Enspire multimode reader (PerkinElmer, USA). The reproducibility, signal stability, and robustness were determined for PPI assay to ensure it was HTS compatible. Also one IN dimerization inhibitor INH5 was used to validate the assay for HTS10.
Library screening for inhibitors of IN dimerization
In total, 1000 compounds were selected at random from ‘The Spectrum Collection’ purchased from National compound resource center. The library, which is composed of 2,320 compounds in 10 mM DMSO solution, lists primarily FDA-approved human therapeutic drugs, drug-like compounds, and natural products. Each compound was diluted in DMSO to reach a concentration of 300 μM, which was used as the 10× compound stock. In all screening campaigns, no-protein negative controls (including GST-IN was omitted or IN-His was omitted or both protein were omitted) and the positive control contained 10% v/v DMSO only. The negative and positive control with 8 replicates wells each located randomly among the 96 wells of each plate. The high-throughput assay was carried out in replicate in 96-well microplates as above.
A counterscreen was carried out as described above, replacing purified IN-His6 and GST-IN with 20 nM His6-tagged GST protein.
Data Analysis
The z′ factor, signal-to-noise ratio (S/N), signal-to-background ratio (S/B), signal window (SW), and coefficient of variation (CV) were calculated and then compared with the minimum pass criteria (z′ > 0.5, S/B > 3, SW > 2, CV < 20%)21. The ‘hits’ for PPI assays were classified as compounds that led to > 60% disruption of a PPI complex. All HTS data were processed using Excel (Microsoft Corp.) and visualized using Prism 5.0 (GraphPad Software).
Additional Information
How to cite this article: Dawei, Z. et al. A novel assay for screening inhibitors targeting HIV-1 integrase dimerization based on Ni-NTA magnetic agarose beads. Sci. Rep. 6, 25375; doi: 10.1038/srep25375 (2016).
Acknowledgments
This research was financially supported by the National Natural Science Foundation of China (Grant Nos. 31170016 and 81202438), Special Program for Applied Research on Super Computation of the NSFC-Guangdong Joint Fund (the second phase) and the Program for Innovative Research Team in IMPLAD (PIRTI-IT1302). The authors thank national compound resource center (Shanghai, China) for supplying the small compounds used in screening.
Footnotes
Author Contributions D.Z., Z.M. and S.G. conceived and designed the experiments. D.Z. performed the experiments. D.Z. and M.L. analyzed the data. D.Z. and H.H. wrote the manuscript. All authors reviewed the manuscript.
References
- Craigie R. & Bushman F. D. H. I. V. DNA integration. Cold Spring Harb Perspect Med. 2, a006890 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craigie R. The molecular biology of HIV integrase. Future Virol. 7, 679–89 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazuda D. J. HIV integrase as a target for antiretroviral therapy. Curr Opin HIV AIDS 7, 383–89 (2012). [DOI] [PubMed] [Google Scholar]
- Malet I. et al. New raltegravir resistance pathways induce broad cross-resistance to all currently used integrase inhibitors. J Antimicrob Chemoth. 69, 2118–2112 (2014). [DOI] [PubMed] [Google Scholar]
- Sichtig N. et al. Evolution of raltegravir resistance during therapy. J Antimicrob Chemother. 64, 25–32 (2009). [DOI] [PubMed] [Google Scholar]
- Steigbigel R. T. et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med. 359, 339–54 (2008). [DOI] [PubMed] [Google Scholar]
- Métifiot M. et al. Elvitegravir overcomes resistance to raltegravir induced by integrase mutation Y143. AIDS 25, 1175–78 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayouka Z. et al. Inhibiting HIV-1 integrase by shifting its oligomerization equilibrium. Proc Natl Acad Sci USA 104, 8316–21 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L., Larue R. C., Slaughter A., Kessl J. J. & Kvaratskhelia M. Curr Top Microbiol Immunol 389, 1–27 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeulemeester J., Tintori C., Botta M., Debyser Z. & Christ F. Development of an AlphaScreen-based HIV-1 integrase dimerization assay for discovery of novel allosteric inhibitors. J Biomol Screen. 17, 618–28 (2012). [DOI] [PubMed] [Google Scholar]
- Tsiang M. et al. Affinities between the binding partners of the HIV-1 integrase dimer-lens epithelium-derived growth factor (IN dimer-LEDGF) complex. J Bio Chem. 284, 33580–99 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantz A. Protein-site targeting. Diversification of the drug discovery process. Nat Biotechnol. 16, 1294 (1998). [DOI] [PubMed] [Google Scholar]
- Jin G. & Wong S. T. C. Toward better drug repositioning: prioritizing and integrating existing methods into efficient pipelines. Drug Discov Today 19, 637–44 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburn T. T. & Thor K. B. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 3, 673–83 (2004). [DOI] [PubMed] [Google Scholar]
- Guiot E. et al. Relationship between the Oligomeric Status of HIV-1 Integrase on DNA and Enzymatic Activity. J Biol Chem. 281, 22707–19 (2006). [DOI] [PubMed] [Google Scholar]
- Jenkins T. M., Engelman A., Ghirlando R. & Craigie R. A. Soluble active mutant of HIV-1 integrase: involvement of both the core and carboxyl-terminal domains in multimerization. J Biol Chem. 271, 7712–18 (1996). [DOI] [PubMed] [Google Scholar]
- Inglese J. et al. High-throughput screening assays for the identification of chemical probes. Nat Chem Biol. 3, 466–79 (2007). [DOI] [PubMed] [Google Scholar]
- Birmingham A. et al. Statistical methods for analysis of high-throughput RNA interference screens. Nat Methods 6, 569–75 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. H., Chung T. D. Y. & Oldenburg K. R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen., 4, 67–73 (1999). [DOI] [PubMed] [Google Scholar]
- Maroun R. G. et al. Peptide inhibitors of HIV-1 integrase dissociate the enzyme oligomers. Biochemistry 40, 13840–48 (2001). [DOI] [PubMed] [Google Scholar]
- Schorpp K. et al. Identification of small-molecule frequent hitters from AlphaScreen high-throughput screens. J Biomol Screen. 19, 715–26 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H. Q. et al. A novel high-throughput format assay for HIV-1 integrase strand transfer reaction using magnetic beads. Acta Pharmacol Sin. 29, 397–404 (2008). [DOI] [PubMed] [Google Scholar]