Abstract
Although negative age stereotypes have been found to predict adverse outcomes among older individuals, it was unknown whether the influence of stereotypes extends to brain changes associated with Alzheimer’s disease. To consider this possibility, we drew on the age stereotypes of dementia-free participants in the Baltimore Longitudinal Study of Aging that had been measured decades before yearly MRIs and brain autopsies were performed. Those with more negative age stereotypes earlier in life had significantly steeper hippocampal-volume loss, and significantly greater accumulation of neurofibrillary tangles and amyloid plaques at autopsy, adjusting for relevant covariates. These findings suggest a new pathway to identifying mechanisms and potential interventions related to the neuropathology of Alzheimer’s disease.
Keywords: age stereotypes, perceptions of aging, social cognition, aging, Alzheimer’s disease, brain, brain pathology
Although negative age stereotypes have been found to predict a number of adverse outcomes among older individuals (e.g., Levy & Leifheit-Limson, 2009; Westerhof et al., 2014), it was unknown whether the influence of the stereotypes extended to brain structure and pathology. As premised by stereotype embodiment theory, age stereotypes assimilated from a diversity of sources in the culture at a younger age can impact physiological and cognitive outcomes in later life when these stereotypes become self-relevant (Levy, 2009). The current study, in which the age stereotypes of dementia free and physically healthy participants were assessed decades before measures of brain structure and pathology, provided the first opportunity to test whether this theory applies to biomarkers of Azheimer’s disease.
Specifically, we examined whether negative age stereotypes are a risk factor for the most frequently observed patterns of Alzheimer’s-disease biomarkers: decline in the volume of the hippocampus, which is a critical brain region for maintenance of intact memory (Study 1); and the accumulation of amyloid plaques, which are protein clusters that build up between brain cells, and neurofibrillary tangles, which are twisted strands of protein that build up within brain cells (Study 2). The identification of a culture-based environmental risk factor that contributes to Alzheimer’s disease could be particularly important because such a factor is potentially modifiable (Levy, Pilver, Chung, & Slade, 2014).
The underlying assumption of the current study, that negative age stereotypes can influence the development of Alzheimer’s-disease-related pathological changes in the brain, is based on research findings that support our expectation of a two-stage pathway. First, these stereotypes can contribute to stress. Second, stress can contribute to pathological changes in the brain.
Consistent with this proposed first stage, an experimental study found that older individuals who were subliminally primed with negative age stereotypes and were then exposed to stressors had a significant increase in cardiovascular reactivity, which is linked to cardiovascular events (Levy, Hausdorff, Hencke, & Wei, 2000). Additionally, the negative age stereotypes directly increased cardiovascular measures even before the stress phase of the study (Levy, et al., 2000). This suggests that the negative age stereotypes both exacerbated the stress of an external challenge and acted as a direct stressor. In contrast, the positive age stereotypes appeared to have a protective effect, since the participants exposed to the positive age stereotypes did not show an increase in cardiovascular-reactivity from baseline.
The internalization of negative age stereotypes makes it likely that the stressfulness they generate in the laboratory would also be found over time. For just as participants were experimentally primed with negative age stereotypes, older individuals in the community who hold more negative age stereotypes tend to be subjected to what is, in effect, serial priming: frequent everyday-life encounters with varied forms of ageism in the environment can repetitively activate the internalized negative age stereotypes (Butler, 2008; Levy, et al., 2014; Palmore, 2005). Age stereotypes encountered in the environment often fit the criteria for the types of stressors that are particularly harmful to health: (1) negative; (2) uncontrollable; (3) unpredictable; and (4) repeated over time (Williams & Mohammed, 2009).
This background to the relationship between negative age stereotypes and stress helps to explain the results of a longitudinal study in which the age stereotypes of participants were measured at baseline, decades before old age (Levy, Zonderman, Slade, & Ferrucci, 2009). Those with more negative age stereotypes had a significantly greater risk of experiencing cardiovascular events up to 38 years later than those with more positive age stereotypes. This outcome accords with the well-established relationship of long-term stress to experiencing cardiovascular events (e.g., Steptoe & Kivimäki, 2012). It is also consistent with the higher rates of diastolic blood pressure that were found among middle-aged Black women who perceived greater day-to-day racial discrimination (Guyll, Matthews, & Bromberger, 2001).
The second stage of the negative-age-stereotypes-to-brain pathway involves stress influencing the brain. Evidence of this stage comes from research that has identified stress- hormone receptors in the hippocampus (Fenchel et al., 2015; McEwen, 1998). Although acute stress can have beneficial effects, cumulative stress can damage the brain (McEwen & Giaranos, 2011; Sapolsky, 1996). A contributor to the damage is the prolonged secretion of stress biomarkers (McEwen, 2012).
The influence of cumulative stress on Alzheimer’s-disease biomarkers has been demonstrated most clearly in animal studies. As an example, a series of studies found that experimentally stressed mice tend to show increases in amyloid plaques and tangles and decreases in hippocampal volume (Ahmadian-Attari et al., 2015; Carroll et al., 2011; Lee, Jarome, Li, Kim, & Helmstetter, 2009). In humans, those who suffer from posttraumatic stress disorder (PTSD), which is associated with the experience of cumulative stress, tend to show a significantly greater decline in hippocampal volume than those without PTSD (Chao, Yaffe, Samuelson, & Neylan, 2014; Woon, Sood, & Hedges, 2010). (Older individuals with more negative age stereotypes tend to experience higher rates of PTSD (Levy, Pilver, & Pietrzak, 2014)).
This array of research findings buttresses our hypotheses that, compared with participants with more positive age stereotypes, participants with more negative age stereotypes would show (a) steeper decline of hippocampal volume over time; and (b) greater combined accumulation of amyloid plaques and neurofibrillary tangles.
Study 1
The first study examined whether participants holding more negative age stereotypes showed greater loss of hippocampal volume over time.
Methods
Participants
The cohort consisted of individuals who enrolled in the brain-neuroimaging program of the Baltimore Longitudinal Study of Aging (BLSA) (Ferrucci, 2008). Enrollees received up to 10 yearly magnetic-resonance-imaging (MRI) scans. Inclusion criteria for this program, at time of first scan, were: free of dementia (defined by the Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition (American Psychiatric Association, 1987)); physically healthy (defined as not having central-nervous-system disease, severe cardiovascular disease, severe pulmonary disease, or metastatic cancer) (Driscoll et al., 2009; O’Brien et al., 2009; Resnick et al., 2000); and willingness to undergo yearly MRI scans. There were 158 participants who elected to enroll in the brain-neuroimaging program (Resnick et al., 2000).
Additional inclusion criteria, for the current study, were: provided baseline data on age-stereotypes and covariates; and remained in the study until at least 60 years old. There were 52 participants who met all the inclusion criteria. The average age of the final cohort was 68.54 (SD=6.93) years at the time of first MRI assessment; 92% completed high school; 31% were women; and at baseline, they tended to have high self-rated health (M=4.58, SD=.54), measured by a Likert scale ranging from 1 (very poor) to 5 (excellent).
Measures
Predictor: Negative Age Stereotypes
Negative age stereotypes were assessed with the 16-item age-stereotype subscale of the BLSA’s Attitudes toward Old People Scale (Tuckman & Lorge, 1953) (e.g., “Old people are absent-minded”), starting in 1968. Scores range from 0 to 16, with a higher score signifying more negative age stereotypes. These subscale items were found to load on a single factor (Hatcher, 1994; Levy, et al., 2009). The subscale has good reliability (Cronbach alpha of .80) and has been found to be valid (Kline, 2000; Levy et al., 2009). It significantly correlated in the expected direction with the Image of Aging Scale and has predicted outcomes in the expected direction (Levy et al., 2009; Levy, Zonderman, Slade, & Ferrucci, 2012).
Outcome: Hippocampal Volume
Hippocampal volume was assessed yearly by MRI’s for up to 10 years. (For details on image quantification, see Driscoll et al., 2009.) Our study participants had a total of 364 MRI assessments, with an average of seven per participant. As longitudinal trajectories did not significantly differ for the right and left hippocampal volumes in the cohort, t(94)=1.13, p>.250, d=.21, 95% CI=(−.16, .57), we averaged the hippocampal volumes at each time point. MRI scans took place an average of 25.08 (SD=5.18) years after the baseline age-stereotype measure.
Covariates
The covariates considered have been found to relate to the predictor and/or the outcome in previous research (Driscoll et al., 2009; Levy, 1996; Levy, 2009; Resnick et al., 2000). These covariates consisted of demographics (i.e., age, sex, and education) and health at time of baseline-age-stereotype assessment (i.e., number of chronic conditions based on medical records, well-being, assessed by a subset of the Chicago Attitude Inventory, which was designed to assess components of “personal adjustment to aging” (Burgess, Cavan, & Havighurst, 1948) and self-rated health). Additional covariates were: neuroticism, measured by the neuroticism dimension of the NEO Personality Inventory (Costa & McCrae, 1992); and cognitive performance, measured by the Benton Visual Retention Test (BVRT) (Benton, 1974). The well-being measure (Burgess, et al., 1948) has good test-retest reliability and has been validated in a number of studies with older individuals (Costa, McCrae & Norris, 1981; Larson, 1978; Shock, 1952). The BVRT was selected because it measures visual recall, which is sensitive to decline with age (Lamar, Zonderman, & Resnick, 2002); and has been found to be valid with older individuals (Giambra, Arenberg, Kawas, Zonderman, & Costa, 1995). We also included intracranial volume as a covariate to ensure that hippocampal-volume findings were above and beyond individual differences in overall brain volume (Driscoll et al., 2009; Resnick, Pham, Kraut, Zonderman, & Davatzikos, 2003).
Statistical Analyses
To examine whether those with more negative age stereotypes had a significantly steeper decline in hippocampal volume, we conducted a multivariate model, with the interaction of time and negative age stereotypes as the predictor of hippocampal volume over time. Both variables were continuous. Nonlinearity was checked by adding an age-squared term to the model. The term was not significant, F=.51, p>.25, d=.077, 95% CI=(−.135, .290). Hence, we used a linear mixed-effects regression model. In the model, a one-tailed test of significance was used to reflect the predicted pattern of results of age stereotypes, based on previous studies of the adverse effect of negative age stereotypes on other outcomes (Levy, 2009; Levy, Slade, Murphy, & Gill, 2012; Meisner, 2012; Sargent-Cox, Anstey, & Luszcz, 2012; Westerhof et al., 2014).
To maintain power in the models, we created a parsimonious model by backward elimination, which removed the covariates that did not reach significance, after the forced inclusion of the demographic and health variables, (p<.05, two tailed) (Harrell, 2001).
To make sure that the models were not influenced by extreme observations, we conducted two outlier analyses. We examined whether any observation was more than three standard deviations from the regression line of the final model and whether any observation was more than one Cook’s Distance (Cook & Weisberg, 1982).
Internal validation was conducted using bootstrap modeling, a resampling with replacement procedure (Efron & Tibshirani,1986). To be conservative, we selected 10,000 samples, each consisting of 52 observations, the same as the original model (Efron & Tibshirani, 1998; Pattengale, Alipour, Bininda-Emonds, Moret & Stamatakis, 2010). The parameter estimate of age stereotypes on hippocampal volume from the original model was within the 95% confidence interval of the median-parameter estimate of age stereotypes on hippocampal volume from the bootstrap procedure.
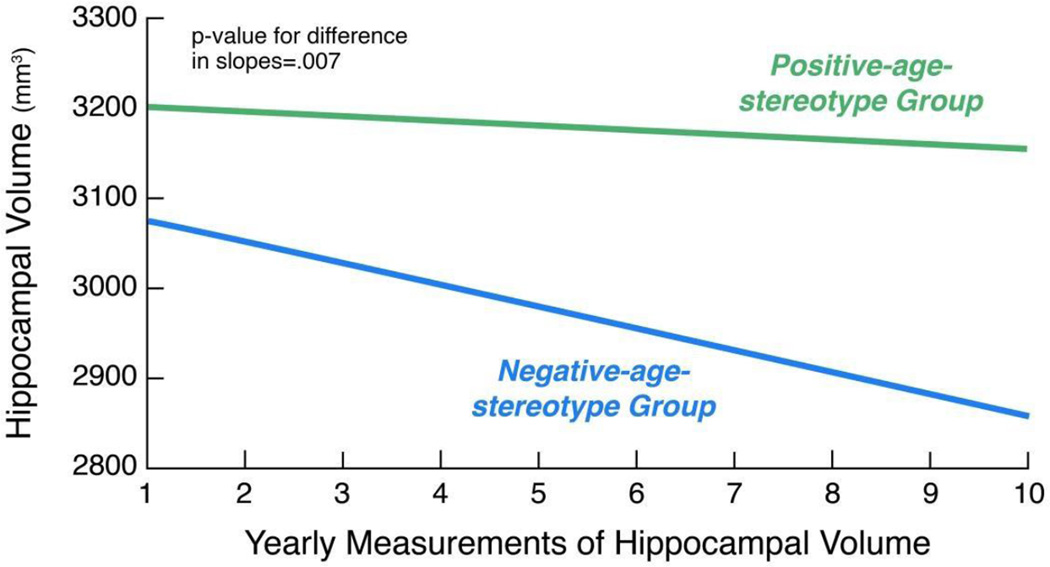
To present the age-stereotype groups for illustration in Figure 1, the sample was dichotomized into those below and those at or above the age-stereotype- measure mean of seven. In the multivariate models, however, the age-stereotype measure was examined as a continuous variable.
Figure 1.
Association of Age Stereotypes with Hippocampal-volume Decline over Time
Note: The figure is based on a model that adjusted for covariates.
Results
As predicted, participants holding more negative age stereotypes had significantly steeper decline in hippocampal volume over time in the full model, F=6.24, p=.007, d=.29, adjusting for age, sex, education, self-rated health, well-being, number of chronic conditions and intracranial volume.
The rate of hippocampal-volume decline in the negative-age-stereotype group was three times the rate of decline in the positive-age-stereotype group (see Figure 1). That is, participants with more negative age stereotypes tended to have the same hippocampal-volume decline in three years that participants with more positive age stereotypes tended to have in nine years.
We determined that the association of negative age stereotypes with greater hippocampal volume was not caused by outliers. All observations of hippocampal volume were within three standard deviations of the regression line, and all-but-one observation had a Cook’s Distance value of less than the Cook’s Distance cut-off criterion of one (Cook & Weisberg, 1982). When we removed this observation that was considered an outlier, it was found that negative age stereotypes continued to predict a decline in hippocampal volume over time, F=4.78, p=.0148, d=.25.
The results of the bootstrap analysis, with 10,000 samples of 52 participants each, showed that the parameter estimate for age stereotypes on hippocampal volume from the original model was stable and robust (Pattengale, et al., 2010). This original age-stereotype-parameter estimate did not differ significantly from the median-parameter estimate for age stereotypes on hippocampal volume that was generated by the bootstrap analysis because the original parameter estimate was within the 95% confidence interval of the bootstrapped-median-parameter estimate (Efron & Tibshirani, 1998)..
Study 2
The second study examined whether participants with more negative age stereotypes would show higher composite-Alzheimer’s-disease-pathology scores.
Methods
Participants
All BLSA participants were invited to join the brain-autopsy program. It aims to compare structural changes due to normal aging with those due to disease (O’Brien et al., 2009; Dolon et al., 2010). In addition to a brain autopsy, our participants met the following criteria: remained in the study until at least 60 years old, and provided baseline data on age-stereotypes and covariates. There were 74 participants who met all the inclusion criteria. The average age of the final cohort was 88.75 (SD=6.45) years at the time of autopsy; 96% completed high school; 26% were women; and at baseline, they tended to have high self-rated health (M=4.51, SD=.51).
Measures
Predictor: Negative Age Stereotypes
The predictor was the same one used in Study 1. It was examined as a continuous variable.
Outcome: Composite-Alzheimer’s-disease-pathology score
For the primary outcome, we used a composite-Alzheimer’s-disease-pathology score that has been validated in previous studies (Dolan et al., 2010; Troncoso et al., 2008). This measure is useful for quantitating the combined effects of amyloid plaques and neurofibrillary tangles in relation to cognitive impairment (Dolan et al., 2010; Troncoso et al., 2008). Investigators at the Johns Hopkins University Alzheimer's Disease Research Center, without knowledge of participants’ age-stereotype scores, identified amyloid plaques and neurofibrillary tangles in five brain regions: superior/middle temporal gyrus, medial frontal lobe, inferior parietal cortex, orbitofrontal cortex, and occipital cortex (Troncoso et al., 2008). Amyloid plaques were graded according to the criteria of the Consortium to Establish a Registry of Alzheimer’s Disease (CERAD), and were then divided into three groups: 1=zero or mild; 2=moderate; and 3=frequent (Mirra et al., 1991). Neurofibrillary tangles were graded according to Braak criteria (Braak & Braak, 1991), and then divided into three groups: 1= preclinical (Braak stages 0, I, and II); 2=moderate (Braak stages III, and IV); and 3=severe (Braak stages V, and VI) (Shoghi-Jadid et al., 2002). The amyloid plaques and neurofibrillary tangles were assessed an average of 28.49 (SD=6.03) years after the baseline age-stereotype measurement.
The sum of the modified CERAD and Braak scores yielded a composite-Alzheimer’s-disease-pathology score ranging from 2 to 6, with a higher score indicating a more advanced condition (Dolan et al., 2010; Troncoso et al., 2008).
Covariates
The covariates considered have been found to relate to the predictor and/ or the outcome in previous research (Driscoll et al., 2009; Levy, 1996; Levy, 2009; Resnick et al., 2000). These covariates consisted of demographics (i.e., age, sex, and education) and health at time of baseline-age-stereotype assessment (i.e., number of chronic conditions based on medical records, well-being (Burgess, et al., 1948), and self-rated health). Additional covariates were: neuroticism, measured by the neuroticism dimension of the NEO Personality Inventory (Costa & McCrae, 1992); and cognitive performance, measured by the Benton Visual Retention Test (BVRT) (Benton, 1974).
Statistical Analyses
To examine whether those with more negative age stereotypes had higher composite-Alzheimer’s-disease-pathology scores, we conducted a generalized linear model with the negative-age-stereotype measure included as a continuous variable and the composite-Alzheimer’s-disease-pathology score as the outcome. A one-tailed test of significance was used to reflect the predicted pattern of how negative age stereotypes were likely to exert their impact (e.g., Levy, 2009; Levy et al, 2012; Meisner, 2012; Sargent-Cox et al., 2012; Westerhof et al, 2014).
To maintain power in the models, we created a parsimonious model by backward elimination, which removed the covariates that did not reach significance, after the forced inclusion of the demographic and health variables, (p<.05, two tailed) (Harrell, 2001).
To make sure that the models were not influenced by extreme observations, we conducted two outlier analyses. We examined whether any observation was more than three standard deviations from the regression line of the final model and whether any observation was more than one Cook’s Distance (Cook & Weisberg, 1982).
Internal validation was conducting using bootstrap modeling (Efron & Tibshirani, 1986). To be conservative, we selected 10,000 samples, each consisting of 74 observations, the same as the original model (Efron & Tibshirani, 1998; Pattengale et al, 2010). We checked to see if the parameter estimate of age stereotypes on the composite-Alzheimer’s-disease-pathology score from the original model was within 95% confidence interval of the median parameter estimate of age stereotypes on the composite-Alzheimer’s-disease-pathology score from the bootstrap procedure.
Results
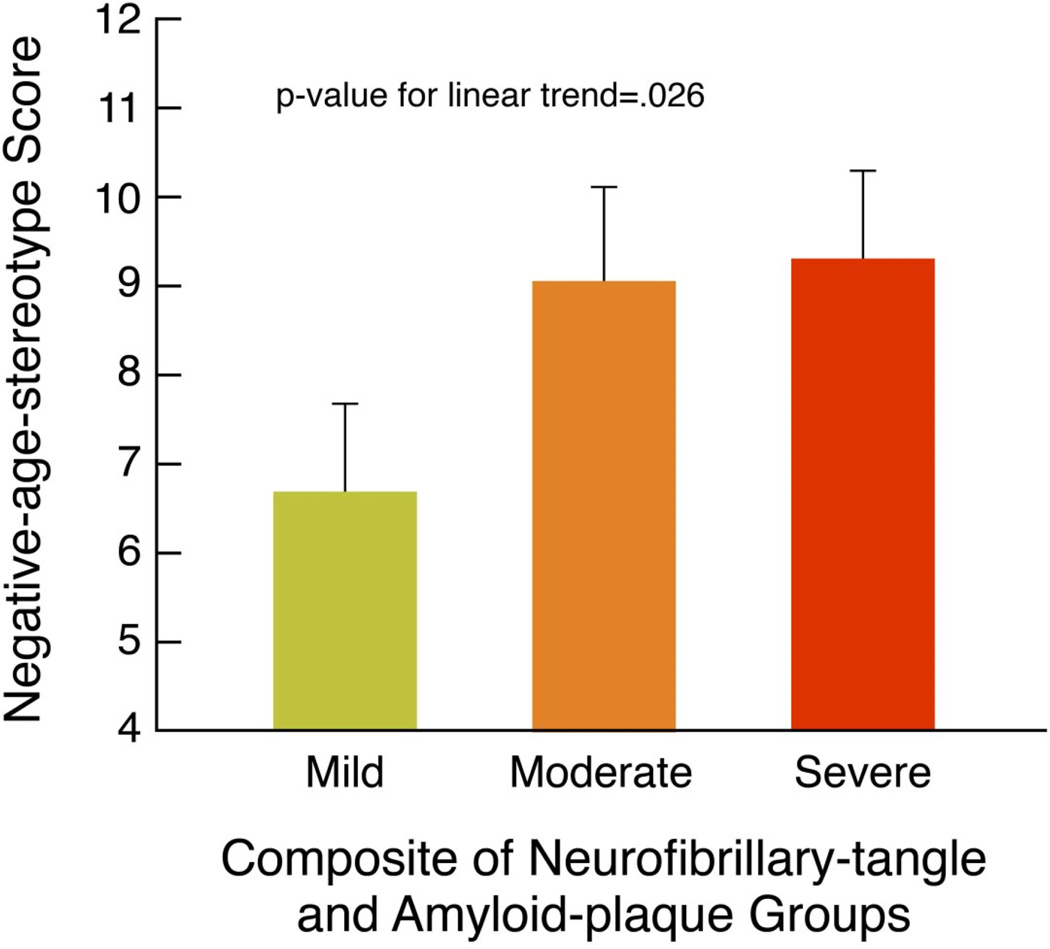
As predicted, participants holding more negative age stereotypes, compared to those with more positive age stereotypes, had significantly higher composite-Alzheimer’s-disease-pathology scores, t(1, 59)=1.71 p=.046, d=.45, adjusting for age, sex, education, self-rated health, well-being, and number of chronic conditions. (See Figure 2).
Figure 2.
More Negative Age Stereotypes Associated with Severity of Neurofibrillary-tangle and Amyloid-plaque Pathology
Note: The mean negative-age-stereotype scores and standard-error bars are adjusted for covariates. The groups are based on the composite-Alzheimer’s-disease-pathology scores of 2 or 3=Mild, 4=Moderate, and 5 or 6=Severe.
The association of negative age stereotypes with higher composite-Alzheimer’s-disease-pathology scores was not caused by outliers. We found that all of the scores were within three standard deviations of the regression line, and all scores were less than the Cook’s Distance cut-off of one for outliers (Cook, & Weisberg, 1982).
The results of the bootstrap procedure showed that the parameter estimate for age stereotypes on the composite-Alzheimer’s-disease-pathology scores from the original model was stable and robust (Pattengale et al., 2010). This age-stereotype-parameter estimate did not differ significantly from the median-parameter estimate for age stereotypes on the composite-Alzheimer’s-disease-pathology scores that were generated by the bootstrap analysis becasuse the original parameter estimate was within the 95% confidence interval of the bootstrapped median parameter estimate (Efron & Tibshirani, 1998).
Discussion
As predicted, participants with more negative age stereotypes had a significantly steeper decline of hippocampal volume than those with more positive age stereotypes. Also as predicted, participants with more negative age stereotypes had a significantly greater accumulation of amyloid plaques and neurofibrillary tangles, compared to those with more-positive age stereotypes.
The robustness of the findings is indicated by the disparateness of the methods used to measure the outcomes: longitudinal MRI scans for hippocampal volume in the first study, whereas the second study relied on brain autopsies to generate the composite measure of amyloid plaques and neurofibrillary tangles. Additional indications of this robustness are: the parallel patterns of negative age stereotypes predicted the biomarkers in the two studies, even though the samples differed in their average ages by 20 years; the finding that these results were not due to the influence of extreme values; and the internal validation procedure of bootstrapping replicated the results from the original models with both hippocampal volume and the composite measure of amyloid plaques and neurofibrillary tangles as the outcomes.
Several factors support our assumption that negative age stereotypes contributed to the Alzheimer’s-disease biomarkers, rather than the reverse direction. First, the baseline measurement of stereotypes occurred more than 20 years before the measurement of hippocampal volume as well as plaques and tangles. Second, all participants were dementia-free at baseline and members of the neuroimaging subsample were dementia-free at the initial MRI scans (O'Brien et al., 2009). Third, previous studies have demonstrated that negative age stereotypes increased stress, but the reverse did not occur; the negativity of age stereotypes held by older individuals did not significantly change over a 10-year period, despite encounters with highly stressful life events (Levy et al., 2000; Levy, Slade, Chung, & Gill, 2014).
A limitation of the otherwise comprehensive data available for our cohort is that it did not include a perceived-stress measure. Perhaps future research will examine stress as a mediator of the relationship of age stereotypes on Alzheimer’s-disease biomarkers.
Our finding that negative age stereotypes held earlier in life predicted brain pathology in later life may shed light on a prior finding that those holding more negative age stereotypes before old age experienced significantly greater memory-performance decline in later life (Levy et al., 2012). (The potential role of brain pathology was not considered in that study.)
The current study is the first to demonstrate that a culture-based risk factor predicts the development of Alzheimer’s-disease-related pathological changes in the brain. This finding provides a basis for interpreting Alzheimer’s-disease data. To illustrate, diet, a previously established environmental factor (Mattson, 2004), has been posited as an explanation for why the rate of Alzheimer’s disease in the United States is five times that of India (Grant, Campbell, Itzhaki, & Savory, 2002). Alternatively, this discrepancy might be explained by a comparison of those two cultures from which age stereotypes are derived: India has a tradition of venerating elders (Bhat & Dhruvarajan, 2001), whereas the United States has a prevalence of negative age stereotypes (Butler, 2008; Levy, Slade, Kunkel & Kasl, 2002). By expanding the boundaries of known environmental influences on amyloid plaques, neurofibrillary tangles, and hippocampal volume, our results suggest a new pathway to identifying mechanisms and potential interventions related to Alzheimer’s disease.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Aging, National Institute of Health; a grant to the Yale School of Public Health (National Institute on Aging R01AG032284); and grants to Johns Hopkins University Alzheimer's Disease Research Center (National Institute on Aging AG 05146, Research and Development Contract HHSN-260-2004-00012C). We thank C. Davatzikos, Johns Hopkins Medical School, for his hippocampal volume analyses and R. J. O’Brien, Duke University School of Medicine, for his autopsy analyses. We also thank the Baltimore Longitudinal Study of Aging participants and staff for their contributions to our studies.
References
- Ahmadian-Attari MM, Dargahi L, Mosaddegh M, Kamalinejad M, Khallaghi B, Noorbala F, Ahmadiani A. Impairment of rat spatial learning and memory in a new model of cold water-induced chronic hypothermia: Implication for Alzheimer's disease. Neurotoxicity Research. 2015;28(2):95–107. doi: 10.1007/s12640-015-9525-0. [Advance online publication]. http://dx.doi.org/10.1007/s12640-015-9525-0. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd. Washington, DC: American Psychiatric Association; 1987. Revised. [Google Scholar]
- Benton AL. The Revised Visual Retention Test: Clinical and experimental applications. New York: Psychological Corporation; 1974. [Google Scholar]
- Bhat AK, Dhruvarajan R. Ageing in India: Drifting intergenerational relations, challenges and options. Ageing and Society. 2001;21:621–640. [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. http://dx.doi.org/10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Burgess EW, Cavan RS, Havighurst RJ. Your attitudes and activities. Chicago: Science Research Associates; 1948. [Google Scholar]
- Butler RN. The longevity revolution: The benefits and challenges of living a long life. New York: Public Affairs; 2008. [Google Scholar]
- Carroll JC, Iba M, Bangasser DA, Valentino RJ, James MJ, Brunden KR, Trojanowski JQ. Chronic stress exacerbates tau pathology, neurodegeneration, and cognitive performance through a corticotropin-releasing factor receptor-dependent mechanism in a transgenic mouse model of tauopathy. The Journal of Neuroscience. 2011;31:14436–14449. doi: 10.1523/JNEUROSCI.3836-11.2011. http://dx.doi.org/10.1523/JNEUROSCI.3836-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Yaffe K, Samuelson K, Neylan TC. Hippocampal volume is inversely related to PTSD duration. Psychiatry Research. 2014;222:119–123. doi: 10.1016/j.pscychresns.2014.03.005. http://www.ncbi.nlm.nih.gov/pubmed/2474292522, 119-23. [DOI] [PubMed] [Google Scholar]
- Cook DR, Weisberg S. Residuals and influence in regression. New York, NY: Chapman & Hall; 1982. [Google Scholar]
- Costa PT, Jr, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) professional manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Costa PT, Jr, McCrae RR, Norris AH. Personal adjustment to aging: Longitudinal prediction from neuroticism and extraversion. Journal of Gerontology. 1981;36:78–85. doi: 10.1093/geronj/36.1.78. http://dx.doi.org/10.1093/geronj/36.1.78. [DOI] [PubMed] [Google Scholar]
- Cook RD, Weisberg S. Residuals and Influence in Regression. New York, NY: Chapman & Hall; 1982. [Google Scholar]
- Dolan D, Troncoso J, Resnick SM, Crain BJ, Zonderman AB, O’Brien RJ. Age, Alzheimer’s disease and dementia in the Baltimore Longitudinal Study of Ageing. Brain: A Journal of Neurology. 2010;133:2225–2231. doi: 10.1093/brain/awq141. http://dx.doi.org/10.1093/brain/awq141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Davatzikos C, An Y, Wu X, Shen D, Kraut M, Resnick SM. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology. 2009;72:1906–1913. doi: 10.1212/WNL.0b013e3181a82634. http://dx.doi.org/10.1212/WNL.0b013e3181a82634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Statistical Science. 1986;1:54–75. http://dx.doi.org/10.1214/ss/1177013815. [Google Scholar]
- Efron B, Tibshirani R. An introduction to the bootstrap. New York: Chapman & Hall/CRC; 1998. [Google Scholar]
- Fenchel D, Levkovitz Y, Vainer E, Kaplan Z, Zohar J, Cohen H. Beyond the HPA-axis: The role of the gonadal steroid hormone receptors in modulating stress-related responses in an animal model of PTSD. European Neuropsychopharmacology. 2015;25:944–957. doi: 10.1016/j.euroneuro.2015.02.004. http://dx.doi.org/10.1016/j.euroneuro.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Ferrucci L. The Baltimore Longitudinal Study of Aging (BLSA): A 50-year-long journey and plans for the future. The Journals of Gerontology. Series A: Biological Sciences and Medical Sciences. 2008;63:1416–1419. doi: 10.1093/gerona/63.12.1416. http://dx.doi.org/10.1093/gerona/63.12.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giambra LM, Arenberg D, Kawas C, Zonderman AB, Costa PT., Jr Adult life span changes in immediate visual memory and verbal intelligence. Psychology and Aging. 1995;10:123–139. doi: 10.1037//0882-7974.10.1.123. http://dx.doi.org/10.1037/0882-7974.10.1.123. [DOI] [PubMed] [Google Scholar]
- Grant WB, Campbell A, Itzhaki RF, Savory J. The significance of environmental factors in the etiology of Alzheimer’s disease. Journal of Alzheimer's Disease. 2002;4:179–189. doi: 10.3233/jad-2002-4308. [DOI] [PubMed] [Google Scholar]
- Guyll M, Matthews KA, Bromberger JT. Discrimination and unfair treatment: Relationship to cardiovascular reactivity among African American and European American women. Health Psychology. 2001;20:315–325. doi: 10.1037//0278-6133.20.5.315. http://dx.doi.org/10.1037/0278-6133.20.5.315. [DOI] [PubMed] [Google Scholar]
- Harrell FE. Regression modeling strategies: With applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. http://dx.doi.org/10.1007/978-1-4757-3462-1. [Google Scholar]
- Hatcher L. A step-by-step approach to using the SAS System for factor analysis and structural equation modeling. Cary, NC: SAS Institute Inc; 1994. [Google Scholar]
- Kline P. The handbook of psychological testing. 2nd. London: Routledge; 2000. [Google Scholar]
- Lamar M, Zonderman AB, Resnick S. Contribution of specific cognitive processes to executive functioning in an aging population. Neuropsychology. 2002;16:156–162. doi: 10.1037//0894-4105.16.2.156. http://dx.doi.org/10.1037/0894-4105.16.2.156. [DOI] [PubMed] [Google Scholar]
- Larson R. Thirty years of research on the subjective well-being of older americans. Journal of Gerontology. 1978;33:109–125. doi: 10.1093/geronj/33.1.109. http://dx.doi.org/10.1093/geronj/33.1.109. [DOI] [PubMed] [Google Scholar]
- Lee T, Jarome T, Li SJ, Kim JJ, Helmstetter FJ. Chronic stress selectively reduces hippocampal volume in rats: A longitudinal magnetic resonance imaging study. Neuroreport. 2009;20:1554–1558. doi: 10.1097/WNR.0b013e328332bb09. http://dx.doi.org/10.1097/WNR.0b013e328332bb09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B. Improving memory in old age through implicit self-stereotyping. Journal of Personality and Social Psychology. 1996;71:1092–1107. doi: 10.1037//0022-3514.71.6.1092. http://dx.doi.org/10.1037/0022-3514.71.6.1092. [DOI] [PubMed] [Google Scholar]
- Levy B. Stereotype embodiment: A psychosocial approach to aging. Current Directions in Psychological Science. 2009;18:332–336. doi: 10.1111/j.1467-8721.2009.01662.x. http://dx.doi.org/10.1111/j.1467-8721.2009.01662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BR, Hausdorff JM, Hencke R, Wei JY. Reducing cardiovascular stress with positive self-stereotypes of aging. The Journals of Gerontology. Series B: Psychological Sciences and Social Sciences. 2000;55:205–213. doi: 10.1093/geronb/55.4.p205. http://dx.doi.org/10.1093/geronb/55.4.P205. [DOI] [PubMed] [Google Scholar]
- Levy BR, Leifheit-Limson E. The stereotype-matching effect: Greater influence on functioning when age stereotypes correspond to outcomes. Psychology and Aging. 2009;24:230–233. doi: 10.1037/a0014563. http://dx.doi.org/10.1037/a0014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BR, Pilver C, Chung PH, Slade MD. Subliminal strengthening: Improving older individuals’ physical function over time with an implicit-age-stereotype intervention. Psychological Science. 2014;25:2127–2135. doi: 10.1177/0956797614551970. http://dx.doi.org/10.1177/0956797614551970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BR, Pilver CE, Pietrzak RH. Lower prevalence of psychiatric conditions when negative age stereotypes are resisted. Social Science & Medicine. 2014;119:170–174. doi: 10.1016/j.socscimed.2014.06.046. http://dx.doi.org/10.1016/j.socscimed.2014.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BR, Slade MD, Chung PH, Gill TM. Resiliency over time of elders’ age stereotypes after encountering stressful events. The Journals of Gerontology. Series B: Psychological Sciences and Social Sciences. 2014 doi: 10.1093/geronb/gbu082. First published online July 5, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BR, Slade MD, Kunkel SR, Kasl SV. Longevity increased by positive self-perceptions of aging. Journal of Personality and Social Psychology. 2002;83:261–270. doi: 10.1037//0022-3514.83.2.261. http://dx.doi.org/10.1037/0022-3514.83.2.261. [DOI] [PubMed] [Google Scholar]
- Levy BR, Slade MD, Murphy TE, Gill TM. Association between positive age stereotypes and recovery from disability in older persons. Journal of the American Medical Association. 2012;308:1972–1973. doi: 10.1001/jama.2012.14541. http://dx.doi.org/10.1001/jama.2012.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BR, Zonderman AB, Slade MD, Ferrucci L. Age stereotypes held earlier in life predict cardiovascular events in later life. Psychological Science. 2009;20:296–298. doi: 10.1111/j.1467-9280.2009.02298.x. http://dx.doi.org/10.1111/j.1467-9280.2009.02298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BR, Zonderman AB, Slade MD, Ferrucci L. Memory shaped by age stereotypes over time. The Journals of Gerontology. Series B: Psychological Sciences and Social Sciences. 2012;67:432–436. doi: 10.1093/geronb/gbr120. http://dx.doi.org/10.1093/geronb/gbr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. http://dx.doi.org/10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. The New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. http://dx.doi.org/10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Brain on stress: How the social environment gets under the skin. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(Suppl 2):17180–17185. doi: 10.1073/pnas.1121254109. http://dx.doi.org/10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annual Review of Medicine. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. http://dx.doi.org/10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisner BA. A meta-analysis of positive and negative age stereotype priming effects on behavior among older adults. The Journals of Gerontology. Series B: Psychological Sciences and Social Sciences. 2012;67:13–17. doi: 10.1093/geronb/gbr062. http://dx.doi.org/10.1093/geronb/gbr062. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. http://dx.doi.org/10.1212/WNL.41.4.479. [DOI] [PubMed] [Google Scholar]
- Mohiyeddini C, Bauer S, Semple S. Neuroticism and stress: The role of displacement behavior. Anxiety, Stress, and Coping. 2015;28:391–407. doi: 10.1080/10615806.2014.1000878. [Epub ahead of print]. http://dx.doi.org/10.1080/10615806.2014.1000878. [DOI] [PubMed] [Google Scholar]
- O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L, Crain BJ, Pletnikova O, Troncoso JC. Neuropathologic studies of the Baltimore Longitudinal Study of Aging (BLSA) Journal of Alzheimer's Disease. 2009;18:665–675. doi: 10.3233/JAD-2009-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmore E. Three decades of research on ageism. Generations (San Francisco, Calif.) 2005;29:87–90. [Google Scholar]
- Pattengale ND, Alipour M, Bininda-Emonds OR, Moret BM, Stamatakis A. How many bootstrap replicates are necessary? Journal of Computational Biology. 2010;17:337–354. doi: 10.1089/cmb.2009.0179. http://dx.doi.org/10.1089/cmb.2009.0179. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Goldszal AF, Davatzikos C, Golski S, Kraut MA, Metter EJ, Zonderman AB. One-year age changes in MRI brain volumes in older adults. Cerebral Cortex. 2000;10:464–472. doi: 10.1093/cercor/10.5.464. http://dx.doi.org/10.1093/cercor/10.5.464. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. The Journal of Neuroscience. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. http://dx.doi.org/10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- Sargent-Cox KA, Anstey KJ, Luszcz MA. The relationship between change in self-perceptions of aging and physical functioning in older adults. Psychology and Aging. 2012;27:750–760. doi: 10.1037/a0027578. http://dx.doi.org/10.1037/a0027578. [DOI] [PubMed] [Google Scholar]
- Shock N. Aging and psychological adjustment. Review of Educational Research. 1952;22:439–458. [Google Scholar]
- Steptoe A, Kivimäki M. Stress and cardiovascular disease. Nature Reviews. Cardiology. 2012;9:360–370. doi: 10.1038/nrcardio.2012.45. http://dx.doi.org/10.1038/nrcardio.2012.45. [DOI] [PubMed] [Google Scholar]
- Troncoso JC, Zonderman AB, Resnick SM, Crain B, Pletnikova O, O’Brien RJ. Effect of infarcts on dementia in the Baltimore longitudinal study of aging. Annals of Neurology. 2008;64:168–176. doi: 10.1002/ana.21413. http://dx.doi.org/10.1002/ana.21413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckman J, Lorge I. Attitudes toward old people. The Journal of Social Psychology. 1953;37:249–260. [Google Scholar]
- Westerhof GJ, Miche M, Brothers AF, Barrett AE, Diehl M, Montepare JM, Wurm S. The influence of subjective aging on health and longevity: A meta-analysis of longitudinal data. Psychology and Aging. 2014;29:793–802. doi: 10.1037/a0038016. http://dx.doi.org/10.1037/a0038016. [DOI] [PubMed] [Google Scholar]
- Williams DR, Mohammed SA. Discrimination and racial disparities in health: Evidence and needed research. Journal of Behavioral Medicine. 2009;32:20–47. doi: 10.1007/s10865-008-9185-0. http://dx.doi.org/10.1007/s10865-008-9185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woon FL, Sood S, Hedges DW. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: A meta-analysis. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010;34:1181–1188. doi: 10.1016/j.pnpbp.2010.06.016. http://dx.doi.org/10.1016/j.pnpbp.2010.06.016. [DOI] [PubMed] [Google Scholar]