Abstract
To further expand the substrate range of the cyclohexylamine oxidase (CHAO) from Brevibacterium oxydans, a library of diverse mutants was created and assayed toward a group of structurally diverse substrates. Among them, mutants T198A and M226A exhibited enhanced activity relative to wt CHAO for most (S)-enantiomers of primary amines and some secondary amines. While mutants T198I, L199I, L199F, M226I and M226T were more active than wt CHAO toward the primary amines, mutants T198F, L199T, Y321A, Y321T, Y321I and Y321F enhanced the enzyme activity toward the secondary amines. In particular, mutant Y321I displayed an enhanced catalytic efficiency toward 1-(4-methoxybenzyl)-1, 2, 3, 4, 5, 6, 7, 8-octahydroisoquinoline (13). Whereas a double mutant, Y321I/M226T, acted on (S)-N-(prop-2-yn-1-yl)-2, 3-dihydro-1H-inden-1-amine [(S)-8]. Since (R)-8 is an irreversible inhibitor of monoamine oxidase and (S)-13 is an intermediate of dextromethorphan, a cough suppressant drug, deracemizations of 8 and 13 were carried out with crude enzyme extracts of the respective mutants. This resulted in 51% and 78% isolated yields of (R)-8 and (S)-13, respectively, each with high enantiomeric excess (93% and 99% ee). The results demonstrated the application potential of the evolved CHAO mutants in drug synthesis requiring chiral secondary amines.
Enantiomerically pure chiral amines are widely used as intermediates for agrochemicals and pharmaceuticals1, and they are of increasing value in organic synthesis. Recently, several kinds of biocatalysts for the synthesis of chiral amines have received significant attention. For example, transaminases2 and ammonia lyases3 are now finding applications in the industrial synthesis of chiral amines. Engineered amino acid dehydrogenases are emerging biocatalysts that are capable of using ketones as substrates for the asymmetric synthesis of chiral amines4. In 2002, Turner et al. reported a novel deracemization strategy for the preparation of optically active chiral amines5. This protocol was applied to the deracemization of primary5,6,7, secondary8,9 and tertiary amines10,11,12 as well as substituted pyrrolidines13 using recombinant monoamine oxidase (MAO-N) mutants obtained by directed evolution. The combination of biocatalytic oxidation with non-stereoselective reduction of the resulting imine by ammonia-borane generally rendered a high enantiomeric excess (ee) of a desired enantiomer from the racemate. In this context, Leisch et al. explored the possibility of a bacterial cyclohexylamine oxidase (CHAO), derived from Brevibacterium oxydans IH-35A, as an alternative biocatalyst for the synthesis of chiral primary amines using either kinetic resolution or deracemization protocol14. The wild-type CHAO (wt CHAO) exhibited low or no activity toward secondary and tertiary amines. We carried out site-specific mutagenesis15, and iterative saturation mutagenesis coupled with functional screening, to afford mutant CHAOs16, which increased the activity toward primary amines15 and extended the substrate specificity to include some secondary amines16. Notably, the substrate 2-methyl-1, 2, 3, 4-tetrahydroquinoline (10) was deracemized by a CHAO triple mutant (T198F/L199S/M226F) leading to the production of (R)-2-methyl-1, 2, 3, 4-tetrahydroquinoline (THQ) with 76% yield and 98% ee. This R-enantiomer is a valuable building block for the synthesis of a variety of THQ derivatives of pharmaceutical or clinical importance17,18,19,20,21,22.
Recently, Vergne-Vaxelaire et al. reported a high throughput orthogonal screening of putative nitrilases against 25 structurally diverse substrates that resulted in over one hundred new nitrilases from a genomic library of prokaryotic origin23. This prompted us to adopt a similar strategy to further explore the substrate specificity of CHAO. Accordingly, a library of site-directed mutants was constructed, and assayed against 18 structurally diverse amines. Among the new mutants, Y321I/M226T and Y321I were applied to deracemization of N-(prop-2-yn-1-yl)-2, 3-dihydro-1H-inden-1-amine (8) and 1-(4-methoxybenzyl)-1, 2, 3, 4, 5, 6, 7, 8-octahydroisoquinoline (13), respectively, as a proof of concept for the synthetic potential of the biocatalysts.
Results
Choice of mutation residues and construction of the mutant library
The solved two-domain structures of CHAO consisting of a cofactor-binding domain and a substrate-binding domain provided a framework for the choice of amino acid residues for mutagenesis24. In the substrate-binding domain, the side chains of Y321, F368 and Y459 form a cage-like structure in which the amine substrate is bound (Fig. 1). This cage-like structure in CHAO is similar to that of the human monoamine oxidase B (MAO B) formed by the side chains of Y398 and Y435 which serve to orient the amine substrates and promote catalysis25. In addition to the substrate-binding cavity, CHAO has a second cavity that is located closer to the protein surface, and possibly serves as a substrate entrance cavity. These two cavities are separated by the side chains of amino acid residues, T198, L199, M226 and F351(Fig. 1)24. Hence, access to the active site from the entrance cavity is blocked by these amino acids and they are postulated to play a “gate-keeping” role in CHAO24.
Figure 1. The active site of cyclohexylamine oxidase (CHAO).
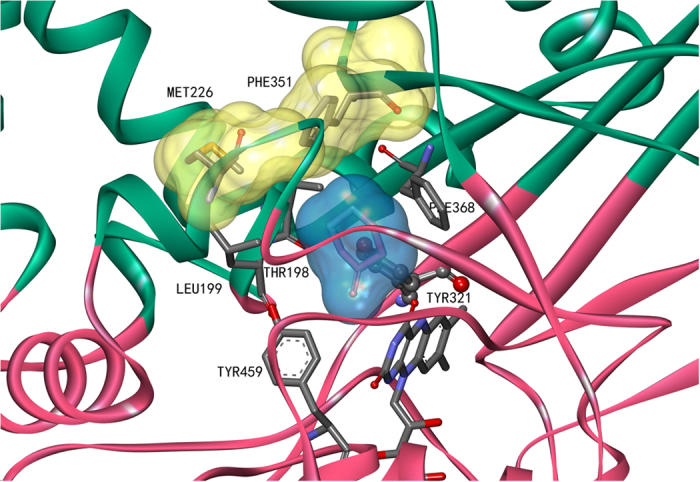
The substrate-binding cavity is shown in blue and the secondary cavity in yellow. The structure according to reference 24 was visualized with Accelrys Discovery Studio 4.1 (Accelrys Inc.).
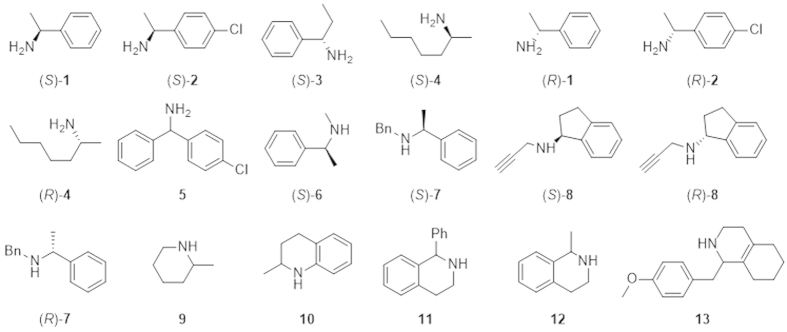
Accordingly, residues T198, L199, M226, Y321, F351, F368 and Y459 were postulated to affect substrate entrance and binding in the catalytic cavity, thus determine the substrate specificity of CHAO. Therefore, these seven amino acids were selected as mutation targets. We adopted the strategy of Verge-Vaxelaire et al. in which the selected residues were mutated to amino acids of different properties to create a library of 51 designed mutants (supplementary Table S1). The mutants were assayed against a group of substrates consisting of 18 structurally diverse amines (Fig. 2).
Figure 2. Substrates used for the activity assay.
Activity assay of the mutant library
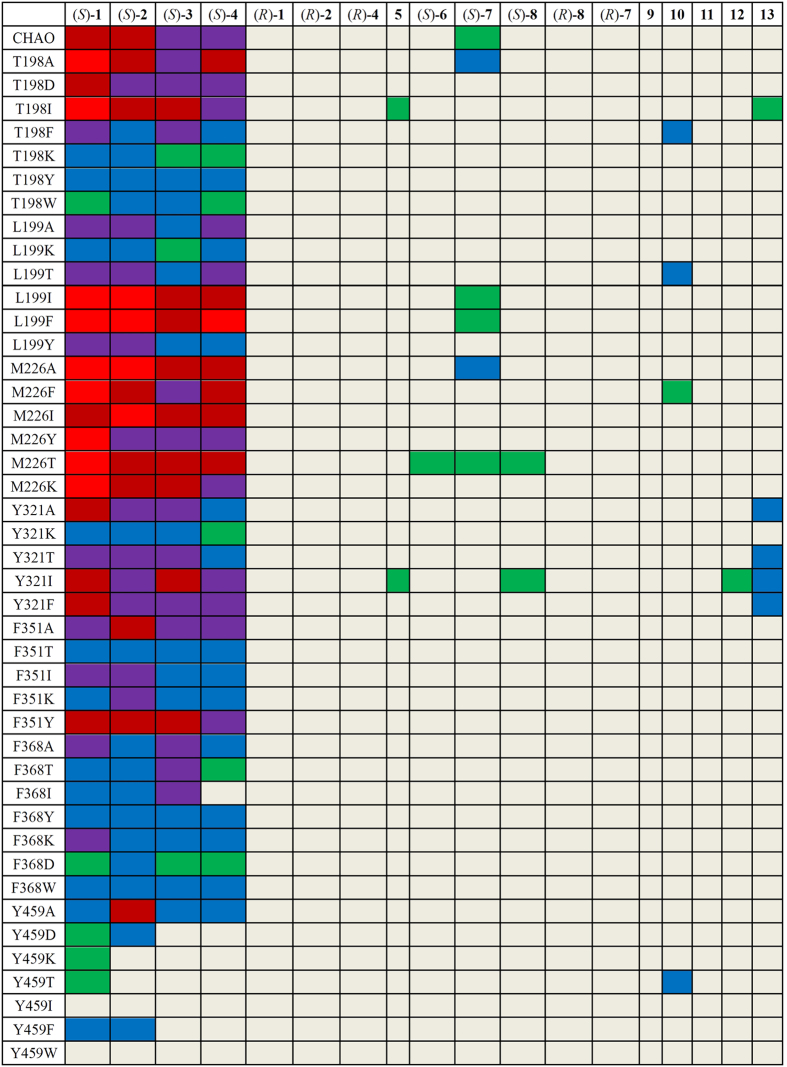
Forty-one of the 51 mutants were produced as soluble proteins in E. coli at 25 °C, but the other mutants (L199D, L199W, M226D, M226W, Y321D, Y321W, F351D, F351W, Y459D and Y459W) were found in inclusion bodies. The trials to solubilize and refolding of the inclusion body proteins were not successful, so these mutants were not investigated further. Equal concentration of the soluble wt CHAO and 41 mutant proteins were used for activity measurements toward the selected group of substrates. These results are shown in Fig. 3 and supplementary Table S2. In general, the mutants showed a higher activity toward (S)-enantiomers than the (R)-counterparts of primary amines. Similar to wt CHAO, the mutants showed higher activity toward primary amines than the secondary ones. However, some obvious differences were observed. For example, almost all mutants of F368 and Y459 exhibited a much lower activity (46-0.1% activity relative to wt CHAO) toward (S)-primary amines with only exception of Y459A toward substrate (S)-2. In contrast, mutation at other selected sites generated two mutants (T198A and M226A) with higher activity than wt CHAO for most (S)-enantiomers of both primary amines (1.04–1.44 fold) and secondary amines (2.62–4.25 fold). Five mutants (T198I, L199I, L199F, M226I and M226T) were up to 2.1-fold more active toward the primary amines compared to the wt enzyme. The other six mutants (T198F, L199T, Y321A, Y321T, Y321I and Y321F) showed greater than 1% relative activity toward the secondary amines, which were not the active substrate for the wt enzyme.
Figure 3. Activity assay results of CHAO and mutants against various amines.
The activities of various mutants are relative to that of wt CHAO toward (S)-1 set as 100% (3.5U/mg).  relative activity >100;
relative activity >100;  100> relative activity >50;
100> relative activity >50;  50> relative activity >10;
50> relative activity >10;  10> relative activity >1;
10> relative activity >1;  1> relative activity > 0.1;
1> relative activity > 0.1; 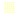 trace activity or no activity (relative activity <0.1).
trace activity or no activity (relative activity <0.1).
Notably, some mutants showed an apparent activity toward bulky primary amines. For example, both mutants T198I and Y321I showed an obvious activity toward (4-chlorophenyl) (phenyl) methanamine (5), toward which wt CHAO was not active. Active variants were also obtained for the following secondary amines: mutant M226T for (S)-N-methyl-1-phenylethanamine ((S)-6); T198A for (S)-N-benzyl-1-phenylethylamine ((S)-7); M226T and Y321I for (S)-N-propynyl-2, 3-dihydro-1 H-inden-1-amine ((S)-8); T198F, L199T, M226T and Y459T for 10; Y321I for 1-methyl-1, 2, 3, 4-tetrahydroisoquinoline (12); and T198I, Y321A, Y321T, Y321F and Y321I for 13.
Construction of double mutants Y321I/M226T and T198I/Y321I and the determination of kinetic parameters
Compared to wt CHAO, which was not active toward secondary amines (S)-8 and 13, some single amino acid substitutions showed activity, e.g., Y321I and M226T for (S)-8, and T198I and Y321I for 13. To create potentially better mutants, double mutants by combining Y321I and M226T for (S)-8, and T198I and Y321I for 13, were constructed by site-directed mutagenesis. The kinetic parameters of the purified proteins were determined to compare the catalytic efficiency of the mutants toward substrates (S)-8 and 13. As shown in Table 1, the double mutant Y321I/M226T displayed an additive effect, showing a higher catalytic efficiency toward (S)-8 (930 min−1M−1 and about 1.7 and 23 fold relative to Y321I and M226T, respectively). In contrast, mutant T198I/Y321I exhibited lower catalytic efficiency compared to Y321I (about 30% relative to Y321I).
Table 1. Kinetic parameters of cyclohexylamine oxidase variants.
| Substrate | Variant | Km (mM) | kcat (min−1) | kcat/Km (min−1 M−1) |
|---|---|---|---|---|
| (S)-8 | Y321I | 2.22 ± 0.32 | 1.21 ± 0.06 | 540 |
| M226T | 5.03 ± 0.46 | 0.20 ± 0.06 | 40 | |
| Y321IM226T | 0.43 ± 0.05 | 0.41 ± 0.02 | 930 | |
| 13 | T198I | n.d. | n.d. | n.d. |
| Y321I | 0.89 ± 0.09 | 0.35 ± 0.01 | 390 | |
| T198IY321I | 4.93 ± 0.75 | 0.68 ± 0.06 | 140 |
n.d: not determined due to negligible activity.
Deracemization of 8 and 13 using crude enzyme extract of mutants Y321I/M226T and Y321I
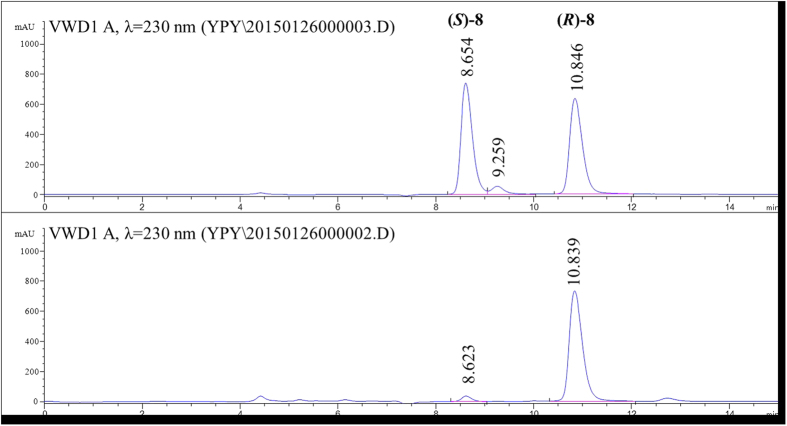
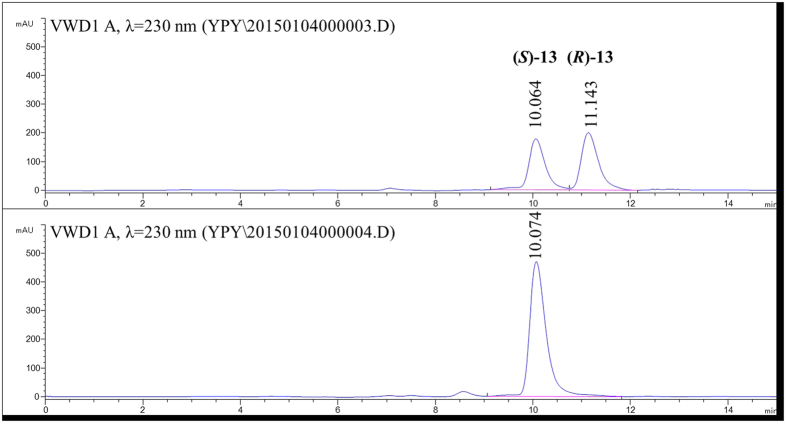
By virtue of the fact that mutants Y321I/M226T and Y321I displayed higher catalytic efficiency toward (S)-8 and 13, respectively, they were tested for the deracemization of 8 and 13. Crude enzyme extracts of mutants Y321I/M226T and Y321I were coupled with the non-selective reducing agent NH3·BH3 to achieve the deracemization of 8 and 13 (Fig. 4). The reactions were fast and linear in the first eight hours, resulting in up to 88% and 96% ee for 8 and 13, respectively. The deracemization process plateaued in the next 12 h, with slight increase in ee to 93% and 99% for 8 and 13, respectively (Fig. 5). As a result, deracemization of 8 gave a 51% isolated yield and 93% ee of (R)-8 in 20 h (Fig. 6). In the same timeframe (S)-13 was prepared with 78% isolated yield and 99% ee (Fig. 7). In either case, the wt CHAO was ineffective for the said deracemization.
Figure 4. Schemes for the deracemization of N-(prop-2-yn-1-yl)-2, 3-dihydro-1H-inden-1-amine (8) or 1-(4-methoxybenzyl)-1, 2, 3, 4, 5, 6, 7, 8-octahydroisoquinoline (13) by coupling an enantioselective oxidation using an amine oxidase mutant Y321I or Y321I/M226T with a nonselective reducing agent NH3·BH3.
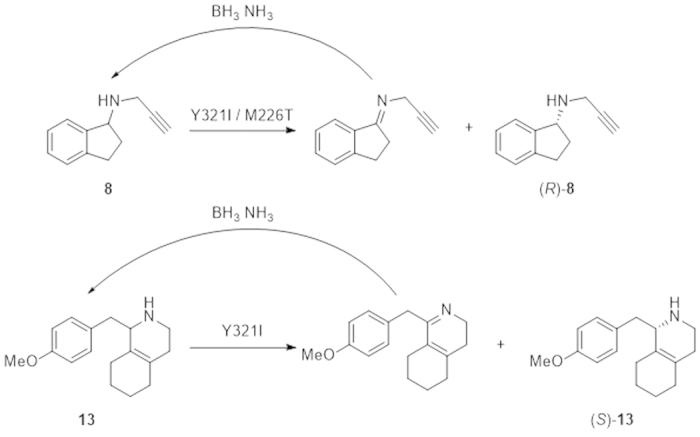
Figure 5. Time course of the deracemization of N-(prop-2-yn-1-yl)-2, 3-dihydro-1H-inden-1-amine (8,  ) and 1-(4-methoxybenzyl)-1, 2, 3, 4, 5, 6, 7, 8-octahydroisoquinoline (13,
) and 1-(4-methoxybenzyl)-1, 2, 3, 4, 5, 6, 7, 8-octahydroisoquinoline (13, ).
).
Figure 6. HPLC spectra of racemic 8 and the product (R)-N-(prop-2-yn-1-yl)-2, 3-dihydro-1H-inden-1-amine ((R)-8).
The analysis was performed on a chiral OJ-H column (4.6 mm × 250 mm, DAICEL CHIRAL TECHNOLOGIES CO.LTD) with a mixture of hexane and isopropanol (0.5% ethanolamine) (9:1) as the eluent at 0.8 mL/min of flow rate and the column temperature being controlled at 30 °C.
Figure 7. HPLC spectra of racemic 13 and the product (S)-1-(4-methoxybenzyl)-1, 2, 3, 4, 5, 6, 7, 8-octahydroisoquinoline ((S)-13).
The analysis was performed on a chiral OJ-H column (4.6 mm × 250 mm, DAICEL CHIRAL TECHNOLOGIES CO.LTD) with a mixture of hexane and isopropanol (0.5% ethanolamine) (9:1) as the eluent at 0.5 mL/min of flow rate and the column temperature being controlled at 30 °C.
Discussion
In the solved structure of CHAO, the side chains of Y321, F368 and Y459 form a cage-like structure in the substrate binding pocket, and amino acid side chains of T198, L199, M226, and F351 separate the substrate-binding cavity from a second cavity that is located closer to the protein surface24. In theory, these residues would affect the substrate binding profile. Indeed, previous results indicated that the amine acid residues T198, L199, M226 and Y459 exerted some effect on the enzyme’s substrate capability15,16. In those studies, five single-amino acid substitution mutants were tested with various substrates to examine the possible effects of the mutations on substrate binding and catalytic activity15. The mutant libraries were screened against a single substrate to identify active mutants for the deracemization of that particular chemical16. Herein, we created a library of diverse mutants and assayed toward a group of structurally diverse substrates to further expand the enzyme’s substrate capability and identify active mutants for deracemization of some interesting amines.
All the mutants exhibited higher activity toward (S)-enantiomer than the (R)-counterpart of primary amines, suggesting the retention of high stereoselectivity of the mutant enzymes. Most mutants of F368 and Y459 showed an obviously lower or no activity toward almost all of the tested substrates. This is consistent with the observation for the corresponding amino acid residues Y398 and Y435 of human MAO-B, which were found in the substrate binding site on the re face of the covalent flavin ring. Y435 in MAO-B was mutated to the amino acids F, H, L, or W, and all the mutant proteins showed an obviously decreased catalytic efficiency than the wt MAO-B25. The side chains of Y321, F368 and Y459 of CHAO form a cage-like structure, and previous studies have indicated that the functions of the “aromatic cage” in MAO-B include recognition of the substrate amine group and increasing the nucleophilicity of the substrate amine moiety25,26. It is possible that mutagenesis of F368 and Y459 may break the cage-like structure and result in a decrease of activity. It has been previously reported that the side chains of residues T198, L199, M226, and F351 separate the entrance cavity and the substrate-binding cavity, and play a “gate-keeping” role in CHAO24. For some mutants (T198A, M226A, M226I, M226T), substitutions of the original amino acids by smaller ones are envisioned to enlarge the “gate”, thus providing a reasonable basis for an enhanced activity relative to the wt CHAO. In other mutants, T198I, L199I and L199F that also displayed increased activity, we propose that those mutations might change the conformation of the “gate” between the two cavities, thus the substrate and product could diffuse more easily in and out of the substrate-binding pocket and the entrance cavity. However, without the crystal structures of these mutant proteins, it is difficult to give definitive explanations for these results.
The kinetic parameters of the mutant Y321I and Y321I/M226T proteins showed a high catalytic efficiency toward 1-(4-methoxybenzyl)-1, 2, 3, 4, 5, 6, 7, 8-octahydroisoquinoline (13) and (S)-N-(prop-2-yn-1-yl)-2, 3-dihydro-1 H-inden-1-amine [(S)-8], respectively. Since (R)-8 is an irreversible inhibitor of monoamine oxidase for Alzheimer disease27, and (S)-13 is a key intermediate for the synthesis of the antitussive agent dextromethorphan28, several methods have been reported for the synthesis of (R)-829,30,31 and (S)-1328,32,33,34. Most methods are based on classical kinetic resolution with chiral acids31,32 or focused on the asymmetric synthesis of (R)-2, 3-dihydro-1-indanamine29,30. Kitamura et al. reported a stereoselective synthesis of (S)-13 with 97% ee and 98% yield catalyzed by a chiral phosphine-Ru complex under high hydrogen pressure of 100 atm34. Broger et al. found that amidophosphine-phosphinites-Ir complex catalyzed asymmetric hydrogenation of the corresponding iminium salt to (S)-13 in up to 86% ee33. Our present study is the first report that optically active (R)-8 and (S)-13 can be synthesized with high ee value and comparable isolated yield by an amine oxidase-catalyzed deracemization, thus providing a new biocatalytic approach to access these chiral amines and demonstrating the application potential of the evolved CHAO mutants in the synthesis of pharmaceutical molecules with chiral secondary amine moiety.
Materials and Methods
Materials
1-(4-methoxybenzyl)-1, 2, 3, 4, 5, 6, 7, 8-octahydroisoquinoline (13) was prepared as described in the literature (supplementary Figs S1 and S2)35. Other reagents were purchased from Sigma-Aldrich, Alfar Aesar (USA), J&K scientific Ltd (Beijing, China), and AccelaChembio Inc., and were used without further purification.
Construction of the mutant library and double mutants Y321I/M226T and T198I/Y321I by site-specific mutagenesis
Choice of the mutation sites (T198, L199, M226, Y321, F351, F368 and Y459) was guided by the solved structure of CHAO24. Site-directed mutagenesis of CHAO-encoding gene in Escherichia coli recombinant plasmid pSD80 vector (Tac promoter)24 was carried out by PCR-based Quick-change method36. Each selected site was mutated to amino acid residues of different property (A, I, F, W, T, Y, D and K). Both of the double mutants Y321I/M226T and T198I/Y321I were constructed using Y321I plasmid as template by PCR-based Quick-change method. The mutagenic primers are shown in supplementary Table S3 and the plasmids were transformed into E. coli Top10. In each case, the sequence of the mutated gene was verified by DNA sequencing (BGI Company, Beijing, China).
Expression of wt CHAO and mutants
E. coli recombinant cells were cultivated in 5 ml LB medium containing ampicillin (100 μg/ml) overnight at 37 °C. Then 1 mL culture was inoculated into 50 mL of LB medium containing ampicillin (100 μg/ml) and grown at 37 °C. At an OD600 of 0.8, gene expression was induced by the addition of IPTG (final concentration 0.5 mmol/L), and then allowed to grow for an additional 10 h at 25 °C. The cells were harvested by centrifugation at 10,000 × g and 4 °C for 15 min, then the bacterial pellet was washed twice with phosphate buffer (50 mM, pH 6.5), and resuspended in a phosphate buffer (50 mM, pH 6.5). The recombinant cells were lysed by sonication and the supernatant was collected by centrifugation at 10,000 × g at 4 °C for 30 min. Then the collected supernatant protein was analyzed by SDS-PAGE (supplementary Fig. S3) and protein concentration was measured by Bradford method37. Based on above results, the CHAO and mutants were diluted to same concentration for activity detection.
Activity Assay
Enzyme activity was determined by a modified procedure of Braun et al.38. The formation of a dye (ε = 29.4 × 103 mol–1 L cm−1) by the action of horseradish peroxidase with liberated hydrogen peroxide from the reaction of the amine and amino oxidase, 4-aminoantipyrine, and 2, 4, 6-tribromo-3-hydroxybenzoicacid was monitored at 510 nm using a SPECTRAMAX M2e (MD, USA) at 30 °C. The standard assay mixture (0.2 mL total volume) contained 184 μL of 50 mmol/L phosphate buffer (pH 6.5), 2 μL of a 2, 4, 6-tribromo-3-hydroxybenzoic acid stock solution [20 mg/mL in dimethyl sulfoxide (DMSO)], 2 μL of 4-aminoantipyrine stock solution (15 mg/mL in H2O), 2 μL of an amine stock solution (1 mol/L in DMSO), and 10 U of horseradish peroxidase. The reaction (0.2 mL) was started by the addition of 10 μL of an appropriate amount of enzyme at 30 °C. For each experiment, enzymatic assays were performed in triplicate with cell-free extract of E. coli Top10 and E. coli Top10 recombinant cells of wt CHAO under same culture condition as control experiments. One enzyme unit (U) was defined as the amount of enzyme that produced 1 μmol of hydrogen peroxide per min. The activity of CHAO for (S)-1-phenylethanamine (3.5 U/mg) was defined as 100%.
Purification of CHAO variants and the determination of kinetic parameters
The recombinant CHAO variants (Y321I, M226T, T198I, Y321I/M226T and T198I/Y321I) were purified by ion interaction chromatography on an AKTA purifier 10 system with DEAE FF crude column (GE, USA), as reported in our previous study15. The purified enzymes were analyzed using SDS-PAGE (supplementary Fig. S4). The kinetic parameters were obtained by measuring the initial velocities of the enzymatic reaction and curve-fitting according to the Michaelis-Menten equation. The activity assay was performed in a mixture containing a varying concentration of (S)-8 (0.1-10 mM) or 13 (0.1-10 mM). All experiments were conducted in triplicate.
General procedure for deracemization of 8 and 13 using crude enzyme extract of mutant Y321I/M226T and Y321I
The plasmids of mutants Y321I or Y321I/M226T were transformed into E. coli BL21(DE3). The cells were cultured and the crude enzyme extracts were prepared as described above and used for biotransformation. The deracemization of 8 or 13 was carried out as follows: 8 (118 mg, 0.75 mmol) or 13 (129 mg, 0.5 mmol) dissolved in DMSO (1 mL), 50 mL crude enzyme extract of mutant Y321I/M226T for 8 or Y321I for 13 (10 g wet cells) in phosphate buffer (50 mM, pH = 6.5) and borane–ammonia complex (124 mg, 4 mmol) were mixed. The mixture was shaken at 200 rpm at 25 °C on an orbital shaker. After 20 h of reaction, the reaction was quenched with 6 M HCl to pH 2 and the pH of the mixture was then adjusted to 10 with 5 M NaOH. The aqueous layer was extracted two times with 25 mL of ethyl acetate and the phase separation was facilitated by centrifugation (5500 × g, 15 min). The combined organic extract was dried over anhydrous sodium sulphate, filtered, and evaporated in vacuum. The product was purified by preparative thin layer chromatography.
Deracemization of 8 gave 60 mg product (51% isolated yield, ee = 93%). Optical rotation for (R)-8: [α]25D = 17.2 (c = 1.7, chloroform). Literature values for (R)-8: [α]25D = 18.8 (c = 1.7, chloroform)39. HPLC analysis was performed on an Agilent 1200 Series using Chiracel OJ-H column (4.6 mm × 250 mm, DAICEL CHIRAL TECHNOLOGIES CO.LTD).A mixture of hexane and isopropanol (0.5% ethanolamine) (9:1) was used as eluent in 0.8 mL/min of flow rate and the column temperature was controlled at 30 °C. The retention times for (S)-8 and (R)-8 were 8.61 and 10.84 min, respectively. 1H NMR (400 MHz, CDCl3): δ 7.31–7.37 (m, 1 H), 7.15–7.24 (m, 3 H), 4.41 (t, J = 6.1 Hz, 1 H), 3.52 (t, J = 2.3 Hz, 2 H), 2.98–3.09 (m, 1 H), 2.78–2.88 (m, 1 H), 2.34–2.45 (m, 1 H), 2.25 (t, J = 2.3 Hz, 1 H), 1.81–1.91 (m, 1 H), 1.71 (s, 1 H). 13C NMR (100 MHz, CDCl3): δ 144.51, 143.84, 127.66, 126.28, 124.89, 124.22, 82.50, 71.42, 61.90, 36.17, 33.34, 30.48 (supplementary Figs S5 and S6).
Deracemization of 13 gave 100 mg product (78% isolated yield, ee > 99%), which was identified as (S)-13 by comparison of its optical rotation with the literature40. Observed values for (S)-13: [α]20D = −130 (c = 1.0, methanol). Literature values for (R)-13: [α]20D = 139 (c = 1.0, methanol). HPLC analysis was performed on an Agilent 1200 Series using Chiracel OJ-H column (4.6 mm × 250 mm, DAICEL CHIRAL TECHNOLOGIES CO.LTD). A mixture of hexane and isopropanol (0.5% ethanolamine) (9:1) was used as eluent in 0.5 mL/min of flow rate and the column temperature was controlled at 30 °C. The retention times for (S)-13 and (R)-13 were 10.06 and 11.14 min, respectively. 1H NMR (400 MHz, CDCl3): δ 7.21 (d, J = 8.6 Hz, 2 H), 6.85 (d, J = 8.6 Hz, 2 H), 3.79 (s, 3 H), 3.52 (br. s., 1 H), 3.08 (dd, J = 14.1, 4.4 Hz, 1 H), 2.93–3.02 (m, 1 H), 2.87 (dt, J = 12.0, 5.9 Hz, 1 H), 2.78 (dd, J = 14.1, 8.4 Hz, 1 H), 1.98–2.15 (m, 3 H), 1.94 (br. s., 2 H), 1.82 (m, 1 H), 1.63–1.77 (m, 2 H), 1.55 (m, 2 H). 13C NMR (100 MHz, CDCl3): δ 158.40, 130.46, 129.88, 128.83, 127.45, 114.06, 57.56, 55.23, 39.57, 37.05, 30.21, 28.72, 27.07, 22.85, 22.53 (supplementary Figs S7 and S8).
Additional Information
How to cite this article: Li, G. et al. New recombinant cyclohexylamine oxidase variants for deracemization of secondary amines by orthogonally assaying designed mutants with structurally diverse substrates. Sci. Rep. 6, 24973; doi: 10.1038/srep24973 (2016).
Supplementary Material
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21302215) and Youth Innovation Promotion Association (CAS). Support by Tianjin “Thousand Talents” Program for Senior International Scientists to P.C.K. Lau is gratefully acknowledged.
Footnotes
Author Contributions G.L. and P.Y. contributed equally to this work. G.L., P.Y., J.F., P.C.K.L., Q.W. and D.Z. conceived and designed experiments and wrote the manuscript. G.L. and P.Y. performed most experiments. P.C., J.R. and L.W. performed a small part of experiments. G.L. and P.Y. analyzed data and prepared the figures. All authors reviewed the manuscript.
References
- Blacker J. & Headley C. E. Dynamic Resolution of Chiral Amine Pharmaceuticals: Turning Waste Isomers into Useful Product, in Green Chemistry in the Pharmaceutical Industry (eds) Dunn P. J., Wells A. & Williams M. T. (John Wiley & Sons, 2010). [Google Scholar]
- Savile C. K. et al. Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture. Science 329, 305–309 (2010). [DOI] [PubMed] [Google Scholar]
- de Lange B. et al. Asymmetric synthesis of (S)−2−indolinecarboxylic acid by combining biocatalysis and homogeneous catalysis. ChemCatChem 3, 289–292 (2011). [Google Scholar]
- Abrahamson M. J., Vázquez-Figueroa E., Woodall N. B., Moore J. C. & Bommarius A. S. Development of an amine dehydrogenase for synthesis of chiral amines. Angew. Chem. Int. Ed. 51, 3969–3972 (2012). [DOI] [PubMed] [Google Scholar]
- Alexeeva M., Enright A., Dawson M. J., Mahmoudian M. & Turner N. J. Deracemization of α-methylbenzylamine using an enzyme obtained by in vitro evolution. Angew. Chem. Int. Ed. 41, 3177–3180 (2002). [DOI] [PubMed] [Google Scholar]
- Alexeeva M., Carr R. & Turner N. J. Directed evolution of enzymes: new biocatalysts for asymmetric synthesis. Org. Biomol. Chem. 1, 4133–4137 (2003). [DOI] [PubMed] [Google Scholar]
- Ghislieri D. et al. Engineering an enantioselective amine oxidase for the synthesis of pharmaceutical building blocks and alkaloid natural products. J. Am. Chem. Soc. 135, 10863–10869 (2013). [DOI] [PubMed] [Google Scholar]
- Carr R. et al. Directed evolution of an amine oxidase for the preparative deracemisation of cyclic secondary amines. ChemBioChem 6, 637–639 (2005). [DOI] [PubMed] [Google Scholar]
- Ghislieri D., Houghton D., Green A. P., Willies S. C. & Turner N. J. Monoamine oxidase (MAO-N) catalyzed deracemization of tetrahydro-β-carbolines: Substrate dependent switch in enantioselectivity. ACS Catal. 3, 2869–2872 (2013). [Google Scholar]
- Dunsmore C. J., Carr R., Fleming T. & Turner N. J. A chemo-enzymatic route to enantiomerically pure cyclic tertiary amines. J. Am. Chem. Soc. 128, 2224–2225 (2006). [DOI] [PubMed] [Google Scholar]
- Schrittwieser J. H. et al. Deracemisation of benzylisoquinoline alkaloids employing monoamine oxidase variants. Catal. Sci. Technol. 4, 3657–3664 (2014). [Google Scholar]
- Rowles I., Malone K. J., Etchells L. L., Willies S. C. & Turner N. J. Directed evolution of the enzyme monoamine oxidase (MAO-N): Highly efficient chemo-enzymatic deracemisation of the alkaloid (+/−)-Crispine A. ChemCatChem 4, 1259–1261 (2012). [Google Scholar]
- Köhler V. et al. Enantioselective biocatalytic oxidative desymmetrization of substituted pyrrolidines. Angew. Chem. Int. Ed. 49, 2182–2184 (2010). [DOI] [PubMed] [Google Scholar]
- Leisch H., Grosse S., Iwaki H., Hasegawa Y. & Lau P. C. K. Cyclohexylamine oxidase as a useful biocatalyst for the kinetic resolution and dereacemization of amines. Can. J. Chem. 90, 39–45 (2012). [Google Scholar]
- Li G. et al. Substrate profiling of cyclohexylamine oxidase and its mutants reveals new biocatalytic potential in deracemization of racemic amines. Appl. Microbiol. Biotechnol. 98, 1681–1689 (2013). [DOI] [PubMed] [Google Scholar]
- Li G. et al. Deracemization of 2-Methyl-1, 2, 3, 4-tetrahydroquinoline using mutant cyclohexylamine oxidase obtained by iterative saturation mutagenesis. ACS Catal. 4, 903–908 (2014). [Google Scholar]
- Wang T. et al. Highly enantioselective hydrogenation of quinolines using phosphine-free chiral cationic ruthenium catalysts: Scope, mechanism, and origin of enantioselectivity. J. Am. Chem. Soc. 133, 9878–9891 (2011). [DOI] [PubMed] [Google Scholar]
- Katritzky A. R., Rachwal S. & Rachwal B. Recent progress in the synthesis of 1, 2, 3, 4,-tetrahydroquinolines. Tetrahedron 52, 15031–15070 (1996). [Google Scholar]
- Jacquemond-Collet I. et al. Antiplasmodial and cytotoxic activity of galipinine and other tetrahydroquinolines from Galipea officinalis. Planta Med. 68, 68–69 (2002). [DOI] [PubMed] [Google Scholar]
- Di Fabio R. et al. Chiral tetrahydroquinoline derivatives as potent anti-hyperalgesic agents in animal models of sustained inflammation and chronic neuropathic pain. Bioorg. Med. Chem. Lett. 17, 1176–1180 (2007). [DOI] [PubMed] [Google Scholar]
- Wallace O. B., Lauwers K. S., Jones S. A. & Dodge J. A. Tetrahydroquinoline-based selective estrogen receptor modulators (SERMs). Bioorg. Med. Chem. Lett. 13, 1907–1910 (2003). [DOI] [PubMed] [Google Scholar]
- Bendale P. et al. Second generation tetrahydroquinoline-based protein farnesyltransferase inhibitors as antimalarials. J. Med. Chem. 50, 4585–4605 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergne-Vaxelaire C. et al. Nitrilase activity screening on structurally diverse substrates: providing biocatalytic tools for organic synthesis. Adv. Synth. Catal. 355, 1763–1779 (2013). [Google Scholar]
- Mirza I. A. et al. Structural analysis of a novel cyclohexylamine oxidase from Brevibacterium oxydans IH-35A. PLos One 8, e60072 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Binda C., Mattevi A. & Edmondson D. E. Functional role of the “aromatic cage” in human monoamine oxidase B: Structures and catalytic properties of Tyr435 mutant proteins. Biochem. 45, 4775–4784 (2006). [DOI] [PubMed] [Google Scholar]
- Binda C., Newton-Vinson P., Hubalek F., Edmondson D. E. & Mattevi A. Structure of human monoamine oxidase B, a drug target for the treatment of neurological disorders. Nat. Struct. Biol. 9, 22–26 (2002). [DOI] [PubMed] [Google Scholar]
- Oldfield V., Keating G. M. & Perry C. M. Rasagiline - A review of its use in the management of Parkinson’s disease. Drugs 67, 1725–1747 (2007). [DOI] [PubMed] [Google Scholar]
- Meyers A. I. & Bailey T. R. An asymmetric synthesis of (+)-morphinans in high enantiomeric purity. J. Org. Chem. 51, 872–875 (1986). [Google Scholar]
- Fonseca T. D. S. et al. Chemoenzymatic synthesis of rasagiline mesylate using lipases. Appl. Catal. A: Gen. 492, 76–82 (2015). [Google Scholar]
- Ma G. Z. et al. A novel Synthesis of Rasagiline via a Chemoenzymatic Dynamic Kinetic Resolution. Org. Process Res. Dev. 18, 1169–1174 (2014). [Google Scholar]
- Allegrini P. et al. Inventors; Dipharma Francis S. R. I, assignee. Crystalline form of rasagiline and process for the preparation thereof. united states patent US 2010029987. 2010 Feb 4. [Google Scholar]
- Divi M. K. P., Pendyala S. R., Ramana & L. V. inventors; Divi’s Laboratories, Ltd., assignee. Resolution of 1-(4-methoxybenzyl)-octahydro-iso-quinoline. United States patent US 8148527. 2012 Apr 3.
- Broger E. A. et al. New amidophosphine-phosphinites (tLANOPs) as chiral ligands for asymmetric hydrogenation reactions. Tetrahedron Asymm. 9, 4043–4054 (1998). [Google Scholar]
- Kitamura R. et al. General asymmetric synthesis of isoquinoline alkaloids. Enantioselective hydrogenation of enamides catalyzed by BINAP-ruthenium(II) complexes. J. Org. Chem. 59, 297–310 (1994). [Google Scholar]
- Mohacsi E. & Leimgruber W. Inventors. Hoffmann-La Roche Inc., assignee. 4-hydroxy-N-substituted morphinan derivatives. United States patent US 3914233. 1975 Oct 21.
- Papworth C., Bauer J., Braman J. & Wright D. Site-directed mutagenesis in one day with >80% efficiency. Strategies 9, 3–4 (1996). [Google Scholar]
- Bradford M. M. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976). [DOI] [PubMed] [Google Scholar]
- Braun M., Kim J. M. & Schmid R. D. Purification and some properties of an extracellular l-amino acid oxidase from Cellulomonas cellulans AM8 isolated from soil. Appl. Microbiol. Biotechnol. 37, 594–598 (1992). [Google Scholar]
- Davies H. M. L. & Reddy R. P. Inventors; The Research Foundation of State University of New York, assignee. Catalysts for use in enantioselective synthesis. United States patent US 7385064. 2008 Jun 10.
- Brossi A. & Schnider O. Hydroxy-morphinane.9. Versuche zur racemisierung optisch aktiver 1-(p-hydroxybenzyl)-1, 2, 3, 4, 5, 6, 7, 8-octahydro-isochinoline. Helv. Chim. Acta 39, 1376–1386 (1956). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.