Abstract
Acute heart failure (AHF) is a complex clinical syndrome characterized by fluid overload and haemodynamic abnormalities (short-term clinical consequences) and the development of end-organ damage (long-term consequences). Current therapies for the treatment of AHF, such as loop diuretics and vasodilators, help to relieve haemodynamic imbalance and congestion, but have not been shown to prevent (and may even contribute to) end-organ damage, or to provide long-term clinical benefit. Serelaxin is the recombinant form of human relaxin-2, a naturally occurring hormone involved in mediating haemodynamic changes during pregnancy. Preclinical and clinical studies have investigated the effects mediated by serelaxin and the suitability of this agent for the treatment of patients with AHF. Data suggest that serelaxin acts via multiple pathways to improve haemodynamics at the vascular, cardiac, and renal level and provide effective congestion relief. In addition, this novel agent may protect the heart, kidneys, and liver from damage by inhibiting inflammation, oxidative stress, cell death, and tissue fibrosis, and stimulating angiogenesis. Serelaxin may therefore improve both short- and long-term outcomes in patients with AHF. In this review, we examine the unique mechanisms underlying the potential benefits of serelaxin for the treatment of AHF, in particular, those involved in mediating end-organ protection.
Keywords: Serelaxin, Acute heart failure, Congestion relief, Organ protection, Long-term outcomes
Introduction
Heart failure (HF) is a chronic condition, punctuated by acute episodes, which affects as many as one in five people aged 70–80 years.1,2 In acute heart failure (AHF), rapid worsening of the signs and symptoms of HF results in the requirement for urgent therapy and, frequently, hospitalization.3 The frequency of AHF episodes increases with disease progression, resulting in high rates of hospitalization and an increased risk of mortality.3 As such, AHF places a significant burden on both patients and healthcare systems.4
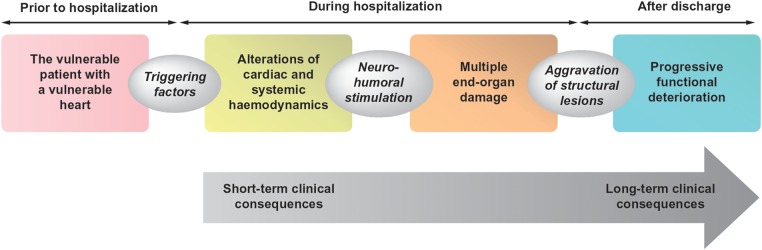
Pathophysiologically, it is known that AHF involves both haemodynamic abnormalities and end-organ damage (Figure 1).5–12 Haemodynamic abnormalities result in early clinical features of congestion,2,13–15 whereas end-organ damage may contribute to long-term morbidity and mortality.16
Figure 1.
The ‘continuum’ of pathophysiological changes associated with acute heart failure that may lead to both short- and long-term effects on the heart and other end organs.5–12
Current therapies for AHF include loop diuretics and vasodilators, agents which stimulate vasodilation and diuresis to relieve haemodynamic abnormalities.4,10,17–19 However, none of these agents have been shown to prevent end-organ damage, and their use may be associated with detrimental effects on numerous organs, thereby contributing to long-term morbidity and mortality.20–22 As a result, new therapies for the treatment of AHF should relieve congestion to improve short-term clinical consequences and provide organ protection to positively impact the long-term clinical consequences of AHF.
Human relaxin-2 is the major form of the hormone relaxin, which has vital roles during pregnancy.23,24 Relaxin-2 binds primarily to relaxin family peptide receptor 1 (RXFP1), located in the heart, kidneys, and vasculature, to activate numerous cellular pathways.16,25–27 Serelaxin has been manufactured as the recombinant form of human relaxin-2 and is currently under investigation for the treatment of AHF.27,28
In this review, we briefly describe the unique mechanisms underlying the ability of serelaxin to relieve congestion and, therefore, mediate short-term beneficial effects in patients with AHF. We also examine, in detail, the novel mechanisms by which serelaxin, unlike current treatments, may limit end-organ damage and thus, provide long-term treatment benefit in patients with AHF.
Serelaxin for the treatment of acute heart failure: key clinical data
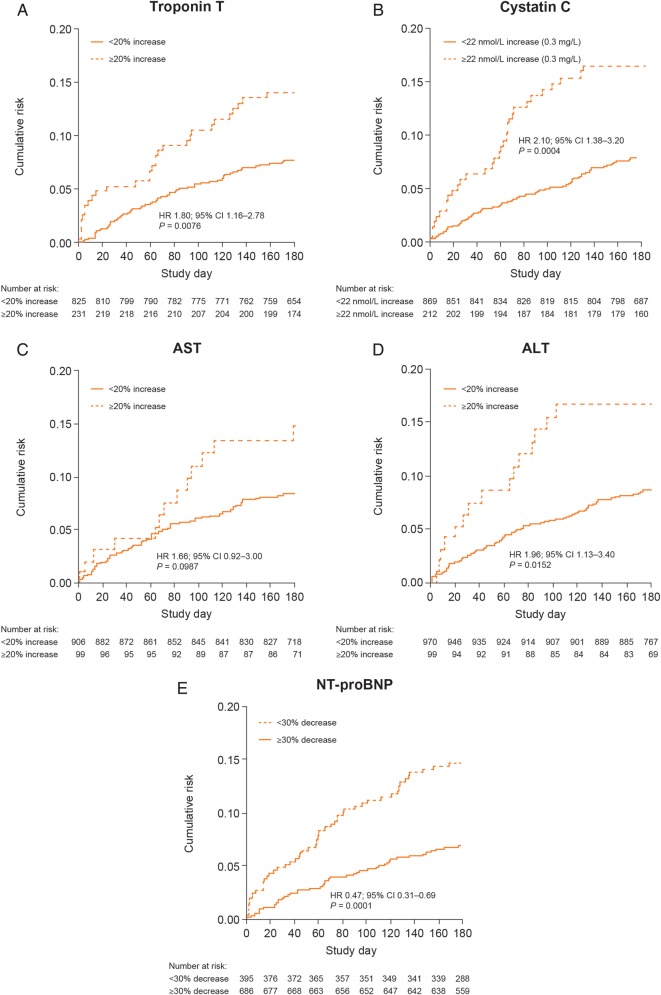
The safety and efficacy of serelaxin for the treatment of patients with AHF has been determined in the preliminary RELAXin in Acute Heart Failure (pre-RELAX-AHF) and RELAXin in Acute Heart Failure (RELAX-AHF) clinical trials. In the phase IIb pre-RELAX-AHF trial, serelaxin (30 µg/kg/day 48-h infusion) resulted in a positive effect on dyspnoea compared with placebo.29 In the phase III RELAX-AHF trial, serelaxin (30 µg/kg/day 48-h infusion), when compared with placebo, significantly improved the primary efficacy endpoint of dyspnoea relief by the visual analogue scale area under the curve to Day 5, with numerical improvement observed in the primary endpoint of dyspnoea as assessed by the Likert scale at 6, 12, and 24 h.30 Serelaxin treatment improved signs and symptoms of congestion and length of hospital stay compared with placebo in the RELAX-AHF study, although, no significant improvement in the two secondary endpoints of days alive and out of hospital, and cardiovascular (CV) death or rehospitalization for HF or renal failure through Day 60 was observed.30 In both studies, serelaxin demonstrated favourable effects on longer-term clinical outcomes, such as CV and all-cause mortality through Day 180 compared with placebo (Figure 2).29–31 In the RELAX-AHF study, elevated levels of troponin T, cystatin C, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and N-terminal pro-B-type natriuretic peptide (NT-proBNP) were associated with an increased risk of all-cause mortality through Day 180 (Figure 3).31 Serelaxin treatment, when compared with placebo, was associated with lower levels of these biomarkers, indicating that serelaxin may protect organs from further damage following AHF hospitalization.31 Overall, serelaxin had a favourable safety and tolerability profile compared with placebo.29,30
Figure 2.
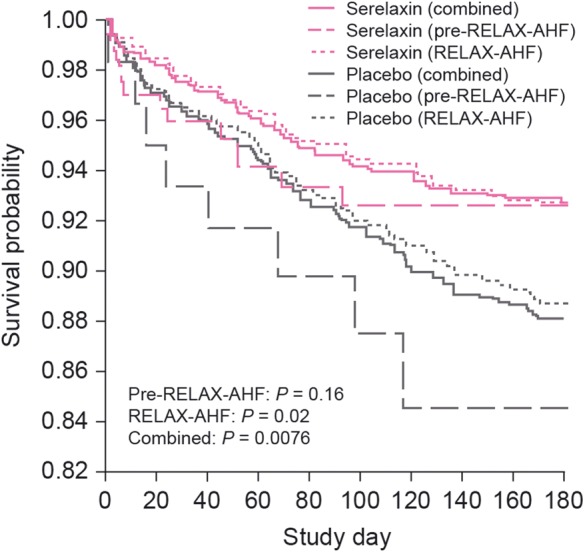
Risk for all-cause mortality through Day 180 in Pre-RELAX-AHF and RELAX-AHF.31 AHF, acute heart failure; RELAX-AHF, RELAXin in Acute Heart Failure; Pre-RELAX-AHF, preliminary RELAXin in Acute Heart Failure. Reproduced under the terms of the Elsevier user license (http://www.elsevier.com/about/open-access/open-access-policies/oa-license-policy/elsevier-user-license) for Metra et al.31
Figure 3.
All-cause mortality through Day 180 in RELAX-AHF by markers of organ damage/dysfunction: troponin T (A); cystatin C (B); AST (C); ALT (D), and NT-proBNP (E).31 ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; HR, hazard ratio; NT-proBNP, N-terminal pro-B-type natriuretic peptide. Reproduced under the terms of the Elsevier user license (http://www.elsevier.com/about/open-access/open-access-policies/oa-license-policy/elsevier-user-license) for Metra et al.31
Although promising, pre-RELAX-AHF and RELAX-AHF studies were not powered to detect changes in mortality, thus adequately designed follow-up studies are needed. A second phase III trial, RELAX-AHF-2, is ongoing and will further investigate the safety and efficacy of serelaxin for the treatment of patients with AHF, including the mortality benefit observed in previous clinical trials.32
Serelaxin and correction of haemodynamic imbalance
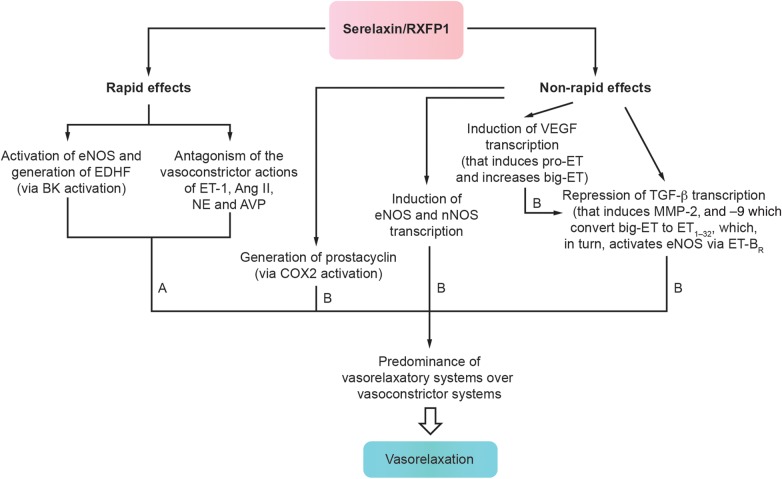
Observations from preclinical and clinical studies indicate that serelaxin acts via multiple mechanisms to correct haemodynamic imbalance and relieve congestion, as described in Table 1.30,33–52 For instance, serelaxin is thought to stimulate vasorelaxatory systems and counteract vasoconstrictor systems, to mediate both rapid and sustained vasorelaxation53 (Figure 4),28,43,54 and thus, improve haemodynamics and alleviate congestion. Evidence suggests that serelaxin also increases arterial compliance40,42 and decreases systemic vascular resistance,35,36,44–46 which could increase capacitance to prevent fluid redistribution to the lungs and improve haemodynamic abnormalities, aiding decongestion in AHF.8 Interestingly, in contrast to vasodilators such as nitroglycerin, which primarily act via direct venodilation,55 the vasorelaxatory action of serelaxin is thought to predominantly affect arteries.45
Table 1.
Effects mediated by serelaxin that may alleviate haemodynamic imbalance and relieve congestion in patients with AHF30,33–52
| Effect mediated by serelaxin | Evidence from preclinical and clinical studies following administration of serelaxina |
||||||
|---|---|---|---|---|---|---|---|
| In vitro | Mice | Rats | Healthy subjects | Patients with CHF | Patients with AHF | Possible clinical consequences | |
| Reduction of cardiac pressures | ↓SBPb33,34 (including porcine relaxin) | ↓DBPc35 ↓PCWPc35 ↓SBPc35 ↓PAPc35 |
↓DBPd36 ↓PCWPd36 ↓SBPd36 ↓PAPd36 ↓JVPd30 |
Improved haemodynamics Relief of congestion Prevention of further stimulation of neurohumoral systems |
|||
| Stimulation of vasorelaxation | Blunted responses of rat mesenteric arteries to vasoconstriction induced by AVP and NE37 (rat relaxin) Vasorelaxation of small human resistance arteries38 ↑Coronary flow/↑NO generation in isolated guinea pig hearts subject to IR injury39 (porcine relaxin) |
↑Arterial compliance40 | Blunted response to vasoconstriction and ↑BP induced by Ang II33,41 (including porcine relaxin) ↓Wall stiffness42 ↑Arterial compliance42 ↑Rapid and sustained BK-mediated vasorelaxation of mesenteric arteries43 |
Improved haemodynamics Relief of congestion Possible prevention of fluid redistribution |
|||
| Reduction of SVR | ↓SVRe44–46 | ↓SVRf35 | ↓SVRd36 | Vasorelaxation Improved haemodynamics Relief of congestion Possible prevention of fluid redistribution |
|||
| Preservation of diuresis and natriuresis | ↑Urinary excretion of sodium47 ↓Salt sensitivityb34 (porcine relaxin) ↑Urinary flow rate47 |
↑Renal clearance, fractional excretion and urinary excretion of sodiumg48 No effect on urinary flow rateg48 |
No effect on urinary excretion of sodium or urinary flow rateh49 | Neutral effect on diuretic responsei50 | Preservation of renal function Improved haemodynamics Possible prevention of fluid redistribution |
||
| Increased RBF and preservation of GFR | ↑GFR41,51,52 ↑RBF41,47,51,52 |
↑RBFg48 No effect on GFRg48 |
↑RBFh,j49 No effect on GFRh49 |
Preservation of renal function Possible long-term renal protection |
|||
| Increased cardiac output | ↑COe44–46 | ↑COf35 | No impact on CId36 | Improved haemodynamics Relief of congestion Prevention of further stimulation of neurohumoral systems |
|||
AHF, acute heart failure; Ang II, angiotensin II; AVP, arginine vasopressin; BK, bradykinin; BP, blood pressure; CHF, chronic heart failure, CI, cardiac index; CO, cardiac output; DBP, diastolic blood pressure; GFR, glomerular filtration rate; IR, ischaemia reperfusion; JVP, jugular venous pressure; NE, norepinephrine; NO, nitric oxide; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; RBF, renal blood flow; SBP, systolic blood pressure; SVR, systemic vascular resistance.
aSerelaxin unless otherwise stated.
bIn rat models of Ang II-induced hypertension and/or salt-sensitive hypertension, reductions in SBP were mediated by the NOS system.
cSerelaxin administered at doses of 10–100 µg/kg/day and/or 960 µg/kg/day for 24 h.
dSerelaxin administered at a dose of 30 µg/kg/day for 20 h.
eIn hypertensive and non-hypertensive rats.
fSerelaxin administered at a dose of 960 µg/kg/day for 24 h.
gAn intravenous bolus of serelaxin (0.2 μg/kg) was administered over 5 min, followed by an infusion of 0.5 μg/kg per hour for 4 h.
hSerelaxin administered at a dose of 30 µg/kg/day for 24 h.
iSerelaxin administered at a dose of 30 µg/kg/day for 48 h.
jImproved RBF observed up to 28 h post-serelaxin dose compared with placebo.
Figure 4.
Time-dependent effects of intravenously administered serelaxin on vasoactive systems that result in vasorelaxation.28,43,54 A, time after serelaxin administration, when the hormone is detectable in the blood ranges from minutes to hours; B, time after serelaxin administration, when the hormone is not detected in the blood ranges from 1 to several days; Ang II, angiotensin II; AVP, arginine vasopressin; BK, bradykinin; COX2, cyclo-oxygenase 2; EDHF, endothelium-derived hyperpolarizing factor; eNOS, endothelial nitric oxide synthase; ET, endothelin; ET-BR, endothelin receptor type B; MMP, metalloproteinase; NE, norepinephrine; nNOS, neuronal nitric oxide synthase; RXFP1, relaxin/insulin-like family peptide receptor 1; TGF-β, transforming growth factor β; VEGF, vascular endothelial growth factor.
In addition to inducing vasorelaxation, serelaxin treatment has been shown to reduce cardiac pressures and to preserve or improve cardiac and renal function,30,33–36,41,44–52 which is likely to help restore haemodynamics, relieve congestion (via mechanisms which may include the prevention of fluid redistribution), and prevent further stimulation of neurohumoral systems in AHF.2,56 In addition, the renal effects of serelaxin may be associated with long-term renal protection, which warrants further investigation.
Serelaxin treatment and the limitation of end-organ damage
Serelaxin interferes with the mechanisms underlying the development of end-organ damage
In patients with AHF, haemodynamic alterations stimulate a number of systemic mechanisms, including the adrenergic system, vasoactive hormones, inflammation, and oxidative stress which, in turn, alter the local mechanisms controlling cell death, tissue repair, and vessel function, contributing to the development of cardiac, renal, hepatic, vascular, and other organ damage.2,6,57–64 The available evidence suggests that serelaxin may interfere with these systemic and local mechanisms to limit end-organ damage.
Serelaxin and inhibition of inflammation
Damage to organs including the heart, kidneys, and liver occurs early in AHF and has long-term consequences.16,65 Inflammatory activation can contribute to organ injury, in addition to vascular dysfunction and fluid overload.8,16 For instance, in patients with newly diagnosed HF, levels of tumour necrosis factor alpha (TNF-α), interleukin (IL)-6, and CD14 were elevated on the third day of initial hospitalization and associated with impaired function of the left atrium and more advanced left ventricular (LV) systolic and diastolic dysfunction.66
Changes in inflammatory pathways have been determined in a number of studies following the administration of serelaxin. In human umbilical vein endothelial cells incubated with serelaxin, TNF-α-induced upregulation of vascular cell adhesion molecule 1 (VCAM-1) and platelet endothelial cell adhesion molecule was diminished, along with C-C chemokine receptor type 2 and monocyte chemotactic protein 1 levels, and monocyte adhesion to the cells.67 In addition, serelaxin inhibited basophil function, via nitric oxide synthase activation, to reduce histamine release and prevent the rise in intracellular calcium that stimulates granule release.68,69
In rats subjected to cardiac, renal, hepatic, or splanchnic ischaemia–reperfusion (IR) injury, treatment with serelaxin or porcine relaxin diminished myeloperoxidase activity, a marker of inflammatory leukocyte infiltration.70–74 Serelaxin treatment decreased expression of inflammatory mediators and adhesion molecules including intercellular adhesion molecule-1 (ICAM-1), IL-1β, IL-18, and TNF-α in rats subjected to renal IR injury,71 while porcine relaxin downregulated expression of adhesion molecules P-selectin, E-selectin, VCAM, and ICAM-1 in a rat model of splanchnic IR injury,70 as well as TNF-α expression in a rat model of renal IR injury.75 In addition, porcine relaxin treatment was associated with a reduction in the number of neutrophils and inhibition of mast cell granule release in a rat model of cardiac IR injury.74 Similarly, reductions in myeloperoxidase levels and cardiac mast cell degranulation were evident following the administration of serelaxin in a pig model of cardiac IR injury.76,77
Inhibiting the inflammatory response in patients with AHF may decrease fluid overload to relieve congestion and positively impact vascular, myocardial, renal, and hepatic injury and dysfunction8,71,73,75,77 and consequently, improve long-term outcomes. The anti-inflammatory actions of serelaxin distinguish this agent from current AHF therapies, such as nitrates, that have not been shown to improve long-term outcomes in patients with AHF21 and are therefore unlikely to inhibit inflammation.
Serelaxin and reduction of oxidative stress
Increased oxidative stress results from the dominance of reactive oxygen species (ROS) such as superoxide over endogenous antioxidant defence mechanisms.78 In patients with AHF, oxidative stress can result in myocardial, renal, and hepatic injury and remodelling.16 Neurohormones contribute to the activation of ROS in AHF, while mitochondrial calcium overload and dysfunction (via leaky type 2 ryanodine receptors) may lead to increased release of ROS in HF78,79 and reperfusion-induced inflammation may contribute to oxidative cardiac tissue injury.24
In vitro studies, animal models and clinical studies have investigated the effects of animal relaxin and serelaxin on oxidative stress. In vitro, porcine relaxin was found to reduce the production of superoxide anions from human neutrophils.68 In rats with renal or splanchnic IR injury, serelaxin treatment was associated with increased levels of the antioxidant enzymes manganese and copper–zinc superoxide dismutase71 and diminished consumption of superoxide dismutase, lipid peroxidation, and markers of deoxyribonucleic acid (DNA) damage including 8-hydroxy-2′-deoxyguanosine and poly-ADP-ribosylated DNA.70 In addition, serelaxin decreased hydrogen peroxide and thiobarbituric acid-reactive substance (TBARs) excretion and consequently, oxidative stress, in rats with angiotensin II-induced hypertension.33 In the same experimental model, serelaxin treatment reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity (i.e. superoxide anion generation) and excretion of TBARs and 8-isoprostane (markers of oxidative stress), and restored nitric oxide (NO) oxidation product excretion.80 Finally, serelaxin was found to decrease levels of malondialdehyde (MDA), a marker of oxygen-free radical-mediated cell damage, in a porcine model of cardiac IR injury.76
In patients with AHF, serelaxin treatment (30 µg/kg/day, 48-h infusion) significantly reduced levels of uric acid, a marker of oxidative stress, compared with placebo.31 This finding reinforces the novel mechanism of action of serelaxin and suggests that this agent may possess antioxidant properties, to prevent excess formation of superoxide, which reacts with NO to form the powerful oxidant peroxynitrite.81 Protecting against oxidative stress could prevent apoptosis/necrosis and, consequently, protect the endothelium and limit the end-organ damage associated with AHF.31,33,70,71 In contrast to serelaxin, current AHF therapies, such as nitrates, do not possess antioxidant properties and may contribute to the development of endothelial dysfunction, via NO-mediated increases in superoxide and thus, peroxynitrite.82–84
Serelaxin and inhibition of cell death
Cardiac wall stress as well as the stimulation of neurohormones, oxidative stress, and release of inflammatory mediators result in cell death via apoptosis and necrosis, and ultimately, organ damage in patients with AHF.2,6,58–63 Previous studies have shown that anti-apoptotic and anti-necrotic effects are associated with end-organ preservation.75,85 Preventing organ damage by protecting cells from apoptosis and/or necrosis is therefore likely to improve long-term outcomes in patients with AHF;31,86 however, evidence suggests that current standard of treatment does not provide such benefit.3,21,22
In vitro, serelaxin has been shown to antagonize apoptosis in neonatal rat cardiomyocytes exposed to hydrogen peroxide87 and high levels of glucose.88 Serelaxin also significantly increased cell viability and diminished apoptosis and nitroxidative damage in both H9c2 rat cardiomyoblasts and primary mouse cardiomyocytes subjected to hypoxia and reoxygenation; these effects were partly due to the upregulation of Notch-1 signalling.89
In vivo studies have demonstrated beneficial effects of serelaxin and animal relaxin on apoptosis and necrosis. In rat models with renal injury, serelaxin treatment has been associated with reduced DNA damage and lipid peroxidation.71 In addition, serelaxin has been shown to protect against IR injury in the rat liver, as demonstrated by lower MDA levels in a model of isolated reperfused rat liver.72,73 Administration of porcine relaxin has resulted in diminished calcium overload and MDA levels74 and lower apoptotic cell counts, as assessed by caspase-3 expression and/or terminal deoxynucleotidyl transferase dUTP nick-end labelling (TUNEL) in rat models of cardiac IR injury, splanchnic IR injury, and renal IR injury, respectively.70,75 Decreased peroxidation products, nitration products, and markers of DNA damage were also reported following porcine relaxin treatment in a rat model of splanchnic IR injury,70 while rat relaxin-3 reduced MDA levels following myocardial injury in rats.90 Similarly, in a mouse model of cardiac IR injury, treatment with serelaxin antagonized apoptosis, as assessed by TUNEL staining.85 Finally, in pig models of cardiac IR injury, tissue calcium overload, tissue caspase-3 activity, TUNEL-positive cardiomyocytes, and mitochondrial swelling in cardiomyocytes were diminished76 and oxidative cardiac tissue injury was inhibited, as demonstrated by decreased MDA levels.77
Serelaxin and inhibition of tissue fibrosis
Induction of fibrosis and remodelling of organs, including the heart, kidneys, and liver, can result from neurohumoral activation, inflammation, and oxidative stress in AHF.16 Increased levels of markers of extracellular matrix turnover, including matrix metalloproteinase (MMP)-2, tissue inhibitor of MMP (TIMP)-1, and procollagen type III N-terminal peptides, have been observed during the first 24 h of hospital admission for HF decompensation.6 In addition, failing hearts, when compared with non-failing hearts, have demonstrated dysregulation of microRNA expression, which is thought to contribute to myocardial remodelling in HF.91
In vitro, serelaxin inhibited transforming growth factor beta (TGF-β) and/or TIMPs in human hepatic stellate cells and human dermal fibroblasts,92,93 and increased expression of MMPs, including MMP-1, -2, -9, and -13, via mechanisms including the NO pathway, in human dermal fibroblasts92,94 and rat renal myofibroblasts.94 Production of collagen was found to decrease in rat atrial and ventricular fibroblasts95,96 and human scleroderma fibroblasts97 following administration of serelaxin. In addition, serelaxin treatment downregulated activation of human renal fibroblasts,98 rat renal fibroblast function,99 and differentiation of rat renal fibroblasts to myofibroblasts,100 to inhibit renal fibrogenesis.
The potential anti-fibrotic and anti-hypertrophic actions of serelaxin have also been assessed in vivo. Serelaxin treatment reduced ventricular collagen accumulation in mice,95 cardiac fibrosis in mouse models of myocardial infarction-induced IR injury,85 and isoprenaline-induced cardiac injury when compared with the angiotensin-converting enzyme inhibitor enalapril.101 In the latter study, combined administration of enalapril and serelaxin diminished cardiac fibrosis two-fold compared with enalapril alone, and the inhibitory effects of serelaxin were mediated by TGF-β downregulation.101 In ageing rats and in rat models of hypertension and diabetic cardiomyopathy, administration of serelaxin decreased LV and kidney collagen content,52,102,103 fibroblast differentiation in the left ventricle,103 and atrial remodelling,104 as well as cardiac hypertrophy via inhibition of extracellular signal-regulated kinase.105 In addition, porcine relaxin diminished renal fibrosis in a rat model of salt-sensitive hypertension34 and rat relaxin-3 ameliorated cardiac fibrosis in rats with isoproterenol-induced myocardial injury.90
Inhibiting fibrosis and hypertrophy is likely to be beneficial in patients with AHF, and may be associated with reduced fibrosis in organs, including the heart, vessels, kidneys, and liver, as well as the limitation of organ damage and improvement of long-term prognosis.16,34,103 The anti-fibrotic effects of serelaxin may differentiate this agent from current treatments for AHF, such as nitrates, that do not protect end organs from further damage,2,21,22 and are therefore unlikely to inhibit tissue fibrosis.
Serelaxin and stimulation of angiogenesis
Using imaging techniques, significant reductions in perfused small microvessels have been demonstrated in tissues from patients with AHF106 compared with control subjects.107 In addition, the peripheral tissue oxygen extraction rate (an inverse index of tissue microvascular perfusion) is increased in patients with AHF compared with those with chronic stable HF.108 Of interest, this parameter improved with AHF therapy, in parallel with the amelioration of congestion and haemodynamic parameters.108 Therefore, alterations in microcirculation may play an important role in organ damage in AHF.
Angiogenesis can facilitate tissue repair and serelaxin may mediate pro-angiogenic effects, unlike current treatments for AHF, as assessed in vitro and in animal models. Serelaxin has been reported to stimulate NO production from, and migration of, human endothelial progenitor cells in vitro, and to increase the number of circulating human endothelial progenitor cells and stimulate vascularization in mice.109 In addition, studies with H2 relaxin and serelaxin have observed increased expression of the angiogenic cytokine vascular endothelial growth factor (VEGF) in a cyclic adenosine monophosphate (cAMP)-dependent manner,110 stimulation of angiogenesis at ischaemic cardiac sites, and induction of expression of VEGF in rodents and pigs.85,111,112 This induction of angiogenesis could minimize further organ damage and repair injury, particularly of the myocardium, in patients with AHF.16
Serelaxin and effective protection of end-organs
As previously mentioned, serelaxin treatment, in contrast to current therapies, interferes with the systemic and local mechanisms underlying the development of organ damage, and thus, may protect end organs in patients with AHF.3,21,22,75,85
Cardiac protection
Early cardiomyocyte injury and stress and LV dysfunction result from AHF.95,113,114 Cardiomyocyte injury and loss can be detected by measuring troponin T levels, which are elevated in HF,60,61 while increased levels of NT-proBNP indicate ventricular wall stress.115 In patients with AHF, increased levels of troponin T may be detected upon hospital admission and in the 6–12 h following admission.86
In vitro studies, animal models and clinical studies have investigated the cardioprotective properties of serelaxin and porcine relaxin. In vitro, administration of porcine relaxin has been reported to diminish IR injury in isolated reperfused guinea pig hearts, as determined by decreased calcium overload and MDA production,39 in addition to infarct size in a rat model of IR injury.74 Serelaxin treatment also reduced markers of cardiomyocyte damage, including troponin T, creatine kinase-MB, and myoglobin, as well as cardiac injury in pig models of IR injury.76,77
In patients with AHF, serelaxin (30 µg/kg/day for 20 or 48 h) decreased levels of troponin T and NT-proBNP.31,36 Similarly, NT-proBNP levels were diminished following serelaxin treatment (10–100 and 960 µg/kg/day for 24 h) in patients with chronic heart failure (CHF).35 These data imply that the unique mechanism of action of serelaxin may be associated with the preservation of cardiac function in patients with AHF. Although further assessment of this hypothesis is needed, this finding contrasts with the effects of nitrate treatment, which is thought to contribute to cardiac injury by reducing blood pressure and organ perfusion.2,22
In addition to protecting cardiomyocytes from injury and death, serelaxin has been reported to modulate ionic currents in cardiac cells.104,116 Although the translation of these findings into the clinic requires further studies, it is interesting to note that recently, in the RELAX-AHF study, serelaxin treatment reduced mortality from other CV causes and sudden deaths, without impact on HF deaths.117
Renal protection
Renal dysfunction is common in patients with AHF62 and may be exacerbated by nitrate treatment, which can cause hypotension and subsequently, renal hypoperfusion and injury.2 Renal damage and dysfunction is a major predictor of poor outcomes in AHF27 and can be detected via increased levels of serum creatinine, cystatin C, uric acid, and blood urea nitrogen (BUN), as well as reduced estimated glomerular filtration rate (GFR).4,16,31,118 Elevated levels of serum creatinine, cystatin C, uric acid, and BUN have been reported in patients with AHF in the 48 h following hospital admission.31,119
Data from preclinical and clinical studies are available concerning the impact of serelaxin treatment on kidney function and protection. For example, in rats, serelaxin treatment increased GFR and renal blood flow, and protected against renal IR injury and glomerular dysfunction,41,47,51,52,71 whereas porcine relaxin decreased levels of creatinine and BUN in rats subjected to renal IR injury.75
In healthy subjects, serelaxin increased renal blood flow, but did not impact GFR,48 an effect also observed following administration of serelaxin (30 µg/kg/day for 24 h) in patients with CHF when compared with placebo, suggesting that serelaxin treatment reduces the increase in filtration fraction to mediate beneficial renal haemodynamic effects.49
In patients with AHF, serelaxin (30 µg/kg/day for 48 h) reduced levels of cystatin C, uric acid, BUN, and serum creatinine,31 and increased creatinine clearance (30 µg/kg/day for 20 h).36 Decreased serum creatinine was also reported after infusion of serelaxin (10–100 and 960 µg/kg/day for 24 h) in patients with CHF.35 Consequently, serelaxin may prevent worsening renal function, a property which differentiates this novel agent from vasodilator treatment in AHF.
Hepatic protection
Hepatic injury and cell death can occur during AHF,58,120 with elevated markers of hepatic dysfunction, including AST and ALT, which are also predictors of mortality and worsening HF, reported within 48 h of hospitalization for AHF.31,121 Studies have demonstrated that serelaxin may mediate hepatic protection, as observed by diminished IR injury in rat liver72,73 and decreased levels of AST and ALT in patients with AHF following serelaxin treatment (30 µg/kg/day for 48 h).31
Vascular and other organ protection
Damage to the vasculature and other organs may occur in patients with AHF57,122 and nitrate therapy may increase endothelial dysfunction further in these patients via increased oxidative stress.82
Organ preservation and vasoprotective properties may distinguish serelaxin from classical vasodilators for the treatment of AHF and improve outcomes in these patients.16,123 For instance, treatment with serelaxin has been associated with improved endothelial function in rat aortic endothelial cells57 and decreases in vessel size, wall thickening, cross-sectional area, and collagen content in spontaneously hypertensive rats,124 while porcine relaxin has provided endothelial protection in a rat model of splanchnic IR injury.70 Furthermore, studies in the rat brain have shown that serelaxin treatment reduced ischaemic cell damage in brain slices, as well as infarct size in vivo, determined 4 h following ischaemia.125–127 In addition, administration of serelaxin has resulted in diminished IR injury in rat lungs.128,129
Conclusions and perspectives
AHF poses a significant burden to patients and healthcare systems. The precise mechanisms underlying this condition are poorly understood, but it is clear that a variety of pathophysiological processes are involved, which result in both haemodynamic abnormalities and end-organ damage. Current therapies available for the treatment of AHF moderately address the haemodynamic changes associated with the short-term effects of this condition, to alleviate congestion. However, no currently approved agent has demonstrated true benefit on the long-term outcomes of AHF. As such, there is an unmet medical need in AHF; a need for therapies that address both the short- and long-term effects of this condition.
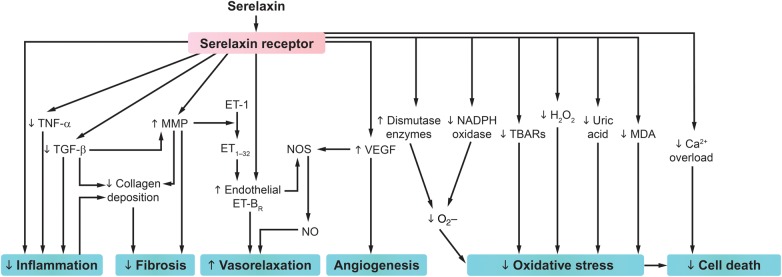
Preclinical and clinical data have highlighted serelaxin as a promising treatment of both the short- and long-term consequences of AHF. In contrast to classical vasodilators, serelaxin may act at the vascular, cardiac, and renal level to improve haemodynamics and effectively relieve congestion. Moreover, available data suggest that serelaxin may provide organ protection via inhibition of inflammation, oxidative stress, cell death, and tissue fibrosis, and induction of angiogenesis (Figure 5),24,31,33,71–74,76,77,80,90 to improve the long-term prognosis of these patients, as observed in clinical trials to date.
Figure 5.
Serelaxin activates multiple pathways to improve haemodynamics and may protect cells and organs via anti-apoptotic/necrotic, anti-inflammatory, anti-fibrotic, antioxidant, and pro-angiogenic effects.24,31,33,71–74,76,77,80,90 ET, endothelin; ET-BR, endothelin receptor type B; MDA, malondialdehyde; MMP, metalloproteinase; NADPH, nicotinamide adenine dinucleotide phosphate-oxidase; NO, nitric oxide; NOS, nitric oxide synthase; TBARs, thiobarbituric acid-reactive substance; TGF-β, transforming growth factor β; TNF-α, tumour necrosis factor α; VEGF, vascular endothelial growth factor. Adapted from Teichman et al.2,4 Reproduced under the terms of the Creative Commons Attribution Non-commercial License for open access.
Additional clinical data are required to confirm the potential benefits of serelaxin for the treatment of AHF. A second phase III study, RELAX-AHF 2, began in September 2013 and will further assess the effects of serelaxin on CV mortality in patients with AHF.32 Future experimental research efforts should aim to establish animal models of AHF, in which the mechanisms underlying the efficacy of serelaxin for the treatment of this condition could be studied. Meanwhile, further preclinical studies are required to investigate the pharmacokinetic and pharmacodynamic properties of serelaxin in this patient population.
Authors' Contributions
J.D. and L.R. designed, jointly reviewed, and revised the initial draft and subsequent versions of the manuscript, and both agreed on the final version submitted for publication.
Funding
The writing/editorial support was funded by Novartis Pharma AG, Basel, Switzerland. The sponsor reviewed the initial draft and subsequent versions of the manuscript for own data accuracy and for proprietary evaluation. Funding to pay the Open Access publication charges for this article was provided by Novartis Pharma AG, Basel, Switzerland.
Conflict of interest: J.D. has served as an advisor and as a speaker for Novartis, Merck, Sharp and Dohme, and Abbvie. L.R. has served as an advisor and as a speaker for Novartis.
Acknowledgements
The authors thank Hannah Birchby and Rebecca Douglas (CircleScience, an Ashfield Company, part of UDG Healthcare plc), for providing writing/editorial assistance, which was funded by Novartis Pharma AG, Basel, Switzerland.
References
- 1.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, Strömberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K; ESC Committee for Practice Guidelines (CPG) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J 2008;29:2388–2442. [DOI] [PubMed] [Google Scholar]
- 2.Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol 2009;53:557–573. [DOI] [PubMed] [Google Scholar]
- 3.Gheorghiade M, De LL, Fonarow GC, Filippatos G, Metra M, Francis GS. Pathophysiologic targets in the early phase of acute heart failure syndromes. Am J Cardiol 2005;96:11G–17G. [DOI] [PubMed] [Google Scholar]
- 4.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A; ESC Committee for Practice Guidelines ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 5.Cotter G, Felker GM, Adams KF, Milo-Cotter O, O'Connor CM. The pathophysiology of acute heart failure—is it all about fluid accumulation? Am Heart J 2008;155:9–18. [DOI] [PubMed] [Google Scholar]
- 6.Biolo A, Fisch M, Balog J, Chao T, Schulze PC, Ooi H, Siwik D, Colucci WS. Episodes of acute heart failure syndrome are associated with increased levels of troponin and extracellular matrix markers. Circ Heart Fail 2010;3:44–50. [DOI] [PubMed] [Google Scholar]
- 7.Bött-Flugel L, Weig HJ, Uhlein H, Nabauer M, Laugwitz KL, Seyfarth M. Quantitative analysis of apoptotic markers in human end-stage heart failure. Eur J Heart Fail 2008;10:129–132. [DOI] [PubMed] [Google Scholar]
- 8.Cotter G, Metra M, Milo-Cotter O, Dittrich HC, Gheorghiade M. Fluid overload in acute heart failure—re-distribution and other mechanisms beyond fluid accumulation. Eur J Heart Fail 2008;10:165–169. [DOI] [PubMed] [Google Scholar]
- 9.Feng Q, Wang S. Cardiomyocytic apoptosis and heart failure. J Geriatr Cardiol 2008;5:1–6. [Google Scholar]
- 10.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW; American College of Cardiology Foundation; American Heart Association 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol 2009;53:e1–e90. [DOI] [PubMed] [Google Scholar]
- 11.Oikonomou E, Tousoulis D, Siasos G, Zaromitidou M, Papavassiliou AG, Stefanadis C. The role of inflammation in heart failure: new therapeutic approaches. Hellenic J Cardiol 2011;52:30–40. [PubMed] [Google Scholar]
- 12.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol 2011;301:H2181–H2190. [DOI] [PubMed] [Google Scholar]
- 13.Gheorghiade M, Filippatos G, De LL, Burnett J. Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. Am J Med 2006;119:S3–S10. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg RJ, Spencer FA, Szklo-Coxe M, Tisminetzky M, Yarzebski J, Lessard D, Gore JM, Gaasch W. Symptom presentation in patients hospitalized with acute heart failure. Clin Cardiol 2010;33:E73–E80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams KF Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP; ADHERE Scientific Advisory Committee and Investigators Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 2005;149:209–216. [DOI] [PubMed] [Google Scholar]
- 16.Díez J. Serelaxin: a novel therapy for acute heart failure with a range of hemodynamic and non-hemodynamic actions. Am J Cardiovasc Drugs 2014;14:275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konecke LL. Clinical trial of bumetanide versus furosemide in patients with congestive heart failure. J Clin Pharmacol 1981;21:688–690. [DOI] [PubMed] [Google Scholar]
- 18.Bolognese L, Sarasso G, Rognoni G, Makmur J, Fornaro G, Perucca A, Rossi P. Sustained beneficial hemodynamic effects of low transdermal nitroglycerin doses compared with placebo in patients with congestive heart failure. Clin Cardiol 1988;11:79–85. [DOI] [PubMed] [Google Scholar]
- 19.Stroobandt R, Dodion L, Kesteloot H. Clinical efficacy of torasemide, a new diuretic agent, in patients with acute heart failure: a double blind comparison with furosemide. Arch Int Pharmacodyn Ther 1982;260:151–158. [PubMed] [Google Scholar]
- 20.Gheorghiade M, Zannad F, Sopko G, Klein L, Pina IL, Konstam MA, Massie BM, Roland E, Targum S, Collins SP, Filippatos G, Tavazzi L; International Working Group on Acute Heart Failure Syndromes Acute heart failure syndromes: current state and framework for future research. Circulation 2005;112:3958–3968. [DOI] [PubMed] [Google Scholar]
- 21.Stough WG, O'Connor CM, Gheorghiade M. Overview of current noninodilator therapies for acute heart failure syndromes. Am J Cardiol 2005;96:41G–46G. [DOI] [PubMed] [Google Scholar]
- 22.Metra M, Teerlink JR, Voors AA, Felker GM, Milo-Cotter O, Weatherley B, Dittrich H, Cotter G. Vasodilators in the treatment of acute heart failure: what we know, what we don't. Heart Fail Rev 2009;14:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sherwood OD. Relaxin's physiological roles and other diverse actions. Endocr Rev 2004;25:205–234. [DOI] [PubMed] [Google Scholar]
- 24.Teichman SL, Unemori E, Teerlink JR, Cotter G, Metra M. Relaxin: review of biology and potential role in treating heart failure. Curr Heart Fail Rep 2010;7:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grossman J, Frishman WH. Relaxin: a new approach for the treatment of acute congestive heart failure. Cardiol Rev 2010;18:305–312. [DOI] [PubMed] [Google Scholar]
- 26.Sarwar M, Samuel CS, Bathgate RA, Stewart DR, Summers RJ. Serelaxin-mediated signal transduction in human vascular cells: bell-shaped concentration-response curves reflect differential coupling to G proteins. Br J Pharmacol 2015;172:1005–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teichman SL, Unemori E, Dschietzig T, Conrad K, Voors AA, Teerlink JR, Felker GM, Metra M, Cotter G. Relaxin, a pleiotropic vasodilator for the treatment of heart failure. Heart Fail Rev 2009;14:321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bathgate RA, Halls ML, van der Westhuizen ET, Callander GE, Kocan M, Summers RJ. Relaxin family peptides and their receptors. Physiol Rev 2013;93:405–480. [DOI] [PubMed] [Google Scholar]
- 29.Teerlink JR, Metra M, Felker GM, Ponikowski P, Voors AA, Weatherley BD, Marmor A, Katz A, Grzybowski J, Unemori E, Teichman SL, Cotter G. Relaxin for the treatment of patients with acute heart failure (Pre-RELAX-AHF): a multicentre, randomised, placebo-controlled, parallel-group, dose-finding phase IIb study. Lancet 2009;373:1429–1439. [DOI] [PubMed] [Google Scholar]
- 30.Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF Jr, Dorobantu MI, Grinfeld LR, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin TM, Metra M; RELAXin in Acute Heart Failure (RELAX-AHF) Investigators Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet 2013;381:29–39. [DOI] [PubMed] [Google Scholar]
- 31.Metra M, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF Jr, Dorobantu MI, Grinfeld L, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Prescott MF, Edwards C, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin T, Teerlink JR; RELAX-AHF Investigators Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the Relaxin in Acute Heart Failure (RELAX-AHF) development program: correlation with outcomes. J Am Coll Cardiol 2013;61:196–206. [DOI] [PubMed] [Google Scholar]
- 32.Clinicaltrials.gov. NCT01870778. Efficacy, safety and tolerability of serelaxin when added to standard therapy in AHF (RELAX-AHF-2). https://clinicaltrialsgov/ct2/show/NCT01870778 (19 May 2015).
- 33.Sasser JM, Molnar M, Baylis C. Relaxin ameliorates hypertension and increases nitric oxide metabolite excretion in angiotensin II but not N(omega)-nitro-l-arginine methyl ester hypertensive rats. Hypertension 2011;58:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshida T, Kumagai H, Suzuki A, Kobayashi N, Ohkawa S, Odamaki M, Kohsaka T, Yamamoto T, Ikegaya N. Relaxin ameliorates salt-sensitive hypertension and renal fibrosis. Nephrol Dial Transplant 2012;27:2190–2197. [DOI] [PubMed] [Google Scholar]
- 35.Dschietzig T, Teichman S, Unemori E, Wood S, Boehmer J, Richter C, Baumann G, Stangl K. Intravenous recombinant human relaxin in compensated heart failure: a safety, tolerability, and pharmacodynamic trial. J Card Fail 2009;15:182–190. [DOI] [PubMed] [Google Scholar]
- 36.Ponikowski P, Mitrovic V, Ruda M, Fernandez A, Voors AA, Vishnevsky A, Cotter G, Milo O, Laessing U, Zhang Y, Dahlke M, Zymlinski R, Metra M. A randomized, double-blind, placebo-controlled, multicentre study to assess haemodynamic effects of serelaxin in patients with acute heart failure. Eur Heart J 2014;35:431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massicotte G, Parent A, St-Louis J. Blunted responses to vasoconstrictors in mesenteric vasculature but not in portal vein of spontaneously hypertensive rats treated with relaxin. Proc Soc Exp Biol Med 1989;190:254–259. [DOI] [PubMed] [Google Scholar]
- 38.Fisher C, MacLean M, Morecroft I, Seed A, Johnston F, Hillier C, McMurray J. Is the pregnancy hormone relaxin also a vasodilator peptide secreted by the heart? Circulation 2002;106:292–295. [DOI] [PubMed] [Google Scholar]
- 39.Masini E, Bani D, Bello MG, Bigazzi M, Mannaioni PF, Sacchi TB. Relaxin counteracts myocardial damage induced by ischemia-reperfusion in isolated guinea pig hearts: evidence for an involvement of nitric oxide. Endocrinology 1997;138:4713–4720. [DOI] [PubMed] [Google Scholar]
- 40.Debrah DO, Debrah JE, Haney JL, McGuane JT, Sacks MS, Conrad KP, Shroff SG. Relaxin regulates vascular wall remodeling and passive mechanical properties in mice. J Appl Physiol 2011;111:260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Danielson LA, Sherwood OD, Conrad KP. Relaxin is a potent renal vasodilator in conscious rats. J Clin Invest 1999;103:525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jelinic M, Leo CH, Post Uiterweer ED, Sandow SL, Gooi JH, Wlodek ME, Conrad KP, Parkington H, Tare M, Parry LJ. Localization of relaxin receptors in arteries and veins, and region-specific increases in compliance and bradykinin-mediated relaxation after in vivo serelaxin treatment. FASEB J 2014;28:275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leo CH, Jelinic M, Parkington HC, Tare M, Parry LJ. Acute intravenous injection of serelaxin (recombinant human relaxin-2) causes rapid and sustained bradykinin-mediated vasorelaxation. J Am Heart Assoc 2014;3:e000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conrad KP, Debrah DO, Novak J, Danielson LA, Shroff SG. Relaxin modifies systemic arterial resistance and compliance in conscious, nonpregnant rats. Endocrinology 2004;145:3289–3296. [DOI] [PubMed] [Google Scholar]
- 45.Debrah DO, Conrad KP, Jeyabalan A, Danielson LA, Shroff SG. Relaxin increases cardiac output and reduces systemic arterial load in hypertensive rats. Hypertension 2005;46:745–750. [DOI] [PubMed] [Google Scholar]
- 46.Debrah DO, Conrad KP, Novak J, Danielson LA, Shroff SG. Recombinant human relaxin (rhRLX) modifies systemic arterial properties in conscious rats irrespective of gender, but in a biphasic fashion. Ann N Y Acad Sci 2005;1041:155–162. [DOI] [PubMed] [Google Scholar]
- 47.Bogzil AH, Eardley R, Ashton N. Relaxin-induced changes in renal sodium excretion in the anesthetized male rat. Am J Physiol Regul Integr Comp Physiol 2005;288:R322–R328. [DOI] [PubMed] [Google Scholar]
- 48.Smith MC, Danielson LA, Conrad KP, Davison JM. Influence of recombinant human relaxin on renal hemodynamics in healthy volunteers. J Am Soc Nephrol 2006;17:3192–3197. [DOI] [PubMed] [Google Scholar]
- 49.Voors AA, Dahlke M, Meyer S, Stepinska J, Gottlieb SS, Jones A, Zhang Y, Laurent D, Slart RH, Navis GJ. Renal hemodynamic effects of serelaxin in patients with chronic heart failure: a randomized, placebo-controlled study. Circ Heart Fail 2014;7:994–1002. [DOI] [PubMed] [Google Scholar]
- 50.Voors AA, Davison BA, Teerlink JR, Felker GM, Cotter G, Filippatos G, Greenberg BH, Pang PS, Levin B, Hua TA, Severin T, Ponikowski P, Metra M; RELAX-AHF Investigators Diuretic response in patients with acute decompensated heart failure: characteristics and clinical outcome-an analysis from RELAX-AHF. Eur J Heart Fail 2014;16:1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Danielson LA, Kercher LJ, Conrad KP. Impact of gender and endothelin on renal vasodilation and hyperfiltration induced by relaxin in conscious rats. Am J Physiol Regul Integr Comp Physiol 2000;279:R1298–R1304. [DOI] [PubMed] [Google Scholar]
- 52.Danielson LA, Welford A, Harris A. Relaxin improves renal function and histology in aging Munich Wistar rats. J Am Soc Nephrol 2006;17:1325–1333. [DOI] [PubMed] [Google Scholar]
- 53.Conrad KP. Unveiling the vasodilatory actions and mechanisms of relaxin. Hypertension 2010;56:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Du XJ, Hewitson TD, Nguyen MN, Samuel CS. Therapeutic effects of serelaxin in acute heart failure. Circ J 2014;78:542–552. [DOI] [PubMed] [Google Scholar]
- 55.Hollenberg SM. Vasodilators in acute heart failure. Heart Fail Rev 2007;12:143–147. [DOI] [PubMed] [Google Scholar]
- 56.Kemp CD, Conte JV. The pathophysiology of heart failure. Cardiovasc Pathol 2012;21:365–371. [DOI] [PubMed] [Google Scholar]
- 57.Dschietzig T, Brecht A, Bartsch C, Baumann G, Stangl K, Alexiou K. Relaxin improves TNF-alpha-induced endothelial dysfunction: the role of glucocorticoid receptor and phosphatidylinositol 3-kinase signalling. Cardiovasc Res 2012;95:97–107. [DOI] [PubMed] [Google Scholar]
- 58.Giallourakis CC, Rosenberg PM, Friedman LS. The liver in heart failure. Clin Liver Dis 2002;6:947–967. [DOI] [PubMed] [Google Scholar]
- 59.Missov E, Calzolari C, Pau B. Circulating cardiac troponin I in severe congestive heart failure. Circulation 1997;96:2953–2958. [DOI] [PubMed] [Google Scholar]
- 60.Januzzi JL Jr, Filippatos G, Nieminen M, Gheorghiade M. Troponin elevation in patients with heart failure: on behalf of the third Universal Definition of Myocardial Infarction Global Task Force: Heart Failure Section. Eur Heart J 2012;33:2265–2271. [DOI] [PubMed] [Google Scholar]
- 61.Kociol RD, Pang PS, Gheorghiade M, Fonarow GC, O'Connor CM, Felker GM. Troponin elevation in heart failure prevalence, mechanisms, and clinical implications. J Am Coll Cardiol 2010;56:1071–1078. [DOI] [PubMed] [Google Scholar]
- 62.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol 2008;52:1527–1539. [DOI] [PubMed] [Google Scholar]
- 63.Ronco C, Cicoira M, McCullough PA. Cardiorenal syndrome type 1: pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J Am Coll Cardiol 2012;60:1031–1042. [DOI] [PubMed] [Google Scholar]
- 64.Santulli G. Adrenal signaling in heart failure: something more than a distant ship's smoke on the horizon. Hypertension 2014;63:215–216. [DOI] [PubMed] [Google Scholar]
- 65.Du XJ, Bathgate RA, Samuel CS, Dart AM, Summers RJ. Cardiovascular effects of relaxin: from basic science to clinical therapy. Nat Rev Cardiol 2010;7:48–58. [DOI] [PubMed] [Google Scholar]
- 66.Chrysohoou C, Pitsavos C, Barbetseas J, Kotroyiannis I, Brili S, Vasiliadou K, Papadimitriou L, Stefanadis C. Chronic systemic inflammation accompanies impaired ventricular diastolic function, detected by Doppler imaging, in patients with newly diagnosed systolic heart failure (Hellenic Heart Failure Study). Heart Vessels 2009;24:22–26. [DOI] [PubMed] [Google Scholar]
- 67.Brecht A, Bartsch C, Baumann G, Stangl K, Dschietzig T. Relaxin inhibits early steps in vascular inflammation. Regul Pept 2011;166:76–82. [DOI] [PubMed] [Google Scholar]
- 68.Masini E, Nistri S, Vannacci A, Bani ST, Novelli A, Bani D. Relaxin inhibits the activation of human neutrophils: involvement of the nitric oxide pathway. Endocrinology 2004;145:1106–1112. [DOI] [PubMed] [Google Scholar]
- 69.Bani D, Baronti R, Vannacci A, Bigazzi M, Sacchi TB, Mannaioni PF, Masini E. Inhibitory effects of relaxin on human basophils activated by stimulation of the Fc epsilon receptor. The role of nitric oxide. Int Immunopharmacol 2002;2:1195–1204. [DOI] [PubMed] [Google Scholar]
- 70.Masini E, Cuzzocrea S, Mazzon E, Muia C, Vannacci A, Fabrizi F, Bani D. Protective effects of relaxin in ischemia/reperfusion-induced intestinal injury due to splanchnic artery occlusion. Br J Pharmacol 2006;148:1124–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Collino M, Rogazzo M, Pini A, Benetti E, Rosa AC, Chiazza F, Fantozzi R, Bani D, Masini E. Acute treatment with relaxin protects the kidney against ischaemia/reperfusion injury. J Cell Mol Med 2013;17:1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boehnert MU, Hilbig H, Armbruster FP. Relaxin as an additional protective substance in preserving and reperfusion solution for liver transplantation, shown in a model of isolated perfused rat liver. Ann N Y Acad Sci 2005;1041:434–440. [DOI] [PubMed] [Google Scholar]
- 73.Boehnert MU, Armbruster FP, Hilbig H. Relaxin as a protective substance in the preserving solution for liver transplantation: spectrophotometric in vivo imaging of local oxygen supply in an isolated perfused rat liver model. Ann N Y Acad Sci 2009;1160:320–321. [DOI] [PubMed] [Google Scholar]
- 74.Bani D, Masini E, Bello MG, Bigazzi M, Sacchi TB. Relaxin protects against myocardial injury caused by ischemia and reperfusion in rat heart. Am J Pathol 1998;152:1367–1376. [PMC free article] [PubMed] [Google Scholar]
- 75.Yoshida T, Kumagai H, Kohsaka T, Ikegaya N. Relaxin protects against renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 2013;305:F1169–F1176. [DOI] [PubMed] [Google Scholar]
- 76.Perna AM, Masini E, Nistri S, Briganti V, Chiappini L, Stefano P, Bigazzi M, Pieroni C, Bani Sacchi T, Bani D. Novel drug development opportunity for relaxin in acute myocardial infarction: evidences from a swine model. FASEB J 2005;19:1525–1527. [DOI] [PubMed] [Google Scholar]
- 77.Nistri S, Cinci L, Perna AM, Masini E, Mastroianni R, Bani D. Relaxin induces mast cell inhibition and reduces ventricular arrhythmias in a swine model of acute myocardial infarction. Pharmacol Res 2008;57:43–48. [DOI] [PubMed] [Google Scholar]
- 78.van Kimmenade RR, Januzzi JL Jr. Emerging biomarkers in heart failure. Clin Chem 2012;58:127–138. [DOI] [PubMed] [Google Scholar]
- 79.Santulli G, Xie W, Reiken SR, Marks AR. Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci USA 2015;112:11389–11394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sasser JM, Cunningham MW Jr, Baylis C. Serelaxin reduces oxidative stress and asymmetric dimethylarginine in angiotensin II induced hypertension. Am J Physiol Renal Physiol 2014;307:F1355–F1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 2007;87:315–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Munzel T, Daiber A, Gori T. Nitrate therapy: new aspects concerning molecular action and tolerance. Circulation 2011;123:2132–2144. [DOI] [PubMed] [Google Scholar]
- 83.Gori T, Parker JD. Nitrate tolerance: a unifying hypothesis. Circulation 2002;106:2510–2513. [DOI] [PubMed] [Google Scholar]
- 84.Daiber A, Mulsch A, Hink U, Mollnau H, Warnholtz A, Oelze M, Münzel T. The oxidative stress concept of nitrate tolerance and the antioxidant properties of hydralazine. Am J Cardiol 2005;96:25i–36i. [DOI] [PubMed] [Google Scholar]
- 85.Samuel CS, Cendrawan S, Gao XM, Ming Z, Zhao C, Kiriazis H, Xu Q, Tregear GW, Bathgate RA, Du XJ. Relaxin remodels fibrotic healing following myocardial infarction. Lab Invest 2011;91:675–690. [DOI] [PubMed] [Google Scholar]
- 86.Metra M, Bettari L, Pagani F, Lazzarini V, Lombardi C, Carubelli V, Bonetti G, Bugatti S, Parrinello G, Caimi L, Felker GM, Dei Cas L. Troponin T levels in patients with acute heart failure: clinical and prognostic significance of their detection and release during hospitalisation. Clin Res Cardiol 2012;101:663–672. [DOI] [PubMed] [Google Scholar]
- 87.Moore XL, Tan SL, Lo CY, Fang L, Su YD, Gao XM, Woodcock EA, Summers RJ, Tregear GW, Bathgate RA, Du XJ. Relaxin antagonizes hypertrophy and apoptosis in neonatal rat cardiomyocytes. Endocrinology 2007;148:1582–1589. [DOI] [PubMed] [Google Scholar]
- 88.Zhang X, Ma X, Zhao M, Zhang B, Chi J, Liu W, Chen W, Fu Y, Liu Y, Yin X. H2 and H3 relaxin inhibit high glucose-induced apoptosis in neonatal rat ventricular myocytes. Biochimie 2015;108:59–67. [DOI] [PubMed] [Google Scholar]
- 89.Boccalini G, Sassoli C, Formigli L, Bani D, Nistri S. Relaxin protects cardiac muscle cells from hypoxia/reoxygenation injury: involvement of the Notch-1 pathway. FASEB J 2015;29:239–249. [DOI] [PubMed] [Google Scholar]
- 90.Zhang J, Qi YF, Geng B, Pan CS, Zhao J, Chen L, Yang J, Chang JK, Tang CS. Effect of relaxin on myocardial ischemia injury induced by isoproterenol. Peptides 2005;26:1632–1639. [DOI] [PubMed] [Google Scholar]
- 91.Wronska A, Kurkowska-Jastrzebska I, Santulli G. Application of microRNAs in diagnosis and treatment of cardiovascular disease. Acta Physiol 2015;213:60–83. [DOI] [PubMed] [Google Scholar]
- 92.Unemori EN, Amento EP. Relaxin modulates synthesis and secretion of procollagenase and collagen by human dermal fibroblasts. J Biol Chem 1990;265:10681–10685. [PubMed] [Google Scholar]
- 93.Williams EJ, Benyon RC, Trim N, Hadwin R, Grove BH, Arthur MJ, Unemori EN, Iredale JP. Relaxin inhibits effective collagen deposition by cultured hepatic stellate cells and decreases rat liver fibrosis in vivo. Gut 2001;49:577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chow BS, Chew EG, Zhao C, Bathgate RA, Hewitson TD, Samuel CS. Relaxin signals through a RXFP1-pERK-nNOS-NO-cGMP-dependent pathway to up-regulate matrix metalloproteinases: the additional involvement of iNOS. PLoS ONE 2012;7:e42714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Samuel CS, Unemori EN, Mookerjee I, Bathgate RA, Layfield SL, Mak J, Tregear GW, Du XJ. Relaxin modulates cardiac fibroblast proliferation, differentiation, and collagen production and reverses cardiac fibrosis in vivo. Endocrinology 2004;145:4125–4133. [DOI] [PubMed] [Google Scholar]
- 96.Mookerjee I, Unemori EN, Du XJ, Tregear GW, Samuel CS. Relaxin modulates fibroblast function, collagen production, and matrix metalloproteinase-2 expression by cardiac fibroblasts. Ann N Y Acad Sci 2005;1041:190–193. [DOI] [PubMed] [Google Scholar]
- 97.Unemori EN, Bauer EA, Amento EP. Relaxin alone and in conjunction with interferon-gamma decreases collagen synthesis by cultured human scleroderma fibroblasts. J Invest Dermatol 1992;99:337–342. [DOI] [PubMed] [Google Scholar]
- 98.Heeg MH, Koziolek MJ, Vasko R, Schaefer L, Sharma K, Müller GA, Strutz F. The antifibrotic effects of relaxin in human renal fibroblasts are mediated in part by inhibition of the Smad2 pathway. Kidney Int 2005;68:96–109. [DOI] [PubMed] [Google Scholar]
- 99.Masterson R, Hewitson TD, Kelynack K, Martic M, Parry L, Bathgate R, Darby I, Becker G. Relaxin down-regulates renal fibroblast function and promotes matrix remodelling in vitro. Nephrol Dial Transplant 2004;19:544–552. [DOI] [PubMed] [Google Scholar]
- 100.Mookerjee I, Hewitson TD, Halls ML, Summers RJ, Mathai ML, Bathgate RA, Tregear GW, Samuel CS. Relaxin inhibits renal myofibroblast differentiation via RXFP1, the nitric oxide pathway, and Smad2. FASEB J 2009;23:1219–1229. [DOI] [PubMed] [Google Scholar]
- 101.Samuel CS, Bodaragama H, Chew JY, Widdop RE, Royce SG, Hewitson TD. Serelaxin is a more efficacious antifibrotic than enalapril in an experimental model of heart disease. Hypertension 2014;64:315–322. [DOI] [PubMed] [Google Scholar]
- 102.Samuel CS, Hewitson TD, Zhang Y, Kelly DJ. Relaxin ameliorates fibrosis in experimental diabetic cardiomyopathy. Endocrinology 2008;149:3286–3293. [DOI] [PubMed] [Google Scholar]
- 103.Lekgabe ED, Kiriazis H, Zhao C, Xu Q, Moore XL, Su Y, Bathgate RA, Du XJ, Samuel CS. Relaxin reverses cardiac and renal fibrosis in spontaneously hypertensive rats. Hypertension 2005;46:412–418. [DOI] [PubMed] [Google Scholar]
- 104.Parikh A, Patel D, McTiernan CF, Xiang W, Haney J, Yang L, Lin B, Kaplan AD, Bett GC, Rasmusson RL, Shroff SG, Schwartzman D, Salama G. Relaxin suppresses atrial fibrillation by reversing fibrosis and myocyte hypertrophy and increasing conduction velocity and sodium current in spontaneously hypertensive rat hearts. Circ Res 2013;113:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dschietzig T, Bartsch C, Kinkel T, Baumann G, Stangl K. Myocardial relaxin counteracts hypertrophy in hypertensive rats. Ann N Y Acad Sci 2005;1041:441–443. [DOI] [PubMed] [Google Scholar]
- 106.Lauten A, Ferrari M, Goebel B, Rademacher W, Schumm J, Uth O, Kiehntopf M, Figulla HR, Jung C. Microvascular tissue perfusion is impaired in acutely decompensated heart failure and improves following standard treatment. Eur J Heart Fail 2011;13:711–717. [DOI] [PubMed] [Google Scholar]
- 107.De Backer D, Creteur J, Dubois MJ, Sakr Y, Vincent JL. Microvascular alterations in patients with acute severe heart failure and cardiogenic shock. Am Heart J 2004;147:91–99. [DOI] [PubMed] [Google Scholar]
- 108.Hogan CJ, Ward KR, Kontos MC, Thacker LR, Pittman R. Peripheral tissue oxygenation improves during ED treatment of acute heart failure. Am J Emerg Med 2012;30:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Segal MS, Sautina L, Li S, Diao Y, Agoulnik AI, Kielczewski J, McGuane JT, Grant MB, Conrad KP. Relaxin increases human endothelial progenitor cell NO and migration and vasculogenesis in mice. Blood 2012;119:629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Unemori EN, Erikson ME, Rocco SE, Sutherland KM, Parsell DA, Mak J, Grove BH. Relaxin stimulates expression of vascular endothelial growth factor in normal human endometrial cells in vitro and is associated with menometrorrhagia in women. Hum Reprod 1999;14:800–806. [DOI] [PubMed] [Google Scholar]
- 111.Formigli L, Perna AM, Meacci E, Cinci L, Margheri M, Nistri S, Tani A, Silvertown J, Orlandini G, Porciani C, Zecchi-Orlandini S, Medin J, Bani D. Paracrine effects of transplanted myoblasts and relaxin on post-infarction heart remodelling. J Cell Mol Med 2007;11:1087–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Unemori EN, Lewis M, Constant J, Arnold G, Grove BH, Normand J, Deshpande U, Salles A, Pickford LB, Erikson ME, Hunt TK, Huang X. Relaxin induces vascular endothelial growth factor expression and angiogenesis selectively at wound sites. Wound Repair Regen 2000;8:361–370. [DOI] [PubMed] [Google Scholar]
- 113.González A, Ravassa S, Beaumont J, Lopez B, Diez J. New targets to treat the structural remodeling of the myocardium. J Am Coll Cardiol 2011;58:1833–1843. [DOI] [PubMed] [Google Scholar]
- 114.Kandasamy AD, Chow AK, Ali MA, Schulz R. Matrix metalloproteinase-2 and myocardial oxidative stress injury: beyond the matrix. Cardiovasc Res 2010;85:413–423. [DOI] [PubMed] [Google Scholar]
- 115.Hall C. NT-ProBNP: the mechanism behind the marker. J Card Fail 2005;11:S81–S83. [DOI] [PubMed] [Google Scholar]
- 116.Han X, Habuchi Y, Giles WR. Relaxin increases heart rate by modulating calcium current in cardiac pacemaker cells. Circ Res 1994;74:537–541. [DOI] [PubMed] [Google Scholar]
- 117.Felker GM, Teerlink JR, Butler J, Hernandez AF, Miller AB, Cotter G, Davison BA, Filippatos G, Greenberg BH, Ponikowski P, Voors AA, Hua TA, Severin TM, Unemori E, Metra M. Effect of serelaxin on mode of death in acute heart failure: results from the RELAX-AHF study. J Am Coll Cardiol 2014;64:1591–1598. [DOI] [PubMed] [Google Scholar]
- 118.Metra M, Cotter G, Gheorghiade M, Dei CL, Voors AA. The role of the kidney in heart failure. Eur Heart J 2012;33:2135–2142. [DOI] [PubMed] [Google Scholar]
- 119.Lassus JP, Nieminen MS, Peuhkurinen K, Pulkki K, Siirila-Waris K, Sund R, Harjola VP; FINN-AKVA study group Markers of renal function and acute kidney injury in acute heart failure: definitions and impact on outcomes of the cardiorenal syndrome. Eur Heart J 2010;31:2791–2798. [DOI] [PubMed] [Google Scholar]
- 120.Nikolaou M, Parissis J, Yilmaz MB, Seronde MF, Kivikko M, Laribi S, Paugam-Burtz C, Cai D, Pohjanjousi P, Laterre PF, Deye N, Poder P, Cohen-Solal A, Mebazaa A. Liver function abnormalities, clinical profile, and outcome in acute decompensated heart failure. Eur Heart J 2013;34:742–749. [DOI] [PubMed] [Google Scholar]
- 121.van Deursen V, Edwards C, Cotter G, Davison BA, Damman K, Teerlink JR, Metra M, Felker GM, Ponikowski P, Unemori E, Severin T, Voors AA. Liver function, in-hospital, and post-discharge clinical outcome in patients with acute heart failure-results from the relaxin for the treatment of patients with acute heart failure study. J Card Fail 2014;20:407–413. [DOI] [PubMed] [Google Scholar]
- 122.de la Torre JC. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc Psychiatry Neurol 2012;2012:367516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bani D. Relaxin as a natural agent for vascular health. Vasc Health Risk Manag 2008;4:515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xu Q, Chakravorty A, Bathgate RA, Dart AM, Du XJ. Relaxin therapy reverses large artery remodeling and improves arterial compliance in senescent spontaneously hypertensive rats. Hypertension 2010;55:1260–1266. [DOI] [PubMed] [Google Scholar]
- 125.Wilson BC, Milne P, Saleh TM. Relaxin pretreatment decreases infarct size in male rats after middle cerebral artery occlusion. Ann N Y Acad Sci 2005;1041:223–228. [DOI] [PubMed] [Google Scholar]
- 126.Wilson BC, Connell B, Saleh TM. Relaxin-induced reduction of infarct size in male rats receiving MCAO is dependent on nitric oxide synthesis and not estrogenic mechanisms. Neurosci Lett 2006;393:160–164. [DOI] [PubMed] [Google Scholar]
- 127.Wilson BC, Rappaport R. An in vitro study of the protective effect of relaxin on brain tissue under ischemic stress. Ann N Y Acad Sci 2009;1160:265–268. [DOI] [PubMed] [Google Scholar]
- 128.Alexiou K, Matschke K, Westphal A, Stangl K, Dschietzig T. Relaxin is a candidate drug for lung preservation: relaxin-induced protection of rat lungs from ischemia-reperfusion injury. J Heart Lung Transplant 2010;29:454–460. [DOI] [PubMed] [Google Scholar]
- 129.Alexiou K, Wilbring M, Matschke K, Dschietzig T. Relaxin protects rat lungs from ischemia-reperfusion injury via inducible NO synthase: role of ERK-1/2, PI3 K, and forkhead transcription factor FKHRL1. PLoS ONE 2013;8:e75592. [DOI] [PMC free article] [PubMed] [Google Scholar]