Abstract
Objective
The main object of this pilot study was to investigate the safety of administering onabotulinumtoxinA (BTA) towards the sphenopalatine ganglion (SPG) in intractable chronic cluster headache. Efficacy data were also collected to provide indication on whether future placebo-controlled studies should be performed.
Method
In a prospective, open-label, uncontrolled study, we performed a single injection of 25 IU (n = 5) or 50 IU BTA (n = 5) towards the SPG in 10 patients with intractable chronic cluster headache with a follow-up of 24 weeks. The primary outcome was adverse events (AEs) and the main efficacy outcome was attack frequency in weeks 3 and 4 post-treatment.
Results
A total of 11 AEs were registered. There was one severe adverse event (SAE): posterior epistaxis. The number of cluster headache attacks (main efficacy outcome) was statistically significantly reduced in the intention-to-treat analysis from 18 ± 12 per week in baseline to 11 ± 14 (p = 0.038) in weeks 3 and 4, and five out of 10 patients had at least 50% reduction of attack frequency compared to baseline. The cluster attack frequency was significantly reduced for five out of six months post-treatment.
Conclusion
Randomised, placebo-controlled studies are warranted to establish the potential of this possible novel treatment of cluster headache.
Keywords: Cluster headache, sphenopalatine ganglion, pterygopalatine ganglion, botulinum toxin, headache
Introduction
Cluster headache (CH) is a primary headache syndrome that often does not respond satisfactorily to pharmacological treatment. Theories of CH pathophysiology posit activation of a positive feedback system involving a reflex arc with the trigeminal system as the afferent limb and parasympathetic nerves, mainly via the sphenopalatine ganglion (SPG), as the efferent limb (1). In this reflex arc, the SPG, localised in the sphenopalatine fossa, is the most available target for interventional treatment.
Attempts to block the SPG with topical agents in CH have a long history, the first by Sluder in 1908 with a cocaine solution (2). Cocaine and short-acting local anaesthetics block Na/K-channels with durations of one to eight hours. No one has convincingly shown that these intranasal topical applications actually reach and hence are able to block the SPG, but several small, uncontrolled studies have explored the effect in CH mainly as acute treatment, and some indicate moderate but short-lasting effect (3). With a transnasal endoscopic technique first described by Prasanna and Murthy in 1993 (4), the pharmacological agents are injected directly into the sphenopalatine fossa to overcome the possible limitations of the topical administration methods described above.
To further explore the potential of SPG blocks, it is of importance to develop an accurate, easy and safe technique to target the SPG, and preferentially with a long-lasting pharmacological agent. In the SPG, pre-ganglionic parasympathetic fibres synapse with postganglionic fibres innervating intracranial vessels using acetylcholine (ACh) as neurotransmitter. OnabotulinumtoxinA (BTA) causes a neural block by inhibiting ACh release. In the autonomic system, based on studies on hyperhidrosis and Frey’s syndrome, the duration of such a block may last three to 12 months (5).
The main object of this study was to investigate the safety of administering two different doses of BTA towards the SPG in 10 patients with intractable chronic CH with an open, uncontrolled design. Secondly, efficacy data were collected to provide an indication on whether future placebo-controlled studies should be performed.
Method
Study design and participants
The study was conducted at St. Olavs Hospital, Trondheim, Norway, between October 2013 and June 2014. The study included a minimum two-week baseline period to register the attack frequency, and a 24-week follow-up. Ten patients with intractable chronic CH were recruited from the Neurology Department and by referrals from collaborating headache experts within Norway. The term intractable has no standardised definition, but the term used in this study has been modified from a paper by Silberstein et al. (6), failing at least two drugs and considered as moderate intractability. The exact limits of our definition are provided in the inclusion criteria. The study was approved by the regional ethics committee (ref. 2012/164) and the Norwegian Medicines Agency (EUDRACT nr: 2012-000248-91) and is registered at ClinicalTrial.gov (NCT02019017). Written informed consent was obtained from all patients. The treatment was performed with a custom-made surgical navigation device under clinical testing (MultiGuide – a safety study: ref. regional ethics committee 2012/2199; ref. Directorate of Health 13/3816).
Inclusion criteria were age 18 to 65 years; chronic CH according to the International Classification of Headache Disorders, third edition (ICHD-3); and unsatisfactory effect, intolerable side effects or contraindications of at least two of the following medications: verapamil, lithium, gabapentin and corticosteroids.
Exclusion criteria were change in dose of prophylactic treatment for CH four weeks prior to inclusion; use of antipsychotics four weeks prior to inclusion; known heart or lung disease; any disease that may complicate treatment or anaesthesia; psychiatric illness preventing full participation; pregnancy, nursing or inability to use contraceptives in fertile women; abuse of any pharmacological substance, narcotics or alcohol; hypersensitivity to short-acting anaesthetics, adrenalin or BTA; and active treatment with pharmacological substances with possible interaction with the study medicament.
Eligible patients underwent physical and neurological examination. Medical history, computed tomography (CT) and magnetic resonance imaging (MRI) of the paranasal sinuses were obtained. Participants were instructed to keep headache diaries from ≥2 weeks before the treatment and through the study recording headache duration, intensity, autonomic symptoms, acute treatment and oxygen, days of sick leave, quality of life (Headache Impact Test-6 (HIT-6)) and adverse events (AEs).
Physician follow-up took place at weeks 4, 12 and 24 and weekly phone interviews were conducted by a study nurse for the first eight weeks, then every four weeks. AEs were registered at follow-up using both open and specified questions. A protocol violator was defined as a participant with less than 60% of diary days registered or change of medication, acute or prophylactic, during the study.
Since there are no data available to determine the correct dosage of the proposed treatment, we decided to explore the safety of two different dosages, 25 IU and 50 IU BTA (Botox®, Allergan Inc, Irvine, CA, USA).
Procedure: BTA treatment of the SPG
We have developed a novel method of injecting BTA towards the SPG. Since BTA is a substance with a low diffusion gradient (8), accurate administration is of paramount importance to reach the desired target. In addition, misplaced injections may cause AEs. To perform the procedure, a custom-made injection device was developed, using a surgical navigation system to ensure accurate administration of the drug.
A single treatment was performed under general anaesthesia using a transnasal approach aided by surgical navigation (Brainlab Vector Vision and Brainlab Kick, version 1, Brainlab AG, Feldkirchen, Germany). The first five patients received 25 IU BTA, the last five received 50 IU. Local anaesthetic was not used. Pre-operative planning of CT and MRI was performed with Brainlab iPlan 3.0 (Brainlab AG, Feldkirchen, Germany). The SPG on the symptomatic side was localised visually and marked on fused MRI and CT scans. The nasal cavity was decongested with adrenalin nasal packing (0.5 ml adrenalin solution 0.1 mg/ml (Takeda Nycomed, Drammen, Norway) suspended in 4.5 ml isotonic saline) for 20 minutes. Aided by surgical navigation and a custom-made device (MultiGuide®), 0.05 mg adrenalin in 5 ml isotonic saline was administered in the sphenopalatine fossa followed by 25 IU or 50 IU of BTA towards the SPG. BTA was suspended in 1 ml isotonic saline for the first three patients, and 0.5 ml for patients 4–10. In patient number 7 the transnasal approach was impossible to perform because of a bony medial wall of the sphenopalatine fossa and a small sphenopalatine foramen; we therefore decided perioperatively to proceed with a percutaneous infrazygomatic (lateral) approach.
Outcome and statistical analysis
AEs (primary outcome) were assessed through tabulation from the treatment procedure to the end of the study. Secondary outcomes were the mean change from baseline in frequency of CH attacks for weeks 3 and 4 (main efficacy outcome); CH intensity; duration of attacks; days with CH; presence of autonomic symptoms; attacks treated with triptans; doses of triptans; acceptability of treatment; and headache-related impact on quality of life measured by the HIT-6. For all efficacy outcomes, weeks 1 and 2 were not included in the analysis of month 1 (according to protocol) since it may take one to two weeks before the BTA block is achieved. For efficacy outcomes we have performed two types of analyses: intention-to-treat (ITT) analysis (n = 9) and per-protocol analysis (n = 7), both without imputing missing data. To better assess the therapeutic gain of the treatment, a responder analysis was performed for each month of the study period. A frequency responder (main efficacy outcome) was pre-defined as at least a 50% reduction of mean attack frequency between baseline and weeks 3 and 4.
Statistical software SPSS, version 21.0 (SPSS Inc, Chicago, IL, USA) was used in the data analyses. Since the study is an exploratory safety study, no power calculation was performed prior to study start. For efficacy measures we used the Wilcoxon signed rank test. A two-sided p < 0.05 was considered statistically significant.
Results
Ten patients were included and all received the treatment. We received safety data on all patients throughout the study. Three patients were defined as ‘protocol violators’. Patient 9 did not deliver headache diaries at all with no reason given and was excluded from the efficacy data analysis. Patient 10 failed to deliver headache diaries for weeks 6–24. Diaries were sent by mail, but never reached the recipient. Patient 8 did not provide headache diary for weeks 5–24 with no reason given.
Table 1.
Demographics and clinical characteristics.
| All patients (n = 10) | |
|---|---|
| Number of females/males | 5/5 |
| Median age, years (range) | 42 (29–64) |
| CH attack laterality, left dominant, % | 70 |
| Mean years since onset of CH (range) | 15 (3–35) |
| Number of preventive treatments faileda (range) | 3,4 (3–6) |
| Concomitant migraine | 2b |
Recommended preventive treatments (7) described for CH with unsatisfactory or intolerable side effects.
Patient 3 and patient 8.
CH: cluster headache.
Safety (primary outcome)
A total of seven patients experienced AEs. Eleven events were registered (Table 2). There was one severe adverse event (SAE), observed four days in hospital after a perioperative posterior epistaxis treated with posterior nasal packing. Investigators classified four AEs as probably related to the effect of BTA. Three patients reported accommodation problems on the ipsilateral eye and one patient reported chewing and gaping difficulties, all resolved within four weeks (range 1–28 days). Patient 5 received a misplaced injection in the mucosa of the nasal cavity due to misinterpretation of image guidance data during the procedure. The treatment was not repeated during the study. Patient 8, with a history of encephalitis before developing CH, had prior to the study experienced sensory disturbances in ipsilateral limbs during cluster attacks, but during the first month after treatment she developed ipsilateral motor weakness of the lower limb, accommodation difficulties and chewing weakness during CH attacks. The neurological exam was normal interictally. The accommodation difficulties and chewing weakness resolved within four weeks, but the ictal lower limb weakness was on-going at the end of the study. Due to the intermittent character of the symptoms, the motor symptoms appearing on the same side as the injection and no obvious relation with the study treatment, except for relation in time, the latter event was registered as an AE probably not related to the study treatment by the principal investigator and sponsor.
Table 2.
Summary table including responder analysis and adverse event data after one single BTA treatment of SPG.
| Efficacy outcome (attack frequency per week) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention |
Reduction in attack frequency from BL (%) |
Adverse event |
|||||||||
| Patient number | Approach | BTA (IU) | BL | M1 | M2 | M3 | M4 | M5 | M6 | Resolved <4 weeks | On-going >4 weeks |
| 1 | Transnasal | 25 | 30.2 | −57% | −3% | −32% | −53% | −28% | −12% | Accommodation problems | |
| 2 | Transnasal | 25 | 15.0 | −57% | −67% | −65% | −82% | −92% | −93% | None | |
| 3 | Transnasal | 25 | 15.2 | −97% | −97% | −85% | −20% | +5% | −3% | Accommodation problems | |
| 4 | Transnasal | 25 | 17.5 | −97% | −99% | −100% | −100% | −100% | −97% | None | |
| 5 | Transnasal | 25 | 3.5 | +43% | +21% | +42% | +22% | +57% | +36% | Temporal headache, misplaced injection | |
| 6 | Transnasal | 50 | 4.5 | −78% | −61% | −56% | −78% | −61% | −67% | Anterior epistaxis | |
| 7 | Lateral | 50 | 14.3 | −23% | −18% | −35% | −18% | −38% | −25% | None | |
| 8 | Transnasal | 50 | 15.4 | −14% | NA | NA | NA | NA | NA | Anterior epistaxis, accommodation and jaw problems | CH attack- related weakness in one foot |
| 9 | Transnasal | 50 | NA | NA | NA | NA | NA | NA | NA | Anterior epistaxis | |
| 10 | Transnasal | 50 | 42.6 | −21% | NA | NA | NA | NA | NA | Posterior epistaxis | |
50% improvement from baseline, depicted in bold.
BTA: onabotulinumtoxinA; SPG: sphenopalatine ganglion; CH: cluster headache; M: month; NA: not available; BL: baseline.
Efficacy outcomes
Main efficacy outcome
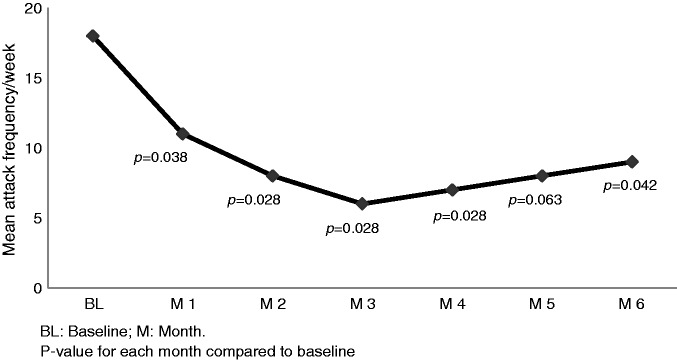
Results on cluster attack frequency (main efficacy outcome) are given in Figure 1 for the ITT analysis (n = 9). In the PP analysis (n = 7) attack frequency per week was reduced from 14 ± 9 in the baseline period to 5 ± 5 (p = 0.028) in month 1; for months 2–6 mean values and significance levels were identical to the ITT analysis. In the PP analysis the average attack reduction from baseline for months 1–3 and months 4–6 was respectively 55% (p = 0.028) and 45% (p = 0.028) (Figure 2), and for the whole follow-up, months 1–6, 51% (p = 0.028).
Figure 1.
Main efficacy measure. Mean cluster attack frequency per week for baseline and after one single BTA treatment of SPG, intention-to-treat analysis (n = 9).
BTA: onabotulinumtoxinA; SPG: sphenopalatine ganglion.
Figure 2.
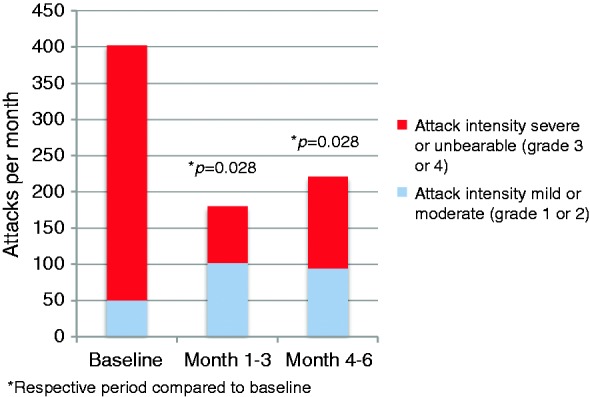
Number of attacks by intensity. Attacks graded mild or moderate and severe or unbearable. Per protocol participants (n = 7).
Secondary efficacy outcomes
Summary statistics for secondary efficacy outcomes for the PP analysis are provided in Table 3. A substantial numerical mean decrease from baseline was observed for all outcomes except mean duration per attack. Five patients were classified as responders for the main efficacy outcome (≥50% reduction of mean attack frequency vs. baseline). Frequency reduction for all months is provided in Table 2.
Table 3.
Secondary efficacy measures for baseline and after one single BTA treatment of SPG. Per protocol analysis (n = 7).
| Baseline | Month 1 | Month 2 | Month 3 | Month 4 | Month 5 | Month 6 | |
|---|---|---|---|---|---|---|---|
| Mean intensity/ attack ± SD (p value) | 3.50 ± 1.05 | 2.66 ± 1.10 (0.043) | 2.56 ± 1.08 (0.043) | 2.44 ± 1.31 (0.043) | 2.46 ± 1.46 (0.043) | 2.38 ± 1.43 (0.028) | 2.56 ± 1.19 (0.043) |
| Mean durationa ± SD (p value) | 1345 ± 793 | 422 ± 691 (0.12) | 528 ± 740 (0.12) | 550 ± 882 (0.12) | 511 ± 792 (0.12) | 666 ± 969 (0.25) | 724 ± 888 (0.50) |
| Mean durationa per attack ± SD (p value) | 35.6 ± 24.8 | 26.0 ± 35.0 (0.25) | 30.5 ± 43.9 (0.60) | 31.0 ± 45.1 (0.89) | 32.1 ± 47.2 (0.69) | 35.6 ± 47.4 (0.89) | 36.2 ± 47.5 (0.89) |
| Days without attacks ± SD (p value) | 4.2 ± 5.9 | 14.6 ± 10.6 (0.046) | 14.3 ± 11.2 (0.075) | 13.6 ± 10.1 (0.063) | 12.3 ± 10.9 (0.12) | 11.7 ± 11.8 (0.25) | 12.1 ± 11.6 (0.23) |
| Headache severity indexb ± SD (p value) | 10.4 ± 14. | 0.98 ± 1.21 (0.075) | 3.60 ± 7.78 (0.075) | 1.78 ± 2.77 (0.12) | 1.51 ± 1.42 (0.17) | 3.38 ± 3.83 (0.46) | 4.25 ± 6.04 (0.46) |
| Mean number of attacks, intensity 3 or 4c ± SD (p value) | 50 ± 38 | 13 ± 16 (0.028) | 11 ± 11 (0.028) | 10 ± 8 (0.043) | 17 ± 20 (0.028) | 18 ± 23 (0.063) | 19 ± 21 (0.042) |
| Triptan doses ± SD (p value) | 91 ± 49 | 35 ± 25 (0.068) | 56 ± 64 (0.068) | 40 ± 37 (0.068) | 42 ± 23 (0.14) | 52 ± 42 (0.14) | 60 ± 54 (0.14) |
| HIT-6 ± SD (p value) | 65.1 ± 2.73 | 51.9 ± 7.76 (0.018) | 56.9 ± 8.86 (0.063) | 54.1 ± 12.2 (0.075) |
P value for each month compared to baseline. BTA: onabotulinumtoxinA; SPG: sphenopalatine ganglion; HIT-6: Headache Impact Test-6.
In minutes.
Duration (min) × intensity × frequency, 106.
Categorical intensity scale: Grade 1; mild, grade 2; moderate, grade 3; severe, grade 4; unbearable.
Headache impact on functioning and health-related quality of life
An improvement of headache impact as measured by HIT-6 was seen during the follow-up, with a mean decrease after four weeks of 13.2 points (p = 0.018), after eight weeks of 8.2 points (p = 0.064) and after 24 weeks of 11.0 points (p = 0.075) (Table 3).
Discussion
BTA injection to the SPG represents a novel approach for the treatment of intractable chronic CH and, to our knowledge, such treatment has not previously been performed.
One patient with posterior epistaxis was reported (SAE), which would be expected performing injections adjacent to the sphenopalatine artery. Four events in three patients may have been caused by BTA. Three patients experienced ipsilateral accommodation problems of the eye. This could be due to diffusion of BTA blocking the inferior rectus muscle of the orbit or the ciliary ganglion, but exhaustive ophthalmological examination could not disclose objective signs of any affliction. Diffusion of BTA blocking the pterygoid muscles may explain the single event of ipsilateral weakened chewing force, but the interictal improvement is not easy to explain on the basis of the action of BTA, and it was not confirmed on clinical examination. In both cases the symptoms lasted much less than expected for a BTA block, and may be explained by local oedema. With the exception of one AE interpreted as not being associated with the treatment, all AEs resolved within four weeks. The AE profile seems acceptable given the level of invalidity of this disorder. While data are scarce, there does not seem to be any clear difference of the AE profile of the group treated with 25 IU BTA compared to the group treated with 50 IU (Table 2).
Due to small sample size, uncontrolled design, and 30% protocol violators, interpretation of efficacy outcomes must be performed with caution. A single intervention and a long follow-up with restriction on acute and preventive medication may explain the high share of protocol violators, also seen in earlier studies (9).
Cluster attack frequency (Figure 1) and attack intensity (Table 3 and Figure 2), were reduced compared to baseline, but there was no change in the mean duration of attacks (Table 3).
Five out of 10 were frequency responders for the main efficacy outcome with an average attack frequency reduction of 77%, responding on average 4.6 months. It seems unlikely that general anaesthesia, placebo or spontaneous remission could explain such long-term improvement after a single intervention in chronic CH that has not responded to any other type of treatment and that has lasted for a minimum of three years. Of the patients not responding in terms of frequency, patient 5 was a misplaced injection and as such did not receive the treatment; patient 10 had a posterior nasal bleeding during the procedure and it is unclear whether he received an effective SPG block; patients 8 and 9 are protocol violators due to missing headache diaries, one claims to have had a ≥50% attack frequency reduction and the other claims to have had no effect. In neither could these be confirmed due to lack of headache diaries.
Patient 5 received a misplaced injection in this study, but responded to post-study treatment with ≥50% attack frequency reduction. Patient 10 did not respond to post-study treatment. Three patients are still frequency responders after >12 months with only one treatment performed. Responders with remission after the first BTA injection also seem to respond well to repetitive treatments.
A major limitation of the treatment is that the transnasal approach requires general anaesthesia. Based on previous experience with BTA targeting autonomic neural structures, one may expect an effect of such treatment to the SPG to last between three and nine months, so the development of a technique performed on an awake patient is warranted. The clinical effect mainly appeared in week 2 in responders, indicating that the treatment also might be suitable for the treatment of episodic CH if proven effective.
While some non-pharmacological options for the treatment of intractable CH exist, they are all based on open studies of limited size, with the notable exception of two small sham-controlled studies. Deep brain stimulation in intractable CH by Fontaine et al. failed to show efficacy (10). Schoenen et al. reported positive results of stimulation of the SPG (11). One common feature of all neurostimulator regimens is the high cost of the treatment, and also there are no known predictors of effect (12). The proposed treatment in this study, if proven effective in intractable CH, may represent a low cost option compared to neurostimulator regimes, without long-lasting AEs and consequently a low threshold for trying it out.
Conclusion
BTA injection to the SPG in intractable chronic CH seems to have an acceptable AE profile. The efficacy data indicate a significant reduction of cluster attack frequency post-treatment, and five out of 10 patients responded to the treatment for the main efficacy outcome with an average attack frequency reduction of 77%. Randomised, placebo-controlled studies are warranted to establish both safety and efficacy of this possible novel treatment of CH.
Clinical implications
In this pilot study sphenopalatine injection of onabotulinumtoxinA in intractable chronic cluster headache seems to have an acceptable adverse event profile.
Efficacy data indicate a significant reduction of cluster attack frequency.
Randomised, placebo-controlled studies are warranted to establish both safety and efficacy.
Acknowledgements
The authors would like to acknowledge the contribution of research nurse Irina Aschehoug for her work during this trial.
Funding
This work was supported by The Liaison Committee between the Central Norway Regional Health Authority and Norwegian University of Science and Technology (grant number 12/9996); Joint Research Unit between St. Olavs Hospital and Norwegian University of Science and Technology (grant number 9885); and NTNU Discovery (grant number 244278).
Declaration of conflicting interests
NTNU and St. Olavs Hospital, Trondheim University Hospital may benefit financially from a commercialisation of the proposed treatment through future possible intellectual properties; this may include financial benefits to authors of this article. Dr Bratbak is co-inventor of the proposed treatment in this study and the intervention device used to perform the treatment, both inventions patent pending, and may benefit financially from a commercialisation of the proposed treatment through future possible intellectual properties. Dr Nordgård is co-inventor of the proposed treatment in this study and the intervention device used to perform the treatment, both inventions patent pending, and may benefit financially from a commercialisation of the proposed treatment through future possible intellectual properties. Dr Tronvik reports a personal fee for one national advisory board meeting and reimbursement for scientific meetings from Allergan; and may benefit financially from a commercialisation of the proposed treatment through future possible intellectual properties. Dr Stovner reports personal fees from GlaxoSmithKline outside the submitted work. Drs Linde, Folvik and Bugten declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.May A, Goadsby PJ. The trigeminovascular system in humans: Pathophysiologic implications for primary headache syndromes of the neural influences on the cerebral circulation. J Cereb Blood Flow Metab 1999; 19: 115–127. [DOI] [PubMed] [Google Scholar]
- 2.Sluder G. The role of the sphenopalatine ganglion in nasal headaches. N Y State J Med 1908; 27: 8–13. [Google Scholar]
- 3.Matharu M. Cluster headache. BMJ Clin Evid 2010; 2010. pii: 1212–1212. [PMC free article] [PubMed] [Google Scholar]
- 4.Prasanna A, Murthy PS. Sphenopalatine ganglion block under vision using rigid nasal sinuscope. Reg Anesth 1993; 18: 139–140. [PubMed] [Google Scholar]
- 5.Naumann M, Lowe NJ. Botulinum toxin type A in treatment of bilateral primary axillary hyperhidrosis: Randomised, parallel group, double blind, placebo controlled trial. BMJ 2001; 323: 596–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silberstein SD, Dodick DW, Pearlman S. Defining the pharmacologically intractable headache for clinical trials and clinical practice. Headache 2010; 50: 1499–1506. [DOI] [PubMed] [Google Scholar]
- 7.May A, Leone M, Afra J, et al. EFNS guidelines on the treatment of cluster headache and other trigeminal-autonomic cephalalgias. Eur J Neurol 2006; 13: 1066–1077. [DOI] [PubMed] [Google Scholar]
- 8.de Almeida AT, De Boulle K. Diffusion characteristics of botulinum neurotoxin products and their clinical significance in cosmetic applications. J Cosmet Laser Ther 2007; 9(Suppl 1): 17–22. [DOI] [PubMed] [Google Scholar]
- 9.Pageler L, Katsarava Z, Lampl C, et al. Frovatriptan for prophylactic treatment of cluster headache: Lessons for future trial design. Headache 2011; 51: 129–134. [DOI] [PubMed] [Google Scholar]
- 10.Fontaine D, Lazorthes Y, Mertens P, et al. Safety and efficacy of deep brain stimulation in refractory cluster headache: A randomized placebo-controlled double-blind trial followed by a 1-year open extension. J Headache Pain 2010; 11: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoenen J, Jensen RH, Lanteri-Minet M, et al. Stimulation of the sphenopalatine ganglion (SPG) for cluster headache treatment. Pathway CH-1: A randomized, sham-controlled study. Cephalalgia 2013; 33: 816–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jürgens TP, Leone M. Pearls and pitfalls: Neurostimulation in headache. Cephalalgia 2013; 33: 512–525. [DOI] [PubMed] [Google Scholar]