Abstract
Obesity promotes breast cancer by enhancing the stiffness of breast adipose tissue through changes in the extracellular matrix (Seo et al., this issue).
Obesity accounts for up to 20% of cancer deaths in women (1). Two-thirds of people in the United States are overweight or obese, and this increase in body mass index (BMI) has been identified as the most important risk factor associated with breast cancer in postmenopausal women. In addition, regardless of menopausal status, obese women (BMI > 30) diagnosed with breast cancer have a worse prognosis than that of women with a normal BMI (2). Obese women generally have larger tumors at the time of diagnosis, with increased risk of lymph node metastases and distant recurrence, relative to nonobese women (3).
This well-established link between obesity and malignancy has driven researchers to investigate the mechanistic underpinnings. Evidence from multiple studies suggests that obesity promotes tumor aggression through locally derived and systemic soluble factors and inflammation. However, in this issue of Science Translational Medicine, Seo et al. show that obesity promotes cancer progression and metastasis by altering the biomechanical microenvironment of fatty adipose tissue in the breast (4). Although this new insight adds to the emerging complexity by which obesity fuels tumor growth and progression, it also may help to integrate the various mechanisms that link obesity-related risk to malignancy—thus providing a basis for clinical interventions.
MODIFYING THE MATRIX
The extracellular matrix (ECM) is a dynamic scaffold of connective tissue—called the stroma—whose composition and biomechanical properties are modified to reflect the physiological state of the adjacent tissue (5). Desmoplasia—changes in the stromal microenvironment, such as the remodeling of fibrous or connective tissue—occurs under pathological conditions, including cancer and wound healing. These changes alter ECM deposition and processing and have lasting effects on the biology of neighboring tumors. Desmoplasia is initiated by the conversion of tissue resident or recruited fibroblasts into myofibroblasts that deposit fibrillar collagen and fibronectins into the surrounding stroma. Desmoplasia is also associated with poor prognosis in multiple different types of cancer and promotes both locally aggressive tumor behavior and metastasis (6). Seo et al. demonstrated that obesity also induces desmoplasia, which similarly alters the extracellular environment of breast adipose tissue, leading to an environment conducive to tumor progression (4).
Whereas the conversion of fibroblasts to myofibroblasts is an important first step in the desmoplastic response, Seo et al. showed that under conditions of obesity, resident adipose stem cells rather than fibroblasts express smooth muscle actin and differentiate down the myofibroblast lineage. In both genetically induced and high-fat–diet mouse models of obesity, adipose stem cell–derived myofibroblasts deposited fibronectin and type 1 collagen within the ECM. Using second harmonic generation imaging, the authors found that the deposited interstitial collagen was more linearized in mammary adipose tissue of both the diet-and genetically induced obesity models, leading to a more rigid ECM consistent with desmoplasia. In addition, the fibronectin deposited into the ECM by the adipose stem cells harbored conformational changes that included partially unfolded fibronectin fibers. These protein modifications translated into enhanced rigidity of adipose stem cell–deposited ECM, and this desmoplasia changed the biomechanical microenvironment of the mammary gland (Fig. 1).
Fig. 1. Link systemically, act locally.
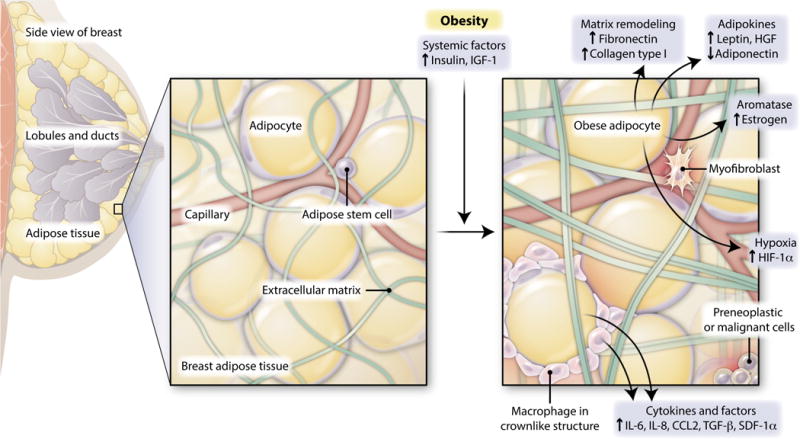
Obesity induces both local and systemic changes implicated in breast cancer progression. Obesity-induced systemic changes include increases in circulating insulin and insulin-like growth factor–1 (IGF-1), which can act within the breast microenvironment to promote cancer progression. Adipocytes in the breast might contribute to breast tumor development through the increased secretion of adipocyte-derived hormones and growth factors (adipokines), including the hormone leptin and paracrine growth factor hepatocyte growth factor (HGF), and the diminished secretion of adiponectin, which is a protein involved in the regulation of glucose levels. Adipocytes also produce aromatase, which can lead to increased local estrogen concentrations in the obese breast. Adipocytes or recruited immune cells (including macrophages), which form characteristic crownlike structures around adipocytes, secrete increased amounts of multiple cytokines and other growth factors in breast adipose depot. Unregulated growth of adipocytes under conditions of obesity also produces hypoxic conditions, which may elevate localized angiogenesis. Now, Seo et al. have identified an additional mechanism by which increased collagen and fibronectin deposition within obese adipose tissue contributes to ECM stiffness, which promotes breast tumor progression.
In multiple organs, transforming growth factor–β (TGF-β) has been shown to induce a fibroblast-to-myofibroblast conversion, resulting in fibrosis and contraction of the ECM. In the new work, Seo et al. showed that differentiation of adipose stem cells into myofibroblasts in obese mammary tissue was not dependent on TGF-β. Rather, using a decellularized matrix from mouse mammary tissue, the authors demonstrated that the physical properties of the ECM provided a direct, TGF-β–independent mechanism that promoted myofibroblast formation in the obese mammary depot. Although we know that TGF-β is not required for this process, it is not clear whether obesity-induced myofibroblasts are induced as a consequence of the increased ECM rigidity, or whether specific components of the ECM are produced under conditions of obesity and are necessary for myofibroblast formation. However, the new findings do suggest that the ECM modification process might provide points of therapeutic intervention for the treatment of obese women diagnosed with breast cancer.
TISSUE STIFFNESS AND MECHANOTRANSDUCTION
Considerable evidence has shown that elevated tissue stiffness induces a reciprocal signaling loop between the ECM and cancer cells that promotes malignant progression. Pathological tissue stiffness can potentiate the ability of untransformed cells to proliferate and transformed cells to become invasive (7). Using decellularized ECM from adipose stem cells of obese mammary glands, Seo et al. showed that the altered biomechanical microenvironment promoted the proliferation and disorganized growth of preneoplastic MCF10AT cells in vitro. Further, the addition of functional blocking antibodies to the α5β1 and αvβ3 integrins (anti-α5β1 and anti-αvβ3, which inhibit the binding of fibronectin to two important integrins that orchestrate communication between cells and ECM) did not alter cellular proliferation, suggesting that these abnormal responses to ECM stiffness do not result from interactions through integrin signaling. Together, these observations suggest that the microenvironment of breast tissue in obese individuals stimulates the growth of preneoplastic lesions within the breast through mechanotransduction—the conversion of mechanical stimuli into chemical or electrical signals—rather than through specific conformational changes of ECM components.
In the context of cancer, Seo et al. also showed that decellularized matrix altered growth properties of cancer cells, suggesting that the obese microenvironment affects not only preneoplastic epithelial cells but also malignant ones. This observation might explain how obesity affects multiple stages of cancer formation and progression.
To determine whether obesity-associated changes in matrix deposition could be found clinically, Seo et al. investigated the relationship between BMI and desmoplasia in human breast tumor specimens. Breast tumors from women with increased BMI harbored an increased expression of smooth muscle actin and fibronectin compared to lean women. These tumors also demonstrated increased collagen fiber thickness and alignment, suggesting that the biomechanical changes that occur within the mammary adipose tissue of obese mice are also present within the human breast tumors. Although the breast cancer samples analyzed in this study represent a small data set, the results from these analyses suggest that neoplasias within the obese microenvironment display increased inflammation and desmoplasia regardless of the tumor subtype. More research is necessary to elucidate the impact of these changes on prognosis for obese women who are diagnosed with various breast cancer subtypes.
How does the biomechanical microenvironment of the obese breast promote tumor formation? One possibility is through altered signaling by the YAP/TAZ transcription factors, which, when activated, translocate to the nucleus in response to changes in cell adhesion and mechanical signals from the surrounding ECM and tissue architecture. During tumorigenesis, aberrantly active YAP/TAZ can foster cancer stem cell behavior and promote tumor progression and metastasis (8). In the new work, Seo et al. observed an increase in the amount of nuclear YAP/TAZ both in MDA-MB-231 cells cultured on decellularized ECM from obese mice and in tumor specimens from obese women. These observations suggest that the biomechanical environment of obesity could promote aggressive tumor behavior through enhanced YAP/TAZ activation. In addition to direct actions of the mechanoenvironment, changes in ECM deposition have been shown to affect epithelial morphogenesis, growth factor and cytokine signaling, and stem cell differentiation (6). Seo et al. also observed that adipose stem cells from obese mice promoted migration of cancer cells in the absence of direct cellular contact, suggesting that soluble growth factors, such as stromal-derived growth factor–1α, might also play a role. Indeed, the mechanisms underlying the promotion of breast cancer are likely to be complex.
How does weight gain promote the initial ECM changes? One possibility is the generation of hypoxic conditions within the adipose tissue, which is critically dependent on angiogenesis for continued growth and development. Another possibility is through the recruitment of inflammatory macrophages, which have been implicated in fibrosis. Currently, it is not clear which evolves first, inflammation or fibrosis, and understanding the relationship between these processes might have important implications for the treatment of obesity (Fig. 1).
Clearly, it will be challenging to dissect the contributions of the mechanical microenvironment, modified ECM, and interactions with inflammatory cells during tumor progression. Do adipose stem cells primed by the obese microenvironment promote the growth of fibrotic tumors? How do inflammatory cells, including macrophages and lymphocytes activated by the obese microenvironment, contribute to the aggressive and other phenotypes of tumors associated with obesity? Studies designed to determine the roles of individual cell types and how they contribute to modifications of the ECM will be necessary to understand how obesity enhances tumor desmoplasia. It is particularly important to understand the origin of desmoplasia in tumors associated with obesity in order to more fully assess therapeutic options.
THERAPEUTIC IMPLICATIONS
As obesity rates continue to rise, it is likely that obesity-associated breast cancer incidence will also increase. Unfortunately, therapeutics specific for obese women with breast cancer are limited. In postmenopausal women, estrogen is mainly produced by adipocytes through activity of the enzyme aromatase. This has led to the hypothesis that aromatase inhibitors may be particularly beneficial for obese women in the treatment of estrogen receptor–positive breast cancer. However, in multiple epidemiologic studies, aromatase inhibitors had similar or reduced benefits compared with that of tamoxifen in obese postmenopausal breast cancer patients, with obese patients potentially demonstrating increased relapse after treatment (9). In addition to elevated estrogen levels, obese women also demonstrate increases in circulating insulin levels. Metformin, a medication currently used to lower insulin levels in diabetic patients, might have promise as an adjuvant cancer therapy. Diabetic women treated with metformin have reduced risk of developing breast cancer, and the addition of metformin to breast cancer treatments in diabetic women improved tumor response (10). Large, clinical trials are now under way to examine the efficacy of metformin in the treatment of obesity-associated breast cancer.
For obese women diagnosed with breast cancer, weight loss is one of the main clinical recommendations after treatment. However, little is known about how elective weight loss alters breast cancer risk. Using calorie restriction, Seo et al. examined the effects of weight loss on a cohort of obese mice. In the weight-loss group, calorie restriction led to significantly reduced numbers of smooth muscle actin–derived myofibroblasts within the mammary gland.
Although adipose stem cell biology appears to be modulated by weight loss, its effects on the ECM are less clear. Fibronectin concentrations were not significantly altered, and it is not clear whether obesity-induced remodeling of the ECM is lasting or whether the effects on ECM though weight loss require a longer period of time for remodeling. A recent study in a large cohort of postmenopausal women suggests that in the short term, elective weight loss may not alter breast cancer risk (3).
Although multiple studies are ongoing to examine the effects of weight loss in breast cancer survivors on long-term survival, few studies examine biomarkers after dietary intervention in order to understand the effects of lifestyle change on the mechanisms linked to breast cancer. Identifying how weight loss contributes to changes in the ECM and ultimately breast cancer risk is critical for advising patient care. Because therapeutics specifically targeted for obese breast cancer patients are lacking and weight reduction is recommended for breast cancer survivors, further research is necessary to determine whether dietary or exercise interventions or both are effective in the amelioration of ECM changes induced by obesity.
Footnotes
Competing Interests: The authors declare that they have no competing financial interests.
REFERENCES AND NOTES
- 1.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 2.Carmichael AR. Obesity as a risk factor for development and poor prognosis of breast cancer. BJOG. 2006;113:1160–1166. doi: 10.1111/j.1471-0528.2006.01021.x. [DOI] [PubMed] [Google Scholar]
- 3.Neuhouser ML, Aragaki AK, Prentice RL, Manson JE, Chlebowski R, Carty CL, Ochs-Balcom HM, Thomson CA, Caan BJ, Tinker LF, Urrutia RP, Knudtson J, Anderson GL. Overweight, obesity, and postmenopausal invasive breast cancer risk: A secondary analysis of the Women’s Health Initiative randomized clinical trials. J Am Med Assoc Oncol. 2015 Jun 11; doi: 10.1001/jamaoncol.2015.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seo BR, Bhardwaj P, Choi S, Gonzalez J, Eguiluz RC Andresen, Wang K, Mohanan S, Morris PG, Du B, Zhou XK, Vahdat LT, Verma A, Elemento O, Hudis CA, Williams RM, Gourdon D, Dannenberg AJ, Fischbach C. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci Transl Med. 2015;7:301ra130. doi: 10.1126/scitranslmed.3010467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hynes RO. The extracellular matrix: Not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15:1243–1253. doi: 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: Hippo signaling and beyond. Physiol Rev. 2014;94:1287–1312. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- 9.Lønning PE, Eikesdal HP. Aromatase inhibition 2013: Clinical state of the art and questions that remain to be solved. Endocr Relat Cancer. 2013;20:R183–R201. doi: 10.1530/ERC-13-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ligibel JA, Strickler HD. Obesity and its impact on breast cancer: Tumor incidence, recurrence, survival, and possible interventions. Am Soc Clin Oncol Educ Book. 2013;2013:52–59. doi: 10.14694/EdBook_AM.2013.33.52. [DOI] [PubMed] [Google Scholar]


