Abstract
We investigate sensing and signaling mechanisms for H+, and CO2 in basilar arteries using out-of-equilibrium solutions. Selectively varying pHo, []o, or pCO2, we find: (a) lowering pHo attenuates vasoconstriction and vascular smooth muscle cell (VSMC) Ca2+-responses whereas raising pHo augments vasoconstriction independently of VSMC [Ca2+]i, (b) lowering []o increases arterial agonist-sensitivity of tone development without affecting VSMC [Ca2+]i but c) no evidence that CO2 has direct net vasomotor effects. Receptor protein tyrosine phosphatase (RPTP)γ is transcribed in endothelial cells, and direct vasomotor effects of are absent in arteries from RPTPγ-knockout mice. At pHo 7.4, selective changes in []o or pCO2 have little effect on pHi. At pHo 7.1, decreased []o or increased pCO2 causes intracellular acidification, which attenuates vasoconstriction. Under equilibrated conditions, anti-contractile effects of CO2/ are endothelium-dependent and absent in arteries from RPTPγ-knockout mice. With CO2/ present, contractile responses to agonist-stimulation are potentiated in arteries from RPTPγ-knockout compared to wild-type mice, and this difference is larger for respiratory than metabolic acidosis. In conclusion, decreased pHo and pHi inhibit vasoconstriction, whereas decreased []o promotes vasoconstriction through RPTPγ-dependent changes in VSMC Ca2+-sensitivity. serves dual roles, providing substrate for pHi-regulating membrane transporters and modulating arterial responses to acid–base disturbances.
Keywords: Vascular biology, basic science, calcium imaging, confocal microscopy, electrophysiology, endothelium, experimental, pH, physiology, receptors, smooth muscle
Introduction
Extracellular acid–base disturbances modify cerebral artery tone and contribute to metabolic regulation of blood flow.1 Arterial tone is influenced by systemic acid–base disturbances, but is especially sensitive to local accumulation of acid equivalents when blood flow is insufficient to meet the metabolic demand. Although Gaskell first described the vascular response to extracellular acid–base disturbances in the late nineteenth century,2 the cellular mechanisms linking altered metabolic state and acid–base disturbances to changes in resistance artery function are still not well understood.
CO2 and constitute the most important extracellular buffer system under physiological circumstances. However, due to the reactions CO2 + H2O ⇄ H2CO3 ⇄ + H+, the concentrations of the individual buffer components cannot be varied independently under equilibrated conditions, and their separate signaling roles in the vasculature have not thus far been resolved. In this study, we use out-of-equilibrium (OOE) CO2/ solutions to determine the separate vasomotor effects of H+, and CO2. The OOE technique is based on rapid mixing and delivery of solutions with different buffer compositions, and exploits the relatively slow kinetics of the reaction CO2 + H2O ⇄ H2CO3.3 Keeping the time delay from the point of mixing to the tissue below 100 ms, the chemical interconversion among CO2/ buffer components is negligible, allowing us to control, accurately and independently, the pH, [], and pCO2 of the delivered solution.3
Investigators have proposed several mechanisms for interactions of acid-base disturbances with vascular tone. Arguing in favor of direct effects of extracellular H+ () on tone development in small arteries, acidosis inhibits active tension development both in the presence and absence of CO2/.4 It should be noted, however, that in the relaxed state as well as during contraction, vascular smooth muscle cells (VSMCs) in mouse small arteries have substantially lower intracellular pH (pHi) when CO2/ is omitted from the extracellular solutions.5 As a consequence, findings obtained under CO2/-free conditions may not apply to physiological or pathophysiological circumstances. Some have argued that CO2 and/or directly affect vasomotor function of both systemic and pulmonary arteries,6,7 although others have suggested that CO2 and affect artery tone only indirectly, through changes in pH.8 Interpreting these studies is complicated, however, by the chemical reactions interlinking the CO2/ buffer components. Because the chemical interconversion between H+, , and CO2 has not always been appreciated6 and because consequences of altering pH, pCO2 and [] are likely to be mutually interdependent, firm conclusions regarding direct vascular effects of the individual CO2/ buffer components cannot be drawn from the existing reports.
A number of candidate molecules involved in acid–base sensing have been suggested, including G-protein coupled receptors (OGR1, GPR4, TDAG8)9 and ion channels (ASICs)10 sensitive to pHo. The activities of several intracellular enzymes (soluble adenylyl cyclase,11 rho-kinase12 and NO-synthase12,13) also depend on [] or pH. Additionally, the receptor protein tyrosine phosphatase (RPTP)γ has been proposed to serve as sensor for extracellular CO2 or in renal proximal tubules.14 RPTPγ contains an extracellular domain with sequence homology to the carbonic anhydrases although the domain is predicted to lack carbonic anhydrase activity.14,15
Considering the complex nature of acid–base disturbances and their substantial clinical implications, in the present study we employ OOE CO2/ solutions to elucidate the individual vasomotor effects of altered pHo, []o, and pCO2, as well as the underlying signaling mechanisms. We demonstrate for the first time that has direct vasomotor effects mediated by changes in VSMC Ca2+-sensitivity, and dependent on RPTPγ. With []o in the physiological range, RPTPγ activates endothelium-dependent vasorelaxant signaling cascades that are inhibited when []o is reduced. We also show that isolated changes in pHo—in the presence of CO2/—have major effects on vasomotor responses, mediated in part through voltage-dependent Ca2+ channels. In addition, the combinations of low pHo and high pCO2 (respiratory acidosis) or low pHo and low []o (metabolic acidosis) cause pHi decreases that inhibit vasoconstriction, mediated by decreased VSMC Ca2+ sensitivity.
Methods
Male C57BL/6 wild-type or RPTPγ-knockout mice (>7 weeks old)—kept in the animal facilities at Aarhus and Case Western Reserve Universities—were euthanized by cervical dislocation. The RPTPγ-knockout mice were generously provided by Dr Joseph Schlessinger, Yale University, USA,16 and back-crossed for six generations with the wild-type line. The animal protocols were approved by the Danish Animal Experiments Inspectorate (2009/562-56) and the Institutional Animal Care and Use Committee at Case Western Reserve University (2013-0172) that conforms to the Guide for the Care and use of Laboratory Animals published by the US National Institutes of Health. The reporting of the animal experiments conforms with the ARRIVE guidelines. Mouse breeding was performed by crossing heterozygous male and female mice in temperature-controlled facilities employing 12-h light/12-h dark cycles with food and drinking water ad libitum.
Mesenteric and basilar arteries (inner diameter approximately 200 µm) were dissected free from surrounding tissue under a stereo microscope. Whenever possible, experiments on arteries from wild-type and RPTPγ-knockout mice were performed in parallel.
Isometric myography and out-of-equilibrium solutions
For studies based solely on equilibrated solutions (i.e. membrane potential recordings, Figures 5(f) and (i) and 6), arteries were mounted on 40-µm stainless steel wires in a one-channel (320A; DMT, Denmark) or four-channel (610M; DMT) wire myograph and investigated under no-flow conditions. The equilibrated CO2/-containing physiological salt solution (PSS) used for these studies was composed (in mM) of 119 NaCl, 22 NaHCO3, 10 HEPES, 1.2 MgSO4, 2.82 KCl, 5.5 glucose, 1.18 KH2PO4, 0.03 ethylenediaminetetraacetic acid (EDTA) and 1.6 CaCl2. Solutions with reduced amounts of, or without, were produced by substituting NaHCO3 with NaCl. Equilibrated CO2/-containing solutions were aerated with a gas mixture of 5 or 10% CO2/balance air, whereas CO2/-free solutions were bubbled with air (i.e. 21% O2 balance N2).
Figure 5.
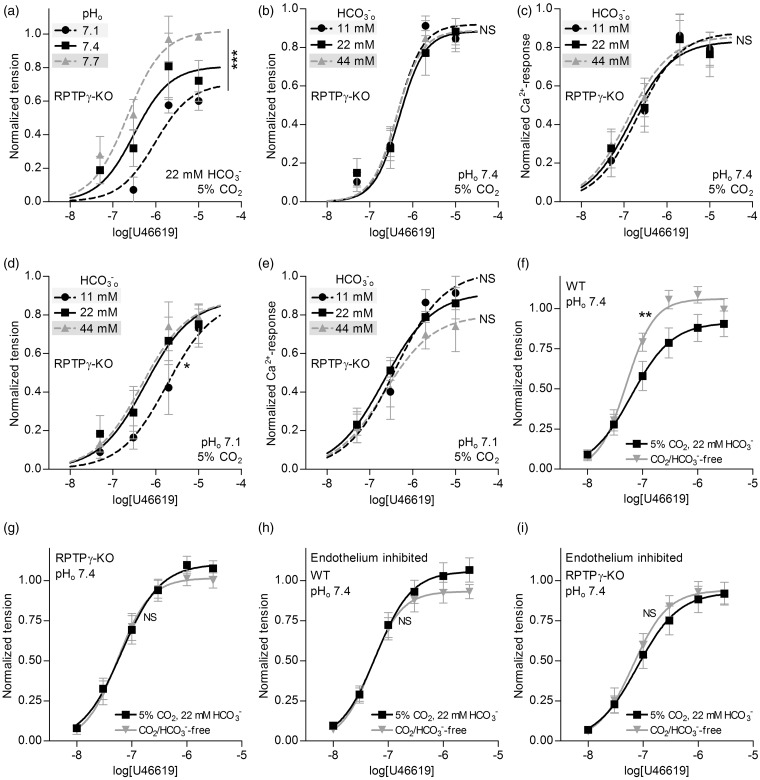
Basilar arteries from RPTPγ-knockout mice are insensitive to changes in []o. (a, b) Effects of selectively varying pHo or []o (maintaining the other at its control level, with CO2 fixed at 5%) on U46619-induced tension development in basilar arteries from RPTPγ-knockout mice (n = 4 in panel (a), n = 8 in panel (b)). (c) Effects of selectively varying []o (maintaining pHo at 7.4, CO2 at 5%) on U46619-induced VSMC Ca2+-responses in basilar arteries from RPTPγ-knockout mice (n = 8). (d) Effects of selectively varying []o at low pHo (maintaining pHo at 7.1, CO2 at 5%) on U46619-induced tension development of basilar arteries from RPTPγ-knockout mice (n = 6). (e) Effects of selectively varying []o at low pHo (maintaining pHo at 7.1, CO2 at 5%) on U46619-induced VSMC Ca2+-responses in basilar arteries from RPTPγ-knockout mice (n = 4). (f-i). Effects of omitting CO2/ on U46619-induced tension development at constant pHo of 7.4. We perform experiments with basilar arteries from wild-type (WT, panels (f) and (h)) and RPTPγ-knockout (KO, panels (g) and (i)) mice. In panel (h) and (i), we inhibit vasorelaxant effects of the endothelium by incubation with 100 µM L-NAME, 3 µM indomethacin, 50 nM apamin and 1 µM TRAM-34. To avoid development of resting tone after inhibition of endothelium-dependent vasorelaxation, we add 600 nM SNAP to achieve an equal [NO] in the tested arteries. The curves are the results of least-squares fits to sigmoidal functions, and we compare them using extra sum-of-squares F-tests. *P < 0.05, **P < 0.01, ***P < 0.001, NS: not significantly different vs. control conditions (panels (a–c) and (f–i): pHo=7.4, []o=22 mM, CO2=5%) or standard acidic conditions (panels (d) and (e): pHo=7.1, []o=22 mM, CO2=5%).
Figure 6.
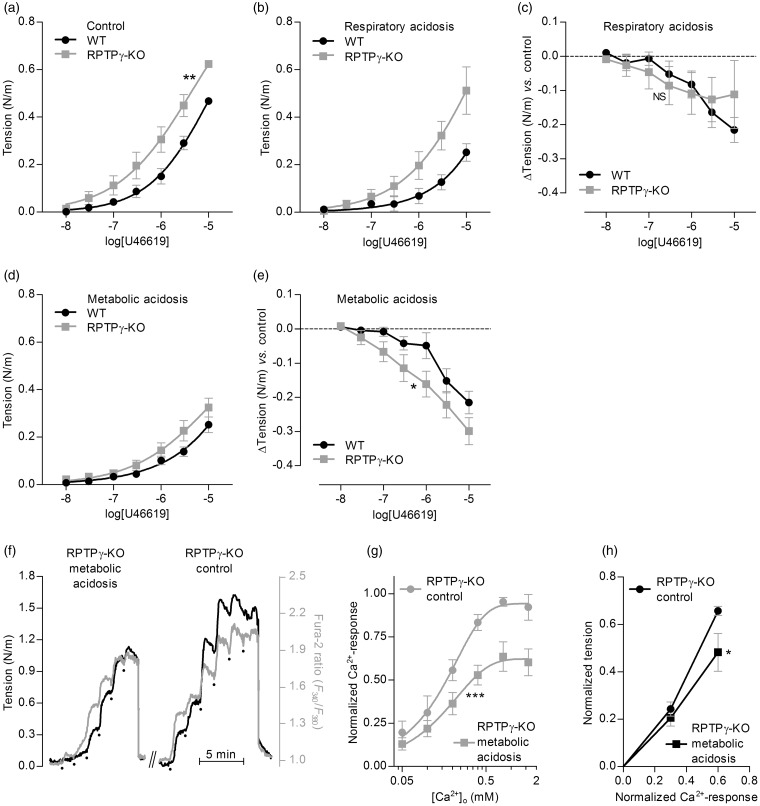
Under equilibrated conditions, RPTPγ is required for sensing—such that basilar arteries from RPTPγ-knockout mice are hypercontractile—and vasorelaxation in response to metabolic acidosis is explained by a combination of attenuated Ca2+ uptake and reduced Ca2+-sensitivity. (a–e) Comparison of U46619-induced contractile responses of basilar arteries from RPTPγ-knockout (KO, n=8) vs. wild-type (WT, n = 10) mice. Panel (a) summarizes experiments under control conditions (pHo=7.4, []o=22 mM, CO2=5%); panel (b), under respiratory acidosis (pHo=7.1, []o=22 mM, CO2=10%); and panel (d), under metabolic acidosis (pHo=7.1, []o=11 mM, CO2=5%). Panels (c) and (e) summarize the average differences in U46619-induced contractile responses in basilar arteries from RPTPγ-knockout and wild-type mice during respiratory (panel c) and metabolic (panel e) acidosis compared to control conditions. (f) Representative time courses of arterial tension development and VSMC Ca2+-dependent fluorescence signal in Ca2+-depleted basilar arteries from RPTPγ-knockout mice in response to step-increases of [Ca2+]o during constant exposure to 10 µM U46619. We perform experiments under control conditions (pHo=7.4, []o=22 mM, CO2=5%) and during metabolic acidosis (pHo=7.1, []o=11 mM, CO2=5%). The dots indicate step-wise increments in [Ca2+]o from 0 to 0.05, 0.1, 0.2, 0.4, 0.8 and 1.6 mM. (g) Average -responses from experiments (n = 5) like that in panel (f). (h) Average tension development of basilar arteries from RPTPγ-knockout mice (n = 5) as a function of the VSMC -response under control (Continued) Figure 6. Continue. conditions (pHo=7.4, []o=22 mM, CO2=5%) and during metabolic acidosis (pHo=7.1, []o=11 mM, CO2=5%). The curves in panels (a), (b), (d) and (g) are the results of least-squares fits to sigmoidal functions; in panels (a) and (g), we compare them using extra sum-of-squares F-tests. The data points in panels (c), (e) and (h) are compared by repeated-measures two-way ANOVA; in panel (h), we report the significance level based on Bonferroni post-tests. *P < 0.05, **P < 0.01, ***P < 0.001, NS: not significantly different vs. wild-type (panels (a), (c) and (e)) or control conditions (panels (g) and (h)).
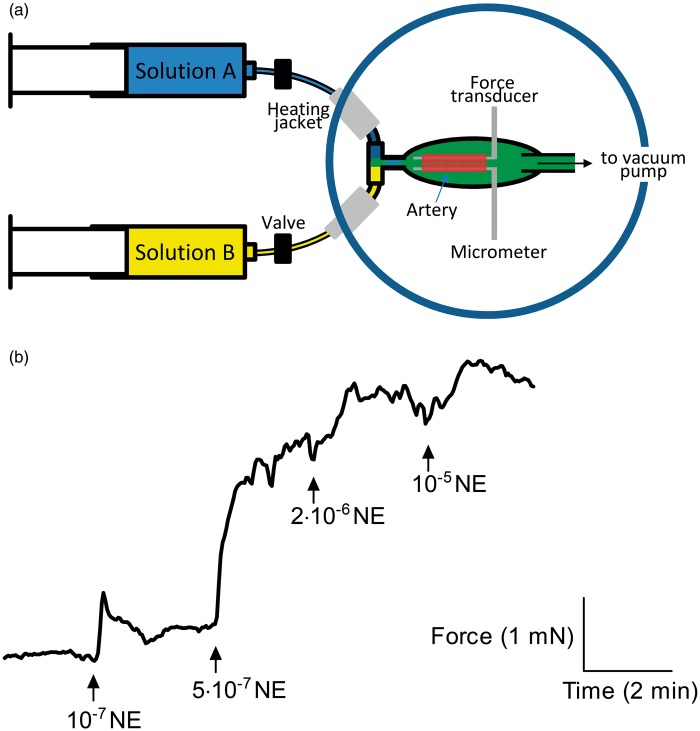
For studies based fully or partly on OOE technology (i.e. Figures 1–3, 5(a–e) and Supplemental Figure 1), the protocol was similar to that described above except for the following. First, OOE solutions3 were created in real time by rapidly mixing two precursor solutions (e.g. solutions 4a and 4b or 5a and 5b, see Supplemental Table 1) with dissimilar CO2//pH compositions. Thus, at the instant of mixing, the CO2//pH composition (e.g. solutions 4Mix or 5Mix) was out of equilibrium. The compositions of the precursor solutions and the instantaneous concentrations after mixing are summarized in Supplemental Table 1. With judicious choice of the “a” and “b” solutions, it is possible to design “Mix” solutions with any CO2//pH combination in the pathophysiological range, making it possible for example to vary pCO2, [], or pH one at a time while holding the other two parameters fixed. The overall reaction CO2 + H2O ⇄ + H+ is so slow that the disequilibrium state degrades only slightly over the course of several hundred milliseconds. Second, arteries were mounted on 50-µm tungsten pins in the heated (37 ℃) stainless steel chamber of a custom-built myograph (CW121; DMT) that was designed to allow rapid mixing of two heated (37 ℃) precursor solutions (e.g. 4a and 4b) through a nylon mesh (see Figure 1a). The transit time from the point of mixing at the mesh to the artery in the chamber was <100 ms. The precursor solutions were delivered through CO2-impermeable Tygon® tubing at a rate of 7 mL/min via Pump 33 dual syringe pumps (Harvard Apparatus; MA, USA), and the mixed solutions were continuously removed by a vacuum pump. Solutions with elevated [K+] were obtained by substituting NaCl with KCl; and when applied to mesenteric arteries, 100 nM of the α1-adrenergic antagonist prazosin was added to avoid effects of norepinephrine released from depolarized nerve endings.
Figure 1.
Application of OOE CO2/ solutions to small-artery myography. (a) Schematic showing generation of OOE solutions by rapid mixing and delivery of differently composed CO2/ solutions. The time delay from the point of mixing to the artery is <100 ms. See Supplemental Methods for details. (b) Force recordings from a mesenteric artery exposed to increasing concentrations of norepinephrine (NE) under OOE conditions (pHo=7.7, []o=22 mM, CO2=5%).
Figure 2.
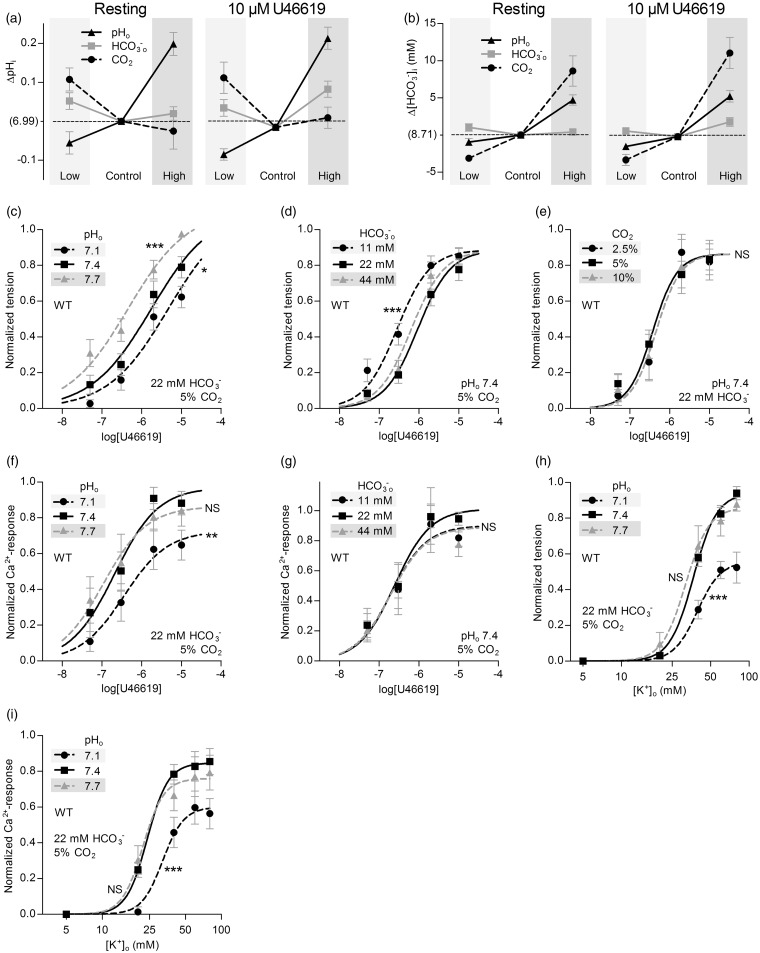
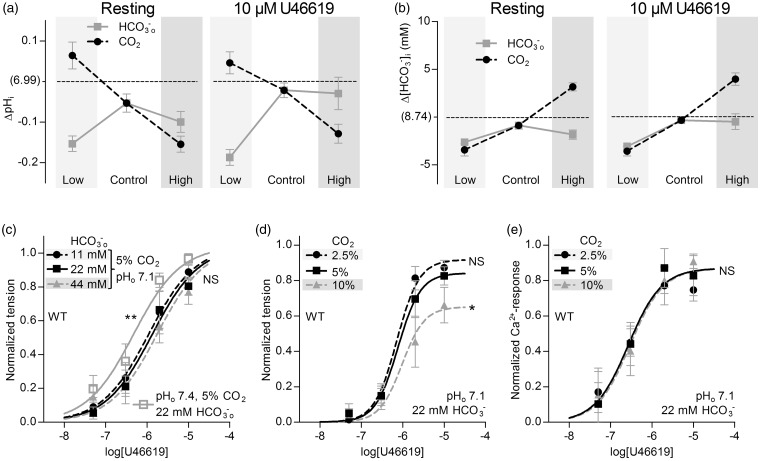
Selective changes in []o or pHo—but not pCO2—directly modulate basilar artery tone under OOE conditions. (a) Effects of selectively varying pHo, []o or pCO2 (maintaining other two at control levels) on VSMC pHi in resting basilar arteries (left panel) or basilar arteries contracted by 10 µM U46619 (right panel). Under “Control” conditions, CO2 is 5%, pHo 7.4, and []o 22 mM. Compared to “Control”, “Low” refers to selective changes in CO2 to 2.5%, []o to 11 mM or pHo to 7.1, and “High” refers to selective changes in CO2 to 10%, []o to 44 mM or pHo to 7.7. Arteries are from wild-type mice (n = 6). (b) Effects of selectively varying pHo, []o or pCO2 (maintaining other two at control levels) on VSMC calculated []i in resting (Continued) Figure 2. Continue. basilar arteries (left panel) or basilar arteries contracted by 10 µM U46619 (right panel). Arteries are from wild-type mice (n = 6). (c-e) Effects of selectively varying pHo, []o, or pCO2 (maintaining other two at control levels) on U46619-induced tension development in basilar arteries from wild-type mice (n = 12 or 13). (f, g) Effects of selectively varying pHo or []o (maintaining unvaried parameters at control levels) on U46619-induced VSMC Ca2+-responses in basilar arteries from wild-type mice (n = 6 for both). (h) Effects of selective changes in pHo ([]o=22 mM, CO2=5%) on depolarization-induced tension development of basilar arteries from wild-type mice (n = 8). We induce depolarization by raising [K+]o. (i) Effects of selective changes in pHo ([]o=22 mM, CO2=5%) on depolarization-induced VSMC Ca2+ responses in basilar arteries from wild-type mice (n = 7). The curves in panels (c) through (i) are the results of least-squares fits to sigmoidal functions, and we compare them using extra sum-of-squares F-tests. *P < 0.05, **P < 0.01, ***P < 0.001, NS: not significantly different vs. control conditions (pHo=7.4, []o=22 mM, CO2=5%).
Figure 3.
Increasing pCO2 at low pHo (pH 7.1) attenuates tension production of basilar arteries under OOE conditions. (a) Effects of selectively varying []o or pCO2 (maintaining the other at its control level, with pHo fixed at 7.1) on VSMC pHi in resting basilar arteries (left panel) or basilar arteries contracted by 10 µM U46619 (right panel). “Low” and “High” refer to half and twice the concentration, respectively, of CO2 and compared to “Control” levels ([]o=22 mM, CO2=5%). The reference pHi value on the y-axis, “(6.99)”, corresponds to control levels of all three parameters (pHo=7.4, []o=22 mM, CO2=5%) in the same arteries from wild-type mice (n = 6). (b) Effects of selectively varying []o or pCO2 (maintaining the other at its control level, with pHo fixed at 7.1) on VSMC calculated []i in resting basilar arteries (left panel) or basilar arteries contracted by 10 µM U46619 (right panel). The reference []i value on the y-axis, “(8.74 mM)”, corresponds to control levels of all three parameters (pHo=7.4, []o=22 mM, CO2=5%) in the same arteries from wild-type mice (n = 6). (c, d) Effects of selectively varying []o or pCO2 (maintaining the other at its control level, with pHo fixed at 7.1) on U46619-induced tension development in basilar arteries from wild-type mice (n = 6 in panel (c), n = 11 in panel (d)). For comparison, in panel (c), we show the reference tension response (solid gray curve) in the same arteries, under conditions that correspond to control levels of all three parameters (pHo=7.4, []o=22 mM, CO2=5%). (e) Effects of selectively varying pCO2 (fixed pHo=7.1, []o=22 mM) on U46619-induced VSMC Ca2+ responses in basilar arteries from wild-type mice (n = 5). The curves in panel (c) through (e) are the results of least-squares fits to sigmoidal functions, and we compare them using extra sum-of-squares F-tests. *P < 0.05, **P < 0.01, NS: not significantly different vs. control acidic conditions (pHo=7.1, []o=22 mM, CO2=5%).
All solutions were heated to 37 ℃ and gassed vigorously for at least 15 min before pH was titrated to the indicated level. Data were acquired using a PowerLab 4/26 A/D converter and LabChart software (ADInstruments, New Zealand). Arteries were normalized to 90% of the internal diameter corresponding to a transmural pressure of 100 mmHg, as previously described.17
Intracellular pH and [Ca2+] measurements
Ca2+ responses and pHi were monitored in VSMCs of mouse mesenteric or basilar arteries using wide-field fluorescence microscopy as previously described.5 Arteries were dye-loaded by addition of 5 µM BCECF-AM (Invitrogen, Denmark) or Fura-2-AM (Invitrogen) to the myograph bath, which has previously been shown to result in loading of VSMCs without detectable loading of endothelial cells (ECs).4 Fluorescence data were acquired using an Olympus IX81 inverted microscope (PA, USA) equipped with a Hamamatsu EM-CCD camera (Japan) or using a Leica DM IRB inverted microscope connected to a PTI Deltascan system (Photon Technology International, NJ, USA). Data acquisition was controlled with Slidebook 5.0 (3i Technology, India) or Felix32 software (Photon Technology International). Arteries were alternately excited at 440 and 495 nm (BCECF) or at 340 and 380 nm (Fura-2) and emission light collected between 510 and 530 nm. Photodamage during BCECF excitation was minimized by reducing light exposure to the last 30 s of each experimental condition.18 The BCECF fluorescence ratio was calibrated to pH values using the high-[K+] nigericin method, as previously described.19 []i was calculated from the Henderson–Hasselbalch equation based on the measured pHi values and the assumption that CO2 is in equilibrium across the cell membrane. Although the spectral properties of Fura-2 are pH-dependent, the KD changes only marginally in the pH range 6.8–7.0 relevant to the current study.20 Thus, no correction of the measured Fura-2 ratio was performed.
Resting steady-state values of force, [Ca2+]i, and pHi were obtained by averaging values during the last 30 s of a 3-min exposure to the solution in question. The stimulated values were obtained by averaging values during the last 30 s of a 2.5-min exposure to the given agonist concentration or to increased [K+]o.
Ca2+ sensitivity
VSMC Ca2+ sensitivity under equilibrated conditions was investigated in basilar arteries from RPTPγ-knockout mice by a modification of a technique previously described by Mulvany and Nyborg.21 Vessels loaded with Fura-2 as described above were first depleted of Ca2+ by a 10-s immersion in Ca2+-free PSS containing 5 mM ethylene glycol tetraacetic acid (EGTA). Subsequently, the arteries were kept in Ca2+-free PSS containing 0.1 mM EGTA and exposed three times to 10 µM U46619 for 2 min. The third stimulation under these conditions did not elicit any increase in force or Fura-2 fluorescence ratio, consistent with depletion of intracellular Ca2+-stores. At this point, arteries were washed to Ca2+-free PSS (no EGTA) and force and Fura-2 fluorescence were simultaneously recorded while [Ca2+]o was increased step-wise up to 1.6 mM in the continuous presence of 10 µM U46619. VSMC Ca2+-sensitivity at pHo 7.1 and 7.4 was compared based on the tension development to 30% and 60% of the maximal intracellular Ca2+-response at pHo 7.4.
β-galactosidase staining
The genetic insert disrupting RPTPγ-expression in the knockout mice contains a promoterless LacZ sequence allowing for β-galactosidase expression under control of the Ptprg promoter.16 We visualized the pattern of Ptprg transcriptional activity using a β-galactosidase-based staining method previously described in detail.22 Paraformaldehyde (PFA)-fixed, stained arteries were investigated either as whole-mount preparations or after paraffin-embedding and processing to 8-µm thick sections. Histological images were acquired using a Leica DMRE bright-field microscope (Denmark) equipped with a Leica DM300 digital camera.
Membrane potential measurements
Membrane potential was measured in VSMCs of isolated basilar arteries under equilibrated conditions using sharp electrodes, as previously described.23
Statistics
Data are expressed as mean±SEM. Unpaired, two-tailed Student's t-test was used for comparison of one variable between two groups. When the same parameter was measured under more than two conditions for the same mice, repeated-measures one-way ANOVA was employed. To evaluate the effects of two variables on the measured variable—measured multiple times for each mouse—we used repeated-measures two-way ANOVA followed by Bonferroni post-tests. Concentration–response relationships were evaluated using least-squares sigmoidal curve fits, and the derived log(EC50)- and maximum values were compared using extra sum-of-squares F-tests. P < 0.05 was considered statistically significant; n equals number of mice. Sample sizes were selected based on previous studies. The investigators were not blinded to the genotype of the mice. Few arteries that did not develop stable tension were excluded from analyses. Data processing and statistical analyses were performed using Microsoft Office Excel 2010 and GraphPad Prism 5.04 software.
Results
We apply OOE CO2/ solutions (Figure 1a) to small arteries in order to elucidate the individual vascular effects of , and CO2. Initially, we vary one of these parameters at a time, while keeping the other two constant at the normal physiological level (pH 7.40, 22 mM , 5% CO2). Figure 1(b) shows a typical isometric force recording obtained under these conditions.
Extracellular pH
We first evaluate the effect of selective changes in pHo—at constant []o and pCO2—on intracellular acid–base control in arteries from wild-type mice. In both basilar (Figure 2a) and mesenteric (Supplemental Figure 1a) arteries, isolated changes in pHo cause parallel changes in VSMC pHi, although the pHi changes are of reduced magnitude compared to those in pHo. Changes in []i also occur (Figure 2(b) and Supplemental Figure 1(b)). The degree of intracellular alkalinization caused by increasing pHo by 0.3 is approximately three times greater than the degree of intracellular acidification induced by decreasing pHo by 0.3 (Figure 2(a) and Supplemental Figure 1(a)), suggesting that VSMCs are better guarded against extracellular acidosis than alkalosis. The patterns of pHi (Figure 2(a) and Supplemental Figure 1(a)) and []i (Figure 2(b) and Supplemental Figure 1(b)) changes are very similar in resting arteries and arteries constricted with vasocontractile agonists (norepinephrine or thromboxane analogue U46619).
Decreasing pHo from 7.4 to 7.1 reduces U46619-induced tension development in basilar arteries (Figure 2c) and, correspondingly, attenuates norepinephrine-induced tension development in mesenteric arteries (Supplemental Figure 1c). Raising pHo to 7.7 enhances U46619-induced tension development in basilar arteries (Figure 2c), whereas norepinephrine-induced tension development is unaffected in mesenteric arteries (Supplemental Figure 1c). We address panels (d), (e), and (g) in the following sections. In both basilar (Figure 2f) and mesenteric (Supplemental Figure 1f) arteries, the responses are smaller at pHo 7.1 than 7.4, suggesting that the reduced tension development at low pHo is at least in part explained by the lower [Ca2+]i, reflecting altered VSMC Ca2+-handling. This interpretation is consistent with previous findings that extracellular acidosis inhibits voltage-dependent Ca2+-channels.24 It is also reinforced by the finding that, in both basilar (Figure 2(h) and (i)) and mesenteric (Supplemental Figure 1(h) and (i)) arteries, selectively decreasing pHo from 7.4 to 7.1 attenuates contractions and responses induced by high [K+]o-mediated depolarization. Agonist-induced VSMC Ca2+ responses in both basilar (Figure 2f) and mesenteric (Supplemental Figure 1f) arteries are unaffected by a selective increase in pHo from 7.4 to 7.7 under OOE conditions. Selectively increasing pHo from 7.4 to 7.7 also does not affect tension development or VSMC Ca2+ responses induced by high [K+]o-mediated depolarization of basilar (Figure 2(h) and (i)) or mesenteric (Supplemental Figure 1(h) and (i)) arteries. Note that, for experiments on mesenteric arteries, we include the α1-adrenergic inhibitor prazosin (100 nM) during the high [K+]o-induced depolarization to inhibit post-synaptic effects of the norepinephrine released from perivascular sympathetic nerves.
In further support of the notion that a selective decrease in pHo inhibits the [Ca2+]i rise in basilar arteries by inhibiting depolarization-induced Ca2+-influx rather than membrane excitability, we see no effect (n = 5; P = 0.98, repeated measures one-way ANOVA) of equilibrated extracellular acidosis on the resting membrane potential, which is −66.6 ± 2.3 mV under control conditions (i.e. pHo 7.40, 22 mM , 5% CO2), −66.7 ± 2.5 mV during metabolic acidosis (i.e. pHo 7.1, 11 mM , 5% CO2) and −67.8 ± 6.8 mV during respiratory acidosis (i.e. pHo 7.1, 22 mM , 10% CO2).
Extracellular
Selective changes in []o—at a constant pHo of 7.4 and CO2 of 5%—have only minor effects on VSMC pHi and []i (Figure 2(a) and (b) and Supplemental Figure 1(a) and (b)). Despite these minor effects on intracellular acid-base balance, reducing []o substantially increases the sensitivity of basilar arteries to U46619 with respect to tension development (Figure 2d), and has a comparable though considerably smaller effect on agonist sensitivity of mesenteric arteries (Supplemental Figure 1d). These findings strongly support the hypothesis that directly affects tension development in basilar arteries. Note that—under our experimental conditions—this sensitivity is in the low-physiological range: reducing []o from 22 to 11 mM paradoxically causes a considerable increase in the agonist-sensitivity of the basilar arteries, whereas an increase in []o from 22 to 44 mM does not affect agonist-induced tension development (Figure 2(d) and Supplemental Figure 1(d)). In contrast to the changes in artery tension development, we observe no effect of changing []o on the VSMC Ca2+ responses in basilar (Figure 2g) or mesenteric (Supplemental Figure 1g) arteries, suggesting that low []o modifies artery tone through changes in VSMC Ca2+ sensitivity.
CO2
A selective decrease in pCO2—at a constant pHo of 7.4 and []o of 22 mM—alkalinizes VSMCs of mesenteric and basilar arteries, both in the resting and the contracted state (Figure 2(a) and Supplemental Figure 1(a)). In contrast, an isolated increase in pCO2 has no significant effect on VSMC pHi under either of these conditions (Figure 2(a) and Supplemental Figure 1(a)). As expected, increased levels of pCO2 cause VSMC []i to increase considerably, whereas reduced levels of pCO2 cause []i to decrease (Figure 2(b) and Supplemental Figure 1(b)).
Contrary to expectations, selective changes in pCO2—at control levels of []o and pHo—have no net effect on active tension development in either basilar (Figure 2e) or mesenteric (Supplemental Figure 1e) arteries.
CO2 and extracellular in basilar arteries at low extracellular pH
Acid–base disturbances in an in vivo, equilibrated system produce combined changes in at least two of the following: pHo, []o and pCO2. To determine whether the above-described cellular responses to changes in individual CO2/ buffer components are interdependent, we next investigate the consequences of selectively changing []o or pCO2 under acidic OOE conditions. Altering []o or pCO2 at pHo 7.1 produces intracellular acid–base responses that differ substantially from those observed at pHo 7.4: during extracellular acidosis, VSMCs regulate pHi less effectively in response to an increase in pCO2 or a decrease in []o (Figure 3a). Moreover, at pHo 7.1, VSMC []i levels (Figure 3b) are generally lower than at pHo 7.4 (Figure 2b). These results are consistent with previous findings from other cell types showing that low pHo inhibits cellular net acid extrusion.25
When selectively varying []o at pHo 7.1, we observe no net effect on tension development of basilar arteries (Figure 3c). Although a selective decrease in pCO2 also has no effect on artery tone at pHo 7.1 (Figure 3d), a selective increase in pCO2 lowers tension development (Figure 3d) with no effect on VSMC Ca2+ dynamics (Figure 3e). The inhibitory effect of intracellular acidification on VSMC Ca2+ sensitivity5,12,18,26 likely contributes to the lower tension development observed at pHo 7.1 compared to 7.4 in the presence of low []o (Figure 3(c) vs. Figure 2(d)) or high pCO2 (Figure 3(d) vs. Figure 2(e)).
RPTPγ expression
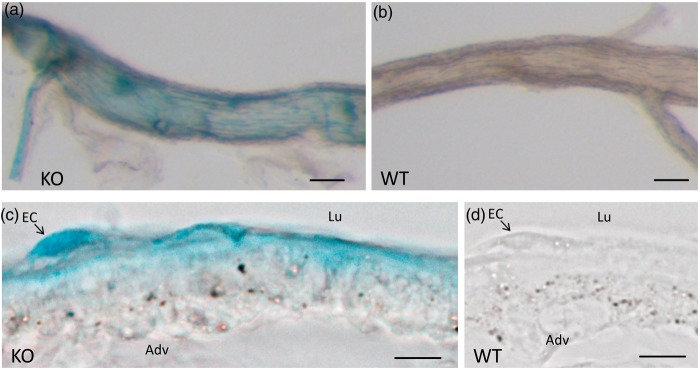
RPTPγ, encoded by Ptprg, has been proposed to serve as extracellular CO2/ sensor in renal proximal tubules.14 Here, we study the pattern of transcriptional activity of Ptprg in vascular tissue of RPTPγ-knockout mice containing a gene trap insertion that brings the LacZ gene, encoding β-galactosidase, under control of the Ptprg promoter.16 In these mice, histochemical staining for β-galactosidase activity is a reporter for Ptprg transcriptional activity.22 We find that basilar arteries actively transcribe Ptprg (Figure 4(a) and (b)), whereas the transcriptional activity in mesenteric arteries is below detection limits (Supplemental Figure 2(a) to (d)). The β-galactosidase staining in basilar arteries is particularly prominent in ECs (Figure 4(c) and (d)).
Figure 4.
Ptprg transcriptional activity is prominent in ECs of basilar arteries. (a, b) Basilar arteries from an RPTPγ-knockout (KO, panel (a)) and a wild-type (WT, panel (b)) mouse, stained histochemically for β-galactosidase activity. Each image is representative of five experiments. (c, d) Histological sections (8 µm thick) of basilar arteries from an RPTPγ-knockout and a wild-type mouse, stained histochemically for β-galactosidase activity. Each image is representative of three experiments. The size bars in panels (a) and (b) represent 100 µm, in (c) and (d) 10 µm. Lu indicates lumen, Adv indicates adventitia and EC indicates endothelial cell.
Role of RPTPγ in basilar arteries under out-of-equilibrium conditions
Using basilar arteries from RPTPγ-knockout mice, we now study the functional importance of RPTPγ for the response to changes in []o. Although U46619-induced tension in arteries from RPTPγ-knockout mice remains sensitive to selective changes in pHo (Figure 5a), neither U46619-induced tension (Figure 5b) nor U46619-induced VSMC Ca2+ responses (Figure 5c) are sensitive to selective changes in []o at pHo 7.4. At pHo 7.1, reducing []o to 11 mM decreases the U46619 sensitivity of tension development in basilar arteries from RPTPγ-knockout mice (Figure 5d), without affecting the response (Figure 5e). This finding further supports the conclusion that at low pHo, reducing []o causes a fall in VSMC pHi (Figure 3a) that in turn reduces VSMC Ca2+-sensitivity.
Role of RPTPγ for sensing in basilar arteries under equilibrated conditions
To further support the role of RPTPγ in sensing, we study the contractile function of basilar arteries from RPTPγ-knockout mice under equilibrated conditions (Figure 5(f) to (i)). Omitting CO2/ from the bath solution—at a constant pHo of 7.4—increases the contractile response to U46619 in basilar arteries from wild-type mice (Figure 5f). This net enhancement of contraction in response to omission of CO2/ is completely abolished in basilar arteries from RPTPγ-knockout mice (Figure 5g).
Role of endothelium-dependent relaxation for RPTPγ-mediated vasomotor effects
Considering the prominent promoter activity for RPTPγ in the endothelium of basilar arteries (Figure 4), we next investigate the role of endothelium-derived vasorelaxant influences for the -dependent vasomotor effects of RPTPγ. We eliminate the relaxant influence of the endothelium by inhibiting NO synthesis (with 100 µM NO-synthase inhibitor L-NAME), prostacyclin production (with 3 µM cyclooxygenase inhibitor indomethacin) and endothelium-derived hyperpolarizations (EDH; with 50 nM apamin and 1 µM TRAM-34 to inhibit small- and intermediate-conductance Ca2+-activated K+-channels, respectively). To avoid development of increased resting tone after inhibition of endothelium-dependent vasorelaxation, we deliver a similar exogenous [NO] level to basilar arteries from wild-type and RPTPγ-knockout mice by incubating them with 600 nM of the NO-donor S-nitro-N-acetyl-DL-penicillamine (SNAP). We find that, following pharmacological inhibition of endothelium-dependent vasorelaxation, U46619-induced vasoconstriction of basilar arteries from wild-type and RPTPγ-knockout mice is similar in the presence and absence of CO2/ (Figure 5(h) and (i)). Together, the experiments performed under equilibrated conditions support that RPTPγ provides a mechanism for endothelium-dependent, -sensitive regulation of cerebral artery tone.
Role of RPTPγ in basilar arteries under metabolic and respiratory acidosis
To further investigate the role of RPTPγ under physiological and pathophysiological circumstances, we next compare basilar arteries from RPTPγ-knockout and wild-type mice under control and acidic conditions (Figure 6). During control conditions (i.e. pHo 7.4, 22 mM , 5% CO2), U46619 causes basilar arteries from RPTPγ-knockout mice to develop more tone than those from wild-type mice (Figure 6a). This effect persists under conditions of extracellular acidosis but is more pronounced during respiratory (i.e. pHo 7.1, 22 mM , 10% CO2; Figure 6b) than metabolic (i.e. pHo 7.1, 11 mM , 5% CO2; Figure 6d) acidosis. These findings confirm that RPTPγ activates vasorelaxant signaling mechanisms in basilar arteries and are consistent with the conclusion that RPTPγ is required for sensing of []o: under physiological conditions, RPTPγ has a vasorelaxant effect that wanes under low-[]o conditions. Looking at the anti-contractile effect of changing from physiological control conditions (i.e. pHo 7.4, 22 mM , 5% CO2) to an equilibrated acidosis, we observe that the vasorelaxant response to respiratory acidosis (i.e. pHo 7.1, 22 mM , 10% CO2) is equivalent in basilar arteries from wild-type and RPTPγ-knockout mice (Figure 6c). In contrast, vasorelaxation in response to metabolic acidosis (i.e. pHo 7.1, 11 mM , 5% CO2) is potentiated in basilar arteries from RPTPγ-knockout compared to wild-type mice (Figure 6e). These findings support that RPTPγ limits vasorelaxation in response to metabolic acidosis.
Effect of metabolic acidosis on VSMC Ca2+-sensitivity in basilar arteries
The OOE data presented above (Figures 2, 3 and 5) support the conclusion that—in addition to direct effects of and on cerebral artery tone—indirect effects caused by changes in pHi also play a role for vasomotor control of basilar arteries. It has been debated to what extent changes in pHi contribute to vasorelaxation in response to extracellular acid–base disturbances,27,28 but previous studies have not been able to take into account potential direct effects of changes in []o. During metabolic acidosis (i.e. pHo 7.1, 11 mM , 5% CO2), VSMC pHi decreases by 0.16 ± 0.03 at rest and 0.18 ± 0.03 during maximal contraction (with 10 µM U46619) compared to control conditions (n = 5). This degree of intracellular acidification is expected to inhibit vasoconstriction by lowering VSMC Ca2+-sensitivity.5,12,18,26 To explore mechanisms of vasomotor control additional to RPTPγ-dependent signaling, we next investigate whether metabolic acidosis interferes with VSMC Ca2+-sensitivity in basilar arteries from RPTPγ-knockout mice (Figure 6(f) and (h)). Because U46619 is expected to increase VSMC Ca2+-sensitivity,29 we determine the contractile response to varying levels of [Ca2+]i during constant exposure to a maximal stimulatory level of U46619 (10 µM). Our approach is to first deplete the arteries of and then to expose them to stepwise increases in [Ca2+]o (Figure 6f). Metabolic acidosis attenuates the VSMC Ca2+ response (Figure 6(f) and (g)), but the inhibitory effect of metabolic acidosis on active tension development is greater than what one would expect from the lower [Ca2+]i levels alone (Figure 6(f) and (h)). Based on these findings, we conclude that—in addition to direct effects of and —extracellular acidosis also affects tone development by lowering VSMC Ca2+-sensitivity, and this mechanism is likely caused by the concomitant decrease in pHi.
Discussion
Metabolic regulation of cerebral artery tone is important in the adaptation of cerebral blood flow to local metabolic demand. Although dilation of systemic resistance arteries in response to extracellular acidosis was first described more than a century ago,2 the complex nature of acid–base disturbances—with concomitant changes in pHo, []o, pCO2 and other buffer systems—has previously prevented a comprehensive mechanistic understanding.
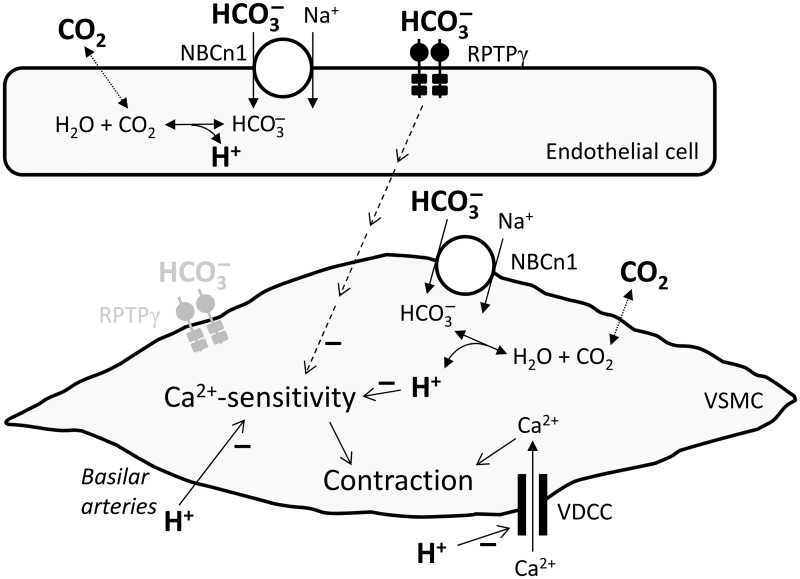
In the present study, we circumvent previous experimental limitations by utilizing OOE CO2/ solutions and thereby determining vascular effects of , , and CO2 one at a time, while holding the other two parameters fixed. We show for the first time that directly modifies arterial tone, and that causes vasorelaxation even with normal pCO2 and []o. Although, under the conditions of the assay, we find no evidence for a direct effect of CO2, we corroborate that secondary changes in pHi induced by extracellular acid-base disturbances modulate arterial tone development. The novel vasomotor response to changes in []o requires a sensing mechanism involving RPTPγ, which—based on its homology to carbonic anhydrases—likely binds and initiates intracellular signaling in response to acid-base disturbances. To investigate physiological and potential pathophysiological roles of RPTPγ, we investigate vasomotor effects of RPTPγ under equilibrated conditions and demonstrate a -sensitive net braking action of RPTPγ on the vasorelaxant response to metabolic acidosis. The important new insights from the current study and the proposed signaling mechanisms controlling metabolic regulation of artery tone are summarized in Figure 7.
Figure 7.
Schematic of proposed signaling pathways affecting cerebral vascular tone in response to acid-base disturbances. Note that in the cases of endothelium-mediated RPTPγ-dependent actions of and the effect of acting on Ca2+-sensitivity (which could occur via a parallel change in pHi), we have only seen effects with reducing the concentrations. RPTPγ in the vascular smooth muscle cell is shown in gray because we have evidence for expression but no function has yet been demonstrated. VDCC, voltage-dependent Ca2+-channels; VSMC, vascular smooth muscle cell.
Extracellular and CO2
In physiological solutions, CO2 and are key buffers and, in addition to H+, they can modify the vascular response to acid–base disturbances. Our findings show that lowering []o directly increases vascular tone (Figures 2(d) and 5(f) and Supplemental Figure 1(d)) via a mechanism that requires RPTPγ (Figure 5(b) and (g)) and elevates VSMC Ca2+ sensitivity (Figure 2(d) and (g) and Supplemental Figure 1(d) and (g)). The regulation of vasomotor tone by []o is endothelium-dependent: under control conditions, omission of CO2/ potentiates vasoconstriction to U46619 in basilar arteries from wild-type (Figure 5f) but not RPTPγ-knockout (Figure 5g) mice; however, following inhibition of well-accepted pathways for endothelium-dependent vasorelaxation (i.e. NO, PGI2 and EDH), omission of CO2/ no longer affects tension development in basilar arteries from either wild-type or RPTPγ-knockout mice (Figure 5(h) and (i)). The endothelium-dependent regulation of VSMC Ca2+-sensitivity by is consistent with previously demonstrated inhibitory effects of the endothelium—and endothelium-derived relaxant factors—on cell signaling pathways for VSMC Ca2+-sensitization.30–33 High transcriptional activity for Ptprg in ECs of basilar compared to mesenteric arteries (Figure 4 vs. Supplemental Figure 2) is reflected in the magnitude of the vasomotor response to lowering []o, which is prominent and RPTPγ-dependent in basilar arteries (Figures 2, 5 and 6) but much less pronounced in mesenteric arteries (Supplemental Figure 1). Selective decreases in []o—induced with OOE solutions at a fixed pHo of 7.4 and CO2 of 5%—do not give rise to statistically significant changes in VSMC pHi or []i (Figure 2(a) and (b) and Supplemental Figure 1(a) and (b)), supporting that the novel pro-contractile effect of lowering []o does not require a change in cellular uptake (Figure 7). Extracellular action of is also supported by the observation that the -dependent vasocontractile effect relies completely on RPTPγ (Figure 5b), which has a carbonic anhydrase-like domain with known extracellular orientation.15
The OOE effect of lowering []o is paradoxical because of the well-known vasorelaxation caused by lowering []o under equilibrated conditions (i.e. metabolic acidosis). In cerebral arteries, this paradoxical action: (a) limits vasorelaxation during metabolic acidosis (Figure 6e) and hence may protect against cerebral edema, and (b) could maintain sufficient Ca2+-sensitivity to permit VSMC responses to Ca2+-dependent modulators even under acidotic conditions. Note that this novel -sensitive, RPTPγ-dependent signaling pathway appears to be fully activated at the control []o of 22 mM. Thus, further increases in []o do not affect tone (Figure 2(d) and Supplemental Figure 1(d)).
In addition to its effects as an extracellular-signaling molecule, also is important as substrate for the electroneutral Na+, -cotransporter NBCn1 (Slc4a7), which maintains a relatively alkaline pHi.34 Lowering []o under OOE conditions at pHo 7.1 has no net effect on tone (Figure 3c) because of the competing (a) RPTPγ-dependent tendency toward vasoconstriction and (b) fall in pHi that inhibits VSMC Ca2+-sensitivity5,12,18,26 and thus tends to cause vasorelaxation. Accordingly, with increased metabolic activity or decreased perfusion, the integrated effect of the resulting metabolic acidosis would be dilation of feed arteries because the low local pHo per se causes vasorelaxation, whereas the accompanying low []o has no net vasomotor effect. In contrast, lowering []o under OOE conditions at pHo 7.4 causes vasoconstriction (Figure 2d), and during a partially compensated whole-body metabolic acidosis (e.g. due to renal or metabolic dysfunction), the low []o would tend to limit the degree of acid-induced cerebral vasodilation and thus allow control of cerebral blood flow by normal autoregulatory mechanisms.
Our results show that selective changes in pCO2 at pHo 7.4 have no net effect on vasoreactivity (Figure 2e). Clearly, CO2 has indirect effects on artery tone by changing pHi, and by changing pHo under equilibrated conditions.
Direct actions of extracellular pH
In both mesenteric and basilar arteries, a selective decrease in pHo inhibits the VSMC Ca2+-response and tone development in response to depolarization (Figure 2(h) and Supplemental Figure 1(h)) and agonist-stimulation (Figure 2(c) and Supplemental Figure 1(c)). These results are consistent with previous findings showing that acidosis inhibits voltage-dependent Ca2+ channels.35 However, while a selective increase in pHo enhances U46619-induced contractions in basilar arteries (Figure 2c), this alkalosis has no effect on norepinephrine-induced contractions in mesenteric arteries (Supplemental Figure 1c) or on depolarization-induced contractions in either vascular bed (Figure 2(h) and Supplemental Figure 1(h)). Because the alkalosis-induced augmentation of agonist-induced contractions of basilar arteries does not involve a change in [Ca2+]i (Figure 2f), our findings suggest that extracellular alkalosis raises VSMC Ca2+-sensitivity in basilar but not mesenteric arteries. The link between increased pHo and increased Ca2+-sensitivity remains unclear, but does not involve RPTPγ, as evidenced by the observation that both increases and decreases in pHo continue to evoke their usual tension responses in basilar arteries from RPTPγ-knockout mice (Figure 5a). It is possible that the effect of high pHo on Ca2+-sensitivity is secondary to the concomitant intracellular alkalinization (Figure 2a).
The role of intracellular pH in the response to extracellular acid-base disturbances
In addition to direct effects of and on vascular tone, our data suggest that extracellular acid–base disturbances also act via secondary changes in VSMC pHi (Figure 7). Investigators have argued both for27 and against28 a major role for VSMC pHi-changes in vascular responses to extracellular acid–base disturbances. Our present study—employing arteries from RPTPγ-knockout mice to eliminate direct effects of —supports the conclusion that part of the vasorelaxant response to metabolic acidosis occurs via changes in VSMC pHi: we find that during extracellular acidification, the pHi of VSMCs is sensitive to changes in []o and pCO2 (Figure 3a) and that VSMC intracellular acidification is accompanied by decreased VSMC Ca2+-sensitivity (Figure 2 vs. Figures 3, 5 and 6). The ability of a decrease in []o to decrease VSMC pHi and VSMC Ca2+-sensitivity at low pHo (Figures 3 and 6) is consistent with previous observations that reduced net uptake consequent to CO2/ omission,5,12,26 siRNA-mediated knockdown5 or knockout12,18 of NBCn1 substantially lowers VSMC pHi and inhibits VSMC Ca2+-sensitivity in murine arteries. During vasoconstriction, NBCn1 in VSMCs is stimulated via Ca2+-dependent activation of calcineurin, which protects against intracellular acidification36 and thereby permits the increased VSMC Ca2+-sensitivity observed during prolonged contraction.5
Conclusion
Our use of OOE CO2/ solutions and RPTPγ-knockout mice allows us to show for the first time that decreases in []o have direct, RPTPγ-dependent vasocontractile effects mediated by increases in VSMC Ca2+-sensitivity. Changes in pHo also modulate vasomotor responses, partly through acid-induced inhibition of voltage-dependent Ca2+-channels. Finally, the combination of low pHo and high pCO2 (respiratory acidosis) or low pHo and low []o (metabolic acidosis) cause pHi decreases that lower VSMC Ca2+-sensitivity and thus inhibit vasoconstriction. We propose that these responses to acid–base disturbances collectively fine-tune vascular tone and adjust blood flow to the local metabolic demand.
Supplementary Material
Acknowledgments
The authors are grateful to Dr. Joseph Schlessinger for the gift of RPTPγ-knockout mice. Jane Rønn and Sukhan Kim are acknowledged for expert technical assistance.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article:
This work was supported by NIH grant DK81567 (to W.F.B.), the Danish Council for Independent Research (grant no. 10-094816 and 4183-00258B to E.B. and 12-126232 to C.A.), Helga og Peter Kornings Fond (to E.B.) and the Lundbeck Foundation (grant no. R93-A8859 to E.B.). Ebbe Boedtkjer was supported by the Danish Ministry of Science, Technology and Innovation.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors' Contributions
EB, CA and WFB conceived the project. EB, DMBB and WFB designed experiments. EB and KBH performed experiments. EB analyzed the data. EB, DMBB, CA and WFB interpreted data. EB drafted the manuscript. All authors revised the manuscript for important intellectual content and approved the final version.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Kontos HA. Regulation of the cerebral circulation. Ann Rev Physiol 1981; 43: 397–407. [DOI] [PubMed] [Google Scholar]
- 2.Gaskell WH. On the tonicity of the heart and blood vessels. J Physiol 1880; 3: 48–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao J, Hogan EM, Bevensee MO, et al. Out-of-equilibrium CO2/ solutions and their use in characterizing a new K/HCO3 cotransporter. Nature 1995; 374: 636–639. [DOI] [PubMed] [Google Scholar]
- 4.Peng HL, Ivarsen A, Nilsson H, et al. On the cellular mechanism for the effect of acidosis on vascular tone. Acta Physiol Scand 1998; 164: 517–525. [DOI] [PubMed] [Google Scholar]
- 5.Boedtkjer E, Praetorius J, Aalkjaer C. NBCn1 (slc4a7) mediates the Na+-dependent bicarbonate transport important for regulation of intracellular pH in mouse vascular smooth muscle cells. Circ Res 2006; 98: 515–523. [DOI] [PubMed] [Google Scholar]
- 6.Vankova M, Snetkov VA, Knock GA, et al. Euhydric hypercapnia increases vasoreactivity of rat pulmonary arteries via transport and depolarisation. Cardiovasc Res 2005; 65: 505–512. [DOI] [PubMed] [Google Scholar]
- 7.Yoon S, Zuccarello M, Rapoport RM. pCO2 and pH regulation of cerebral blood flow. Front Physiol 2012; 3: 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kontos HA, Raper AJ, Patterson JL. Analysis of vasoactivity of local pH, PCO2 and bicarbonate on pial vessels. Stroke 1977; 8: 358–360. [DOI] [PubMed] [Google Scholar]
- 9.Seuwen K, Ludwig MG, Wolf RM. Receptors for protons or lipid messengers or both? J Recept Signal Transduct Res 2006; 26: 599–610. [DOI] [PubMed] [Google Scholar]
- 10.Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: Advances, questions and therapeutic opportunities. Trends Neurosci 2006; 29: 578–586. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Cann MJ, Litvin TN, et al. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 2000; 289: 625–628. [DOI] [PubMed] [Google Scholar]
- 12.Boedtkjer E, Praetorius J, Matchkov VV, et al. Disruption of Na+, -cotransporter NBCn1 (slc4a7) inhibits NO-mediated vasorelaxation, smooth muscle Ca2+-sensitivity and hypertension development in mice. Circulation 2011; 124: 1819–1829. [DOI] [PubMed] [Google Scholar]
- 13.Fleming I, Hecker M, Busse R. Intracellular alkalinization induced by bradykinin sustains activation of the constitutive nitric oxide synthase in endothelial cells. Circ Res 1994; 74: 1220–1226. [DOI] [PubMed] [Google Scholar]
- 14.Skelton LA, Boron WF, Zhou Y. Acid-base transport by the renal proximal tubule. J Nephrol 2010; 23: S4–S18. [PMC free article] [PubMed] [Google Scholar]
- 15.Barnea G, Silvennoinen O, Shaanan B, et al. Identification of a carbonic anhydrase-like domain in the extracellular region of RPTPγ defines a new subfamily of receptor tyrosine phosphatases. Mol Cell Biol 1993; 13: 1497–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamprianou S, Vacaresse N, Suzuki Y, et al. Receptor protein tyrosine phosphatase γ is a marker for pyramidal cells and sensory neurons in the nervous system and is not necessary for normal development. Mol Cell Biol 2006; 26: 5106–5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res 1977; 41: 19–26. [DOI] [PubMed] [Google Scholar]
- 18.Thomsen ABK, Kim S, Aalbaek F, et al. Intracellular acidification alters myogenic responsiveness and vasomotion of mouse middle cerebral arteries. J Cereb Blood Flow Metab 2014; 34: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aalkjaer C, Cragoe EJ., Jr Intracellular pH regulation in resting and contracting segments of rat mesenteric resistance vessels. J. Physiol 1988; 402: 391–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Negulescu PA, Machen TE. Lowering extracellular sodium or pH raises intracellular calcium in gastric cells. J Membr. Biol 1990; 116: 239–248. [DOI] [PubMed] [Google Scholar]
- 21.Mulvany MJ, Nyborg N. An increased calcium sensitivity of mesenteric resistance vessels in young and adult spontaneously hypertensive rats. Br J Pharmacol 1980; 71: 585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boedtkjer E, Praetorius J, Fuchtbauer EM, et al. Antibody-independent localization of the electroneutral Na+- cotransporter NBCn1 (slc4a7) in mice. Am J Physiol Cell Physiol 2008; 294: C591–C603. [DOI] [PubMed] [Google Scholar]
- 23.Boedtkjer E, Kim S, Aalkjaer C. Endothelial alkalinisation inhibits gap junction communication and endothelium-derived hyperpolarisations in mouse mesenteric arteries. J. Physiol 2013; 591: 1447–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klockner U, Isenberg G. Calcium channel current of vascular smooth muscle cells: extracellular protons modulate gating and single channel conductance. J Gen Physiol 1994; 103: 665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roos A, Boron WF. Intracellular pH. Physiol Rev 1981; 61: 296–434. [DOI] [PubMed] [Google Scholar]
- 26.Boedtkjer E, Damkier HH, Aalkjaer C. NHE1 knockout reduces blood pressure and arterial media/lumen ratio with no effect on resting pHi in the vascular wall. J Physiol 2012; 590: 1895–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Austin C, Wray S. Extracellular pH signals affect rat vascular tone by rapid transduction into intracellular pH changes. J Physiol 1993; 466: 1–8. [PMC free article] [PubMed] [Google Scholar]
- 28.Tian R, Vogel P, Lassen NA, et al. Role of extracellular and intracellular acidosis for hypercapnia-induced inhibition of tension of isolated rat cerebral arteries. Circ Res 1995; 76: 269–275. [DOI] [PubMed] [Google Scholar]
- 29.Jiang MJ, Chan CF, Chang YL. Intracellular calcium and myosin light chain phosphorylation during U46619-activated vascular contraction. Life Sci 1994; 54: 2005–2013. [DOI] [PubMed] [Google Scholar]
- 30.Lehen'kyi VV, Zelensky SN, Stefanov AV. Ca2+-sensitivity and cGMP-independent effects of NO in vascular smooth muscle. Nitric Oxide 2005; 12: 105–113. [DOI] [PubMed] [Google Scholar]
- 31.Sauzeau V, Le JH, Cario-Toumaniantz C, et al. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem 2000; 275: 21722–21729. [DOI] [PubMed] [Google Scholar]
- 32.Sawada N, Itoh H, Yamashita J, et al. cGMP-Dependent Protein Kinase Phosphorylates and Inactivates RhoA. Biochem Biophys Res Commun 2001; 280: 798–805. [DOI] [PubMed] [Google Scholar]
- 33.Weigand L, Shimoda LA, Sylvester JT. Enhancement of myofilament calcium sensitivity by acute hypoxia in rat distal pulmonary arteries. Am J Physiol - Lung Cell Molecul Phys 2011; 301: L380–L387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aalkjaer C, Boedtkjer E, Choi I, et al. Cation-coupled bicarbonate transporters. Compr Physiol 2014; 4: 1605–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Irisawa H, Sato R. Intra- and extracellular actions of proton on the calcium current of isolated guinea pig ventricular cells. Circ Res 1986; 59: 348–355. [DOI] [PubMed] [Google Scholar]
- 36.Danielsen AA, Parker MD, Lee S, et al. Splice cassette II of NBCn1 (slc4a7) interacts with calcineurin A: Implications for transporter activity and intracellular pH control during rat artery contractions. J Biol Chem 2013; 288: 8146–8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.