Abstract
Introduction
Obesity is associated with cognitive impairment and bariatric surgery has been shown to improve cognitive functioning. Rapid improvements in glycemic control are common after bariatric surgery and likely contribute to these cognitive gains. We examined whether improvements in glucose regulation are associated with better cognitive function following bariatric surgery.
Method
A total of 85 adult bariatric surgery patients underwent computerized cognitive testing and fasting blood draw for glucose, insulin, and glycated hemoglobin (HbA1c) at baseline and 12 month post-operatively.
Results
Significant improvements in both cognitive function and glycemic control were observed among patients. After controlling for and baseline factors, 12-month homeostatic model assessment of insulin resistance HOMA-IR predicted 12-month digits backward (β = −.253, p < .05), switching of attention- A (β = .156, p < .05), and switching of attention-B (β = −.181, p < .05). Specifically, as HOMA-IR decreased over time, working memory, psychomotor speed, and cognitive flexibility improved. Decreases in HbA1c were not associated with post-operative cognitive improvements. After controlling for baseline cognitive test performance, changes in BMI were also not associated with 12-month cognitive function.
Conclusions
Small effects of improved glycemic control on improved aspects of attention and executive function were observed following bariatric surgery among severely obese individuals. Future research is needed to identify the underlying mechanisms for the neurocognitive benefits of these procedures.
Keywords: Cognitive function, bariatric surgery, glycemic control, obesity, memory
Obesity is a leading cause of preventable death and is associated with numerous medical problems (Bray, 2004) including neurological conditions such as dementia and stroke (Hu et al., 2007; Whitmer, Gunderson, Quesenberry, Zhou, & Yaffee, 2007). Obesity is also associated with cognitive impairment long prior to the onset of these conditions (Gunstad et al., 2007; Gunstad, Lhotsky, Wendell, Ferucci, & Zonderman, 2010; Waldstein & Katzel, 2006). Previous work has shown that nearly a quarter of bariatric surgery candidates demonstrate clinically significant impairment (defined as > 1.5 standard deviation (SD) below the mean) on neuropsychological testing and approximately 40% demonstrate more subtle deficits (> 1 SD below the mean) (Gunstad et al., 2011). Cognitive deficits have been observed in all domains, but are most common in the areas of attention, executive function, and memory (Gunstad et al., 2011; Waldstein & Katzel, 2006). Fortunately, obesity-related cognitive impairments may be at least partly reversible, as recent research demonstrates improved cognitive functioning 12-weeks after bariatric surgery (Gunstad et al., 2011), with cognitive gains persisting at least three years post-operatively (Alosco et al., 2013; 2014; Miller et al., 2013).
The exact mechanisms for obesity-related cognitive dysfunction and cognitive gains after bariatric surgery are poorly understood but are likely to be multifactorial. For example, obesity is associated with a number of medical conditions known that are associated with cognitive impairment such as sleep apnea (Gelir et al., 2014), and cardiac disease (Singer, Trollor, Baune, Sachdev, & Smith, 2014). These conditions often improve following bariatric surgery and may play an important role in the relationship between obesity and cognitive function. Similarly, psychological conditions such as depression and anxiety are also common among obese individuals and related to cognitive deficits, but can improve post-operatively (Castellini et al., 2014).
Another important mechanism likely involves improved glucose regulation. Peripheral glucose dysregulation (i.e., T2DM, pre-diabetes) is common among persons with obesity (Golay & Elber, 1994; Mayega et al., 2013) and has been associated with high rates of cognitive impairment and decline in other samples (Convit, Wolf, Tarshish, & de Leon, 2003; Espeland et al., 2011; Fontbonne, Berr, Ducimetière, & Alpérovitch, 2001). Weight loss has been shown to reduce rates of T2DM and improve insulin resistance and glucose regulation (Buchwald et al., 2009; Kelley et al., 2004; Unick et al., 2011). Importantly, improvements in glucose regulation and insulin sensitivity are observed following bariatric surgery and can occur as soon as one month post-operatively (Kashyap et al., 2010; Nestvold, Nielsen, & Lappegard, 2013). When combined with findings that improved glycemic control leads to better cognitive function in in other patient populations (Hewer, Mussell, Rist, Kulzer, & Bergis, 2003), it appears likely that improved glucose regulation following bariatric surgery may provide cognitive benefits.
No study has examined the possible contribution of glucose regulation to improved cognitive function after bariatric surgery. We examined whether improved glucose regulation, as measured by insulin sensitivity (homeostatic model assessment of insulin resistance (HOMA-IR)) and glycated hemoglobin (HbA1c) levels, would be related to improved cognitive functioning one year following bariatric surgery.
Method
Trial Design and Participants
Participants were 85 individuals recruited into a multi-site National Institutes of Health prospective study examining the effects of bariatric surgery on cognitive function. All patients were part of the Longitudinal Assessment of Bariatric Surgery (LABS) parent project and were recruited from existing LABS sites (Columbia, Cornell, and Neuropsychiatric Research Institute) (Belle et al., 2007). For study inclusion, bariatric surgery patients were required to be enrolled in LABS, between 20–70 years of age, and English-speaking. Exclusion criteria included history of neurological disorder or injury (e.g., dementia, seizures), moderate or severe head injury (defined as >10 minutes loss of consciousness), history of or current severe psychiatric illness (e.g. schizophrenia, bipolar disorder), history of or current alcohol or drug abuse (defined by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria), history of a learning disorder or developmental disability (defined by DSM-IV criteria), or impaired sensory function. All but one patient underwent Roux-en-Y gastric bypass (RYGB) and thus no comparisons for type of surgery were conducted. The present sample of individuals has also been included in previous work by our group examining cognitive changes following bariatric surgery (Alosco et al., 2013; 2014; Gunstad et al., 2011; Miller et al., 2013). All participants in the current sample had complete cognitive and metabolic data at each time point. A total of 3 outliers were removed for baseline HOMA-IR (n = 2), 12 month HOMA-IR (n = 1), resulting in a final sample of 82 individuals. Table 1 presents demographic/clinical characteristics of the sample.
Table 1.
Demographic and Medical Characteristics of the Sample (N = 82)
| Variable | Baseline Mean (SD) or % | 12-Month Mean (SD) or % | F/Chi-Square |
|---|---|---|---|
| Age | 43.55 (10.21) | -- | -- |
| Years of Education | 13.71 (1.30) | -- | -- |
| Female | 81.7% | -- | -- |
| Body Mass Index | 46.32(5.51) | 30.18 (5.25) | 961.30*** |
| Hypertension | 46.3% | 38.4% | 3.80* |
| Type 2 diabetes | 23.1% | 17.8% | 0.32 |
| Sleep Apnea | 36.6% | 16.7% | 1.95 |
| HOMA-IR | 6.69 (5.03) | 1.68(1.27) | 89.79*** |
| HbA1c | 5.74 (1.02) | 5.25 (0.76) | 38.67*** |
Note.
p< .05,
p< 0.01,
p < .001;
Sample size varies for baseline body mass index (n = 81) and education (n = 77) due to missing data;
HOMA-IR – homeostasis model assessment of insulin resistance; hbA1c – glycated hemoglobin
Interventions and Clinical Follow-Up
The Institutional Review Board at each site approved all procedures, and participants provided written informed consent prior to study involvement. All bariatric surgery patients underwent blood draw after fasting for eight hours and completed a computerized cognitive test battery within 30 days prior to and 12 months following surgery. Height and weight were measured at each time point to calculate body mass index (BMI). Medical and demographic characteristics were ascertained via self-report and corroborated by medical record review.
Outcomes
Laboratory measures
Participants completed fasting blood draw and all values were quantified by the LABS Central Laboratory using standardized procedures. Values obtained included fasting glucose, fasting insulin, and hbA1c. Insulin sensitivity was estimated using the homeostasis model assessment (HOMA): HOMA IR = (fasting glucose (mg/dl) × fasting insulin (µU/mL)/ 405 (Matthews et al., 1985). The HOMA model was first described in 1985 and was constructed using experimental data on physiological responses of glucose uptake and insulin production. The HOMA model correlates well the euglycemic clamp which is considered to be the “gold standard” for measuring insulin resistance (Matthews et al., 1985). HOMA-IR and insulin levels were highly correlated in this sample (r = .97, p < .001), essentially representing the same construct; thus, only HOMA-IR was examined for the present study.
Cognitive function
The IntegNeuro cognitive test battery is a computerized battery that assesses cognitive function in multiple domains. It demonstrates excellent psychometric properties (Paul et al., 2005; Williams et al., 2005). An alternate version of the IntegNeuro at each follow-up was also utilized to minimize the possibility of practice effects.
Digit span
There are two parts to this test, forward, which is a measure of auditory attentional capacity, and backward, which is a measure of working memory. Participants are presented with a series of digits on the touch-screen, separated by a one-second interval. The participant immediately enters the digits on a numeric keypad on the touch-screen in a forward or backward sequence from the order presented depending on the test. The number of digits in each sequence is gradually increased from 3 to 9, with two sequences at each level. The two dependent variables were the total number of correct forward and backward trials.
Switching of attention
This test is a computerized adaptation of the Trail Making Test A and B (Reitan, 1958). First, participants are asked to touch a series of 25 numbers in ascending order as quickly as possible. This is followed by the presentation of an array of 13 numbers (1–13) and 12 letters (A–L) that participants must alternately touch in ascending order. The first part of this test assesses attention and psychomotor speed and the second part assesses executive function. Time to completion was the dependent variable.
Verbal interference
This task taps into the ability to inhibit automatic and irrelevant responses and is similar to the Stroop Color Word Test (Golden, 1978). Participants are presented with colored words one at a time. Below each colored word is a response pad with the four possible words displayed in black and in fixed format. The subject is required to name the color of each word as quickly as possible, assessing executive functioning. Total number of words correctly identified was the dependent variable.
Verbal list-learning
Participants are read a list of 12 words a total of 4 times and asked to recall as many words as possible after each trial. Following presentation and recall of a distraction list, participants are asked to recall words from the original list. After a 20-minute filled delay, participants are asked to freely recall the learned list and perform a recognition trial comprised of target words and non-target words. Dependent variables were total words recalled for all four trials, after a short delay, and after a long delay. Given the high intercorrelation among these three variables (r’s ranged from .71 to .82), a memory composite score was created by averaging the scores on these variables.
Letter fluency
Participants are asked to generate words beginning with a given letter of the alphabet for 60 seconds. A different letter is used for each of the three trials. Total number of correct words was the dependent variable.
Animal fluency
In this task, participants generate as many animal names as possible in 60 seconds. Total number of correct words was the dependent variable.
Statistical Analysis
Descriptive statistics and histograms were used to examine continuous variables for univariate normality and outliers (> 3 SD above or below the mean). Multivariate outliers were examined for each regression model separately using Mahalanobis D2 and were removed if p < .001. In order to characterize impairment in the sample, raw scores of neuropsychological measures were transformed to T-scores using normative data correcting for age, gender, and IQ. Frequency analyses examined rates of cognitive impairment (>1.5 SD below the mean of the normative data) at baseline and 12-months in the two groups. Chi-square analyses examined differences in impairment between baseline and 12-months in each of the groups.
For the remainder of the analyses, raw scores for the cognitive variables were used. Bivariate correlational analyses were utilized to examine the relationships among demographic variables and cognitive test performance. Repeated measures ANOVAs were then conducted to examine changes in test performance and HOMA-IR from baseline to 12-months following surgery. There were no cases with missing data and thus repeated measures ANOVA was performed with the full sample size of 85. For all analyses, examination of correlations among predictor variables was performed to examine the possible presence of multicollinearity. Regression-based assumptions (e.g., normality, homoscedasticity) were also examined via P-P plots and histograms. To determine the effects of post-operative changes in glycemic control on each cognitive test at the 12-month follow-up, a series of separate regression based models were performed for each index of glycemic control as the predictor variable (e.g., HbA1c and HOMA-IR). Age was entered as a covariate into all models. Additional covariates (i.e., education, sex) were determined by their correlations with baseline test performance and were entered into each model for which they were significant. For models examining changes in HOMA-IR, block 1 included age, baseline test performance of the respective cognitive test and baseline HOMA-IR levels. Block 2 included 12-month HOMA-IR to determine its incremental predictive validity on 12-month cognitive function. This approach was repeated for HbA1c. The adjustment for baseline in a regression framework was performed because it accounts for the variance of baseline glycemic control and cognitive function and thus isolates the residual change among these variable at the follow-up time point. Change scores are less reliable and sensitive to regression towards the mean and therefore this approach to examine longitudinal data was not performed.
Results
Sample Characteristics
At baseline, average body mass index (BMI) was 46.82 (SD = 6.09) kg/m2. High rates of comorbid medical conditions such as hypertension, type 2 diabetes mellitus (T2DM), and sleep apnea were observed at baseline. BMI and rates of medical comorbidities decreased significantly 12-month follow-up. At baseline, insulin resistance was common, as the sample demonstrated elevated HOMA-IR (M = 6.69, SD = 5.03). As would be expected, high levels of HbA1c at baseline (M = 5.74, SD = 1.02) were associated with the presence of T2DM. Repeated measures revealed significant decreases in HOMA-IR (F(1,81) = 89.79, p < .001; ηp2= .53) and HbA1c levels (F(1,81) = 38.67, p < .001; ηp2 = .32) from baseline to 12-month follow-up.
Refer to Table 2 for a correlation matrix among baseline glycemic, demographic, medical, and cognitive variables. Notably, higher HbA1c levels were associated with older age. Elevated HOMA-IR was associated with type 2 diabetes. HbA1c was associated with hypertension, sleep apnea, and type 2 diabetes. Regression analyses controlling for baseline factors also showed that post-operative changes in BMI did not correspond to changes in HOMA-IR at the follow-up (p > .05 for all).
Table 2.
Baseline Associations among glycemic, demographic, medical, and cognitive variables (n = 82)
| HOMA-IR | HbA1c | BMI | Educ | Age | Sex | HTN | T2DM | SA | |
|---|---|---|---|---|---|---|---|---|---|
| HOMA-IR | -- | -- | -- | - | -- | -- | -- | -- | -- |
| HbA1c | .52*** | -- | -- | -- | -- | -- | -- | -- | -- |
| BMI | <.001 | −.01 | -- | -- | -- | -- | -- | -- | -- |
| Educ | −.09 | −.10 | .07 | -- | -- | -- | -- | -- | -- |
| Age | .10 | .46*** | −.04 | −.07 | -- | -- | -- | -- | -- |
| Sex | −.07 | −.10 | −.08 | −.11 | −.22* | -- | -- | -- | -- |
| HTN | .15 | .42*** | −.07 | <.01 | .47*** | −.22 | -- | -- | -- |
| T2DM | .41** | .45*** | .01 | −.25* | .43** | −.06 | .41*** | -- | -- |
| SA | −.09 | .42*** | .11 | .10 | .32* | −.12 | .10 | .07 | -- |
| Digits-F | −.13 | −.21 | .10 | .13 | −.26* | .06 | −.15 | −.15 | −.13 |
| Digits-B | −.06 | −.08 | .01 | .15 | −.20 | .15 | −.20 | −.26* | −.02 |
| SOA-A | .15 | .23* | .01 | −.24* | .31** | −.24* | .14 | .15 | .09 |
| SOA-B | .12 | .33** | .04 | −.35** | .44*** | −.19 | .17 | .35** | .29** |
| Stroop | <.001 | −.17 | .07 | .03 | −.29* | .15 | −.11 | −.20 | −.03 |
| Memory | −.27* | −.38*** | .13 | .13 | −.46*** | .42*** | −.31** | −.24* | −.23 |
| Letters | −.16 | −.15 | .07 | .17 | −.02 | .13 | −.10 | −.09 | <.01 |
| Animals | −.14 | −.11 | .01 | .19 | −.11 | .08 | −.18 | −.11 | −.20 |
Note
p < 0.05;
p < 0.01;
Sample sizes vary for education and medical variables due to missing data.
Abbreviations: Educ-Education, Digits-F = Digits forward, Digits-B = Digit Span Backward, SOA= Switching of Attention (A = numbers only, B = numbers and letters), BMI = body mass index; HTN = hypertension; T2DM = type 2 diabetes mellitus; SA = sleep apnea; higher score for SOA tests are worse; all other cognitive tests higher scores are better. HTN, T2DM, and SA coded 0 (absence) 1 (presence) of the condition.
Cognitive Function Improves Following Bariatric Surgery
Cognitive impairment at baseline (defined as 1.5 SD below the normative mean) was most common for letter fluency (17.6%) and memory (16.5%). At 12 months, rates of impairment apparently decreased for most of the cognitive tests. Given that many of the expected cell sizes were less than 5, nonparametric tests were required to compare the rates of impairment from baseline to 12 months on each test. Related-samples McNemar tests revealed significant reductions in impairment for memory. Rates of cognitive impairment at baseline and 12 months are presented in Table 3.
Table 3.
Percentage of Impairment Compared to Normative Data at Baseline and 12-months for Neuropsychological Tests (N = 82)
| Test | Baseline | 12-month | Exact Sig. |
|---|---|---|---|
| Digits Forward | 7 (8.5%) | 4 (4.9%) | .508 |
| Digits Backward | 5 (6.1%) | 4 (4.9%) | 1.00 |
| SOA-A | 3 (3.7%) | 2 (2.4%) | 1.00 |
| SOA-B | 6 (7.3%) | 6 (7.6%) | 1.00 |
| Verbal Interference | 6 (7.3%) | 1 (1.2%) | .06 |
| Memory | 13 (15.9%) | 2(2.4%) | .003 |
| Letter Fluency | 15 (18.3%) | 9 (11.0%) | .180 |
| Animal Fluency | 5 (6.1%) | 5 (6.1%) | 1.00 |
Note: McNemar test; Exact significance (2-tailed);
SOA-A = Switching of Attention Letters, SOA-B = Switching of Attention Letters and Numbers
Repeated measures ANOVA examined changes in cognitive function pre- to post-surgery. There were no violations in statistical assumptions (e.g., univariate normality) associated with repeated measure ANOVA design. Analyses showed significant improvements in cognitive function over 12-months for all cognitive tests except letter and animal fluency. Table 4 presents full neuropsychological test performance for baseline and 12-month follow-up.
Table 4.
Neuropsychological Test Performance Means (Standard Deviations) among Bariatric Surgery Patients (N = 82)
| Test | Baseline | 12-month | F | ηp2 |
|---|---|---|---|---|
| Digits Forward | 7.23(2.36) | 8.06(2.73) | 6.90** | .08 |
| Digits Backward | 4.28(2.86) | 5.40(3.07) | 7.72** | .09 |
| SOA-A | 20.31(7.18) | 17.50(5.71) | 23.05*** | .22 |
| SOA-B | 47.30(18.47) | 40.35(14.97) | 21.47*** | .21 |
| Verbal Interference | 11.67 (3.87) | 13.72(3.57) | 21.99*** | .21 |
| Memory Composite | 15.04(3.04) | 16.84(2.89) | 49.01*** | .38 |
| Letter Fluency | 14.42(3.84) | 14.70(3.73) | 1.06 | .01 |
| Animal Fluency | 23.28(5.29) | 23.798(5.68) | 1.00 | .01 |
Note.
p<.05,
p<.01,
p<.001;
Abbreviations—Digits Forward = Digit Span Forward, Digits Backward = Digit Span Backward, SOA= Switching of Attention (A = numbers only, B = numbers and letters); LDFR = Long Delay Free Recall; higher scores reflect better performance except for SOA-A and SOA-B for which lower scores reflect better performance.
Effects of Glycemic Control on Cognitive Function
Bivariate correlations showed significant relationships between baseline HOMA-IR and memory (r = −.27, p < .05), and between HbA1c and switching of attention-A (r = .23, p < .05), switching of attention –B (r = .33, p < .01), and memory (r = −.38, p < .001).
Linear regression models were used to examine the effects of improved glycemic control on improvements in each cognitive test. Covariates were entered into the first block and were determined by baseline correlations with each test. Sample sizes varied due to missing data on education (n = 5) for models including education as a covariate and due to the removal of multivariate outliers.
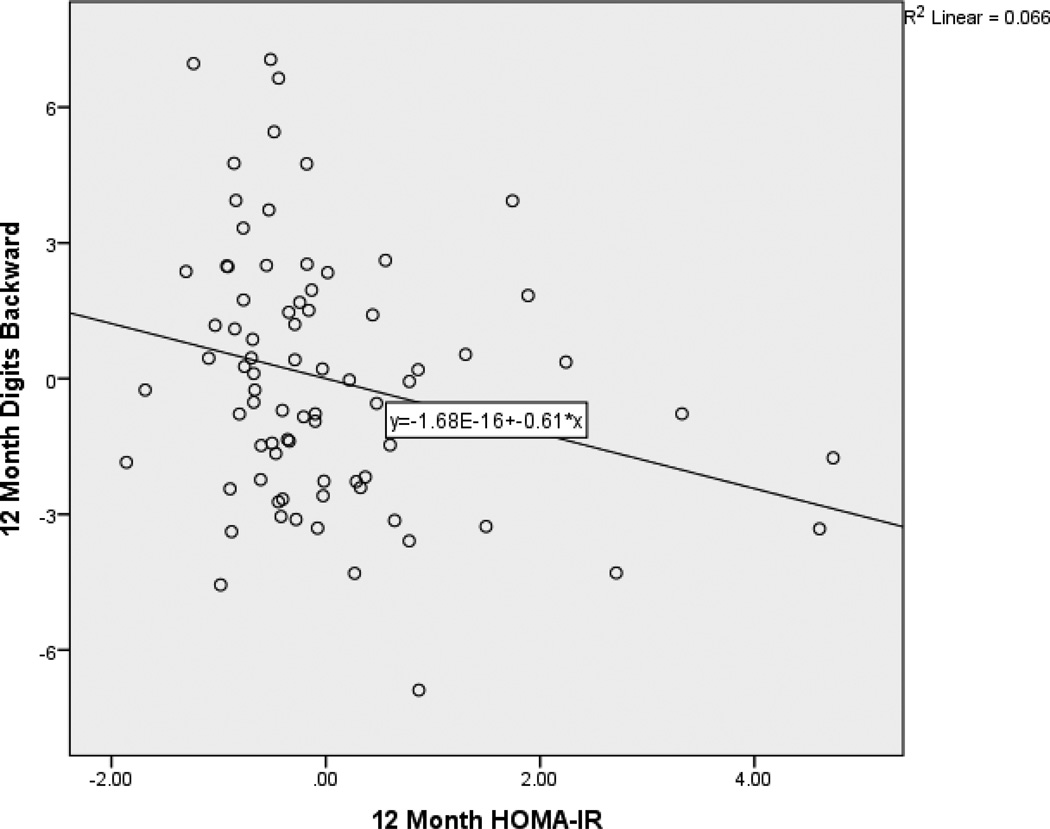
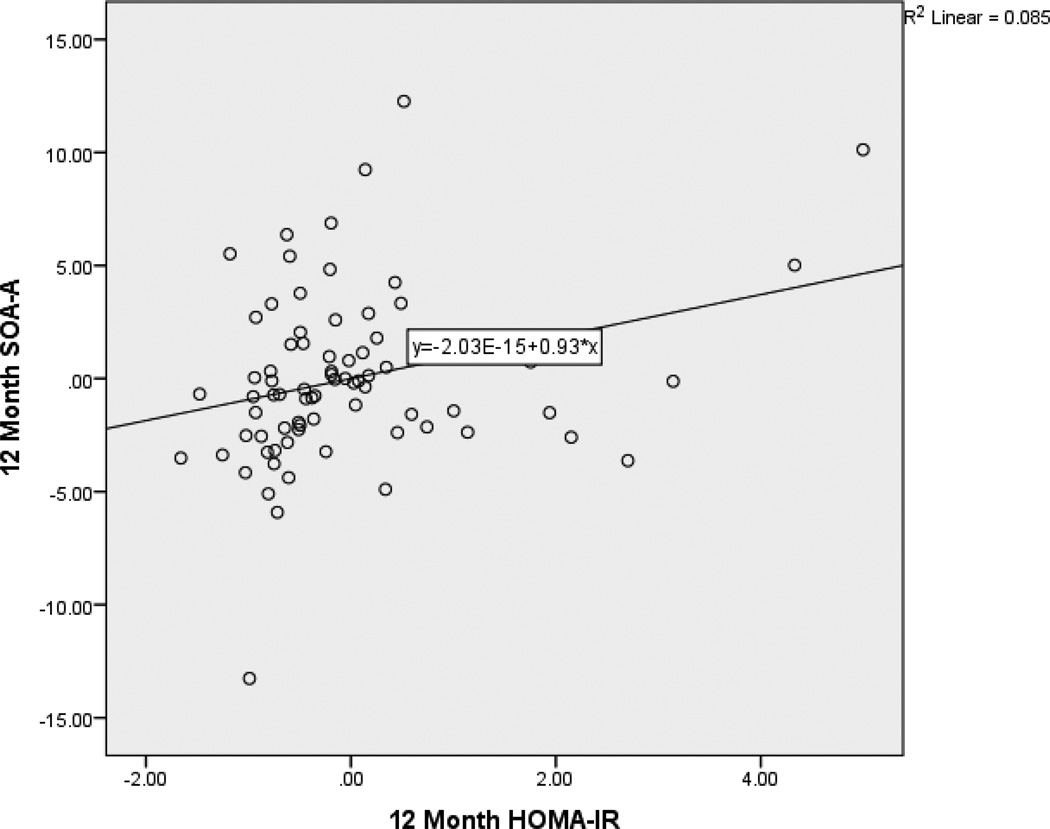
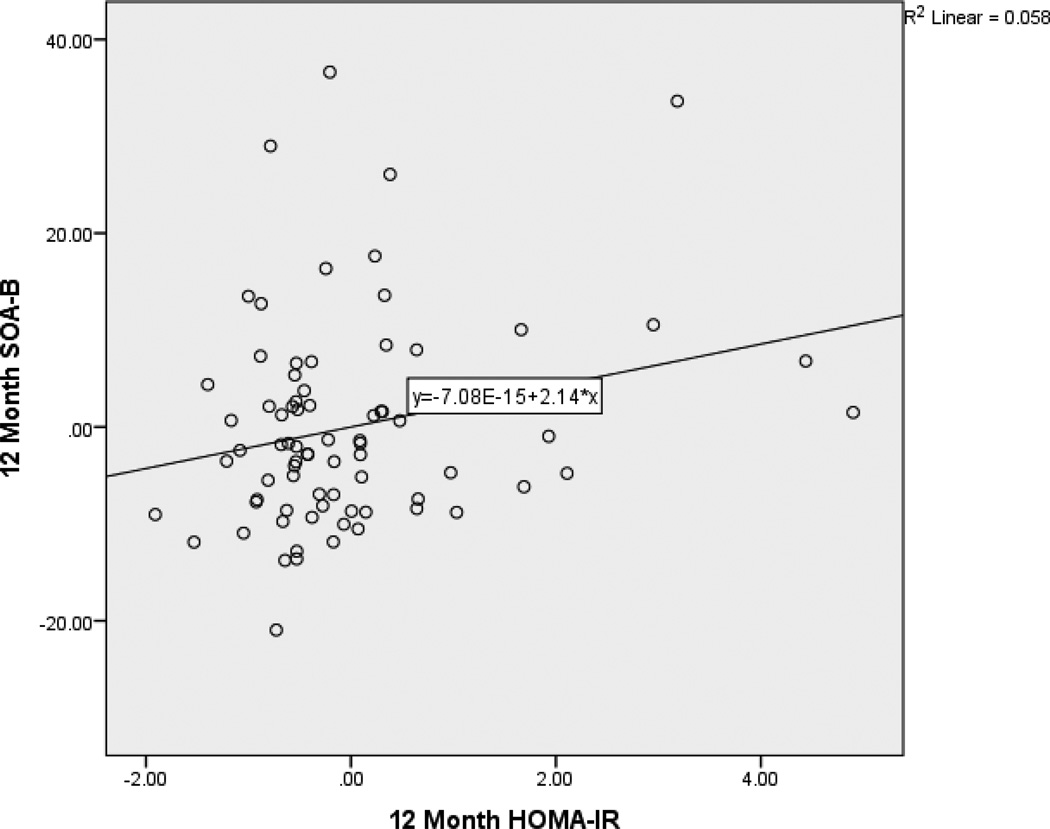
After controlling for age and baseline factors, 12-month HOMA-IR predicted 12-month digits backward (β = −.253, p < .05). Specifically, as HOMA-IR decreased over time, working memory improved. After controlling for age, education, and sex, 12-month HOMA-IR predicted SOA-A (β = −.156, p < .05). Specifically, as HOMA-IR decreased over time, time to completion for SOA-A also decreased reflecting an improvement in performance. After controlling for age and education, 12-month HOMA-IR predicted SOA-B (β = −.181, p < .05). Specifically, as HOMA-IR decreased over time, time to completion for SOA-B also decreased reflecting an improvement in performance. Partial regression plots (Figures 1, 2, and 3) provide a graphical depiction of the association between and 12-month HOMA-IR and 12-month digits backward, SOA-A and SOA-B. Full regression results are presented in Table 5.
Figure 1.
Partial Regression Plot of the Relationship between 12-Month HOMA-IR and digits backward. This plot depicts the nature of the relationship between 12-month HOMA-IR and 12-month digits backward after accounting for baseline values of these variables and age. Higher performance on digits backward is better.
Figure 2.
Partial Regression Plot of the Relationship between 12-Month HOMA-IR and switching of attention - letters. This plot depicts the nature of the relationship between 12-month HOMA-IR and 12-month switching of attention - letters after accounting for baseline values of these variables, age, and education. Lower performance on switching of attention - letters is better.
Figure 3.
Partial Regression Plot of the Relationship between 12-Month HOMA-IR and switching of attention – letters and numbers. This plot depicts the nature of the relationship between 12-month HOMA-IR and 12-month switching of attention - letters after accounting for baseline values of these variables and age. Lower performance on switching of attention - letters and numbers is better.
Table 5.
Hierarchical Linear Regressions Examining Changes in HOMA-IR Predicting Changes Cognitive Function from Baseline to 12 Months
| B | SE B | β | ΔR2 | ΔF | |
|---|---|---|---|---|---|
| Digit Span Forward (n = 79) | .00 | .01 | |||
| Age | −.04 | .03 | −.140 | ||
| BL HOMA-IR | <.001 | .07 | .006 | ||
| BL Digits Forward | .37 | .12 | .335** | ||
| 12 Month HOMA-IR | .03 | .27 | .013 | ||
| Digit Span Backward (n = 78) | .06 | 5.17* | |||
| Age | −.07 | .03 | −.227* | ||
| BL HOMA-IR | <.001 | .07 | −.007 | ||
| BL Digits Backward | .31 | .12 | .292** | ||
| 12 Month HOMA-IR | −.61 | .27 | −.253* | ||
| Switching of Attention-A (n = 75) | .02 | 5.45* | |||
| Age | .05 | .04 | .080 | ||
| Education | −.58 | .29 | −.132* | ||
| BL HOMA-IR | −.05 | .08 | −.047 | ||
| BL Switching A | .76 | .07 | .743*** | ||
| 12 Month HOMA-IR | .74 | .32 | .162* | ||
| Switching of Attention-B (n = 77) | .03 | 5.14* | |||
| Age | .08 | .13 | .054 | ||
| BL HOMA-IR | −.48 | .25 | −.163 | ||
| BL Switching B | .54 | .07 | .673*** | ||
| 12 Month HOMA-IR | 2.31 | 1.02 | .195* | ||
| Stroop (n = 81) | .001 | .16 | |||
| Age | −.09 | .04 | −.265* | ||
| BL HOMA-IR | −.05 | .07 | −.065 | ||
| BL Stroop | .33 | .09 | .362** | ||
| 12 Month HOMA-IR | −.11 | .29 | −.04 | ||
| Memory (n = 80) | .005 | .79 | |||
| Age | −.05 | .03 | −.165 | ||
| BL HOMA-IR | .06 | .05 | .111 | ||
| BL Memory | .63 | .09 | .660*** | ||
| 12 Month HOMA-IR | .17 | .19 | .075 | ||
| Letters (n = 76) | .002 | .36 | |||
| Age | −.04 | .03 | −.107 | ||
| Education | .56 | .20 | .195** | ||
| BL HOMA-IR | −.01 | .05 | −.008 | ||
| BL Letters | .75 | .07 | .760*** | ||
| 12 Month HOMA-IR | .13 | .21 | .043 | ||
| Animals | .009 | 1.32 | |||
| Age | −.05 | .05 | −.08 | ||
| BL HOMA-IR | .09 | .10 | .08 | ||
| BL Animals | .72 | .09 | .666*** | ||
| 12 Month HOMA-IR | .46 | .40 | .103 |
Note:
p< .05,
p<.01,
p<.001;
higher scores are better for all tests except SOA-A and SOA-B.
Abbreviations: BL = Baseline, HOMA-IR = homeostasis model of assessment for insulin resistance, Switching = Switching of Attention. These results represent the final model in which 12 month HOMA-IR is entered into the second block.
Changes in HbA1c did not predict improvements in any of the cognitive tests. Full regression results are presented in Table 6.
Table 6.
Hierarchical Linear Regressions Examining Changes in HbA1c Predicting Changes Cognitive Function from Baseline to 12 Months
| B | SE B | β | ΔR2 | ΔF | |
|---|---|---|---|---|---|
| Digit Span Forward (n = 82) | .02 | 1.77 | |||
| Age | −.04 | .03 | −.132 | ||
| BL HBA1C | −.39 | .41 | −.144 | ||
| BL Digits Forward | .40 | .13 | .345** | ||
| 12 Month HBA1C | .72 | .54 | .200 | ||
| Digit Span Backward (n = 78) | <.001 | .02 | |||
| Age | −.06 | .04 | −.183 | ||
| BL HBA1C | −.60 | .49 | −.167 | ||
| BL Digits Backward | .27 | .12 | .252* | ||
| 12 Month HBA1C | −.08 | .64 | −.017 | ||
| Switching of Attention-A (n = 77) | <.001 | .002 | |||
| Age | .05 | .05 | .081 | ||
| Education | −1.03 | .36 | −.234** | ||
| Sex | −3.98 | 1.30 | −.265*** | ||
| BL HBA1C | .31 | .67 | .055 | ||
| BL Switching A | .42 | .08 | 0.526*** | ||
| 12 Month HBA1C | −.04 | .96 | −.005 | ||
| Switching of Attention-B (n = 77) | <.001 | .05 | |||
| Age | .16 | .15 | .11 | ||
| Education | −1.50 | 1.06 | .61*** | ||
| BL HBA1C | −.21 | 1.84 | −.015 | ||
| BL Switching B | .49 | .08 | .613*** | ||
| 12 Month HBA1C | −.56 | 2.47 | −.028 | ||
| Stroop (n = 82) | .002 | .25 | |||
| Age | −.09 | .04 | −.242* | ||
| BL HBA1C | −.52 | .51 | −.148 | ||
| BL Stroop | .33 | .09 | .357** | ||
| 12 Month HBA1C | .33 | .66 | .071 | ||
| Memory (n = 81) | .002 | .34 | |||
| Age | −.05 | .03 | −.170 | ||
| BL HBA1C | −.10 | .34 | −.034 | ||
| BL Memory | .60 | .34 | .628*** | ||
| 12 Month HBA1C | .26 | .44 | .068 | ||
| Letters (n = 76) | <.001 | .03 | |||
| Age | −.04 | .03 | −.110 | ||
| Education | .55 | .20 | .193** | ||
| BL HBA1C | .003 | .37 | .001 | ||
| BL Letters | .75 | .07 | .760*** | ||
| 12 Month HBA1C | .09 | .50 | .018 | ||
| Animals (n = 81) | .003 | .39 | |||
| Age | −.07 | .05 | −.133 | ||
| BL HBA1C | .55 | .70 | .099 | ||
| BL Animals | .72 | .09 | .669*** | ||
| 12 Month HBA1C | .57 | .92 | .077 |
Note:
p< .05,
p<.01,
p<.001;
higher scores are better for all tests except SOA-A and SOA-B.
Abbreviations: BL = Baseline, HBA1C = homeostasis model of assessment for insulin resistance, Switching = Switching of Attention. These results represent the final model in which 12 month HBA1C is entered into the second block.
Discussion
Consistent with previous work, bariatric surgery was associated with significant improvements in both cognitive function (Alosco et al., 2013; 2014;Gunstad et al., 2011; Miller et al., 2013) and medical status, including weight loss, resolution or improvement of comorbidities, and decreases in HOMA-IR, and HbA1c levels (Buchwald et al., 2009; Hewer et al., 2003; Netvold et al., 2013). Importantly, there was a small effect of changes in HOMA-IR on improvements in working memory, psychomotor speed, and cognitive flexibility, suggesting that improved glycemic control may play a small role in the observation of cognitive improvement, following bariatric surgery, particularly for attention and executive function. Several aspects of these finding warrant brief discussion.
Consistent with past studies, the current results demonstrate that bariatric surgery has beneficial effects on insulin sensitivity. Insulin resistance, which is common among persons with severe obesity, is an established risk factor for adverse neurocognitive outcomes, including Alzheimer’s disease and vascular dementia (Hu et al., 2007; Gunstad et al., 2007; 2011; Waldstein & Katzel, 2006; Whitmer et al., 2007) and has also been linked to brain changes independent of these conditions, including reduced brain volume and accelerated atrophy over time (Willette et al., 2013).
However, past research shows inconsistent effects for the possible cognitive benefits of improved glycemic control. The current findings demonstrate a pattern of improved glycemic control leading to better cognitive function which is similar previous studies which have found improved glycemic control through pharmacological treatment (Ryan et al., 2006) and aerobic exercise (Baker et al., 2010) lead to improved cognitive function. In contrast, there have been other recent studies that have failed to show cognitive benefit from improving glycemic control (Kawamura, Umemura, & Hotta, 2012; Launer et al., 2011; Moore et al., 2013; Ng et al., 2014). Future studies are needed to clarify this phenomenon, particularly the current findings that improved HOMA-IR was related to performance on measures of attention/executive function but not memory. This pattern is particularly surprising given that participants improved on all nearly cognitive tests, highlighting an urgent need to examine other factors such as improved cardiovascular function, decreased systemic inflammation, changes in the concentration of circulating biomarkers like leptin and ghrelin (King et al., 2012; Iannelli et al., 2013) as possible mechanisms for post-operative cognitive improvement. A better understanding of these mechanisms may provide key insight into the mechanisms that link obesity to neurological outcomes such as stroke and Alzheimer’s disease.
Contrary to expectations, post-operative improvements in HbA1c levels did not correspond to changes in cognitive function. Although past work has linked HbA1c levels to cognitive impairment in persons with type 2 diabetes mellitus (Umegaki et al., 2011) and chronic kidney disease (Seidel et al., 2013), this pattern is not consistent across studies. For example, recent findings from the ACCORD-MIND trial found that intensive glycemic therapeutic intervention (targeting HbA1c < 6%) was not associated with better cognitive function compared to a standard intervention (targeting HbA1c – 7–7.9%) in patients with T2DM (Launer et al., 2011). Past work also shows an inverse relationship between HbA1c and cognitive function in some samples of very old adults (van den Berg, de Craen, Biessels, Gussekloo, & Westendorp, 2006), even raising the possibility that the hypoglycemic events found in some post-surgical patients (Ritz & Hanaire, 2011; Joseph et al., 2007) may adversely impact cognitive function. Further research on the possible contribution of both hyper- and hypoglycemic on neurocognitive outcomes in bariatric surgery patients will help to clarify these mechanisms and long-term outcomes.
The results of this study must be viewed in light of a number of limitations. Most notably, while the current study utilized normative data to help characterize cognitive status at each time point, we did not have access to test data from a matched control group. This concern can be at least attenuated through examination of the literature, as cognitive improvements in this sample of obese persons re in contrast to the accelerated cognitive decline in epidemiological studies (Gunstad et al., 2010). Nevertheless, future studies that implement a severely obese control group that do not undergo bariatric surgery are needed to elucidate the effects of post-surgery glucose regulation on cognitive function.
Another important limitation involves having two time points and relatively modest sample size, as they preclude implementation of more advanced statistical analyses (e.g., structural equation modeling, linear mixed effects modeling) to determine the exact linear structure of change in cognition and glucose indices over time after bariatric surgery. In addition to quantification of linear structure (e.g., linear, cubic, non-linear, etc.), such statistical methods also account for random intercepts, slope variability, and inter- and intra-individual changes over time. As such, future work that implements larger samples and several time points are much needed in order to implement advance statistical modeling to better understand the effects of bariatric surgery on cognition and glycemic control.
Several additional limitations deserve further mention. Although following patients for 12 months can provide some insight into possible mechanisms, future studies that monitor post-operative changes in glucose regulation and cognitive function for a longer period of time (i.e. 5–10 years) are much needed to better examine these possible mechanisms. Similarly, examining cognitive function at longer intervals will also clarify the possibility that bariatric surgery may attenuate the rapid cognitive decline found in persons with obesity (Sellbom & Gunstad, 2012) and ultimately reduce risk of conditions like Alzheimer’s disease. Bariatric surgery procedures are most commonly performed in middle-aged persons (Santry, Gillen, & Lauderdale, 2005), prior to the usual onset of neurodegenerative disorders; it is possible that persons with obesity that undergo weight loss surgery may exhibit a shallower slope of cognitive decline with advancing age. Such studies will also begin to clarify the possible effect of weight regain on the relationship between glycemic control and cognitive impairment over time, as weight regain is common among bariatric surgery patients (Karlsson, Taft, Ryden, Sjöström, & Sullivan, 2007). Future studies should also utilize more sophisticated measures of glucose regulation (e.g. oral glucose tolerance testing) and neurocognitive outcomes (e.g., structural and functional MRI) to provide greater insight into the contribution of glycemic control to neurological outcomes in this population. Additionally, we did not adjust for the possible influence of insulin or other diabetic medications on study outcomes in order to emphasize ecological validity in this study. However, future studies are needed to clarify the contribution of measures of glucoregulation to cognitive function in diabetes and non-diabetes populations.
In addition, in order to reduce practice effects, alternate forms of the Integneuro tests were used at the follow-up evaluation. Unfortunately, these versions of Integneuro were not counterbalanced in the current study and no specific estimates for possible effects of repeated assessments have been published for this measure. As a result, it is possible that some of the effects may be related to differences in the versions of the tests. However, given the magnitude of improvements in performance from baseline to 12-months across tests, it is unlikely that potential differences in the test versions could solely account for these changes. Lastly, although patients were excluded for a clinical history of or current drug or alcohol abuse to minimize confounding factors, their exclusion limits the generalizability of our findings, given the high rates of alcohol and drug use among persons with obesity. Furthermore, since these disorders are often diagnosed during the preoperative period, it is possible that alcohol and drug use disorders were present after follow up in the current sample, which is a confounding factor. Future research should use additional measures, such as blood testing, to screen for these disorders.
In brief summary, the current findings suggest that improvements in insulin sensitivity may play a small role in improved cognitive function one year after bariatric surgery Examination of these effects at more distant follow-up visits will clarify whether improved glycemic control has neuroprotective effects in severely obese persons.
Footnotes
Conflict of Interest Statement
Rachel Galioto declares no conflicts of interest.
Michael Alosco declares no conflicts of interest.
Mary Beth Spitznagel declares no conflicts of interest.
Gladys Strain declares no conflicts of interest.
Michael Devlin declares no conflicts of interest.
Ross Crosby declares no conflicts of interest.
Ronald Cohen declares no conflicts of interest.
John Gunstad declares no conflicts of interest.
James Mitchell declares no conflicts of interest.
References
- Alosco ML, Galioto R, Spitznagel MB, Strain G, Devlin M, Cohen R, Gunstad J. Cognitive function following bariatric surgery: Evidence for Improvement 3 years post-surgery. American Journal of Surgery. 2013 doi: 10.1016/j.amjsurg.2013.05.018. S0002-9610(13)00514-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, Spitznagel MB, Strain G, Devlin M, Cohen R, Paul R, Gunstad J. Improved memory function two years after bariatric surgery. Obesity (Silver Spring) 2014;22:32–38. doi: 10.1002/oby.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Craft S. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer's disease. Journal of Alzheimer’s Disease. 2010;22:569–579. doi: 10.3233/JAD-2010-100768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belle SH, Berk PD, Courcoulas AP, Flum DR, Miles CW, Mitchell JE, Yanovski SZ. Safety and efficacy of bariatric surgery: Longitudinal Assessment of Bariatric Surgery. Surgery for Obesity and Related Diseases. 2007;3:116–126. doi: 10.1016/j.soard.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray GA. Medical Consequences of Obesity. Journal of Clinical Endocrinology and Metabolism. 2004;89:2583–2589. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. American Journal of Medicine. 2009;122:248–256. e5. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- Castellini G, Godini L, Amedei SG, Faravelli C, Lucchese M, Ricca V. Psychological effects and outcome predictors of three bariatric surgery interventions: a 1-year follow-up study. Eating and Weight Disorders. 2014;19:217–214. doi: 10.1007/s40519-014-0123-6. [DOI] [PubMed] [Google Scholar]
- Convit A, Wolf OT, Tarshish C, de Leon MJ. Reduced glucose tolerance is associated with poor memory performance and hippocampal atrophy among normal elderly. Proceedings of the National Academy of Sciences USA. 2003;100:2019–2022. doi: 10.1073/pnas.0336073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland MA, Miller ME, Goveas JS, Hogan PE, Coker LH, Williamson J, Resnick SM. Cognitive function and fine motor speed in older women with diabetes mellitus: results from the women's health initiative study of cognitive aging. Journal of Women’s Health. 2011;20:1435–1443. doi: 10.1089/jwh.2011.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontbonne A, Berr C, Ducimetière P, Alpérovitch A. Changes in cognitive abilities over a 4-year period are unfavorably affected in elderly diabetic subjects: results of the Epidemiology of Vascular Aging Study. Diabetes Care. 2001;24:366–370. doi: 10.2337/diacare.24.2.366. [DOI] [PubMed] [Google Scholar]
- Gelir E, Basaran C, Bayrak S, Yağcıoğlu S, Budak MT, Firat H, Ungan P. Electrophysiological assessment of the effects of obstructive sleep apnea on cognition. PLoS One. 2014;9:90647. doi: 10.1371/journal.pone.0090647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golay A, Felber JP. Evolution from obesity to diabetes. Diabetes and Metabolism. 1994;20:3–14. [PubMed] [Google Scholar]
- Golden C. Stroop color and word task: a manual for clinical and experimental uses. Chicago: Stoeling; 1978. [Google Scholar]
- Gunstad J, Lhotsky A, Wendell C, Ferrucci L, Zonderman A. Longitudinal examination of obesity and cognitive function: results from the Baltimore longitudinal study of aging. Neuroepidemiology. 2010;34:222–229. doi: 10.1159/000297742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunstad J, Paul R, Cohen R, Tate D, Spitznagel MB, Gordon E. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Comprehensive Psychiatry. 2007;48:57–61. doi: 10.1016/j.comppsych.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Gunstad J, Strain G, Devlin MJ, Wing R, Cohen RA, Paul RH, Mitchell JE. Improved memory function 12 weeks after bariatric surgery. Surgery for Obesity and Related Diseases. 2011;7:465–472. doi: 10.1016/j.soard.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewer W, Mussell M, Rist F, Kulzer B, Bergis K. Short-term effects of improved glycemic control on cognitive function in patients with type 2 diabetes. Gerontology. 2003;49:86–92. doi: 10.1159/000067947. [DOI] [PubMed] [Google Scholar]
- Hu G, Tuomilehto J, Silventoinen K, Sarti C, Männistö S, Jousilahti P. Body mass index, waist circumference, and waist-hip ratio on the risk of total and type-specific stroke. Archives of Internal Medicine. 2007;167:1420–1427. doi: 10.1001/archinte.167.13.1420. [DOI] [PubMed] [Google Scholar]
- Iannelli A, Martini F, Rodolphe A, Schneck AS, Gual P, Tran A, Gugenheim J. Body composition, anthropometrics, energy expenditure, systemic inflammation, in premenopausal women 1 year after laparoscopic Roux-en-Y gastric bypass. Surgical Endoscopy. 2013;28:500–507. doi: 10.1007/s00464-013-3191-1. [DOI] [PubMed] [Google Scholar]
- Joseph B, Genaw J, Carlin A, Jordan J, Talley J, Rubinfeld I. Perioperative tight glycemic control: the challenge of bariatric surgery patients and the fear of hypoglycemic events. The Permanente Journal. 2007;11:36–39. doi: 10.7812/tpp/06-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson J, Taft C, Ryden A, Sjöström L, Sullivan M. Ten year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. International Journal of Obesity (London) 2007;31:1248–1261. doi: 10.1038/sj.ijo.0803573. [DOI] [PubMed] [Google Scholar]
- Kashyap SR, Daud S, Kelly KR, Gastaldelli A, Win H, Brethauer S, Schauer PR. Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. International Journal of Obesity (London) 2010;34:462–471. doi: 10.1038/ijo.2009.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T, Umemura T, Hotta N. Cognitive impairment in diabetic patients: can. diabetic control prevent cognitive decline? Journal of Diabetes Investigation. 2012;3:413–423. doi: 10.1111/j.2040-1124.2012.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DE, Kuller LH, McKolanis TM, Harper P, Mancino J, Kalhan S. Effects of moderate weight loss and orlistat on insulin resistance, regional adiposity, and fatty acids in type 2 diabetes. Diabetes Care. 2004;27:33–40. doi: 10.2337/diacare.27.1.33. [DOI] [PubMed] [Google Scholar]
- King WC, Hsu JY, Belle SH, Courcoulas AP, Eid GM, Flum DR, Wole BM. Pre- to postoperative changes in physical activity: report from the longitudinal assessment of bariatric surgery-2 (LABS-2) Surgery for Obesity and Related Diseases. 2012;8:522–532. doi: 10.1016/j.soard.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launer LJ, Miller ME, Williamson JD, Lazar RM, Gerstein HC, Murray AM, Bryan RN. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurology. 2011;10:969–977. doi: 10.1016/S1474-4422(11)70188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and B-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Mayega RW, Guwatudde D, Makumbi F, Nakwagala FN, Peterson S, Tomson G, Ostenson CG. Diabetes and pre-diabetes among persons aged 35 to 60 years in Eastern Uganda: Prevalence and associated factors. PloS One. 2013;8:e72554. doi: 10.1371/journal.pone.0072554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LA, Crosby RD, Galioto R, Strain G, Devlin MJ, Wing R, Gunstad J. Bariatric surgery patients exhibit improved memory function 12 months postoperatively. Obesity Surgery. 2013;23:527–535. doi: 10.1007/s11695-013-0970-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Mander AG, Ames D, Kotowicz MA, Carne RP, Brodaty H, Watters DA. Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care. 2013;36:2981–2987. doi: 10.2337/dc13-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestvold TK, Nielsen EW, Lappegard KY. Bariatric surgery reduces risk factors for development of type 2 diabetes mellitus in morbidly obese, nondiabetic patients. Metabolic Syndrome and Related Disorders. 2013;11:441–446. doi: 10.1089/met.2013.0085. [DOI] [PubMed] [Google Scholar]
- Ng T, Feng L, Yap K, Lee TS, Tan CH, Winblad B. Long-term metformin. usage and cognitive function among older adults with diabetes. Journal of Alzheimer’s Disease. 2014;41:61–68. doi: 10.3233/JAD-131901. [DOI] [PubMed] [Google Scholar]
- Paul RH, Lawrence J, Williams LM, Richard CC, Cooper N, Gordon E. Preliminary validity of “integneuro”” a new computerized battery of neurocognitive tests. International Journal of Neuroscience. 2005;115:1549–1567. doi: 10.1080/00207450590957890. [DOI] [PubMed] [Google Scholar]
- Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- Ritz P, Hanaire H. Post-bypass hypoglycaemia: a review of current findings. Diabetes and Metabolism. 2011;37:274–281. doi: 10.1016/j.diabet.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Ryan CM, Freed MI, Rood JA, Cobitz AR, Waterhouse BR, Strachan MW. Improving metabolic control leads to better working memory in adults with type 2 diabetes. Diabetes Care. 2006;29:345–351. doi: 10.2337/diacare.29.02.06.dc05-1626. [DOI] [PubMed] [Google Scholar]
- Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. Journal of the American Medical Association. 2005;294:1909–1917. doi: 10.1001/jama.294.15.1909. [DOI] [PubMed] [Google Scholar]
- Seidel UK, Gronewold J, Volsek M, Todica O, Kribben A, Bruck H, Hermann DM. The prevalence, severity, and association with HbA1c and fibrinogen of cognitive impairment in chronic kidney disease. Kidney International. 2014;85:693–702. doi: 10.1038/ki.2013.366. [DOI] [PubMed] [Google Scholar]
- Sellbom KS, Gunstad J. Cognitive function and decline in obesity. Journal of Alzheimer’s Disease. 2012;30:S89–S95. doi: 10.3233/JAD-2011-111073. [DOI] [PubMed] [Google Scholar]
- Singer J, Trollor JN, Baune BT, Sachdev PS, Smith E. Arterial stiffness, the brain and cognition: A systematic review. Ageing Research Reviews. 2014;15:16–27. doi: 10.1016/j.arr.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Stanek KM, Strain G, Devlin M, Cohen R, Paul R, Crosby RD, Gunstad J. Body mass index and neurocognitive functioning across the adult lifespan. Neuropsychology. 2013;27:141–151. doi: 10.1037/a0031988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umegaki H, Kawamura T, Kawano N, Umemura T, Kanai A, Sano T. Factors associated with cognitive decline in elderly diabetics. Dementia and Geriatric Cognitive Disorders Extra. 2011;1:1–9. doi: 10.1159/000323188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unick JL, Beavers D, Jakicic JM, Kitabchi AE, Knowler WC, Wadden TA, Wing RR. Effectiveness of lifestyle interventions for individuals with severe obesity and type 2 diabetes: results for the Look AHEAD Trial. Diabetes Care. 2011;34:2152–2157. doi: 10.2337/dc11-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg E, de Craen AJ, Biessels GJ, Gussekloo J, Westendorp RG. The impact of diabetes mellitus on cognitive decline in the oldest of the old: a prospective population-based study. Diabetologia. 2006;49:2015–2023. doi: 10.1007/s00125-006-0333-1. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Katzel LL. Interactive relations of central versus total obesity and blood pressure to cognitive function. International Journal of Obesity (London) 2006;30:201–207. doi: 10.1038/sj.ijo.0803114. [DOI] [PubMed] [Google Scholar]
- Whitmer R, Gunderson E, Quesenberry CP, Jr, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Current Alzheimer Research. 2007;4:103–109. doi: 10.2174/156720507780362047. [DOI] [PubMed] [Google Scholar]
- Willette AA, Xu G, Johnson SC, Birdsill AC, Jonaitis EM, Sager MA, Bendlin BB. Insulin resistance, brain atrophy, and cognitive performance in late middle-aged adults. Diabetes Care. 2013;36:443–449. doi: 10.2337/dc12-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Simms E, Clark CR, Paul RH, Rowe D, Gordon E. The test-retest reliability of a standardized neurocognitive and neurophysiological test battery: “neuromarker”. International Journal of Neuroscience. 2005;115:1605–1630. doi: 10.1080/00207450590958475. [DOI] [PubMed] [Google Scholar]