Summary
The transcription factor p53 responds to DNA double strand breaks by increasing in concentration in a series of pulses of fixed amplitude, duration, and period. How p53 pulses influence the dynamics of p53 target gene expression is not understood. Here we show that in bulk cell populations, patterns of p53 target gene expression cluster into groups with stereotyped temporal behaviors, including pulsing and rising dynamics. These behaviors correlate statistically with the mRNA decay rates of target genes: short mRNA half-lives produce pulses of gene expression. This relationship can be recapitulated by mathematical models of p53-dependent gene expression in single cells and cell populations. Single-cell transcriptional profiling demonstrates that expression of a subset of p53 target genes is coordinated across time within single cells; p53 pulsing attenuates this coordination. These results help delineate how p53 orchestrates the complex DNA damage response and give insight into the function of pulsatile signaling pathways.
Introduction
Cells use complex signaling pathways to detect environmental stimuli and execute appropriate responses. As methods for quantifying intracellular signaling have improved, several signaling pathways have been found to transmit information using signals that pulse in time (Dalal et al., 2014; Levine et al., 2013). Pulsatile signaling has been found to serve diverse purposes in a biological context, including enforcing proportionality in gene expression (Cai et al., 2008), implementing timer processes (Levine et al., 2012), generating different gene expression or phosphorylation patterns (Ashall et al., 2009; Batchelor et al., 2009; Hansen and O’Shea, 2014; Hao and O’Shea, 2012; Nelson et al., 2004; Purvis et al., 2012; Tay et al., 2010; Toettcher et al., 2013), and improving signaling fidelity (Selimkhanov et al., 2014).
The transcription factor p53 is a key stress-response regulator that exhibits pulsatile dynamics (Batchelor et al., 2009; Purvis et al., 2012). In response to DNA double-strand breaks (DSBs), p53 levels in the nucleus increase in pulses with a fixed amplitude, duration, and period; the mean number of pulses increases with DNA damage (Lev Bar-Or et al., 2000; Lahav et al., 2004). p53 regulates the expression of over 100 target genes involved in a range of cellular stress responses including apoptosis, cell cycle arrest, senescence, DNA repair, and changes in metabolism (Aylon and Oren, 2011; Riley et al., 2008). p53 pulsing directly impacts p53 function: altering p53 dynamics by pharmacologically inhibiting p53 degradation changes patterns of target gene expression and cell fate (Purvis et al., 2012).
While p53 pulsing serves important biological functions, it is less clear how it accomplishes these functions mechanistically. We previously proposed two possibilities for how p53 dynamics impact its activity as a transcription factor. First, p53 pulsing could be a mechanism to generate a range of target gene expression dynamics wider than would be possible if p53 levels simply rose over time (Batchelor et al., 2009), thereby producing a greater variety of possible downstream responses. Second, p53 pulsing could coordinate expression of its target genes (Batchelor et al., 2009; Levine et al., 2013). Such coordination has been observed in the pulsatile response mediated by the S. cerevisiae transcription factor Crz1 (Cai et al., 2008). We therefore sought to determine the impact of p53 pulsing on the dynamics and coordination of target gene expression.
Results
p53 target genes display a range of expression dynamics
To identify the range of expression dynamics with which p53 target genes respond to p53 pulsing, we treated MCF-7 breast carcinoma cells expressing p53-Venus (Batchelor et al., 2008) with neocarzinostatin (NCS), a radiomimetic drug that generates DNA DSBs. Following treatment, we measured the expression dynamics of a large panel of p53 target genes by RT-qPCR. Genes in the panel were well-characterized, direct p53 targets (Riley et al., 2008) representing a wide range of downstream functions (Table S1). To identify p53-dependent responses, we compared target gene dynamics with those in MCF-7 sh-p53 cells, in which p53 is knocked down by an shRNA (Figure S1A–L, Data Set S1) (Brummelkamp et al., 2002).
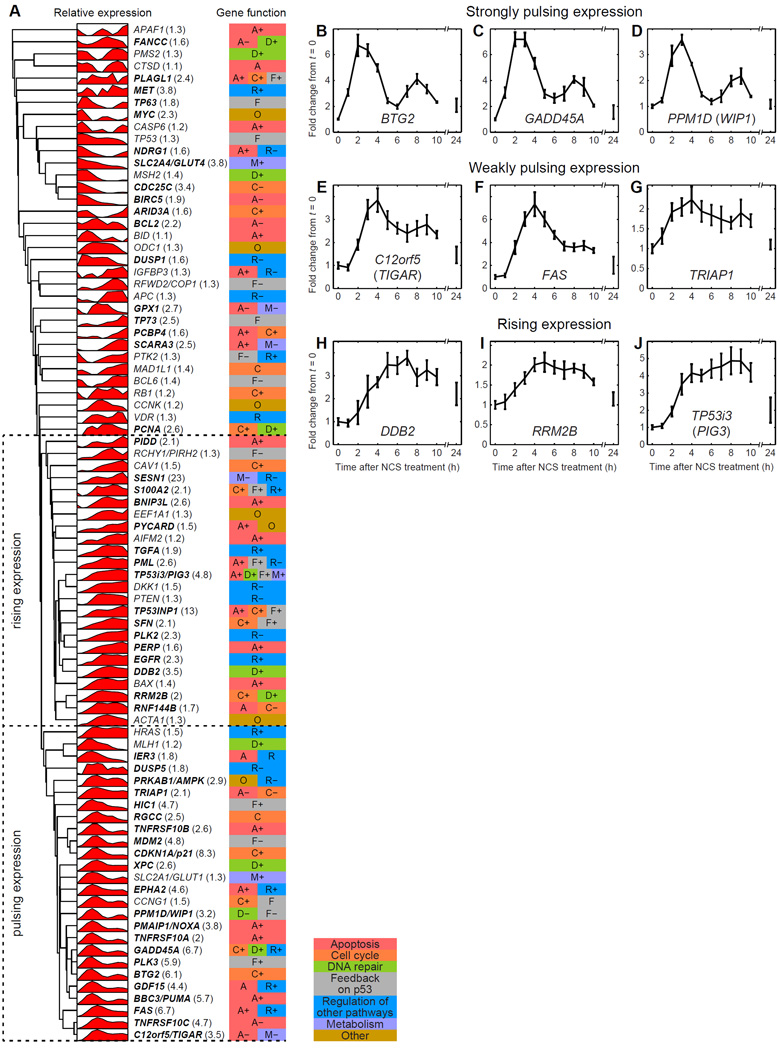
To identify patterns in p53 target gene expression, we performed unsupervised hierarchical clustering analysis (Supplemental Experimental Procedures) on the experimentally measured target gene expression profiles (Figure 1A). The clustering revealed two major dynamical expression patterns, which we termed “pulsing” and “rising.” Pulsing expression profiles were observed for a cluster of 26 target genes (Figure 1A–G, Figure S1A–L, Data Set S1). These genes shared a common peak of expression around 3–4 h post-treatment and in most cases (21 out of 26) a second peak around 8–9 h. Some genes with “strongly pulsing” expression had a very clear second expression peak (Figure 1B–D); others with “weakly pulsing” expression had less pronounced second peaks (Figure 1E–G). The timing of peaks was consistent with a delay with respect to the timing of p53 protein peaks (Geva-Zatorsky et al., 2006). Consistent with population measurements of p53 dynamics (Lev Bar-Or et al., 2000), the pulses of target gene expression were damped; this was likely due to the fraction of cells with active p53 decreasing over time as well as de-synchronization of the p53 response between individual cells in the population (Geva-Zatorsky et al., 2006; Lahav et al., 2004). 24 targets displayed rising expression dynamics (Figure 1A, H–J; Figure S1A–L; Data Set S1), in which mRNA levels increased during the first pulse of p53 and then remained elevated without perceived pulsing. Of the genes with rising expression, many reached an expression plateau around 4–5 h, following the first pulse of p53. mRNA levels of these targets decreased after this plateau, again likely due to eventual DNA damage repair and decreased p53 activity across the population of cells (Figure 1A, H–J). The major dynamical expression patterns did not correlate with specific known gene functions (Figure 1A). For a limited subset of p53 target genes, we confirmed that the major classes of expression dynamics observed at the mRNA level were reflected in the dynamics of the protein products (Figure S1M–Q) (Batchelor et al., 2008; Lahav et al., 2004).
Figure 1.
p53 target genes show different temporal patterns of expression in response to DNA double strand breaks. (A) Clustering analysis of gene expression time courses (n=4, averaged, filtered) identifies groups of genes that show pulsing and rising expression patterns. Gene expression level is shown relative to the maximum and minimum expression levels of each gene. Fold-differences between maximum and minimum expression for each gene are shown in parentheses next to each gene name. Genes with fold-differences of at least 1.5 are shown in bold. Gene function (Table S1) is indicated by the colored box on the right. “+” indicates a positive effect in the specified functional pathway, “−” indicates a negative effect. (B–J) Sample gene expression time courses (averaged, unfiltered) for three genes with strongly pulsing expression (B–D), three with weakly pulsing expression (E–G), and three with rising expression (H–J). Error bars represent standard error of the mean (n=4). See also Figure S1 and Data Set S1.
mRNA decay rate determines target gene expression dynamics in response to p53
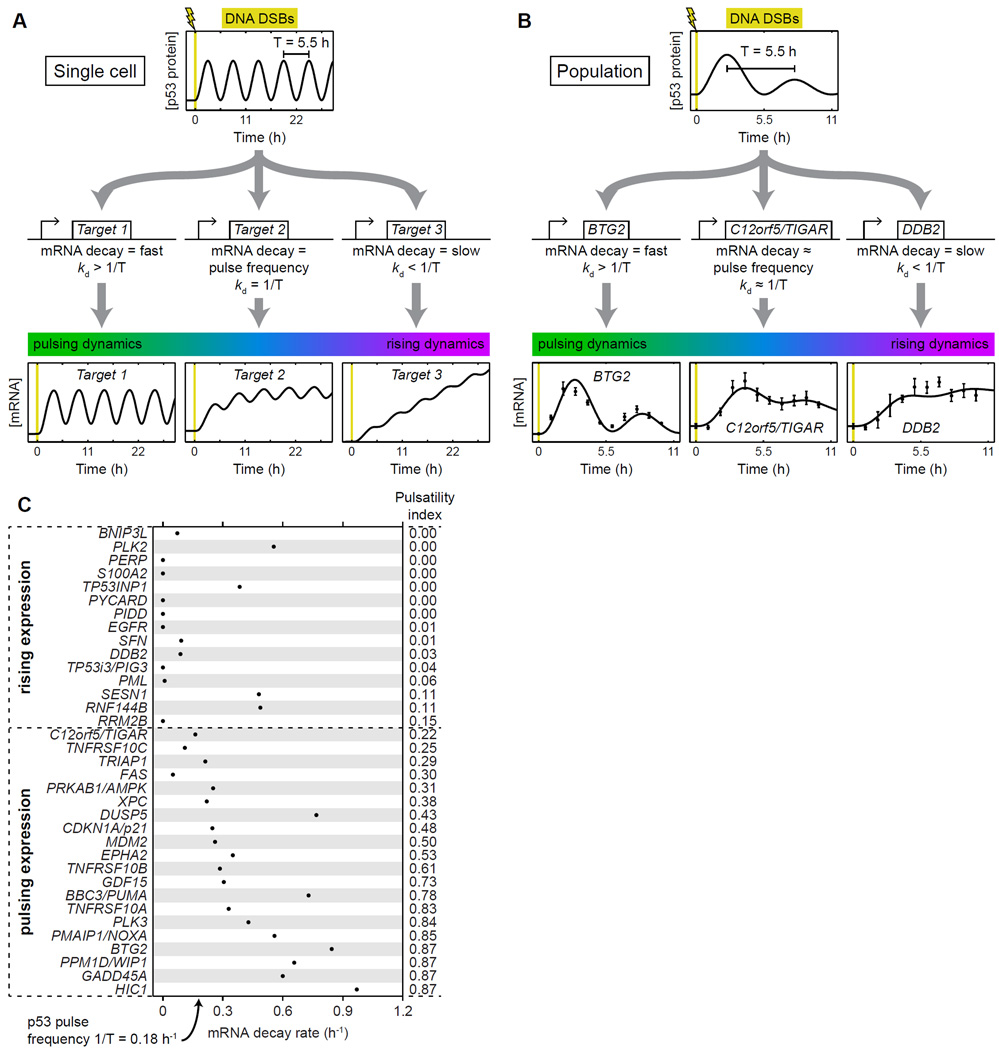
To identify mechanisms that could generate both pulsing and rising expression dynamics, we constructed a simple mathematical model of p53 target gene activation in a single cell undergoing a pulsatile p53 response to DNA DSBs (Experimental Procedures). In this model, mRNA of a target gene is produced as a function of the p53 protein level and decays at a rate proportional to its abundance. We found that qualitatively distinct dynamics resulted from different target gene mRNA decay rates (Figure 2A). For a fast decay rate, the simulated target gene response is pulsatile, tracking p53 levels. For a slow decay rate, the response rises over time, approximately integrating p53 levels. A continuous transition between these qualitatively different responses occurs around the decay rate equal to the frequency of p53 pulsing.
Figure 2.
Gene expression dynamics correlate with mRNA decay rate. (A) A simple model predicts that pulses of p53 expression in a single cell can generate different target gene expression dynamics for different mRNA decay rates. For Target 1, the mRNA decay rate kd = 1.0 h−1; for Target 2, kd = 0.18 h−1, the frequency of p53 pulsing; for Target 3, kd = 0.01 h−1. (B) A model of p53 dynamics and target gene expression in a population of cells fits measurements of p53 target gene expression (Figure 1). Model fits are shown for representative target genes with strongly pulsing (BTG2), weakly pulsing (C12orf5/TIGAR), and rising (DDB2) expression dynamics. Error bars on gene expression measurements represent standard error of the mean (n=4). (C) Measured mRNA decay rates are predictive of p53 target gene expression dynamics. mRNA decay rates for nascent transcripts were measured for the indicated genes in MCF-7 p53-Venus cells in response to treatment with 400 ng/mL NCS for 3 h. The pulsatility index was calculated for each target gene by fitting the model of population-level target gene expression dynamics to measurements of the same (Figure 1). Pulsatility index is correlated with mRNA decay rate (Spearman’s ρ = 0.69, p = 4.9×10−6). See also Figure S2 and Data Set S2.
To describe a mixed population of cells such as that measured in our transcriptional profiling experiments, in which the p53 response ceases in some cells and desynchronizes in the remaining cells as time goes on, we modified the single-cell model with a p53 pulse amplitude that decays over time (Supplemental Experimental Procedures). The target gene expression profiles produced by this population model (Figure 2B) resembled those observed in our experiments on populations of cells (Figure 1). Slow mRNA decay rates yielded rising gene expression dynamics. As mRNA decay rates increased, gene expression dynamics became increasingly pulsatile (Figure 2B). These observations demonstrate that even a simple model of p53 activity can recapitulate much of the observed diversity of p53 target gene dynamics.
Using this population-level model, we tested our prediction that greater pulsatility of gene expression in vivo correlates with a faster mRNA decay rate. To quantify the pulsatility of target gene expression beyond the categories determined by clustering, we developed a rigorous mathematical metric of the pulsatility of each target’s expression dynamics (Figure 1A). We first fit our population-level model (Figure 2B, Figure S2, Data Set S2) to our measurements of target gene expression (Figure 1) and determined the parameters that provided an optimal match between model and experiment for each target gene. We then computed a “pulsatility index” for each gene expression profile; this was defined as the ratio of the pulse amplitude to the increase in average mRNA level in cells with active p53 (Supplemental Experimental Procedures). To directly measure mRNA decay rates for p53 target genes whose expression was identified as pulsing or rising, we labeled newly synthesized RNA with a uridine analog following induction of DNA DSBs, then washed cells with fresh medium and measured persistence of labeled mRNA using RT-qPCR (Supplemental Experimental Procedures). As predicted, we found a strong positive association between a gene’s mRNA decay rate and the pulsatility index of its expression profile (Figure 2C; Spearman’s ρ = 0.69, p = 4.9×10−6). The pulsatility index was consistent with the categorization of gene expression dynamics in our clustering analysis (Figure 1A); all genes identified by clustering as having pulsing expression also had pulsatility indices ≥ 0.22, while all genes identified by clustering as having rising expression also had pulsatility indices ≤ 0.15 (Figure 2C). Moreover, the transition between pulsing and rising dynamics occurred at an mRNA decay rate near the p53 pulse frequency (~1/5.5 h−1), as predicted by our model.
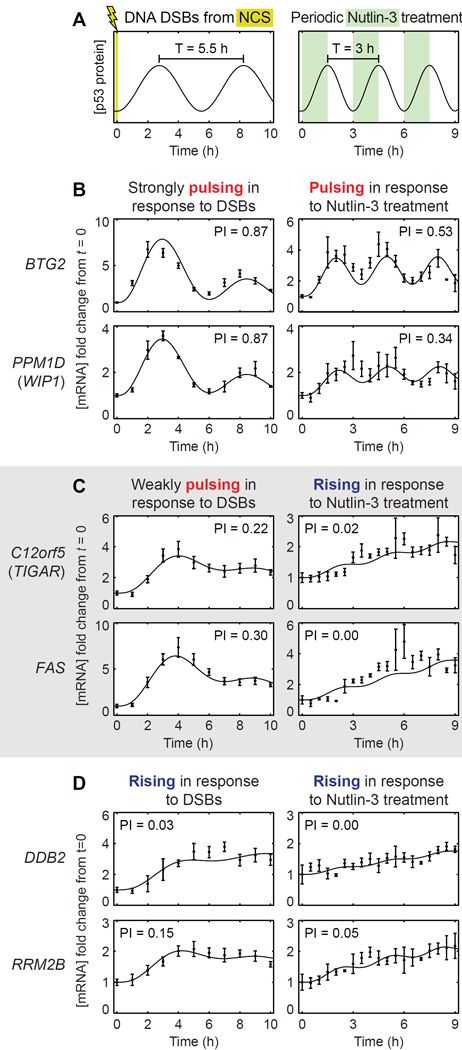
Our findings suggest that altering the p53 pulse frequency would generate large qualitative changes in expression dynamics for a subset of target genes. Specifically, increasing the p53 pulse frequency should convert weakly pulsing gene expression to rising expression. To test this prediction, we created p53 pulses of higher frequency by treating cells with the small molecule Nutlin-3, as the p53 pulse frequency induced by DNA DSBs is relatively fixed (Toettcher et al., 2010). Nutlin-3 inhibits the interaction between p53 and its E3 ubiquitin ligase Mdm2 (Vassilev et al., 2004), thereby both stabilizing p53 and enhancing its transcriptional activity (Allen et al., 2014). We applied a periodic dose of 5 µM Nutlin-3 to MCF-7 cells expressing p53-Venus to generate pulses of p53 with a period of 3 h (Figure 3A), as confirmed by Western blot analysis (Figure S3). Following periodic Nutlin-3 treatment, we measured the expression dynamics of p53 target genes by RT-qPCR.
Figure 3.
Changing the p53 pulse frequency changes the set of genes showing pulsatile expression. (A) p53 pulses occur with a period of ~5.5 h in response to DNA DSBs. p53 pulses with a period of 3 h were generated by periodic treatment of cells with 5 µM Nutlin-3. (B) p53 target genes BTG2 and PPM1D (WIP1), which have strongly pulsing expression dynamics in response to DNA DSBs, retained pulsatile expression in response to periodic Nutlin-3 treatment. (C) C12orf5 (TIGAR) and FAS, which have weakly pulsing expression dynamics in response to DNA DSBs, switched to rising expression in response to periodic Nutlin-3 treatment. (D) DDB2 and RRM2B, which have rising expression dynamics in response to DNA DSBs, retained rising expression in response to periodic Nutlin-3 treatment. The pulsatility index (PI) for each expression profile is indicated. Error bars represent standard error of the mean (n=4 NCS-treated samples, n=3 Nutlin-3-treated samples). See also Figure S3.
Based on our model, we predicted that periodic Nutlin-3 treatment to create a faster p53 pulse frequency would decrease the pulsatility of expression of all target genes from that observed in the DNA DSB response. While genes with rising expression in the DNA DSB response would continue to have rising expression in response to periodic Nutlin-3 treatment, strongly pulsing gene expression would become weaker, and weakly pulsing gene expression would convert to rising expression. Indeed, we observed this among the p53 target genes whose expression was induced by Nutlin-3 treatment. Genes such as DDB2 and RRM2B that showed rising expression in response to DNA DSBs continued to show rising expression in response to periodic Nutlin-3 treatment (Figure 3D). In contrast, genes such as BTG2 and PPM1D (WIP1), which have high mRNA decay rates and strongly pulsatile expression in response to DNA DSBs, showed less pulsatile expression in response to periodic Nutlin-3 treatment (Figure 3B). The largest qualitative changes occurred for genes such as C12orf5 (TIGAR) and FAS, which have mRNA decay rates near the natural p53 pulse frequency and showed weakly pulsatile expression in response to DNA DSBs. The expression dynamics of these genes switched from pulsatile to rising in response to periodic Nutlin-3 treatment (Figure 3C). Taken together, our results suggest that the dynamics of p53 target gene expression depend on the relationship between the mRNA decay rate of the gene and the p53 pulse frequency.
A subset of p53 target genes are coordinated in expression
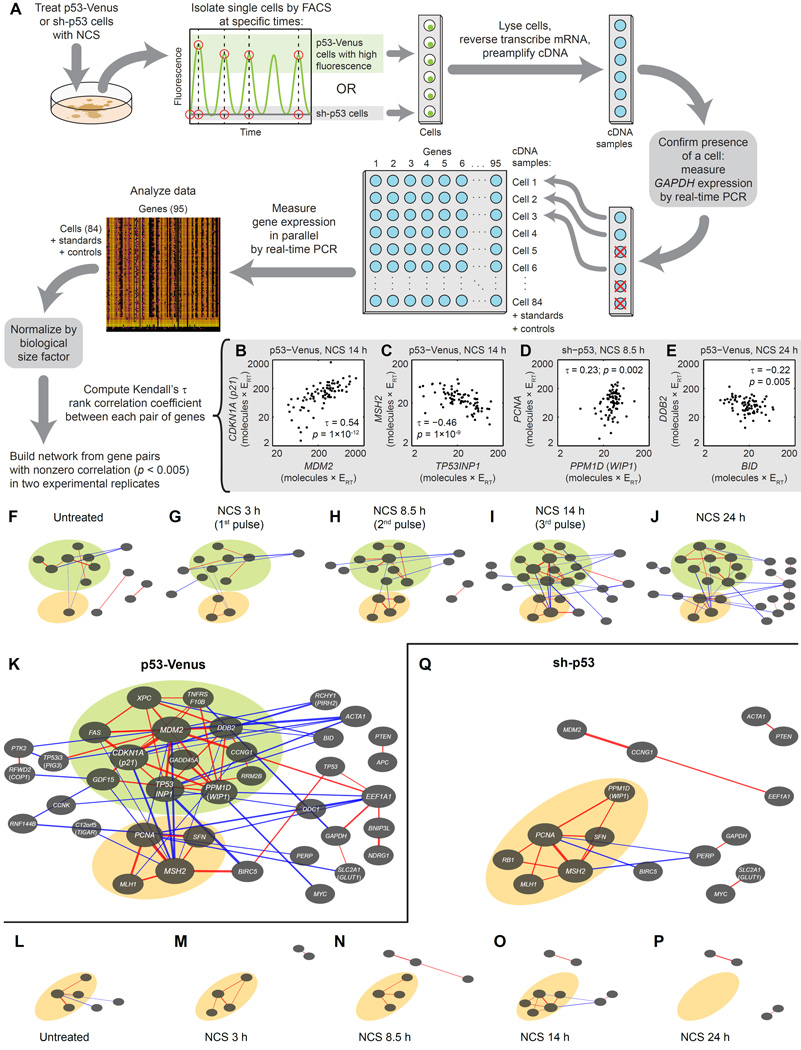
We next determined whether p53 coordinates the expression of its target genes, that is, whether it activates them in concert in individual cells, as other pulsatile transcription factors have been observed to do (Cai et al., 2008). Population-level measurements preclude measures of coordinated regulation because one cannot identify whether genes are being activated in the same subset of cells in the population; therefore, we measured target gene expression in individual cells. We treated MCF-7 cells expressing p53-Venus with NCS to induce DNA DSBs, then isolated the 15% brightest individual cells by fluorescence-activated cell sorting at times corresponding to the first, second, third, and fifth pulse peaks of p53 (Figure 4A). These criteria for p53-Venus intensity and time following NCS treatment corresponded to conditions for which nearly all cells showed the desired number of p53 pulses, as determined by analysis of time-lapse fluorescence microscopy movies (Supplemental Experimental Procedures) (Loewer et al., 2010). For comparison, we treated MCF-7 sh-p53 cells, in which p53 is knocked down, with NCS and sorted individual cells with low fluorescence at the same time points. In each of 1680 individual cells, we quantified transcript levels from the same set of p53 target genes as before using single-cell RT-qPCR (Figure S4A–B, Data Set S3). We verified the reproducibility of transcript measurements by calculating the discrepancy between technical replicates, from which we determined a median percent error of 6.95% (Figure S4C).
Figure 4.
Single-cell transcriptional profiling shows that p53 target genes are co-regulated in subnetworks in response to DNA DSBs. (A) MCF-7 cells expressing p53-Venus or with p53 knocked down (sh-p53) were treated with NCS, then sorted by FACS at specific times into lysis buffer. mRNA of 95 genes was reverse-transcribed, and the resulting cDNA was preamplified. Presence of a sorted cell was verified by measuring GAPDH amplification by real-time PCR. In samples passing this test, expression of 95 target genes was measured by real-time PCR. Measurements were normalized by the biological size factor for each cell (Experimental Procedures). (B–E) Examples of gene pairs with different correlation coefficients (Kendall’s τ), representing a relatively strong positive (B), strong negative (C), weak positive (D), and weak negative (E) correlation. Each point represents normalized gene expression in a single cell. All units are molecules × RT efficiency (ERT). p-values test the null hypothesis of no correlation. (F–J) Network diagrams of correlations between expression of genes (p < 0.005 in each of two biological replicates) in MCF-7 p53-Venus cells treated with NCS. Networks are shown for untreated cells (F) or cells sorted during the first (G), second (H), third (I) or fifth (J) pulse of p53. Each node in the network represents a gene, whose identity is shown in (K). Red and blue edges represent positive and negative correlations, respectively. Line thickness indicates strength of correlation as measured by Kendall’s τ. Node size represents the sum of correlations for a given gene. (K) Network diagram showing total of gene expression correlations for all time points (F–J). Green and orange regions represent sets of genes whose members are positively correlated with at least two other member genes and negatively correlated with nonmember genes based on analysis of all time points. (L–P) Network diagrams of correlations between expression of genes in MCF-7 sh-p53 (p53 knockdown) cells treated with NCS. Networks are shown for untreated cells (F) or cells sorted at times (M–P) corresponding to those in (G–J). Each node represents a gene, whose identity is shown in (Q). (Q) Network diagram showing total of gene expression correlations for all time points (L–P). Orange region represents a set of genes whose members are positively correlated with at least two other member genes and negatively correlated with nonmember genes based on analysis of all time points. See also Figure S4 and Data Set S3.
If p53 coordinates the expression of all its target genes, we would expect homogeneity in the gene expression patterns of individual cells with high p53 levels, that is, we would not expect to be able to identify subpopulations of these cells responding in fundamentally different ways. To assess the degree of homogeneity among p53-responsive cells, we performed unsupervised hierarchical clustering on the target gene expression profile for each cell (Supplemental Experimental Procedures, Figure S4D). Individual MCF-7 p53-Venus cells clustered separately from individual MCF-7 sh-p53 cells for the first three pulses following DNA damage; however, no distinct subpopulations were identified within p53-Venus or sh-p53 cells, consistent with the hypothesis that p53 coordinates expression of all its target genes.
Furthermore, if p53 coordinates expression of all its target genes, we would expect that much of the variation in gene expression between individual cells could be represented in a single dimension whose magnitude directly reflects p53 activity. Using principal component analysis (Supplemental Experimental Procedures), we found that the variance in gene expression, particularly during pulses subsequent to the first, could not be accounted for by a single principal component (Figure S4E–H). This suggests that p53 does not simply coordinate the expression of all its target genes; rather, any pattern of target gene coordination is more complex.
To look for complex patterns of p53 target gene coordination, we used our measurements of gene expression in individual cells to generate network diagrams showing pairwise correlations in gene expression. First, we normalized gene expression measurements by a biological size factor to account for variation due to cell size (Experimental Procedures). Next, we computed Kendall’s τ rank correlation coefficient between expression measurements from each pair of genes (examples shown in Figure 4B–E). Pairs of genes that had nonzero correlation coefficients (p < 0.005) with the same sign in two replicates of a given experiment were used to generate network diagrams (Figure 4F–Q).
Within these p53 target gene correlation networks, specific subsets of p53 targets were coordinated in expression. For each cell line, we identified subnetworks of genes whose members were positively correlated with at least two other member genes and negatively correlated with nonmember genes based on the analysis of all time points (Figure 4K, Q). In the p53-Venus cells, one such subnetwork (green in Figure 4F–K) was composed of functionally unrelated genes that primarily displayed pulsatile expression dynamics (9 of 12 genes, Figure 1A). This subnetwork was p53-dependent, as we did not identify it in sh-p53 cells (Figure 4Q). In p53-Venus cells, the time courses of mean expression of these genes fell into two categories: those whose expression peaked with the first pulse of p53 and then fell (8 of 12 genes, Figure S4I) and those whose expression rose with the first pulse of p53 and stayed high (4 of 12 genes). Correlation between expression of genes in this set was present even before DNA damage (5 edges), decreased during the first pulse of p53 (2 edges), increased during the second and third pulses (9 edges and 12 edges, respectively), and persisted 24 h after treatment (10 edges). Thus, the common feature of these genes is strong expression with the first pulse of p53 followed by strong coordination with successive pulses. This diversity of behavior stands in contrast to the total coordination of S. cerevisiae target genes downstream of the pulsatile transcription factor Crz1 (Cai et al., 2008).
We also identified a second subnetwork of correlated p53-target genes (orange in Figure 4F–K) primarily composed of genes involved in DNA repair (3 out of 4 genes). In contrast to the first subnetwork, this second subnetwork had a counterpart in sh-p53 cells with a similar set of genes (orange in Figure 4L–Q). This suggests that correlations between genes within this second subnetwork are independent of p53 in the DNA damage response. Notably, in p53-Venus cells, the first p53-dependent (pulsing) subnetwork became negatively correlated with the second p53-independent (DNA repair) subnetwork by the third pulse of p53. This suggests that after a sufficiently long (three-pulse) p53 response, coordinated DNA damage repair becomes opposed to other p53-dependent processes including apoptosis, cell cycle arrest, and senescence.
p53 pulsing attenuates target gene coordination
Having determined that specific subsets of p53 target genes are coordinated in response to DNA DSBs, we asked whether p53 pulsing contributes to this coordination. To alter p53 dynamics in the presence of DNA DSBs, we treated MCF-7 cells expressing p53-Venus with NCS, then followed NCS treatment with a sequence of Nutlin-3 treatments designed to yield a constant high level of p53 (Purvis et al., 2012). We confirmed by Western blotting that this treatment indeed eliminated p53 pulsing (Figure S5A–B). After subjecting cells to this treatment for 14 h, we measured expression of p53 target genes in individual cells (Figure 4A, Figure S4A–B, Data Set S3) and generated a network diagram of gene expression correlations (Figure 5).
Figure 5.
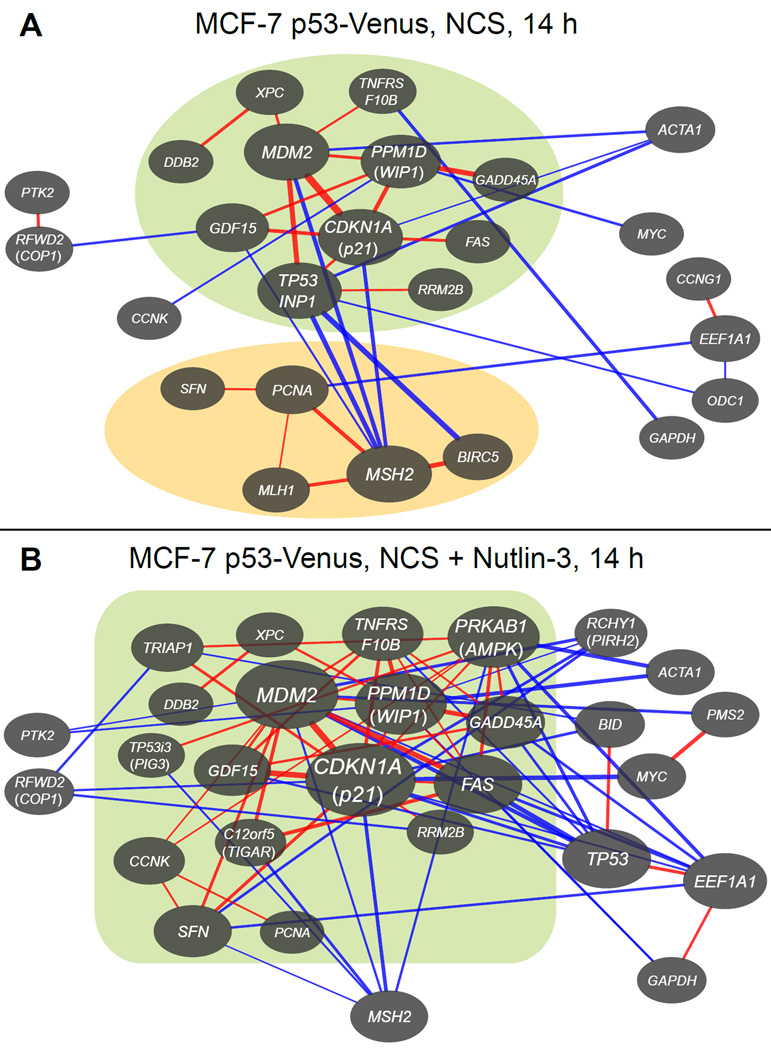
Sustained high p53 expression changes patterns of target gene co-regulation. Network diagrams of genes with correlated expression as determined by single-cell transcriptional profiling were generated as in Figure 4 for MCF-7 p53-Venus cells (A) treated with NCS for 14 h and (B) treated with NCS followed by sequential doses of Nutlin-3 designed to hold p53 at a high level. Green and orange regions represent sets of genes whose members are positively correlated with each other and negatively correlated with nonmember genes. See also Figure S5.
Holding p53 at a constant high level after DNA damage gave rise to an enlarged p53-dependent subnetwork. The number of coordinated genes in this subnetwork increased from 11 in response to p53 pulses to 17 in response to constant p53 levels (Figure 5). While the overall subnetwork density was approximately equivalent under both treatments (0.076 vs. 0.081, Experimental Procedures), the network clustering coefficient, or local density, was ~58% higher (0.134 vs. 0.085) when p53 was held constant. Of the 11 genes in the p53-dependent subnetwork produced in response to p53 pulses, 10 were retained in the subnetwork when p53 was held at a high level, and 6 of those were expressed at least 30% higher (Figure S5C). In contrast, 3 of the 5 genes in the p53-independent subnetwork were expressed at least 30% lower when p53 was held at a high level (Figure S5C), and the p53-independent subnetwork was eliminated (Figure 5). Together, these results suggest that p53 pulsing generates greater specificity in the p53-dependent subnetwork of the DNA DSB response while simultaneously enabling the coordination of a p53-independent subnetwork.
Discussion
p53 target genes perform low-pass filtering on p53 dynamics
Our results, taken together, show that p53 pulsing generates diverse target gene expression dynamics and that this diversity is governed by the value of the mRNA decay rate relative to the p53 pulse frequency. These results are consistent with our simple model of p53 transcriptional activity and similar to what has been observed in the NF-κB system (Hao and Baltimore, 2009; Tay et al., 2010).
Effectively, p53 target genes can act as low-pass filters for p53 dynamics with their mRNA decay rates as their cutoff frequencies. When the mRNA decay rate for a target gene is greater than the p53 pulse frequency, the expression profile of the gene pulses as a reflection of the frequency content of the p53 signal. By contrast, when a target gene’s mRNA decay rate is lower than the p53 pulse frequency, the pulsing component of p53 dynamics is filtered out, and gene expression levels rise more slowly over time due to a higher time-averaged level of p53. A similar filtering mechanism is likely to operate at the level of protein dynamics, as suggested by the variety of expression dynamics observed for p53 targets (Figure S1M–Q) (Batchelor et al., 2008; Lahav et al., 2004). Biological systems have been known to use low-pass filters to attenuate noise (Rao et al., 2002) and, in particular, to prevent transient inputs from activating a signaling pathway (Ashall et al., 2009; Toettcher et al., 2013). In the p53 system, low-pass filtering acts on the output of the p53 signaling pathway, decoding p53 dynamics to potentially impact cell fate decisions, similar to what has been observed downstream of the Ras/Erk pathway (Toettcher et al., 2013).
The dependence of target expression dynamics on mRNA decay rate implies that cells could readily evolve small changes to the p53 stress response via mutations affecting the mRNA decay rates of particular target genes, changing the pulsatility of the gene expression dynamics and changing the behavior of downstream components. In contrast, evolving a new p53 pulse frequency would be a large and likely undesirable change, as it would potentially alter the expression dynamics of many p53 target genes at once. This may explain why, in cells that show a pulsatile p53 response to DNA DSBs, the p53 pulse frequency has relatively low observed variability (Geva-Zatorsky et al., 2006) and is invariant to modifications in the network structure (Toettcher et al., 2010).
The list of transcription factors known to display pulsatile dynamics is rapidly growing (Dalal et al., 2014; Levine et al., 2013), and there are likely more such transcription factors to be identified. Transcription factors often regulate dozens of targets, some of which are regulated by multiple factors, forming what has been termed a dense overlapping regulon (Shen-Orr et al., 2002). If different transcription factors in a dense overlapping regulon, each pulsing at a different frequency, target the same gene, they could give rise to qualitatively different transcriptional profiles depending on whether their pulse frequencies exceed the mRNA decay rate of the gene. By such a mechanism, a single gene could generate different downstream responses to different transcription factors without the need for additional regulatory components. Future studies are required to determine whether such diversity in regulation is achieved by larger systems of pulsatile transcription factors.
p53 pulsing limits the coordination of the response to DNA DSBs
In contrast to the pulsatile transcription factor Crz1 in yeast, which coordinates the expression of the majority of its target genes in response to extracellular calcium (Cai et al., 2008), p53 coordinates expression of only a limited subset of its targets in response to DNA DSBs, and this coordination occurs in a complex pattern involving multiple subnetworks (Figure 4K). The different mechanistic consequences of p53 and Crz1 pulsing may arise from differences in the time scale of pulses relative to that of the overall responses. In the Crz1 system, each fixed-amplitude transcription factor pulse leads to the production of a fixed amount of mRNA for each target gene. Consequently, Crz1 target genes are expressed at constant ratios irrespective of pulse frequency or promoter differences between genes. Variation in pulse amplitude or noise in promoter function would distort the amounts of mRNA produced and thus distort the constant expression ratios for a given pulse, but these relationships still hold on average over a response comprising many short-duration pulses (~1–3 min each, Cai et al. (2008)). In the p53 system, however, pulses are of longer duration, and expression of many target genes reaches a maximum even before the end of the first pulse (Figure 1A, Figure S4I). These conditions strongly suggest that the physiologically relevant DNA damage response begins after very few p53 pulses or even before the first p53 pulse is completed. In this time frame, cells do not have the benefit of averaging the results of many pulses; thus, the relatively long pulse duration and the considerable variation in the p53 pulse amplitude (Geva-Zatorsky et al., 2006) cause genes to be expressed in different ratios between cells. This explanation is consistent with our observation that coordination of p53 target genes increases with successive p53 pulses following DNA damage (Figure 4G–J).
What, then, does p53 pulsing contribute to coordination of target gene expression? We found that eliminating p53 pulsing by holding p53 levels high with Nutlin-3 leads to a single, larger, more densely connected subnetwork of coordinated target genes, in contrast to the two separate subnetworks found when p53 is pulsing (Figure 5). Thus, p53 pulses confer greater selectivity in the coordination of downstream targets than would occur if p53 were activated with a constant amplitude. This supports the idea that the network downstream of p53 has a complex sub-network architecture, which is likely to be necessary for p53 to orchestrate the complex decisions inherent to the DNA damage response.
Our results establish that specific subnetworks of p53 target genes are correlated in expression at the level of individual cells, but they do not establish causality. The correlations could be generated by p53, by another factor regulated by p53 that in turn regulates other target genes, or by target genes influencing each other’s expression. Identifying specific modes of regulation for members of the target gene subnetworks that we have identified will likely depend on more targeted studies tracking specific subnetwork components over time in individual cells. Future work refining our understanding of the dynamic coordination of p53 targets in specific subnetworks, the impact of target gene dynamics on cell fate decisions, and the disruption of those dynamics in specific disease contexts will likely provide novel strategies for therapeutic manipulation of the p53 response in cancer treatment.
Experimental Procedures
Cell lines and culture
MCF-7 breast carcinoma cells expressing fluorescently tagged p53 (p53-Venus) (Batchelor et al., 2008) or with p53 knocked down by an shRNA (sh-p53) (Brummelkamp et al., 2002) were maintained at 37°C and 5% CO2 in RPMI containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, 100 µg/mL streptomycin, and 250 ng/mL amphotericin B (Corning 30-004-CI).
Population-level gene expression measurements
4×105 p53-Venus cells or 8×105 sh-p53 cells were plated on a 6-cm dish two days prior to treatment. Cells were treated with 400 ng/mL neocarzinostatin (Sigma, N9162), which induces DNA double-strand breaks within 2–4 min (Shiloh et al., 1983), and/or Nutlin-3 (Sigma, N6287), which inhibits the interaction between p53 and its E3 ubiquitin ligase Mdm2 (Vassilev et al., 2004). At indicated times following treatment, cells were harvested by scraping and frozen in a dry ice-ethanol bath. RNA was extracted using QIAshredder and RNeasy Kits (Qiagen). For each sample, RNA concentration was determined using a UV spectrophotometer, and 604.8 ng of RNA was used to generate complementary DNA in a 20 µL reaction using an Invitrogen High Capacity cDNA Reverse Transcription Kit (Life Technologies, 4374966). Expression of 95 genes (Table S1) was then measured in duplicate for each sample by qPCR using a 96.96 Dynamic Array on a BioMark™ system (Fluidigm) according to the manufacturer’s instructions (Fluidigm, 100-4109 B1). Samples and assays were subjected to thermal mixing (70°C for 40 min, 60°C for 30 s), hot start (95°C for 1 min), and 35 cycles of PCR (96°C for 5 s, 64°C for 20 s), followed by melt curve acquisition (64–95°C with 0.5°C resolution).
qPCR measurements were processed using Fluidigm Real-Time PCR Analysis software. For each gene, samples with a melt peak greater than 0.5°C from the median of melt peaks were discarded. Relative expression values were computed from Ct values assuming a PCR efficiency of 100%. Duplicate measurements of gene expression were normalized to measurements of GAPDH expression and averaged.
Mathematical model of transcription in single cells
We modeled transcription of a p53 target gene by the following differential equation:
Briefly, target gene mRNA is produced as a cooperative function of p53 concentration and decays at a rate proportional to its own concentration. In our simulations we used kp = 1, K = 3, and n = 2. Simulations with different kd values were performed using MATLAB (MathWorks, Natick, MA).
Fluorescence-activated cell sorting
4×105 MCF-7 p53-Venus cells or 8×105 MCF-7 sh-p53 cells were plated on a 6-cm dish two days prior to sorting. After treatment as described above, cells were harvested by trypsinization approximately 45 minutes prior to cell sorting and resuspended in PBS containing 2% FBS. Single cells were sorted into 96-well PCR plates with a BD FacsAria I, a BD FacsAria IIu, or a BD Influx (Becton Dickinson, Franklin Lakes, NJ). For treated p53-Venus cells, the 15% most fluorescent cells were sorted; for untreated (control) p53-Venus cells and for all sh-p53 cells, the 50% least fluorescent cells were sorted. Cells were sorted directly into 9 µL of lysis buffer consisting of 5 µL of CellsDirect™ 2× Reaction Mix, 0.2 µL of SuperScript® III RT/Platinum® Taq Mix (Life Technologies, 11753-500), and 3.8 µL of water; this was supplemented with 55.6 nM of each primer (Table S1), 0.05 U of SUPERase-IN™ RNase Inhibitor (Life Technologies, AM2694), 6.2 pg of E. coli genomic DNA (Affymetrix, 14380 10 MG), 3.3 mM Tris, and 33 µM EDTA, pH 8.0. Each plate was centrifuged for 30 s (2500 RCF, 4°C) after sorting. Cell lysates were subjected to reverse transcription (50°C for 15 min), hot start (95°C for 2 min), and 20 cycles of PCR for cDNA preamplification (95°C for 15 s, 64°C for 4 min). To remove excess primers, each sample was then supplemented with 3.6 µL of exonuclease mix consisting of 0.72 µL Exonuclease I, 0.36 µL Exonuclease I Reaction Buffer (New England BioLabs, M0293L), and 2.52 µL of water, then subjected to exonuclease digestion (37°C for 30 min) and inactivation (80°C for 15 min). Finally, each sample was diluted with TE Buffer to a final volume of 45 µL and stored at −20°C.
Single-cell gene expression measurements
To verify the presence of a sorted cell in each well, levels of GAPDH expression in each sample were measured by qPCR and compared with the standards on each plate. Samples with GAPDH amplicon exceeding 50 molecules × RT efficiency were marked for further analysis. For each cell line, treatment, and biological replicate, expression of 95 genes (Table S1) was measured in 84 single-cell samples plus standards by qPCR using a 96.96 Dynamic Array on a BioMark™ system (Fluidigm) according to the manufacturer’s instructions (Fluidigm, 100-4109 B1). Samples and assays were subjected to the same thermal cycling as described under “Population-level gene expression measurements.”
qPCR measurements were processed using Fluidigm Real-Time PCR Analysis software. For each gene, a target melt peak temperature was computed as the median of melt peak temperatures of standards flagged as passing by the software. Samples with a melt peak more than 0.5°C from this target temperature were discarded. PCR efficiency for each gene was computed from the Ct values of standards flagged as passing; genes with PCR efficiency > 115% or R2 < 0.98 were discarded. PCR efficiencies were used to estimate the expression of each gene in each sample in molecules × RT efficiency. Genes with fewer than 3 unique standard measurements flagged as passing were discarded. Measurements below 1 molecule × RT efficiency were considered to be zero, and samples with fewer than 30 (out of 95 possible) passing nonzero measurements were discarded. Samples with a GAPDH measurement below 30 molecules × RT efficiency were also discarded.
Computation of correlation networks
For computing correlation networks, genes with more than 80% of their measurements missing in any experiment and genes that had all zero measurements in any experiment were not considered. To compensate for differences in cell size, we computed the biological size factor for each cell, which estimates its overall level of gene expression, and normalized all gene expression measurements by this factor (Anders and Huber, 2010; Brennecke et al., 2013; Robinson and Oshlack, 2010). For each experiment, normalized gene expression measurements were transformed to log scale and used to compute Kendall’s τ rank correlation coefficient between each pair of genes. All calculations were performed using custom software in MATLAB (MathWorks, Natick, MA). Network diagrams were generated using Cytoscape (Shannon et al., 2003).
Supplementary Material
Acknowledgments
We thank W. Telford and V. Kapoor in the Center for Cancer Research (CCR) ETIB Flow Cytometry Core for aid in single cell sorting, as well as M. Raffeld and the CCR Molecular Diagnostics Unit and J. Zhu and the NHLBI DNA Sequencing and Genomics Core for help in single cell transcriptional profiling. We also thank D. Levens and the Levens lab, A. Moody, A. Sun, T. Przytycka, C. Parent, J. Oberholtzer, A. Loewer, L. Goentoro, J. Hansen, J. Stommel, and J. Luo for helpful discussions.
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, CCR.
The authors declare no competing financial interests.
Footnotes
Author Contributions
J.P. and E.B. conceived the study and designed experiments. J.P., B.F., and E.B. performed experiments. J.P. and E.B. analyzed data and wrote the paper.
References
- Allen MA, Andrysik Z, Dengler VL, Mellert HS, Guarnieri A, Freeman JA, Sullivan KD, Galbraith MD, Luo X, Kraus WL, et al. Global analysis of p53-regulated transcription identifies its direct targets and unexpected regulatory mechanisms. eLife. 2014;3:e02200+. doi: 10.7554/eLife.02200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106+. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashall L, Horton CA, Nelson DE, Paszek P, Harper CV, Sillitoe K, Ryan S, Spiller DG, Unitt JF, Broomhead DS, et al. Pulsatile Stimulation Determines Timing and Specificity of NF-κB-Dependent Transcription. Science. 2009;324:242–246. doi: 10.1126/science.1164860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylon Y, Oren M. New plays in the p53 theater. Curr. Opin. Genet. Dev. 2011;21:86–92. doi: 10.1016/j.gde.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev Bar-Or R, Maya R, Segel LA, Alon U, Levine AJ, Oren M. Generation of oscillations by the p53-Mdm2 feedback loop: A theoretical and experimental study. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11250–11255. doi: 10.1073/pnas.210171597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor E, Mock CS, Bhan I, Loewer A, Lahav G. Recurrent initiation: a mechanism for triggering p53 pulses in response to DNA damage. Mol. Cell. 2008;30:277–289. doi: 10.1016/j.molcel.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor E, Loewer A, Lahav G. The ups and downs of p53: understanding protein dynamics in single cells. Nat. Rev. Cancer. 2009;9:371–377. doi: 10.1038/nrc2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennecke P, Anders S, Kim JK, Kolodziejczyk AA, Zhang X, Proserpio V, Baying B, Benes V, Teichmann SA, Marioni JC, et al. Accounting for technical noise in single-cell RNA-seq experiments. Nat. Methods. 2013;10:1093–1095. doi: 10.1038/nmeth.2645. [DOI] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R. A System for Stable Expression of Short Interfering RNAs in Mammalian Cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Cai L, Dalal CK, Elowitz MB. Frequency-modulated nuclear localization bursts coordinate gene regulation. Nature. 2008;455:485–490. doi: 10.1038/nature07292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal CK, Cai L, Lin Y, Rahbar K, Elowitz MB. Pulsatile Dynamics in the Yeast Proteome. Curr. Biol. 2014;24:2189–2194. doi: 10.1016/j.cub.2014.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geva-Zatorsky N, Rosenfeld N, Itzkovitz S, Milo R, Sigal A, Dekel E, Yarnitzky T, Liron Y, Polak P, Lahav G, et al. Oscillations and variability in the p53 system. Mol. Sys. Biol. 2006;2 doi: 10.1038/msb4100068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AS, O’Shea EK. Promoter decoding of transcription factor dynamics involves a trade-off between noise and control of gene expression. Mol. Sys. Biol. 2014;9:704. doi: 10.1038/msb.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao N, O’Shea EK. Signal-dependent dynamics of transcription factor translocation controls gene expression. Nat. Struct. Mol. Biol. 2012;19:31–39. doi: 10.1038/nsmb.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S, Baltimore D. The stability of mRNA influences the temporal order of the induction of genes encoding inflammatory molecules. Nat. Immunol. 2009;10:281–288. doi: 10.1038/ni.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahav G, Rosenfeld N, Sigal A, Geva-Zatorsky N, Levine AJ, Elowitz MB, Alon U. Dynamics of the p53-Mdm2 feedback loop in individual cells. Nat. Genet. 2004;36:147–150. doi: 10.1038/ng1293. [DOI] [PubMed] [Google Scholar]
- Levine JH, Fontes ME, Dworkin J, Elowitz MB. Pulsed Feedback Defers Cellular Differentiation. PLoS Biol. 2012;10:e1001252+. doi: 10.1371/journal.pbio.1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JH, Lin Y, Elowitz MB. Functional Roles of Pulsing in Genetic Circuits. Science. 2013;342:1193–1200. doi: 10.1126/science.1239999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewer A, Batchelor E, Gaglia G, Lahav G. Basal Dynamics of p53 Reveal Transcriptionally Attenuated Pulses in Cycling Cells. Cell. 2010;142:89–100. doi: 10.1016/j.cell.2010.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DE, Ihekwaba AEC, Elliott M, Johnson JR, Gibney CA, Foreman BE, Nelson G, See V, Horton CA, Spiller DG, et al. Oscillations in NF-κB Signaling Control the Dynamics of Gene Expression. Science. 2004;306:704–708. doi: 10.1126/science.1099962. [DOI] [PubMed] [Google Scholar]
- Purvis JE, Karhohs KW, Mock C, Batchelor E, Loewer A, Lahav G. p53 dynamics control cell fate. Science. 2012;336:1440–1444. doi: 10.1126/science.1218351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao CV, Wolf DM, Arkin AP. Control, exploitation and tolerance of intracellular noise. Nature. 2002;420:231–237. doi: 10.1038/nature01258. [DOI] [PubMed] [Google Scholar]
- Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat. Rev. Mol. Cell Biol. 2008;9:402–412. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- Robinson MD, Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010;11:R25+. doi: 10.1186/gb-2010-11-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selimkhanov J, Taylor B, Yao J, Pilko A, Albeck J, Hoffmann A, Tsimring L, Wollman R. Accurate information transmission through dynamic biochemical signaling networks. Science. 2014;346:1370–1373. doi: 10.1126/science.1254933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Orr SS, Milo R, Mangan S, Alon U. Network motifs in the transcriptional regulation network of Escherichia coli. Nat. Genet. 2002;31:64–68. doi: 10.1038/ng881. [DOI] [PubMed] [Google Scholar]
- Shiloh Y, Schans GP, van der, Lohman PHM, Becker Y. Induction and repair of DNA damage in normal and ataxiatelangiectasia skin fibroblasts treated with neocarzinostatin. Carcinogenesis. 1983;4:917–921. doi: 10.1093/carcin/4.7.917. [DOI] [PubMed] [Google Scholar]
- Tay S, Hughey JJ, Lee TK, Lipniacki T, Quake SR, Covert MW. Single-cell NF-κB dynamics reveal digital activation and analogue information processing. Nature. 2010;466:267–271. doi: 10.1038/nature09145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toettcher JE, Mock C, Batchelor E, Loewer A, Lahav G. A synthetic–natural hybrid oscillator in human cells. Proc. Natl. Acad. Sci. U. S. A. 2010;107:17047–17052. doi: 10.1073/pnas.1005615107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toettcher JE, Weiner OD, Lim WA. Using Optogenetics to Interrogate the Dynamic Control of Signal Transmission by the Ras/Erk Module. Cell. 2013;155:1422–1434. doi: 10.1016/j.cell.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, et al. In Vivo Activation of the p53 Pathway by Small-Molecule Antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.