Abstract
Aims
Cenderitide is a novel dual natriuretic peptide (NP) receptor chimeric peptide activator, which targets the particulate guanylyl cyclase B (pGC-B) receptor and pGC-A unlike native NPs. Cenderitide was engineered to retain the anti-fibrotic properties of C-type natriuretic peptide (CNP)/pGC-B with renal-enhancing actions facilitated by fusion to the carboxyl terminus of Dendroaspis NP (DNP), a pGC-A agonist, to CNP. Here, we address significance of the DNP carboxyl terminus in dual pGC receptor activation and actions of cenderitide compared with CNP on renal function and cyclic guanosine monophosphate (cGMP) in vivo and ex vivo in normal canines.
Methods and results
In vitro, only cenderitide and not CNP or three CNP-based variants was a potent dual pGC-A/pGC-B activator of cGMP production (from 5 to 237 pmol/mL) in human embryonic kidney (HEK) 293 cells overexpressing human pGC-A while in pGC-B overexpressing cells cenderitide increased cGMP production (from 4 to 321 pmol/mL) while the three CNP-based variants were weak agonists. Based upon our finding that the DNP carboxyl terminus is a key structural requirement for dual pGC-A/pGC-B activation, we defined in vivo the renal-enhancing actions of cenderitide compared with CNP. Cenderitide increased urinary cGMP excretion (from 989 to 5977 pmol/mL), net generation of renal cGMP (821–4124 pmol/min), natriuresis (12–242 μEq/min), and glomerular filtration rate (GFR) (37–51 mL/min) while CNP did not. We then demonstrated the transformation of CNP ex vivo into a renal cGMP-activating peptide which increased cGMP in freshly isolated glomeruli eight-fold greater than CNP.
Conclusion
The current study establishes that dual pGC-A and pGC-B activation with CNP requires the specific carboxyl terminus of DNP. In normal canines in vivo and in glomeruli ex vivo, the carboxyl terminus of DNP transforms CNP into a natriuretic and GFR-enhancing peptide. Future studies of cenderitide are warranted in cardiorenal disease states to explore its efficacy in overall cardiorenal homeostasis.
Keywords: C-type natriuretic peptide, Chimeric natriuretic peptide, CD-NP, canine
Introduction
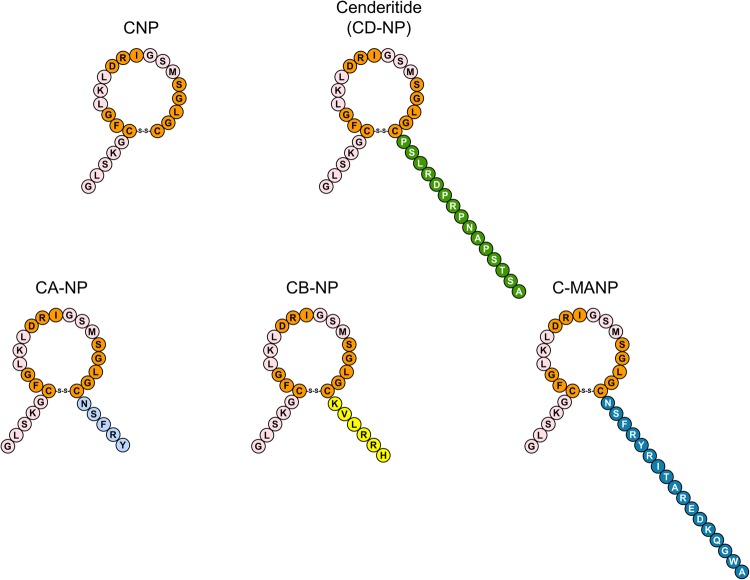
Cenderitide (CD-NP) is a novel chimeric natriuretic peptide (NP) currently in clinical trials for heart failure (HF).1–3 As a chimeric peptide, cenderitide is a single-chemical entity that possesses two separate functions.4 Specifically, this novel peptide was engineered unlike native NPs to uniquely co-activate the two particulate guanylyl cyclase (pGC) receptors (pGC-A and pGC-B) so as to take advantage of distinct receptor mediated actions through 3′5′ cyclic guanosine monophosphate (cGMP).5 In studies by Dickey et al., Cenderitide activated pGC-A but less than ANP and was closely equivalent to C-type natriuretic peptide (CNP) in activating pGC-B. Further in vivo, CD-NP like ANP, BNP, and Dendroaspis NP (DNP), and unlike CNP, possesses renal-enhancing actions through pGC-A/cGMP activation.1 Specifically, cenderitide compared with CNP is a 200-fold greater activator of pGC-A and 5-fold less potent activator of GC-B. Compared with ANP, Cenderitide had 50% potency in activating pGC-A and 40-fold greater GC-B-activating actions also compared with ANP. In contrast, CNP has potent anti-fibrotic properties through pGC-B activation and cGMP generation without renal-enhancing actions.6,7 Therefore, to achieve the renal-enhancing and anti-fibrotic properties of dual receptor activation, we designed cenderitide by fusing the 22 amino acid (AA) structure of CNP together with the 15-AA carboxyl terminus of the potent pGC-A agonist DNP, derived from venom of the Dendroaspis angusticeps (eastern green mamba) snake (Figure 1).8–10
Figure 1.
Structures and amino acid sequences of CNP, Cenderitide, CA-NP, CB-NP, and C-MANP.
To date, studies have established that cenderitide is more potent than CNP or BNP in inhibiting collagen type 1 gene and protein expressions in cultured human cardiac fibroblasts in vitro, and is natriuretic in normal canines in vivo and has plasma and urinary cGMP-activating properties in normal human volunteers.1,3,11 Further, Dickey and co-workers demonstrated that cenderitide is more resistant to neprilysin (NEP) degradation compared with the native NPs, ANP, BNP, and CNP, in vitro.12 Thus, cenderitide represents a first in class dual pGC-A/pGC-B-activating chimeric peptide, which does not exist in nature.
The current study was designed with two major goals. First, recognizing the novel structure of cenderitide, we addressed the hypothesis that the 15-AA carboxyl terminus of DNP, which is fused to CNP, uniquely facilitates dual pGC-A and pGC-B activation. To address this hypothesis, we defined the actions of cenderitide on cGMP activation in vitro in human embryonic kidney (HEK) 293 cells selectively overexpressing human pGC-A or pGC-B and compared cenderitide with native CNP and three variants that we designed and synthesized. Specifically, for the variants, we replaced the carboxyl terminus of cenderitide with the carboxyl terminus of ANP (CA-NP), BNP (CB-NP), or MANP (C-MANP), a designer pGC-A activator which is currently in clinical trials for resistant hypertension (Figure 1).13 Our second major goal was to further define the renal actions of cenderitide compared with CNP in vivo and ex vivo using normal canines. First, we compared in vivo the renal actions of cenderitide to CNP in normal canines with a special focus on urinary and renal generation of cGMP, natriuresis, and glomerular filtration rate (GFR). Here we hypothesized that cenderitide would result in greater increases in urinary and renal cGMP generation, natriuresis, and GFR compared with CNP. We also compared cGMP generation in response with cenderitide or CNP in freshly isolated canine glomeruli where pGC-A is highly expressed and where haemodynamic and circulating hormonal influences are not present.14 We tested the hypothesis that cenderitide would be superior to CNP in activating glomerular cGMP production consistent with the transformation of CNP into a novel NP with renal actions. Thus, these studies were designed to advance our understanding of cenderitide as a novel chimeric NP targeting two distinct NP receptors with therapeutic implications for cardiorenal disease.
Methods
Peptide synthesis
Cenderitide, CNP, CA-NP, CB-NP, and C-MANP were synthesized by Fmoc solid phase chemistry on a Liberty Peptide Synthesizer (CEM Corp.), as previously described.1 The structure of each peptide was confirmed by mass spectrometry, and HPLC analysis confirmed the purity of each peptide to be >90%.
Human embryonic kidney 293 cells and cyclic guanosine monophosphate generation in vitro
Human embryonic kidney 293 cells were stably transfected with either human pGC-A or pGC-B using Lipofectamine (Invitrogen, Grand Island, NY, USA) as previously reported.15 Transfected cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% foetal bovine serum, 100 U/mL penicillin, 100 U/mL streptomycin, and 250 µg/mL G418 (all reagents from Invitrogen). Cells were plated in 6-well plates and treated. Briefly, cells were incubated in Hank's balanced salt solution (Invitrogen, Carlsbad, CA, USA) containing 20 mmol/L N-[2-hydroxyethyl]piperazine-N′[2-ethanesulfonic acid], 0.1% bovine serum albumin, and 0.5 mmol/L 3-isobutyl-1-methylzanthine (Sigma, St. Louis, MO, USA). Treated cells received 10−6 M of all peptides including cenderitide, CNP, CA-NP, CB-NP, or C-MANP for 10 min. Note: for control purposes, non-transfected HEK293 cells were treated with ANP, BNP, and CNP (10−6 M) to assure no activation of cGMP occurred. Cells were lysed in 300 µL 6% trichloroacetic acid (TCA) and sonicated for 10 min. The samples were ether extracted four times in four volumes of ether, dried, and reconstituted in 300 µL cGMP assay buffer. The samples were assayed using a competitive RIA cGMP kit (Perkin-Elmer, Boston, MA, USA) as previously described.1
In vivo renal function
Male mongrel canines were studied in accordance with the Animal Welfare Act and with the approval of the Mayo Clinic Institutional Animal Care and Use Committee. Dogs were maintained on a Na+-controlled diet (Hill's i/d® canine diet, Hill's Pet Nutrition, Inc., Topeka, KS, USA).
On the evening before in vivo experiments, canines were fasted with ad lib access to water and also received 300 mg lithium carbonate for measurement of tubular sodium handling using the lithium clearance technique.16 On the day of the experiment, canines were anaesthetized with pentobarbital sodium and fentanyl, and were intubated and mechanically ventilated (Harvard Apparatus, Holliston, MA, USA) with 5 L/min of O2 (tidal volume 15 mL/kg, 12 cycles/min). The right femoral artery was cannulated for blood pressure monitoring and for blood sampling. The right femoral vein was cannulated for infusion of inulin and normal saline. A saphenous vein was cannulated for peptide infusion. The left kidney was exposed via a flank incision. The ureter was cannulated for timed urine collection. An electromagnetic flow probe was placed on the renal artery for measuring renal blood flow. A weight-adjusted bolus of inulin was given, followed by an inulin infusion (1 mL/min) to achieve plasma levels of 40–60 mg/dL, for measuring GFR by inulin clearance. Normal saline was infused (1 mL/min) and was temporarily discontinued during peptide infusion (1 mL/min).
Following a 60-min equilibration period, a 30-min pre-infusion clearance was obtained. This was followed by a 75-min continuous infusion of cenderitide (n = 10) or CNP (n = 8) at equimolar concentrations (32 mmol/kg/min). After a 15-min lead-in period, two 30-min clearances were obtained during the last 60 min of peptide infusion. Following a 30-min washout, a post-infusion clearance was obtained. Haemodynamic parameters, urine and blood samples were collected during each clearance. Blood was collected at mid-clearance and was replaced with an equal volume of normal saline. The lithium clearance technique (CLLi) was used to assess proximal and distal fractional sodium reabsorption (PFRNa and DFRNa, respectively) as follows: PFRNa = [1 – (CLLi/GFR)] × 100 and DFRNa = [(CLLi – CLNa)/CLLi] × 100, where CLLi = [urine Li+] × urine flow/[plasma Li+] and CLNa = [urine Na+] × urine flow/[plasma Na+].16 Net renal production of cGMP was estimated as: (urinary cGMP × urine flow rate) – (plasma cGMP × GFR).17
Ex vivo cyclic guanosine monophosphate activation in freshly isolated canine glomeruli
For studies of isolated glomeruli, kidneys were immediately harvested and glomeruli were isolated from normal canines (n = 3) as previously described by Supaporn et al.18 Briefly, the renal cortex was isolated, minced, forced through a 19-gauge needle and centrifuged with Krebs buffer. The pellet was resuspended and washed through sieves with pore sizes of 250 and 212 µm several times to remove tubular fragments. This was followed by further washing through 60 µm sieves. The glomeruli were collected, resuspended, and centrifuged at 2500 rpm. The pellet was finally suspended in ice-cold Krebs buffer (pH 7.4), containing (in mM) NaCl 118.3, KCl 4.7, NaHCO3 25, K2HPO4 1.2, CaCl2 2.5, Ca disodium versenate 0.026, glucose 11.1, and MgSO4 1.2 (equilibrated with 95% O2 and 5% CO2). The yield and the quality of the isolated glomeruli were confirmed by examination under light microscopy. Typically, >90% of glomeruli and <5% tubular contamination was observed in an aliquot of the final centrifugation. For quantification of cGMP response to the study peptides, aliquots of glomeruli (300 µL, suspended in Krebs buffer) were incubated with cenderitide or CNP (final concentration 10−5 M) for 10 min at 37°C (following an initial 10 min of pre-incubation) in the presence of isobutylmethylxanthine (0.3 mM) in a final volume of 500 µL. The controls consisted of the same composition with the exception that Krebs buffer was used instead of glomeruli suspended in Krebs buffer. The reaction was terminated by the addition of TCA (300 µL of ice-cold TCA) and centrifuged. An 800 µL supernate aliquot was extracted with ether for cGMP assay and the remaining supernate was neutralized with 1 N NaOH and analysed in a protein assay (BCA protein assay, Pierce Biotechnology, Rockford, IL, USA).
Assays for in vivo studies
Cyclic GMP was measured by radioimmunoassays (RIA).1 All blood and urine samples were placed immediately on ice after collection. Urine for cGMP measurement was heated to >90°C to eliminate phosphodiesterase activity prior to storage at −80°C. Following centrifugation (2500 rpm, 4°C), plasma was aliquoted and stored at −80°C until analysis. Urine volume, sodium, and cGMP were measured using a competitive RIA cGMP kit (Perkin-Elmer, Boston, MA, USA) as previously described.1 Inulin was determined by the anthrone method.19
Data analysis
Results are reported as mean ± SEM. For cell culture studies, each experiment was performed in triplicate. Differences between groups were made using unpaired t-tests. Physiologic parameters in study groups were compared with one-way analysis of variance (ANOVA) or repeated measures ANOVA. The normal distribution was tested with uninvariate analysis. Specifically, clearances from 16th to 45th min and from 46th to 75th min following initiation of peptide infusion are denoted by ‘30’ and ‘60 min’, respectively. Within each group, parameters at 30 and 60 min of peptide infusion, and post-infusion were compared with pre-infusion values by one-way ANOVA for repeated measurements followed by post hoc Dunnett's multiple comparison test, where applicable. Comparisons between groups were made by two-way ANOVA followed by Bonferroni post-test. GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA) was used for statistical analysis.
Results
In vitro cyclic guanosine monophosphate generation in human embryonic kidney 293 particulate guanylyl cyclase A or particulate guanylyl cyclase B overexpressing cells
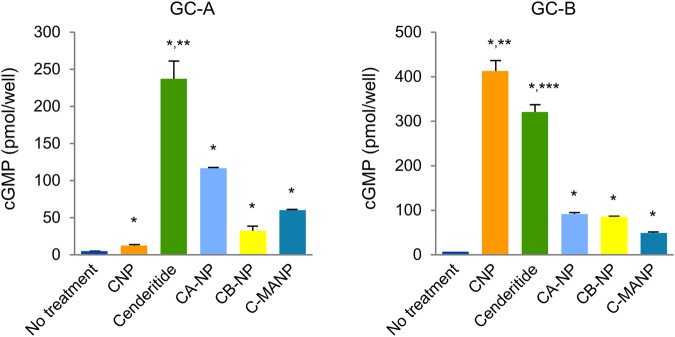
Human HEK 293 cells, which are devoid of endogenous pGC receptors, were stably transfected with human pGC-A or pGC-B. Cells were treated with CNP, cenderitide, CA-NP, CB-NP, or C-MANP and assessed for cGMP production and compared with no treatment (Figures 1 and 2). Our results confirm previous reports that cenderitide activates both human pGC-A and pGC-B and significantly increases cGMP. Indeed, cenderitide augmented cGMP in pGC-A overexpressing cells ∼20-fold greater than CNP while Cenderitide's activation of cGMP in pGC-B cells was ∼80% of that stimulated by CNP, a potent and selective pGC-B activator. The importance of the carboxyl terminus of DNP in providing Cenderitide's ability to co-activate pGC-A/pGC-B is revealed using three variants with differing carboxyl termini. CA-NP, the variant in which the DNP carboxyl terminus was replaced with the 5 AA of ANP increased cGMP production but the increase was reduced by ∼60% in pGC-A cells. CA-NP modestly, although significantly, activated cGMP in pGC-B cells. However, this activation was at an 80% reduced level compared with cenderitide. CB-NP, which possesses the 6 AA carboxyl terminus of BNP, significantly but modestly, increased cGMP in pGC-A cells when compared with no treatment. CB-NP, similar to CA-NP, modestly but significantly increased cGMP in pGC-B cells. C-MANP, like cenderitide, possesses a novel and long 17 AA carboxyl terminus derived from MANP, which is a potent pGC-A agonist and is now in clinical trials for resistant hypertension. C-MANP also modestly increased cGMP production in both pGC-A or pGC-B cells when compared with no treatment but markedly less compared with cenderitide. Thus, these in vitro studies demonstrate that the carboxyl terminus of DNP, which is fused to native CNP, facilitates dual pGC-A and pGC-B activation and cannot be reproduced by alternative carboxyl-terminal AA sequences of other test pGC-A agonists.
Figure 2.
Generation of cyclic guanosine monophosphate. In vitro cyclic guanosine monophosphate generation in human embryonic kidney 293 cells stably transfected with either the particulate guanylyl cyclase A or particulate guanylyl cyclase B receptor in response to a 10−6 M dose of CNP, Cenderitide, CA-NP, CB-NP, and C-MANP compared with no treatment. Values are mean ± SEM. *P < 0.05 vs. no treatment; **P < 0.05 vs. CNP, CA-NP, CB-NP, and C-MANP; ***P < 0.05 vs. CA-NP, CB-NP, and C-MANP.
In vivo renal function
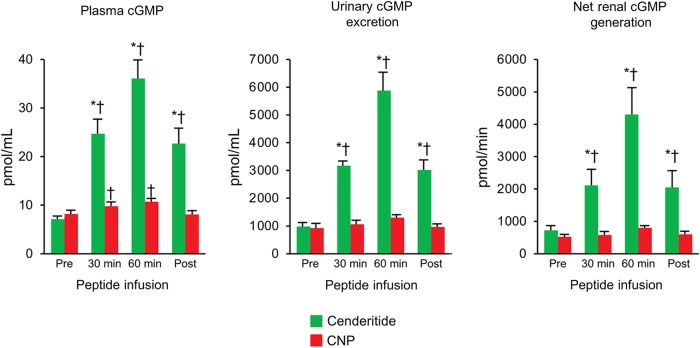
Figure 3 reports plasma cGMP, urinary excretion of cGMP, and net renal generation of cGMP in response to cenderitide or CNP in normal canines. Cenderitide significantly increased plasma cGMP and urinary cGMP excretion compared with CNP ∼5-fold. Cenderitide also significantly increased net renal generation of cGMP, a measure of cGMP production in the kidney also ∼5-fold. In contrast, CNP modestly increased plasma cGMP while urinary cGMP excretion and net renal generation of cGMP were unchanged.
Figure 3.
Plasma cyclic guanosine monophosphate, urinary cyclic guanosine monophosphate excretion, and net renal generation of cyclic guanosine monophosphate at pre-infusion, at 30 and 60 min of peptide infusion, and post-infusion. Values are mean ± SEM. *P < 0.05 vs. CNP and †P < 0.05 vs. pre.
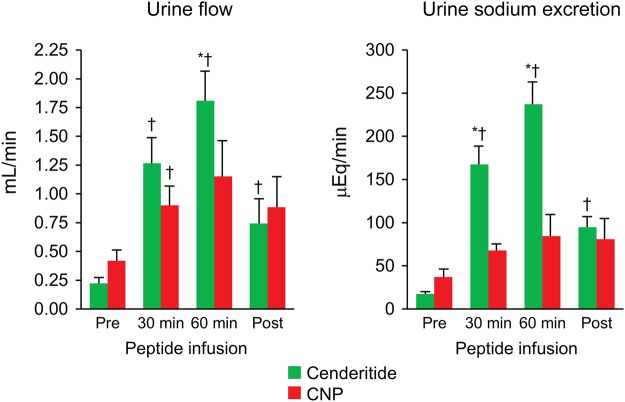
Renal excretory and haemodynamic function is reported in Figure 4 and Table 1. Cenderitide resulted in significantly greater increases in urine flow when compared with CNP, with significant differences between groups (Figure 4). With regarding to urinary sodium excretion, cenderitide increased natriuresis ∼8-fold greater than CNP (Figure 4). Both proximal fractional reabsorption of sodium (PFRNa) and distal fractional reabsorption of sodium (DFRNa) significantly decreased in the cenderitide group but not in the CNP group (Table 1). Glomerular filtration rate increased with cenderitide but not with CNP (Table 1); however, GFR at baseline was lower in the cenderitide group. There was no significant change in renal blood flow with either peptide. Mean arterial pressure was unchanged with infusion of cenderitide or CNP.
Figure 4.
Urinary flow and urinary sodium excretion during pre-infusion, at 30 and 60 min of peptide infusion and post-infusion. Values are mean ± SEM. *P < 0.05 vs. CNP and †P < 0.05 vs. pre.
Table 1.
Renal haemodynamic and excretory function
| Pre-I | 30 min I | 60 min I | Post-I | |
|---|---|---|---|---|
| MAP (mmHg) | ||||
| Cenderitide | 127 ± 4 | 124 ± 5 | 122 ± 6 | 126 ± 7 |
| CNP | 123 ± 4 | 127 ± 4 | 128 ± 4 | 128 ± 4 |
| GFR (mL/min) | ||||
| Cenderitide | 36.8 ± 1.7‡ | 47.5 ± 3.2† | 50.6 ± 3.3† | 53.3 ± 3.5† |
| CNP | 52.3 ± 5.1 | 52 ± 6.7 | 49.5 ± 4.2 | 48.8 ± 5.6 |
| RBF (mL/min) | ||||
| Cenderitide | 229 ± 29 | 244 ± 28 | 265 ± 27 | 261 ± 33 |
| CNP | 273 ± 26 | 294 ± 31 | 278 ± 29 | 270 ± 25 |
| PFRNa (%) | ||||
| Cenderitide | 75 ± 2 | 63 ± 2†‡ | 57 ± 3†‖ | 69 ± 3 |
| CNP | 80 ± 3 | 73 ± 2 | 72 ± 1 | 73 ± 2 |
| DFRNa (%) | ||||
| Cenderitide | 98 ± 0.2 | 92 ± 3†‡ | 92 ± 1†‡ | 96 ± 0.5 |
| CNP | 98 ± 0.6 | 97 ± 0.4 | 97 ± 0.9 | 97 ± 0.8 |
Pre-I, prior to infusion; 30 min I: 30 min of infusion; 60 min I: second 30 min infusion; post-I: post-infusion period. Values are means ± SEM. Comparisons within group vs. pre-infusion. Pre-I (mean ± SE, P < 0.01†) and between groups (P < 0.05‡, <0.001‖).
MAP, mean arterial pressure; GFR, glomerular filtration rate; RBF, renal blood flow; PFRNa, proximal fractional reabsorption of Na+; DFRNa, distal fractional reabsorption of Na+.
Activation of cyclic guanosine monophosphate ex vivo in isolated canine glomeruli
As enhancing renal function such as GFR is an important mechanism of natriuresis as well as a therapeutic goal in renal protection in HF, we sought to determine the direct acute actions of cenderitide compared with CNP upon cGMP generation in freshly isolated canine glomeruli in the absence of systemic cardiovascular haemodynamics and circulating neurohumoral mediators. Our findings of cGMP generation in freshly isolated normal canine glomeruli are illustrated in Figure 5. CNP (10−5 M) significantly but modestly increased cGMP in isolated canine glomeruli compared with control (Blank). At equimolar concentrations cenderitide markedly activated cGMP eight-fold greater than CNP. Thus, cenderitide in isolated glomeruli is a more potent activator of renal cGMP than CNP consistent with the ability of cenderitide to activate pGC-A, which is highly expressed in glomeruli.
Figure 5.
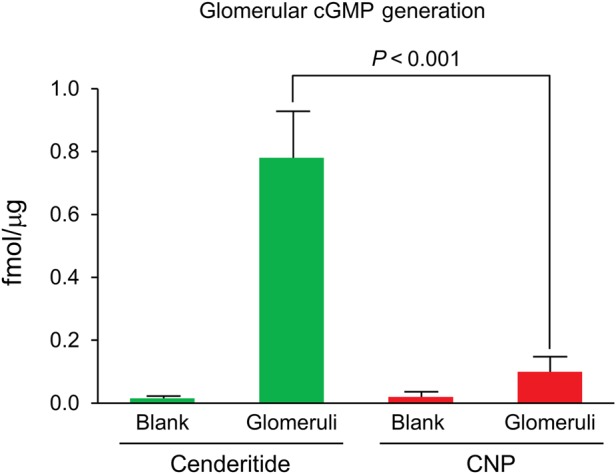
Cyclic guanosine monophosphate response in isolated canine glomeruli to CNP or Cenderitide 10−5 M.
Discussion
Cenderitide represents a novel chimeric NP currently in clinical trials for HF.1,3 The present study confirms the ability of cenderitide to co-activate both pGC-A and pGC-B receptors in vitro.5,15 Importantly, our studies demonstrated that the carboxyl terminus of DNP, derived from venom of the eastern green mamba snake, optimizes dual pGC-A and pGC-B activation, which cannot be mimicked by carboxyl termini from other pGC-A activators. Moreover, in freshly isolated canine glomeruli free of the influence of systemic haemodynamics or circulating hormones, cenderitide was 8-fold greater than CNP in increasing glomerular production of cGMP, which is consistent with the concept that the carboxyl terminus of DNP successfully transforms CNP into a renal-enhancing peptide. Our in vivo studies in normal canines demonstrated cenderitide increased plasma cGMP 5-fold greater than CNP and markedly increased urinary cGMP excretion and net renal generation of cGMP. This renal activation of the cGMP system was associated with natriuresis and diuresis and an increase in GFR with cenderitide infusion.
The endogenous NPs are genetically distinct but share structural similarities. ANP, BNP, CNP, and DNP all possess a 17-AA disulphide ring with distinct and common AAs in each ring.20 Unlike the 17-AA disulphide bridge rings, these four NPs possess heterogeneous amino and carboxyl termini. These termini in part provide resistance to degradation to NEP.21 Importantly, the current study supports a key role of the carboxyl terminus of DNP in interacting with the pGC-A receptor. A key property of cenderitide is the ability of this CNP ring-based peptide to co-activate pGC-A in addition to pGC-B. One may conclude that the carboxyl terminus of cenderitide, represented by the 15-AA carboxyl terminus of DNP, mediates activation by the CNP ring of the pGC-A receptor. Indeed in seminal studies, Ogawa advanced the concept that the carboxyl terminus of ANP interacted with the extracellular domain of pGC-A to optimize receptor activation.22 The ability of cenderitide to activate pGC-A therefore suggests that the CNP ring can activate pGC-A, but such activation must require a carboxyl terminus to facilitate pGC-A binding by a CNP ring as suggested by the lack of or minimal cGMP activation by the three variants.
In the current studies, we designed and synthesized three variants of CNP. These three variants were CA-NP, CB-NP, and C-MANP, which respectively were fusion peptides in which the carboxyl termini of ANP, BNP, or MANP were fused to CNP. CA-NP, CB-NP, and C-MANP were weak activators of both pGC-A and pGC-B. Thus cenderitide represents a novel dual pGC-A and pGC-B receptor activator that requires the specific structural AA and/or length of the carboxyl terminus of DNP. Further structural and biological studies should be pursued to understand the unique interactions of cenderitide with pGC-A and pGC-B and it's favourable cardiorenal protective properties.
The in vivo renal actions of cenderitide, compared with CNP also support the transformation of CNP into a renal-activating peptide. CNP lacked renal actions with no increase in urinary cGMP excretion or augmentation of net renal generation of cGMP. In clear contrast, cenderitide possesses potent cGMP-activating properties in the kidney with greater increases in urinary cGMP excretion and renal generation of cGMP in vivo. This is complemented by significantly greater increases in plasma cGMP with cenderitide, which may reflect pGC-A activation systemically, and/or spillover of cGMP from the renal venous effluent into the general circulation. While cenderitide-induced natriuresis may be secondary to the increase in filtered load of sodium as GFR increased, cenderitide also reduced fractional reabsorption of sodium both at the proximal and distal nephron as determined by the lithium clearance technique. In contrast, CNP was unassociated with changes in sodium reabsorption at these two nephron sites. This result is consistent with the human study that reported the lack of natriuresis with short-term infusion of CNP.7 Thus, the fusion of the carboxyl terminus of DNP to CNP successfully transforms CNP into cenderitide, a potent renal-enhancing and cGMP-activating chimeric NP.
With regard to the kidney, Lisy et al. previously compared the renal response in vivo between cenderitide and BNP. While both BNP and cenderitide are natriuretic, cenderitide increased GFR greater than BNP and was less hypotensive.1 In the current study, we sought to establish that cenderitide activates the cGMP system better than CNP. Our findings demonstrate that cenderitide is 8 fold more potent in activating glomerular cGMP production than CNP and such results are consistent with transforming CNP into a pGC-A activating and a renal acting peptide. The greater activation of cGMP by cenderitide in glomeruli also has physiological significance as cenderitide in vivo increased GFR and urinary sodium excretion, while no change in GFR or sodium excretion was observed with CNP.
The current studies have physiological and therapeutic implications. CNP is thought to be the oldest member in the evolution of a family of NPs although CNP lacks renal actions.23 Its structure is the simplest in terms of lacking a carboxyl terminus, which renders it highly selective for pGC-B and studies suggest that its lack of a carboxyl terminus results in it being highly susceptible to enzymatic degradation by NEP.24 Its physiological actions are linked via pGC-B to bone growth, inhibition of fibrosis, and endothelial regeneration.6,20,25 Indeed, Sangaralingham and co-workers have reported that circulating CNP decreases with age and a relative CNP deficiency may play therefore play a permissive role in the fibrogenesis of aging.26 In contrast, pGC-A, which is activated by ANP, BNP, and DNP, plays a more central role in body fluid homeostasis promoting natriuresis, vasodilation, and reductions in intravascular volume.27,28 Cenderitide therefore emerges as a true first-in-class designer peptide that takes advantage of properties of both pGC-B and pGC-A activation, the latter as clearly demonstrated in the current study. In addition, as reported by Dickey et al., cenderitide is highly resistant to degradation by NEP. Specifically, CD-NP is more resistant to degradation by NEP than ANP, BNP or CNP which adds another important attractive therapeutic property as NEP inhibitors have proven efficacy in cardiovascular disease and this high resistance to NEP degradation could contribute to the enhanced biological actions of cenderitide.12,29
The current study also has relevance to the emerging therapeutic strategy of multivalency. Multivalency is built upon the concept of chimeric technology in which a peptide like cenderitide or a small molecule like LCZ696 (entresto) targets more than one pathway.30 As cenderitide targets two pGC receptors, it optimizes cGMP-dependent actions beyond what selective native peptides such as ANP (carperitide), which is for the treatment of HF in Japan, and BNP (nesiritide) that is approved in the USA and Canada for HF.31,32 Entresto, which has recently been FDA approved for HF with reduced ejection fraction, simultaneously targets the AT1 receptor and NEP to antagonize the deleterious actions of angiotensin II and promote the beneficial properties of the native endogenous NPs (ANP, BNP, and CNP).29,33 Indeed, such multivalency is emerging in metabolic disease with the development an innovative new glucagon and GLP-1 co-agonists for obesity.34
The current studies have limitations. We did not compare the cGMP-activating properties of cenderitide to ANP and BNP in HEK293 cells; however, this comparison has been previously reported.5 We also did not compare cenderitide to ANP and/or BNP in glomeruli nor in normal canines, although we have reported the comparison of BNP with cenderitide on enhancing GFR with less hypotension.1 Additional studies that more clearly differentiate native and designer peptides are needed.
In conclusion, cenderitide represents a new generation first-in-class chimeric NP engineered to co-activate both pGC-A and pGC-B, unlike the native NPs thus maximizing the activation of this important cGMP system. Importantly, our studies also establish that dual pGC-A and pGC-B activation with CNP, evolution's oldest endogenous NP, can be achieved with fusion to the carboxyl terminus of DNP which is derived from the venom of the eastern green mamba snake. Importantly, fusion of the carboxyl terminus of ANP, BNP, or another pGC-A-specific designer MANP to CNP fails to mimic the dual receptor activation achieved with cenderitide. In vivo in normal canines cenderitide transforms CNP into a natriuretic and GFR-enhancing peptide with renal cGMP activation. In freshly isolated canine glomeruli, ex vivo Cenderitide activates cGMP eight-fold greater than CNP. Future studies of cenderitide are clearly warranted in cardiorenal disease states to explore its efficacy in cardiorenal protection and in overall cardiorenal homeostasis.
Funding
Supported by the National Institutes of Health (R01 HL36634, HL83231 and P01 HL76611) and the Mayo Foundation.
Conflict of interest: Mayo Clinic and Foundation has licensed Cenderitide to Capricor Therapeutics. J.C.B. and O.L. are co-inventors of Cenderitide.
References
- 1.Lisy O, Huntley BK, McCormick DJ, Kurlansky PA, Burnett JC Jr. Design, synthesis, and actions of a novel chimeric natriuretic peptide: CD-NP. J Am Coll Cardiol 2008;52:60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Francis GS. From B-type natriuretic peptide to green mambas: the process of discovery. J Am Coll Cardiol 2008;52:69–70. [DOI] [PubMed] [Google Scholar]
- 3.Lee CY, Chen HH, Lisy O, Swan S, Cannon C, Lieu HD, Burnett JC Jr. Pharmacodynamics of a novel designer natriuretic peptide, CD-NP, in a first-in-human clinical trial in healthy subjects. J Clin Pharmacol 2009;49:668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKie PM, Sangaralingham SJ, Burnett JC Jr. CD-NP: an innovative designer natriuretic peptide activator of particulate guanylyl cyclase receptors for cardiorenal disease. Curr Heart Fail Rep 2010;7:93–99. [DOI] [PubMed] [Google Scholar]
- 5.Dickey DM, Burnett JC Jr, Potter LR. Novel bifunctional natriuretic peptides as potential therapeutics. J Biol Chem 2008;283:35003–35009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horio T, Tokudome T, Maki T, Yoshihara F, Suga S, Nishikimi T, Kojima M, Kawano Y, Kangawa K. Gene expression, secretion, and autocrine action of C-type natriuretic peptide in cultured adult rat cardiac fibroblasts. Endocrinology 2003;144:2279–2284. [DOI] [PubMed] [Google Scholar]
- 7.Igaki T, Itoh H, Suga SI, Hama N, Ogawa Y, Komatsu Y, Yamashita J, Doi K, Chun TH, Nakao K. Effects of intravenously administered C-type natriuretic peptide in humans: comparison with atrial natriuretic peptide. Hypertens Res 1998;21:7–13. [DOI] [PubMed] [Google Scholar]
- 8.Schweitz H, Vigne P, Moinier D, Frelin C, Lazdunski M. A new member of the natriuretic peptide family is present in the venom of the green mamba (Dendroaspis angusticeps). J Biol Chem 1992;267:13928–13932. [PubMed] [Google Scholar]
- 9.Singh G, Maguire JJ, Kuc RE, Skepper JN, Fidock M, Davenport AP. Characterization of the snake venom ligand [125I]-DNP binding to natriuretic peptide receptor-A in human artery and potent DNP mediated vasodilatation. Br J Pharmacol 2006;149:838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vink S, Jin AH, Poth KJ, Head GA, Alewood PF. Natriuretic peptide drug leads from snake venom. Toxicon 2012;59:434–445. [DOI] [PubMed] [Google Scholar]
- 11.Ichiki I, Huntley B, Soon P, Sangaralingham J, Sandberg S, Burnett JC Jr. Cardiac fibrosis in end-stage human heart failure and the cardiac natriuretic peptide guanylyl cyclase system: regulation and therapeutic implications. J Mol Cell Cardiol 2014;75:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickey DM, Potter LR. Dendroaspis natriuretic peptide and the designer natriuretic peptide, CD-NP, are resistant to proteolytic inactivation. J Mol Cell Cardiol 2011;51:67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKie P, Cataliotti A, Huntley B, Martin F, Olson T, Burnett JC Jr. A human atrial natriuretic peptide gene mutation reveals a novel peptide with enhanced blood pressure lowering, renal enhancing and aldosterone suppressing actions. J Am Coll Card 2009;54:1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogawa Y, Mukoyama M, Yokoi H, Kasahara M, Mori K, Kato Y, Kuwabara T, Imamaki H, Kawanishi T, Koga K, Ishii A, Tokudome T, Kishimoto I, Sugawara A, Nakao K. Natriuretic peptide receptor guanylyl cyclase-A protects podocytes from aldosterone-induced glomerular injury. J Am Soc Nephrol 2012;23:1198–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin FL, Sangaralingham SJ, Huntley BK, McKie PM, Ichiki T, Chen HH, Korinek J, Harders EJ, Burnett JC Jr. CD-NP: a novel engineered dual guanylyl cyclase activator with anti-fibrotic actions in the heart. PLoS ONE 2012;7:e52422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomsen K, Holstein-Rathlou NH, Leyssac PP. Comparison of three measures of proximal tubular reabsorption: lithium clearance, occlusion time, and micropuncture. Am J Physiol 1981;241:F348–F355. [DOI] [PubMed] [Google Scholar]
- 17.Marqulies KB, Heublein DM, Perrella MA, Burnett JC Jr. ANF-mediated renal cGMP generation in congestive heart failure. Am J Physiol 1991;260:F562–F568. [DOI] [PubMed] [Google Scholar]
- 18.Supaporn T, Sandberg SM, Borgeson DD, Heublein DM, Luchner A, Wei CM, Dousa TP, Burnett JC Jr. Blunted cGMP response to agonists and enhanced glomerular cyclic 3′,5′-nucleotide phosphodiesterase activities in experimental congestive heart failure. Kidney Int 1996;50:1718–1725. [DOI] [PubMed] [Google Scholar]
- 19.Kaczmarczyk J, Kruttgen CJ. Eine einfache colorimetrischemethode zur inulinbestimmung für Nierenclearanceuntersuchungen bei Stoffwechselgesunden und. Diabetikern Kim Wochenschr 1955;33:729–730. [DOI] [PubMed] [Google Scholar]
- 20.Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol 2009;(191):341–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pankow K, Schwiebs A, Becker M, Siems WE, Krause G, Walther T. Structural substrate conditions required for neutral endopeptidase-mediated natriuretic peptide degradation. J Mol Biol 2009;393:496–503. [DOI] [PubMed] [Google Scholar]
- 22.Ogawa H, Qiu Y, Ogata C, Misono K. Crystal structure of hormone-bound atrial natriuretic peptide receptor extracellular domain: rotation mechanism for transmembrane signal transduction. J Biol Chem 2004;279:28625–28631. [DOI] [PubMed] [Google Scholar]
- 23.Van Kimmenade RR, Januzzi JL Jr. The evolution of the natriuretic peptides – current applications in human and animal medicine. J Vet Cardiol 2009;11:S9–21. [DOI] [PubMed] [Google Scholar]
- 24.Kenny AJ, Bourne A, Ingram J. Hydrolysis of human and pig brain natriuretic peptides, urodilatin, C-type natriuretic peptide and some C-receptor ligands by endopeptidase-24.11. Biochem J 1993;291:83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doi K, Ikeda T, Itoh H, Ueyama K, Hosoda K, Ogawa Y, Yamashita J, Chun TH, Inoue M, Masatsugu K, Sawada N, Fukunaga Y, Saito T, Sone M, Yamahara K, Kook H, Komeda M, Ueda M, Nakao K. C-type natriuretic peptide induces redifferentiation of vascular smooth muscle cells with accelerated reendothelialization. Arterioscler Thromb Vasc Biol 2001;21:930–936. [DOI] [PubMed] [Google Scholar]
- 26.Sangaralingham SJ, Huntley BK, Martin FL, McKie PM, Bellavia D, Ichiki T, Harders GE, Chen HH, Burnett JC Jr. The aging heart, myocardial fibrosis, and its relationship to circulating C-type natriuretic peptide. Hypertension 2011;57:201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burnett JC Jr, Granger JP, Opgenorth TF. Effects of synthetic atrial natriuretic factor on renal function and renin release. Am J Physiol 1984;247:F863–F866. [DOI] [PubMed] [Google Scholar]
- 28.Holtwick R, Gotthardt M, Skryabin B, Steinmetz M, Potthast R, Zetsche B, Hammer RE, Herz J, Kuhn M. Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc Natl Acad Sci USA 2002;99:7142–7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 30.Letts G, Loscalzo J. Frontiers in nephrology: targeting inflammation using novel nitric oxide donors. J Am Soc Nephrol 2007;18:2863–2869. [DOI] [PubMed] [Google Scholar]
- 31.Suwa M, Seino Y, Nomachi Y, Matsuki S, Funahashi K. Multicenter prospective investigation on efficacy and safety of carperitide for acute heart failure in the “real world” of therapy. Circ J 2005;69:283–290. [DOI] [PubMed] [Google Scholar]
- 32.O'Connor C, Starling R, Hernandez A. Nesiritide in acute decompensated heart failure. N Engl J Med 2011;365:32–43. [DOI] [PubMed] [Google Scholar]
- 33.Packer M, McMurray JJ, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile M, Andersen K, Arango JL, Arnold JM, Bĕlohlávek J, Böhm M, Boytsov S, Burgess LJ, Cabrera W, Calvo C, Chen CH, Dukat A, Duarte YC, Erglis A, Fu M, Gomez E, Gonzàlez-Medina A, Hagège AA, Huang J, Katova T, Kiatchoosakun S, Kim KS, Kozan Ö, Llamas EB, Martinez F, Merkely B, Mendoza I, Mosterd A, Negrusz-Kawecka M, Peuhkurinen K, Ramires FJ, Refsgaard J, Rosenthal A, Senni M, Sibulo AS Jr, Silva-Cardoso J, Squire IB, Starling RC, Teerlink JR, Vanhaecke J, Vinereanu D, Wong RC; PARADIGM-HF Investigators and Coordinators Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015;131:54–61. [DOI] [PubMed] [Google Scholar]
- 34.Day JW, Ottaway N, Patterson JT, Gelfanov V, Smiley D, Gidda J, Findeisen H, Bruemmer D, Drucker DJ, Chaudhary N, Holland J, Hembree J, Abplanalp W, Grant E, Ruehl J, Wilson H, Kirchner H, Lockie SH, Hofmann S, Woods SC, Nogueiras R, Pfluger PT, Perez-Tilve D, DiMarchi R, Tschöp MH. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat Chem Biol 2009;5:749–757. [DOI] [PubMed] [Google Scholar]