Abstract
Objective
Evaluate the association between pre-treatment and during-treatment weight change. Evaluate differences in self-regulation between those who gain weight, remain weight stable, and lose weight pre-treatment.
Methods
Data from the first six months of a behavioral weight loss study were used. Participants (n=283) were weighed at two assessment points (screening visit and baseline) prior to the start of treatment and at every treatment session. Participants were divided into those who gained weight, remained weight stable, or lost weight between screening visit and the first treatment session.
Results
Pre-treatment weight change was not significantly associated with during-treatment change. Weight change from screening visit to month six was significantly different by category, with losses of 11% and 7% for those who lost and gained weight pre-treatment respectively. Weight change from first treatment session to month six was not different by category. Poorer self-regulation was associated with pre-treatment weight gain and better self-regulation with pre-treatment weight loss.
Conclusions
Pre-treatment weight change may not relate to success during behavioral weight loss treatment. Researchers should carefully consider when the “baseline” assessment takes place to reduce bias introduced by weight change during pre-treatment. Poorer self-regulation may place individuals at risk for weight gain prior to treatment.
Keywords: Outcomes, Obesity Treatment, Weight Variability, Psychosocial
Introduction
The period of time between the formation of an intention to lose weight through a behavioral weight loss program and the start of treatment has received little attention. While one prior study by West and colleagues suggests that there is striking variability in pre-treatment weight change before treatment, and that pre-treatment weight change may predict change during a behavioral weight loss treatment (1), this has not been well-established and warrants further investigation. There may be important methodological implications of pre-treatment variability, as large weight gains or weight losses may bias outcomes reported by clinical trials. Furthermore, an increased understanding of psychological processes associated with pretreatment weight change may help to identify individuals at risk for weight gain in the pretreatment period.
Typical protocol for behavioral weight loss trials is to take anthropometric measurements at a separate baseline appointment completed with some latency to treatment start (2, 3). This methodology implicitly assumes that the time between assessment and treatment start is negligible and/or weight will be static during this time period. One study that evaluated this time period had an average wait of 50 (± 30) days between baseline measurement and treatment start, during which more than half of individuals displayed what was considered a clinically significant weight change (1). However, there is no standard for reporting or accounting for pre-treatment variability. Thus, change that occurs between assessment and treatment start is attributed to treatment and has the potential to bias reported outcomes. While West and colleagues' initial paper examining pre-treatment weight changes began to suggest methodological change, it is important to replicate their findings in order to understand the degree of variability in pre-treatment weight change and how to best account for this when reporting outcomes.
Pre-treatment weight change may also be an indicator of the effectiveness of an individual's weight-related self-regulatory processes, and thus be an important predictor of success. One study suggests those who lose weight during the pretreatment period also lose significantly more weight during treatment than those who gain weight or remain weight stable (1). However, among individuals undergoing bariatric surgery, studies have had mixed results, indicating that better weight loss success at 3-months post-surgery may be predicted by weight gain, the so-called “last supper effect (4),” or pre-treatment weight loss (5). Given the few prior studies examining the association between pre-treatment weight change and treatment outcome in a behavioral weight loss-seeking population, and the conflicting results, it remains unclear how pre-treatment weight change is associated with during-treatment weight change.
Understanding what differentiates those who gain weight, remain weight stable, and lose weight prior to treatment may be important for improving treatment and better addressing the specific needs of these individuals. Self-regulation, which enables individuals to override prepotent responses, is theorized to play a central role in the implementation of goal-directed behavior (6). When applied to restriction of energy intake, self-regulation may be influenced by internal (e.g., hunger, emotions) as well as external (availability of palatable foods) factors (7). Many psychological factors may influence risk of self-regulatory failure during a weight loss attempt (e.g., use of strategies to reduce intake, responsivity to food in the environment, emotional or uncontrolled eating), and such factors may be of particular relevance in the pretreatment period, as formal instruction in weight management has not been introduced. Indeed, research has indicated that individuals with higher levels of restrained eating (i.e., attempt to control intake but have factors that make such attempts more difficult, such as disinhibition) and emotional eating may have difficulty self-regulating eating when anticipating deprivation (8-10), such as what may be experienced prior to behavioral weight loss program. Moreover, an individual's perception of their ability to regulate their eating (i.e., self-efficacy for weight control behaviors) may be associated with pre-treatment weight change, with those higher in self-efficacy being better able to control intake without the structure of treatment.
The current study aimed to add to the body of literature concerning pre-treatment weight change in a sample of individuals who are overweight or obese and seeking behavioral weight loss treatment. First, general patterns of pre-treatment change were evaluated. Second, the association between pre-treatment and during-treatment weight change was evaluated. Third, differences in variables related to self-regulation were examined between those who gained weight, remained weight stable, or lost weight pre-treatment.
Methods
Participants
Adults who were overweight or obese (n = 283) were recruited through radio advertisements, flyers, and health care providers to participate in a study of behavioral weight loss treatment. Eligible participants had a BMI of 27 to 45 kg/m2, were between the ages of 18 and 70 years, were able to walk at least 2 blocks without stopping for rest, and completed a 7-day food diary. Individuals were excluded if pregnant or planning to become pregnant in the next 2 years, planning to move away from Philadelphia in the next 2 years, recently began a course of or changed the dosage of medication that may cause significant change in weight, lost more than 5% of their weight within the past six months, or planned to participate in another weight loss program in the next 2 years. The institutional review board at Drexel University approved this study.
Procedures
Participants went through a series of standard enrollment procedures in order to qualify for the study (see Figure 1). Participants who inquired about the study were screened by phone to assess initial eligibility. Those who met criteria attended a group orientation session to obtain more detailed information about the study. Interested and eligible participants completed baseline measurements over two visits, a “screening visit” and a “baseline visit,” in order to reduce the burden of one longer visit on participants. At the screening visit, participants' eligibility was verified, informed consent obtained, and assessment begun. Participants were asked to complete several measures prior to and during the baseline visit. After the successful completion of the baseline visit, participants were randomized to one of three treatment conditions (treatment condition was not revealed until the first treatment session). The next assessment visit occurred at month six.
Figure 1.
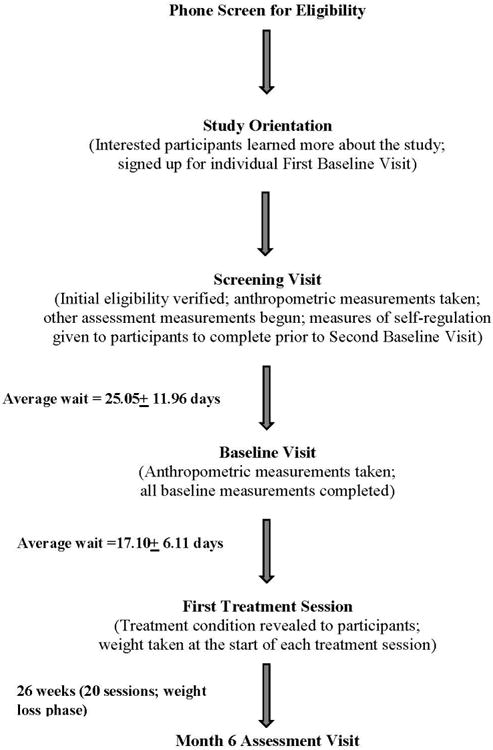
Progression of enrollment and assessment points.
All treatment conditions were based on standard behavioral treatment adapted from the Diabetes Prevention Program and Look AHEAD (2, 11). Each condition was given similar behavioral goals, such as self-monitoring, calorie goals based on the standard balanced deficit diet guidelines, and physical activity prescription increasing gradually to 250 minutes per week (12, 13). All conditions met for 75 minutes per session weekly between months one through four and biweekly in months five and six. This phase of the program was designed to help participants reach a weight loss of approximately 10%. Treatments differed in the emphasis placed on certain skills (i.e., presence or absence of focus on changing environmental determinants of eating and activity and developing acceptance-based psychological skills). These differences were present throughout treatment, but were designed to primarily impact maintenance of lost weight. As expected, there were no differences between conditions in weight change at month six, F (2, 280) = .03, p = .99.
Measures
Anthropometric measurements included body weight using a Seca® scale accurate to 0.1 kg (measured in street clothes) and height using the built-in height rod. Height in meters and weight in kilograms were measured at each assessment point, and weight was measured additionally at each treatment session. BMI was calculated as (weight in kg)/(height in m)2.
Self-regulation of eating behaviors was measured by self-report questionnaires completed at home between the screening visit and baseline appointment. The Three Factor Eating Questionnaire revised assessed uncontrolled eating, cognitive restraint, and emotional eating (14). The TFEQ-18 has a strong factor structure and adequate reliability (14). The Power of Food Scale (PFS) assessed hedonic hunger, or the extent to which the availability of highly palatable foods influences a person's food-related thoughts and feelings (15). The PFS has adequate internal and test-retest reliability and convergent discriminant validity (15). The Weight Efficacy Lifestyle Questionnaire assessed self-efficacy for self-regulating food intake across several situational factors (negative emotions, availability, social pressure, physical discomfort, and positive activities) (16). The WEL has demonstrated good validity and test-retest reliability (17).
Data Analysis
Participants were categorized as having gained more than 1.15% of weight, having remained weight stable within 1.15%, or having lost more than 1.15% of weight between screening visit and first treatment session. The cutoff of 1.15% of weight was selected to note clinically significant pre-treatment weight change identical to the study conducted by West and colleagues (1). As per West and colleagues (1), that criterion was chosen because it was similar to a previously used cut-off for clinical significance (1.25%)(18) and approximately half of what was considered successful weight loss maintenance (2.3 kg, which would translate to 2.3% change for a 100kg individual) in Wing and colleagues' (19) study of weight regain.
Measured weight at six month was missing for 12.7% of participants. Data were not missing significantly differently between weight change categories (χ2=5.75, p=.06, see Table 1). The trend effect observed, however, is unsurprising given prior evidence that poorer early weight loss is predictive of dropout (20). Missing data in weight loss studies has been shown to be effectively handled by multiple imputation (21) and the results of several evaluations of different methods of handling missing data have encouraged studies of weight loss to utilize multiple imputation (e.g., (22, 23)). Thus, missing data were handled with multiple imputation with five iterations. Results from the five imputed datasets were combined based on Rubin's rule using the R software package MICE (24). Pooled means with confidence intervals and pooled test statistics are reported.
Table 1. Between-category differences in demographic and baseline psychological variables.
| Lost Weight Pre-treatment (n = 47) |
Weight Stable Pre-treatment (n = 152) |
Gained Weight Pre-treatment (n = 84) |
p value | Tukey post-hoc tests | |
|---|---|---|---|---|---|
| Weight change, screening visit to first treatment session (average lbs, SD; range) | -4.6 (2.1); -13.4 to -2.4 |
0.2 (1.3); -2.8 to 3.2 |
5.2 (2.5); 2.2 to 14.8 |
< .001 | |
| Weight change, first treatment session to six months (average lbs, 95% CI) | -19.1; (-23.2 to -15.1) |
-20.5; (-22.8 to -18.2) |
-21.7; (-24.8 to -18.5) |
.77 | |
| Days between screening visit and week one of treatment (mean number of days, SD) | 45.2 (13.9) | 41.5 (14.2) | 41.4 (11.5) | .22 | |
| Number of sessions attended (out of 20) | 18.5 (3.9) | 18.2 (4.1) | 18.3 (4.4) | .93 | |
| Percent attrition at month six | 2.1 | 14.5 | 15.5 | .06 | |
| Demographic Variables | |||||
| Age (years); M (SD) | 53.4 (10.9) | 53.8 (9.4) | 52.0 (9.4) | .41 | |
| Baseline BMI; M (SD) | 34.1 (4.1) | 35.2 (5.1) | 35.5 (5.1) | .34 | |
| % Men | 19.1 | 17.3 | 29.3 | .10 | |
| % Caucasian | 57.4 | 65.3 | 72.0 | .24 | |
| Baseline Psychosocial Variables | |||||
| Power of Food Scale (hedonic hunger) | 37.6 (12.8) | 42.0 (13.2) | 44.6 (12.5) | .02 | Lost < Gain * |
| Three-Factor Eating Questionnaire | |||||
| Uncontrolled Eating | 18.1 (4.9) | 20.3 (5.2) | 20.9 (4.0) | .006 | Lost < Stable* Lost < Gain** |
| Emotional Eating | 7.2 (2.7) | 7.8 (2.4) | 8.4 (2.2) | .02 | Lost < Gain* |
| Cognitive Restraint | 14.3 (2.6) | 14.0 (3.1) | 14.1 (2.8) | .85 | |
| Weight Efficacy Lifestyle Questionnaire (self-efficacy for regulating eating) | 113.1 (31.8) | 99.4 (31.8) | 96.5 (30.8) | .01 | Lost > Gain* Lost > Stable * |
p < .01;
p < .05
Results
Participants were 78.9% women, 65.8% Caucasian, had an average age of 53.2 ± 9.7 years and an average BMI of 35.2 ± 5.0 kg/m2. There were no significant demographic differences between pre-treatment weight change categories (see Table 1). We observed no interaction between pre-treatment weight change category and treatment condition, F (4, 274) = .03, p = .78, η2 = .006. While it is possible that the sample size did not provide adequate power to test statistical significance, the near-zero effect size (less than what is considered a “small effect”) indicates that it is unlikely that participants with different pre-treatment weight change trajectories benefitted differentially by treatment condition. Treatment conditions were thus collapsed for the remainder of analyses.
Between the screening visit and first treatment session, 16.6% of participants lost more than 1.15% of their weight (2.17 ± .92; range = 6.33% loss to 1.19% loss). Just over half (53.7%) of participants remained within 1.15% of their screening visit weight at the first treatment session (0.12 ± 0.58; range = 1.09% loss to 1.12% gain). Finally, 29.7% of participants gained at least 1.15% of their weight (2.38 ± 0.98; range = 1.15% gain to 5.30% gain). These values are reported in pounds in Table 1, and are similar to the distribution observed by West and colleagues (16%, 61% and 23% respectively). Days between screening visit and first treatment session and attendance at groups did not differ by weight change category; thus, weight changes were not accounted for by differential latency to treatment start or session attendance (see Table 1).
Weight changes prior to treatment (i.e., between screening visit and first treatment session) were unrelated to weight losses during treatment (r = -.09, P = .15), indicating that these are distinct time periods for weight change. Weight change for the whole sample from first treatment session to month six (9.5% loss) was significantly greater than weight change from screening visit to month six (9.2% loss; t(11679) = -3.95, p < .001) and weight change from baseline visit to month six (9.2% loss; t(2771) = -5.21, p < .001) Pairwise comparisons between weight losses at six months from screening visit, baseline visit, and week one of treatment revealed significant differences between all measurements for each pre-treatment weight change category (see Table 2).
Table 2. Comparison of weight losses between baseline and month six and week one of treatment and month 6.
| Screening to month six change Pooled mean |
Baseline to month six change Pooled mean |
Week one of treatment to month six change Pooled mean |
Pairwise comparisons | |
|---|---|---|---|---|
| Lost Weight Pre-treatment | -11.2 | -9.9 | -9.2 | Screening < Baseline** Baseline < Week one** Screening < Week one** |
| Weight Stable Pre-treatment | -9.4 | -9.3 | -9.6 | Screening < Baseline* Baseline < Week one** Screening < Week one** |
| Gained Weight Pre-treatment | -7.7 | -8.7 | -9.8 | Screening < Baseline** Baseline < Week one** Screening < Week one** |
p < .01;
p < .05
Change in weight from screening visit to month six was significantly different between those who gained weight, remained weight stable, and lost weight prior to treatment (see Figure 2; F(2, 717.85) = 4.90, p = .007,). Individuals who lost weight pretreatment had net average weight change between screening visit and month 6 of treatment of 11.2% loss (95% CI, 12.93 to 9.46% loss); those who remained weight stable had average weight changes of 9.4% loss (95% CI, 10.43 to 8.45% loss); and those who gained weight pretreatment had average weight changes of 7.7% loss (95% CI, 9.06 to 6.30% loss). The differences between pre-treatment weight change categories was attenuated as the measurement used for the initial weight became proximally closer to treatment start, with non-significant between-category differences in change between baseline visit and month six (F(2, 15073.53) = .54, p= .59) and first treatment session and month six (F(2, 3286.45) = 0.13, p = .88).
Figure 2.
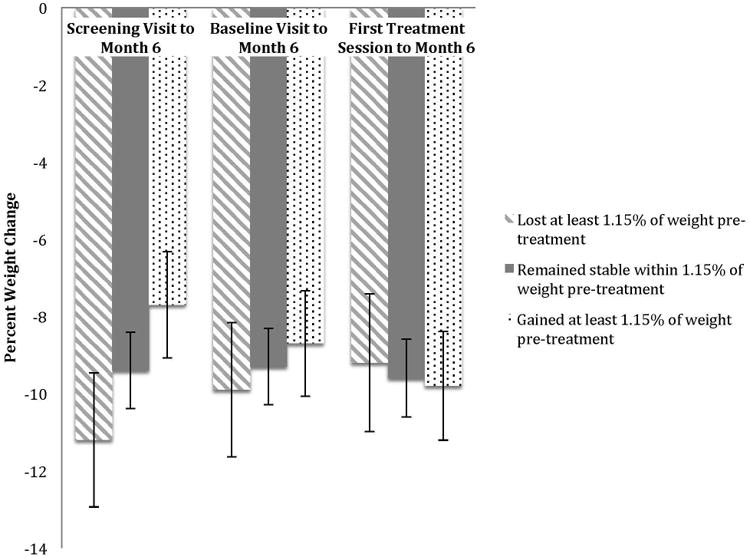
Between-category differences in mean weight loss outcome.
Evaluation of baseline characteristics of pre-treatment weight change revealed a pattern consistent with hypotheses across measures, such that those who lost weight before treatment had lower levels of hedonic, emotional, and uncontrolled eating, and higher levels of self-efficacy (see Table 1) compared to those who remained weight stable or gained weight. Those who gained weight prior to treatment displayed the opposite pattern of results, with higher levels of hedonic, emotional, and uncontrolled eating, and lower self-efficacy. Cognitive restraint was not different between categories.
Discussion
Significant differences in weight outcomes were present between pre-treatment weight change categories when the weight measured at the screening visit was used in analyses. Weight losses observed between screening or baseline visit and month six either significantly over (for those who lost weight pre-treatment) or under (for those who remained weight stable or gained weight pre-treatment) estimated the percent of weight lost during treatment. Pretreatment weight variability was not associated with treatment success. Additionally, pre-treatment weight change categories were markedly different in their reported self-regulation, indicating that pre-treatment weight changes may be predictable and reflect relatively greater or worse self-regulatory ability.
We observed no differences between pre-treatment weight change categories in weight change that occurred between initiation of treatment and the end of the weight loss phase of treatment (i.e., six months). In fact, pre-treatment weight change was unrelated to change during treatment. This finding differs from West and colleagues' findings, although it is noted that in that study, individuals who lost weight pre-treatment were over-represented in the condition that promoted the most weight loss (1, 25). While pre-treatment weight change did not confound treatment results (1), the possibility remains that individuals who lost weight pre-treatment experienced more weight loss during treatment due to differences in treatment modality (i.e., that the association between pre-treatment change and during-treatment change was actually moderated by treatment modality). In the present study, treatment modality and contact frequency were constant across treatment conditions, perhaps providing a clearer understanding of the association between pre- and during-treatment change. Additionally, it is unclear how West and colleagues (1) handled missing data. It may be that accounting for missing data in a different way yielded a different pattern of results. It is also possible, however, that West and colleagues, with a greater sample size, were simply better able to detect differences, though our near-zero effect sizes indicate that it is unlikely that we would have observed a significant effect with a larger sample.
Weight changes were significantly different between weight change categories when including pre-treatment change in month six outcome (i.e., measuring change at six months from screening visit). While those who lost weight pre-treatment exceeded programmatic weight loss goals of 10% (losing an average of 11.2%), those who remained weight stable were slightly under that goal (9.4%) and those who gained weight reached just under three-quarters of the ultimate weight loss goal (7.7%). This finding highlights the importance of when the “pre-treatment” weight measurement takes place in research studies. Similar to what was observed in West and colleagues' study, if the baseline weight is taken several weeks prior to treatment, a potentially significant portion of weight change that is captured may not be a true effect of treatment, but of change that occurs before treatment (2). Even a proximally closer initial weight (such as the baseline in the present study) may over- or under-estimate the amount of weight actually lost during treatment. Consistent with West and colleagues' findings and conclusions, unequal randomization of pre-treatment weight trajectories to treatment condition could confound treatment outcomes reported. Researchers should carefully consider when the initial weight is measured, possibly adopting a standard of taking the initial starting weight on the day of treatment initiation in order to eliminate that variability and the potential confound that follows.
Observed differences in psychological variables at baseline indicated that individuals who gained weight prior to treatment experienced greater difficulties with disinhibition and response to food cues (i.e., difficulties that may make self-regulation of food intake more difficult) than individuals who remain weight stable or lost weight. This pre-treatment weight gain may, in part, be caused by increased stress or anticipation of deprivation caused by “dieting” (8-10). In comparison, less difficulty with disinhibition and response to food cues may allow individuals to exert better self-regulation and initiate behavioral weight loss strategies even if treatment contact is not immediately initiated. Interestingly, there were no differences between categories in cognitive restraint, indicating that all individuals reported attempting to exert the same type of behavioral control over eating (items such as “I do not eat some foods because they make me fat”(14)). Thus, the difference between categories is not due to differential attempts to control intake, but to differential responsivity to food that may make it easier or more difficult to enact or sustain these types of behavioral self-regulation strategies when confronted with internal or external cues for eating (measured by items such as “When I see a real delicacy, I often get so hungry that I have to eat it right away” (TFEQ; (14)) or “Just before I taste a favorite food, I feel intense anticipation” (PFS; (15)). Additionally, marked differences were observed in individuals' perceived ability to utilize self-regulatory skills to manage weight control, such that those who gained weight were less confident and those who lost weight were more confident in their ability. It may be that greater confidence helps those who lose weight to engage self-regulatory processes, or that individuals have accurate perceptions of their self-regulatory ability. Taken together, these results indicate that those who gain weight, remain weight stable, or lose weight are distinct groups with specific deficits or strengths in areas related to self-regulation. The structure and accountability of group may help those with more difficulty self-regulating intake, equalizing weight losses during treatment.
Several limitations of the current findings warrant consideration. Weight change was only evaluated during the first six months of treatment. Thus, it is unknown whether there are long-term differences in weight loss outcomes between pre-treatment weight change categories. Additionally, our sample was composed primarily of women, limiting the generalizability of our results to men. We examined only constructs related to self-regulation between pre-treatment weight change categories. There may be additional constructs which warrant similar examination, such as motivation (e.g., stage of change, motivation to engage in specific weight control behaviors), intention (e.g., some individuals may intentionally engage in a “last supper” prior to beginning a weight loss program), or knowledge and history of effective weight control (e.g., prior experience may help individuals to better control weight prior to beginning a program). Finally, while we sought to replicate and extend the findings in West and colleagues' study, it is noted that our sample size was significantly smaller (283 compared to 480). We highlighted effect size in several places in order to acknowledge our potentially limited power and draw appropriate conclusions; however we cannot rule out the possibility that results may change with a larger sample.
In the future, researchers should carefully consider when the “baseline” weight is obtained, perhaps using the first weight obtained in treatment as the initial weight. Additionally, research should examine whether early intervention for individuals with lower self-regulatory ability can help to prevent this “last supper” effect, helping individuals at risk for weight gain obtain the same benefit as those who are able to lose or maintain weight prior to treatment. Researchers should examine whether pretreatment change predicts weight loss outcome as group session frequency declines or as individuals enter weight loss maintenance. It may be that those who are able to lose weight before treatment starts are also better able to maintain weight losses as treatment contact diminishes. Researchers should also consider whether these pre-treatment weight changes could be used to better match treatment to individual need. For example, a lower-intensity treatment (e.g., fewer treatment contacts, internet-based intervention) may be sufficient to promote continued behavior change in those able to self-initiate weight loss prior to treatment, while more intensive interventions are needed only for those with more difficulty implementing behavior change independently. Finally, exploration of the association between pre-treatment weight change and other potentially salient variables (such as those discussed above) may help to further delineate these pre-treatment weight change categories from one another.
Study Importance Questions.
- - What is already known about this subject?
- There may exist some degree of variability in weight change prior to treatment start.
- Pre-treatment weight loss may be associated with improved treatment outcome
- - What does your study add?
- Evidence suggests that pre-treatment weight changes were not associated with treatment outcome.
- Weight change outcomes were significantly different depending on the time point for initial weight that was chosen.
- Measures related to self-regulation (e.g. disinhibition, emotional eating, hedonic hunger) are different between pre-treatment weight change categories.
Acknowledgments
Funding Source: NIH R01 DK 092374
Footnotes
Disclosure: Dr. Forman has a patent planned (Smartphone app system to prevent dietary lapses) and a patent issued (System and Method for Managing Binge Eating Disorders,” U.S. 2014/049772) which is licensed to Daylan Digital.
All other authors declare no conflicts of interest.
References
- 1.West DS, Harvey-Berino J, Krukowski RA, Skelly JM. Pretreatment weight change is associated with obesity treatment outcomes. Obesity. 2011;19(9):1791–5. doi: 10.1038/oby.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Look AHEAD Research Group. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Controlled clinical trials. 2003;24(5):610–28. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 3.Pellegrini CA, Verba SD, Otto AD, Helsel DL, Davis KK, Jakicic JM. The Comparison of a Technology-Based System and an In-Person Behavioral Weight Loss Intervention. Obesity. 2012;20(2):356–63. doi: 10.1038/oby.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ochner CN, Puma LM, Raevuori A, Teixeira J, Geliebter A. Effectiveness of a Prebariatric Surgery Insurance-required Weight Loss Regimen and Relation to Postsurgical Weight Loss. Obesity. 2010;18(2):287–92. doi: 10.1038/oby.2009.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alami RS, Morton JM, Schuster R, Lie J, Sanchez BR, Peters A, et al. Is there a benefit to preoperative weight loss in gastric bypass patients? A prospective randomized trial. Surgery for Obesity and Related Diseases. 2007;3(2):141–5. doi: 10.1016/j.soard.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Bandura A. Health promotion from the perspective of social cognitive theory. Psychology and health. 1998;13(4):623–49. [Google Scholar]
- 7.Herman CP, Polivy J. The self-regulation of eating: Theoretical and practical problems. 2004 [Google Scholar]
- 8.Eldredge KL, Agras WS, Arnow B. The last supper: emotional determinants of pretreatment weight fluctuation in obese binge eaters. International Journal of Eating Disorders. 1994;16(1):83–8. doi: 10.1002/1098-108x(199407)16:1<83::aid-eat2260160109>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 9.Hill AJ. Does dieting make you fat? British Journal of Nutrition. 2004;92(S1):S15–S8. doi: 10.1079/bjn20041135. [DOI] [PubMed] [Google Scholar]
- 10.Urbszat D, Herman CP, Polivy J. Eat, drink, and be merry, for tomorrow we diet: effects of anticipated deprivation on food intake in restrained and unrestrained eaters. Journal of abnormal psychology. 2002;111(2):396. doi: 10.1037//0021-843x.111.2.396. [DOI] [PubMed] [Google Scholar]
- 11.Group DPPR. The Diabetes Prevention Program (DPP) description of lifestyle intervention. Diabetes care. 2002;25(12):2165–71. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Gastroenterological Association. American Gastroenterological Association medical position statement on Obesity. Gastroenterology. 2002;123(3):879. doi: 10.1053/gast.2002.35513. [DOI] [PubMed] [Google Scholar]
- 13.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1081. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 14.Karlsson J, Persson LO, Sjöström L, Sullivan M. Psychometric properties and factor structure of the Three-Factor Eating Questionnaire (TFEQ) in obese men and women. Results from the Swedish Obese Subjects (SOS) study. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2000;24(12):1715–25. doi: 10.1038/sj.ijo.0801442. [DOI] [PubMed] [Google Scholar]
- 15.Lowe MR, Butryn ML, Didie ER, Annunziato RA, Thomas JG, Crerand CE, et al. The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite. 2009;53(1):114–8. doi: 10.1016/j.appet.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 16.Clark MM, Abrams DB, Niaura RS, Eaton CA, Rossi JS. Self-efficacy in weight management. Journal of consulting and clinical psychology. 1991;59(5):739. doi: 10.1037//0022-006x.59.5.739. [DOI] [PubMed] [Google Scholar]
- 17.Navidian A, Abedi M, Baghban I, Fatehizade M, Poursharifi H. Reliability and validity of the weight efficacy lifestyle questionnaire in overweight and obese individuals. Journal of Behavioral Sciences. 2009;3(3):217–22. [Google Scholar]
- 18.Carels RA, Darby L, Cacciapaglia HM, Konrad K, Coit C, Harper J, et al. Using motivational interviewing as a supplement to obesity treatment: a stepped-care approach. Health Psychology. 2007;26(3):369. doi: 10.1037/0278-6133.26.3.369. [DOI] [PubMed] [Google Scholar]
- 19.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. New England Journal of Medicine. 2006;355(15):1563–71. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 20.Moroshko I, Brennan L, O'Brien P. Predictors of dropout in weight loss interventions: a systematic review of the literature. Obesity reviews. 2011;12(11):912–34. doi: 10.1111/j.1467-789X.2011.00915.x. [DOI] [PubMed] [Google Scholar]
- 21.Batterham MJ, Tapsell LC, Charlton KE. Analyzing weight loss intervention studies with missing data: Which methods should be used? Nutrition. 2013;29(7):1024–9. doi: 10.1016/j.nut.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Elobeid MA, Padilla MA, McVie T, Thomas O, Brock DW, Musser B, et al. Missing data in randomized clinical trials for weight loss: scope of the problem, state of the field, and performance of statistical methods. PloS one. 2009;4(8):e6624. doi: 10.1371/journal.pone.0006624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gadbury G, Coffey C, Allison D. Modern statistical methods for handling missing repeated measurements in obesity trial data: beyond LOCF. Obesity Reviews. 2003;4(3):175–84. doi: 10.1046/j.1467-789x.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 24.Buuren S, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. Journal of statistical software. 2011;45(3) [Google Scholar]
- 25.Harvey-Berino J, West D, Krukowski R, Prewitt E, VanBiervliet A, Ashikaga T, et al. Internet delivered behavioral obesity treatment. Preventive medicine. 2010;51(2):123–8. doi: 10.1016/j.ypmed.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


