Abstract
We have recently shown that intracranial pressure (ICP) increases dramatically 24 h after minor intraluminal thread occlusion with reperfusion, independent of edema. Some of the largest ICP rises were observed in rats with the smallest final infarcts. A possible alternate mechanism for this ICP rise is an increase of cerebrospinal fluid (CSF) volume secondary to choroid plexus damage (a known complication of the intraluminal stroke model used). Alternatively, submaximal injury may be needed to induce ICP elevation. Therefore, we aimed to determine (a) if choroid plexus damage contributes to the ICP elevation, (b) if varying the patency of an important internal collateral supply to the middle cerebral artery (MCA), the anterior choroidal artery (AChA), produces different volumes of ischemic penumbra and (c) if presence of ischemic penumbra (submaximal injury) is associated with ICP elevation. We found (a) no association between choroid plexus damage and ICP elevation, (b) animals with a good internal collateral supply through the AChA during MCAo had significantly larger penumbra volumes and (c) ICP elevation at ≈24 h post-stroke only occurred in rats with submaximal injury, shown in two different stroke models. We conclude that active cellular processes within the ischemic penumbra may be required for edema-independent ICP elevation.
Keywords: Collaterals, intracranial pressure, middle cerebral artery occlusion, penumbra, photothrombosis
Introduction
In recent studies, we have shown that there is a dramatic intracranial pressure (ICP) elevation occurring 18–24 hafter temporary middle cerebral artery occlusion (MCAo) in rats, which is edema independent.1,2 A possible alternate mechanism for this ICP rise is an increase of cerebrospinal fluid (CSF) volume.3 Ischemia of the anterior choroidal artery (AChA) and subsequently the choroid plexus is a known complication of the thread occlusion model.4 Choroid plexus produces CSF, and hence we hypothesised that choroid plexus damage may increase CSF production, leading to edema-independent ICP elevation. To test this hypothesis, our first aim was to measure ICP following cortical photothrombotic (PT) stroke, without choroid plexus damage to determine if choroid plexus injury is necessary for ICP elevation 24 h post-stroke. While developing this model, we discovered that ICP only appeared to increase following submaximal, not maximal injury. This chance finding led us to our primary hypothesis that submaximal injury (penumbra) is necessary for the delayed ICP elevation following stroke. To test this hypothesis, we aimed to induce PT stroke with varying degrees of injury in order to determine if submaximal injury is necessary for ICP elevation at 24 h post-stroke.
ICP is not routinely measured in patients who have small infarcts, due to the invasiveness of ICP monitoring equipment. Hence, we do not know whether a transient ICP increase occurs in patients with minor stroke as we see in rats.1,2 However, recent acute stroke imaging studies indicate that the neurological deterioration in patients with delayed infarct expansion most commonly occurs at a very similar time interval.5 Moreover, deterioration is rarely accompanied by worsening or recurrence of the arterial occlusion as was previously supposed.5 Rather, it is associated with failure of leptomeningeal collateral supply, in patients with stable proximal arterial occlusion, excellent collaterals and large penumbra at baseline imaging.6 In our recent studies, we showed that ICP elevation reduces collateral blood flow.7 This suggests that ICP elevation may be the cause of the collateral failure. Since a large penumbra at baseline imaging was one of the defining features of patients who developed stroke-in-progression, we wished to try to replicate the situation of such patients as far as possible in our experimental model and determine whether it resulted in a significant ICP rise. The presence of penumbra in the PT model remains controversial. Therefore, we wished to use a model with more clear-cut penumbra. We recently demonstrated the importance of an internal collateral vessel, the AChA, to infarct volume in the rat thread occlusion model. Preserved patency of the AChA resulted in significantly smaller final infarct volumes.8 The patency of the AChA is manipulable by altering the silicon tip length of the thread occluder to either occlude or maintain patency of the AChA.9 Therefore, we also sought to determine whether animals with patent AChA had larger volumes of acute ischemic penumbra. Our final aim was to determine whether such rats with permanent MCAo (pMCAo) with excellent collaterals (mimicking the scenario of patients with delayed stroke progression) had 24-h ICP elevation.
Materials and methods
Ethics statement
All animal experiments were performed on male outbred Wistar rats (aged 7–12 weeks, body weight: 300–500 g; ASU breeding facility, University of Newcastle, Australia). All animals were housed in cages in an animal-holding facility in the University of Newcastle with rat chow and water available ad libitum. Experiments were approved by the Animal Care and Ethics Committee of the University of Newcastle (Protocol # A-2011-112) and were in accordance with the requirements of the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. The studies were conducted and the manuscript prepared in accordance with the ARRIVE guidelines.10
Experimental protocols
Study I
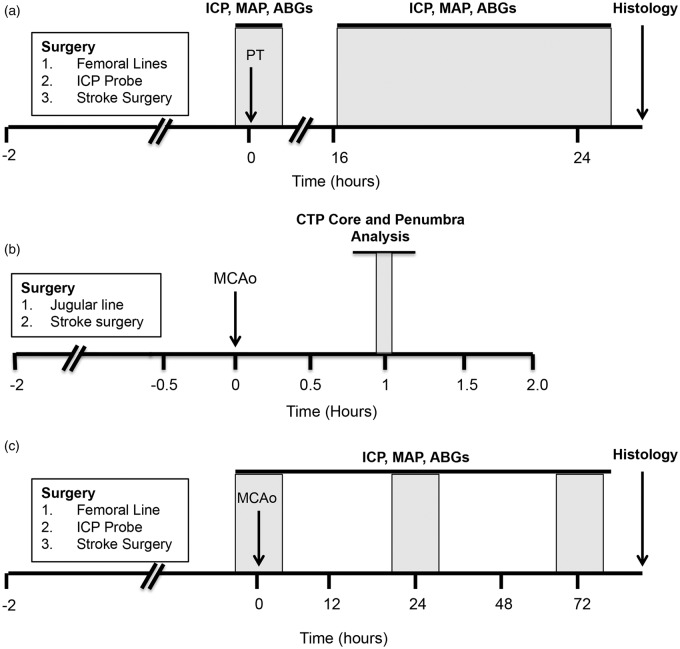
The aim of study I was to investigate whether choroid plexus ischemia or submaximal stroke is required for ICP elevation post-stroke. Baseline ICP and mean arterial pressure (MAP) were monitored for 1 h in all animals. PT stroke was induced by intravenous infusion of 10 mg/kg Rose Bengal (Sigma-Aldrich, Sydney, Australia) followed by illumination of the right parietal bone with a cold focal light source (n = 16). Animals were randomised by sealed numbered envelope to receive either low light exposure (LLE) or standard light exposure (SLE). The skull was illuminated with a 12-inch optic fibre articulating arm light pipe with 6 mm diameter light tip either for 2 min at 0.13 W/cm2 (LLE, to produce submaximal injury) or 20 min at 0.3 W/cm2 (SLE, to produce maximal injury). Sham animals (n = 2) received the same light exposure as the standard exposure group, but Rose Bengal was not administered. ICP and MAP were monitored continuously from 18 h to 26 h post-stroke (Figure 1a).
Figure 1.
Experimental timelines. (a) Study I. ICP was monitored at baseline and between 18 and 26 h after PT stroke. (b) Study II. CTP imaging was carried out to measure whole brain perfusion changes during MCAo. For the purposes of this study, scans taken 1 h post-MCAo were used to create core and penumbra maps using previously established CTP-defined CBF thresholds for this model. (c) Study III. ICP was monitored at baseline, 24 and 72 h after permanent MCAo. ABG = arterial blood gas; CTP: computed tomography perfusion; ICP: intracranial pressure; MAP: mean arterial pressure; MCAo: middle cerebral artery occlusion; PT: photothrombotic stroke.
Study II
The aim of study II was to determine whether the patency of the AChA, would produce different volumes of ischemic penumbra 1 h following MCAo in Wistar rats, using computed tomography perfusion (CTP) imaging. Animals were subject to intraluminal pMCAo (n = 28).11 CTP scans were taken at baseline and 1 h after MCAo (Figure 1b).
Study III
In study III, we aimed to investigate the association between the presence of penumbra and ICP elevation. We altered the length of the silicone occluder tip to produce different degrees of collateral perfusion through the AChA and hence penumbra. Baseline ICP and MAP were monitored for 2 h in all animals. All rats received intraluminal pMCAo.11 Just prior to thread insertion, rats were randomized by sealed numbered envelope to a short-length (1.5 mm) silicon tip on the occluding thread to maintain patency of the AChA during MCAo, or to a 4-mm tip-length thread to simultaneously occlude the origin of the AChA (n = 6/group). ICP and MAP were monitored for 2 h at 24 h and 72 h post-stroke (Figure 1c), respectively. As per our usual practice, lack of laser Doppler flowmetry (LDF) drop >50 % during MCAo or evidence of subarachnoid hemorrhage were prespecified exclusion criteria.7
Anesthesia and monitoring
The anesthetic and monitoring protocols were as previously reported.12 Rats were anesthetised with 5% isoflurane in O2/N2 (1:3) and maintained with 1–2% isoflurane. Animals spontaneously breathed through a laboratory-manufactured low dead-space nose cone. Core temperature was maintained at 37℃ by a thermocouple rectal probe and warming plate. Incision sites were shaved, cleaned and injected subcutaneously with 2 mg/kg 0.05 % Bupivacaine (Pfizer, Sydney, Australia). Blood gases were monitored at baseline and 24 h in Study I and at baseline, 24 and 72 h in Study III. Blood samples (0.1 ml) were taken from a femoral arterial line. This line was also used for continuous arterial pressure monitoring. Heart rate and respiratory rate were calculated from the clearly discernible cardiac and respiratory waveforms on the arterial pressure tracing.13 After stroke surgery, animals were injected subcutaneously with saline (2 × 2.5 ml) to prevent dehydration and returned to their cages with free access to softened laboratory chow and water.
ICP measurement
Epidural ICP was measured using a fibre optic pressure transducer (420LP, SAMBA Sensors, Sweden, accuracy = ±0.4 mmHg) according to our published method.1,2,7,13 Briefly, a hollow poly-ethyl ether ketone screw (Solid Spot LLC, Santa Clara, CA, USA) of 2 mm diameter × 5 mm length was inserted into the left parietal bone. Our experience indicates that the side of probe placement does not influence ICP readings. The sensor was placed above the dura and sealed in place with a caulking material (Silagum, DMG Dental, Hamburg, Germany). Recent studies indicate negligible differences between ICP measurements taken epidurally or intraparenchymally in rats, and the epidural method avoids brain trauma and the risk of creating a CSF leak.14,15 Probe location was validated by ensuring clear cardiac and respiratory waveforms and responsiveness of signal to abdominal compression.13 Cerebral perfusion pressure (CPP) was calculated using the formula CPP = MAP − Mean ICP.
Histological analysis
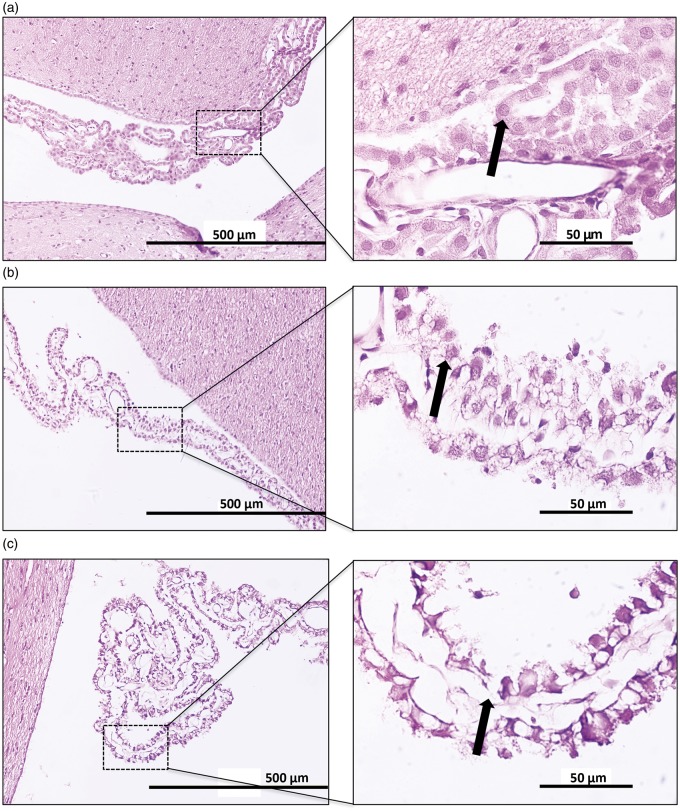
Histological analysis was performed according to our published method.12 After 24 h (Study I) and 72 h (Study III), animals were sacrificed and underwent transcardiac perfusion with saline followed by 4% paraformaldehyde in 0.2 M phosphate buffer. Brains were then fixed in neutral buffered formalin before being processed and paraffin embedded. Coronal sections of 5 µm were cut and stained with hematoxylin and eosin. Images were scanned using a digital slide scanner (Aperio Technologies, Vista, CA, USA) and analyzed by an investigator blind to experimental group allocation. Infarct (corrected for edema) was calculated by subtracting the measured interhemispheric volume difference (edema volume, ipsilateral minus contralateral) from the measured infarct volume for each slice. Lateral ventricle choroid plexus damage was assessed morphologically on three brain slices (1.3, 2.3 and 3.3 mm caudal to bregma) using the following scale; normal morphology (0) (Figure 2a), cellular shrinkage or vacuolation (1) (Figure 2b) and epithelial desquamation (2) (Figure 2c). The scores from each bregma level were summed to give a total score out of 6.
Figure 2.
Choroid plexus damage scoring. Hematoxylin and eosin staining of lateral ventricle choroid plexus at 20× (left panels) and 100× (right panels). (a) Normal choroid plexus histology (given a score of 0) showing a continuous epithelial lining of the choroidal villus with healthy epithelial cells (arrow). (b) Moderate choroid plexus damage (given a score of 1) showing an intact epithelial lining with shrunken and vacuolated epithelial cells (arrow). (c) Severe choroid plexus damage (given a score of 2) showing areas of epithelial desquamation (arrow).
CTP image acquisition, processing and infarct core and penumbra volume analysis
Detailed methods and CTP data have previously been reported for this cohort of animals;8,12,16 however, the CTP-derived infarct core and penumbra volumes 1 h after stroke were not previously analyzed. In brief, imaging was performed using a Siemens 64 slice CT scanner (Siemens, Erlangen, Germany) with a 512 × 512 matrix, 50 mm field of view with twelve 2.4 mm slices. Radio-opaque contrast for perfusion imaging was injected through a jugular venous catheter.12 Post-processing of perfusion data was performed using MIStar imaging software (Apollo Medical Imaging Technology, Melbourne, Australia) as previously described12 with some minor modifications. Animals had already been classified as having MCAo alone (MCAo, n = 19) or MCAo with concomitant AChA occlusion (MCAo + AChAo, n = 9) based on cerebral blood volume (CBV) maps in our previous publication.8 Infarct core and penumbra volumes were determined in this cohort of animals by applying our previously established cerebral blood flow (CBF) thresholds for core and penumbra (<55 % and <75 % of contralateral CBF, respectively) to all CBF maps.16 The total volume for each parameter for each animal was calculated using MiStar imaging software.
Statistics
Statistical tests were performed using Graphpad Prism 5.04 (La Jolla, USA) unless otherwise stated. Sample sizes calculated for between-group differences in ICP, based on pilot studies, were <4. Slightly larger cohorts were used to allow for the possibility of outliers, given the novelty of the study. D’Agostino and Pearson omnibus normality tests were performed on all data; appropriate statistical tests were performed based on the normality of the data.
Student’s t-tests were used to compare differences between groups (unpaired t-test) or changes from baseline (paired t-test). In Study III, ICP was measured at baseline, 24 and 72 h, therefore an analysis of covariance (ANCOVA) was performed using SPSS 21.0 (IBM, Armonk, USA) to test whether thread length had a significant effect on the primary outcome of change in ICP. Post-hoc Bonferroni’s t-tests were performed to test the differences in ICP between groups at each time-point. Mann–Whitney U tests were performed to compare choroid plexus damage scores between groups. Choroid plexus damage scores are presented as median (25th–75th interquartile range; IQR). Significant differences were accepted at the p < 0.05 level. Data are presented as mean ± standard deviation (SD) unless otherwise stated.
Results
Study I
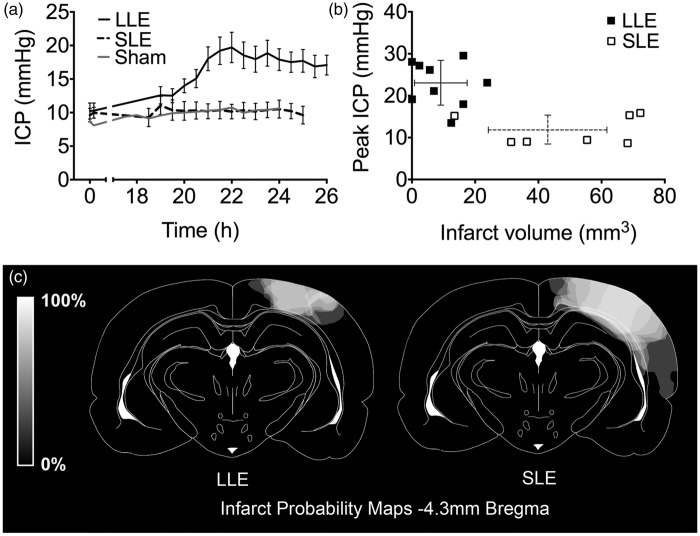
Three animals in total were excluded, due to epidural hemorrhage from ICP catheter insertion (1 LLE and 1 SLE) and equipment difficulties preventing collection of ICP data on day 2 (1 SLE). Physiological variables are presented in Table 1. In animals subjected to LLE, mean ICP rose significantly above baseline (10.2 ± 3.6 mmHg baseline, 23.1 ± 5.4 mmHg peak ICP, p < 0.001, n = 9; Figure 3a) and above the highest mean ICP in the SLE group (11.8 ± 3.7 mmHg, p < 0.01; 9.9 ± 2.4 mmHg baseline, n = 7). ICP did not rise in sham light exposure animals (Figure 3a). LLE animals had significantly smaller 24-h infarct volumes compared to those with SLE (9 ± 8 mm3 vs. 43 ± 18 mm3, respectively, p < 0.01; Figure 3b). Sham animals had no signs of infarction. Representative infarct size from each group is shown in Figure 3c. Edema volumes in both groups were very small and not significantly different (LLE: 0.2 ± 0.4 mm3 vs. SLE: 2.8 ± 3.8 mm3, p > 0.05). There was no evidence of significant choroid plexus injury in any animals in either group. Three animals in the LLE group and three animals in the SLE group had scores suggesting minor injury, however median (IQRs) in both groups were 0 (0–1).
Table 1.
Physiological parameters for Study I.
| Study I | Baseline |
During peak ICP |
24 h post-stroke |
|||
|---|---|---|---|---|---|---|
| LLE | SLE | LLE | SLE | LLE | SLE | |
| MAP (mmHg) | 102 ± 13 | 94 ± 14 | 96 ± 9 | 94 ± 14 | 96 ± 9 | 91 ± 11 |
| CPP (mmHg) | 91 ± 13 | 85 ± 16 | 73 ± 13## | 82 ± 12 | 80 ± 145 | 81 ± 12 |
| RR (BPM) | 71 ± 11 | 71 ± 14 | 70 ± 18 | 63 ± 18 | 68 ± 10 | 55 ± 9 |
| HR (BPM) | 402 ± 58 | 399 ± 36 | 409 ± 27 | 380 ± 38 | ||
| pCO2 (mmHg) | 56 ± 7 | 50 ± 5 | – | – | 55 ± 87 | 58 ± 12 |
| pO2 | 208 ± 90 | 155 ± 27 | – | – | 188 ± 37 | 170 ± 54 |
| pH | 7.36 ± 0.05 | 7.39 ± 0.04 | – | – | 7.39 ± 0.05 | 7.39 ± 0.06 |
| Temp (℃) | 37.6 ± 0.6 | 37.8 ± 0.9 | 37.8 ± 0.6 | 37.5 ± 0.6 | ||
Values for MAP, CPP, RR, HR, pCO2, pO2, pH and temperature. CPP: cerebral perfusion pressure; HR: heart rate; LLE: low light exposure; MAP: mean arterial pressure; pCO2: partial pressure of carbon dioxide; pO2: partial pressure of oxygen; SLE: standard light exposure. #p < 0.001 and ##p < 0.0001 versus baseline and *p < 0.001 versus LLE peak ICP. p values are for illustrative purposes and uncorrected for multiple comparisons.
Figure 3.
Study I – ICP, CPP and infarct volume following photothrombotic stroke. (a) ICP 0–0.5 h and 18–26 h post-photothrombotic stroke in Wistar rats using either LLE (black line; n = 9) or SLE (broken black line; n = 7) and sham animals (grey lines; n = 2), mean ± SEM. Each data point represents ICP averaged over 10 min, at 30-min time intervals. (b) Peak ICP versus 24-h infarct volume for LLE (filled squares, unbroken error bars) or SLE (open squares, broken error bars). (c) Infarct probability map at −4.3 mm Bregma, where the maximal extent of the photothrombotic lesions were located. Lighter regions represent areas more commonly infarcted. CPP: cerebral perfusion pressure; ICP: intracranial pressure; LLE: low light exposure; SEM: standard error mean; SLE: standard light exposure.
Study II
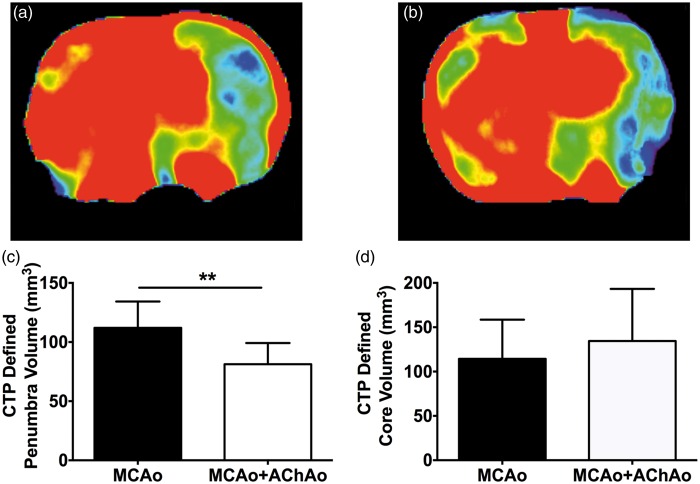
Physiological variables for this cohort of animals have been reported previously.7 Representative 1-h CBF maps for MCAo and MCAo + AChAo groups are shown in Figure 4a and b, respectively. Penumbra volume was significantly larger in the MCAo group compared to the MCAo + AChAo group (112 ± 22 mm3 vs. 81 ± 6 mm3, respectively, p < 0.01; Figure 4c). There was no significant difference in core volume between MCAo and MCAo + AChAo groups (114 ± 44 mm3 vs. 134 ± 20 mm3, p > 0.05; Figure 4d).
Figure 4.
Study II – CTP core and penumbra volume analysis in animals with or without concomitant AChAo during intraluminal MCAo. Representative 1-h CBF maps for animals with MCAo (a) or MCAo + AChA (b). Penumbra volume (c), core volume (d) at 1 h post-MCAo in animals with MCAo only (black bars) and animals with MCAo + AChAo (white bars). **p < 0.01. AChA: anterior choroidal artery; AChAo: anterior choroidal artery occlusion; CTP: computer tomography perfusion; MCAo: middle cerebral artery occlusion.
Study III
Twelve animals were excluded in total, due to technical difficulties during surgery (three long thread), mortality during surgery (one prior to randomization and one short thread), subarachnoid hemorrhage (two short and one long thread), or lack of LDF drop (two short and two long thread). One short thread animal died following 24-h ICP monitoring. It was included in ICP but not infarct volume analysis. Physiological variables are reported in Table 2.
Table 2.
Physiological parameters for Study III.
| Study III | Baseline |
24 h post-stroke |
72 h post-stroke |
|||
|---|---|---|---|---|---|---|
| Short thread | Long thread | Short thread | Long thread | Short thread | Long thread | |
| MAP (mmHg) | 104 ± 11 | 103 ± 13 | 103 ± 8 | 106 ± 12 | 97 ± 13 | 93 ± 7 |
| CPP (mmHg) | 97 ± 11 | 92 ± 17 | 87 ± 15 | 103 ± 7* | 88 ± 14 | 88 ± 8 |
| RR (BPM) | 67 ± 12 | 71 ± 3 | 59 ± 7 | 59 ± 10 | 56 ± 8 | 55 ± 4# |
| HR (BPM) | 434 ± 65 | 468 ± 31 | 411 ± 41 | 460 ± 26 | 373 ± 48 | 394 ± 58# |
| pCO2 (mmHg) | 46 ± 6 | 43 ± 4 | 49 ± 5 | 43 ± 8 | 49 ± 6 | 44 ± 6 |
| pO2 | 213 ± 10 | 203 ± 27 | 216 ± 15 | 207 ± 37 | 223 ± 16 | 209 ± 28 |
| pH | 7.41 ± 0.04 | 7.42 ± 0.03 | 7.39 ± 0.03 | 7.45 ± 0.05 | 7.34 ± 0.16 | 7.41 ± 0.03 |
| Temp (℃) | 37.7 ± 0.9 | 38.3 ± 0.9 | 37.4 ± 0.6 | 38.2 ± 1.1 | 37.3 ± 0.5 | 37.3 ± 0.5 |
MAP, CPP, RR, HR, pCO2, pO2, pH and temperature. CPP: cerebral perfusion pressure; HR: heart rate; LLE: low light exposure; MAP: mean arterial pressure; pCO2: partial pressure of carbon dioxide; pO2: partial pressure of oxygen; SLE: standard light exposure #p < 0.05, ##p < 0.01 and ###p < 0.001 versus baseline and *p < 0.05, **p < 0.01 versus short thread. p values are for illustrative purposes, uncorrected for multiple comparisons.
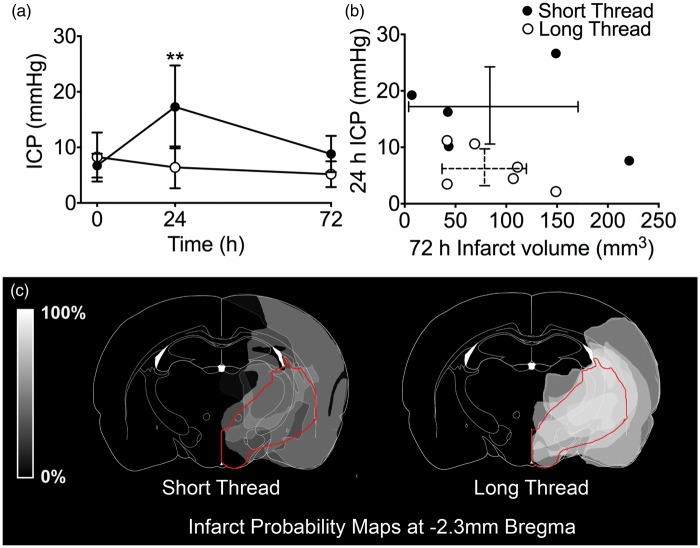
There was a significant main effect of thread length on ICP, F (1,3) = 6.33, p = 0.023, n = 6 per group. In the short thread group, baseline ICP was 6.7 ± 2.2 mmHg and increased to 17.3 ± 7.5 mmHg at 24 h and subsequently decreased to 8.8 ± 3.3 mmHg at 72 h. In the long thread group, ICP was 8.3 ± 4.4 mmHg at baseline, 6.4 ± 3.8 mmHg at 24 h and 5.2 ± 2.3 mmHg at 72 h (Figure 5a). ICP in the short thread group was significantly higher than the long thread group at 24 h post-stroke (p < 0.01, Bonferroni’s t-test). One short thread animal was observed to have severe stroke signs shortly after recovery from anesthesia. This animal subsequently had a large infarct volume at 72 h (221 mm3) and had no 24-h ICP elevation. Infarction was seen within the AChA territory. This animal was included in the short thread (excellent collaterals group) in all analysis, according to original group allocation.
Figure 5.
Study III – ICP, infarct volume and infarct probability following pMCAo in Wistar rats. (a) ICP at baseline, 24 h and 72 h post-pMCAo. Strokes were induced with intraluminal threads with silicon tip length designed either to maintain AChA patency (1.5 mm tips, short thread group, closed circles; n = 6) or to occlude the AChA (4 mm tips, long thread group, open circles; n = 6). Main effect of thread length on ICP, F (1,3) = 6.33, p = 0.023; ANCOVA. **p < 0.01 between groups; post-hoc Bonferroni t-tests (b) Relationship between 72-h infarct volume and 24-h ICP in the short thread group (closed circles, unbroken error bars) and the long thread group (open circles, broken error bars). (c) Infarct probability map at −2.3 mm Bregma. Lighter regions represent areas more commonly infarcted. The region supplied by the AChA is outlined in red.17 AChA: anterior choroidal artery; ANCOVA: analysis of covariance: ICP: intracranial pressure; pMCAo: permanent middle cerebral artery occlusion.
There were no significant differences in infarct or edema volumes between short- and long thread groups. (Infarct: 93 ± 89 mm3 vs. 87 ± 43 mm3, Figure 5b; edema: 1.0 ± 2.8 mm3 vs. 2.0 ± 2.7 mm3, respectively; p > 0.05). There was a noticeable difference in distribution of infarct volumes between groups with a dichotomous distribution in the short thread group. Infarct probability maps revealed that there was a greater probability of infarction within the region supplied by the AChA in the long thread group compared to the short thread group (Figure 5c). There was no significant difference in the degree of lateral ventricle choroid plexus damage scores between the short thread group, median score of 1 (0–1.5) and the long thread group, median score of 0 (0–1.25), p = 0.11.
Discussion
This study indicates that ICP elevation at 24 h post-stroke is not a product of the choroid plexus ischemia that has previously been reported in the thread occlusion model, since we saw ICP elevation with purely cortical PT strokes. Our results also indicate that a region of submaximal injury is required to trigger the recently described edema-independent ICP elevation 24 h after onset of ischemic stroke. We suggest that active cellular processes within the ischemic penumbra are required for ICP elevation, since no ICP rise was seen in animals with larger, more complete infarction, in which cellular products would be expected to be released from necrotic cells.
We found no association between the presence of choroid plexus damage and 24-h ICP elevation in both cortical PT and intraluminal MCAo stroke models. Somewhat surprisingly, we saw minimal damage to the choroid plexus following thread occlusion, which is known to occlude the AChA and has been shown to cause acute damage to the choroid plexus in Sprague-Dawley rats.4 This was despite the long thread group having a higher frequency of infarction within the region of the sub-cortex supplied by the AChA compared to the short thread group, indicating a higher frequency of AChA occlusion in the long thread group.17 A potential explanation for the lack of choroid plexus damage is that choroid plexus histology was assessed three days after MCAo, giving no indication of the degree of ischemia and choroid plexus damage at the time of MCAo or at 24 h when ICP was elevated. Acute damage to the choroid plexus may not be visible at three days due to regenerative capacity of the choroid plexus (occurring within 12–24 h after forebrain ischemia).18 Alternatively, perfusion to the choroid plexus may have been maintained in the presence of AChA occlusion by the posterior choroidal artery, which arises from the posterior cerebral artery.19 Regardless of the mechanism of preserved choroid plexus morphology following MCAo, the lack of choroid plexus damage and the 24-h ICP rise after cortical PT stroke (at a distance from the subcortical choroidal circulation), and the greater ICP rise in the short thread group with less AChA territory infarction, all indicate that choroid plexus damage is not necessary to cause 24-h ICP rise.
ICP elevation was observed following PT stroke, but only after stroke designed to produce submaximal lesions. In our PT model, the area of the cortex exposed to the light was the same between the two groups; only light intensity and duration were varied. Only the SLE produced stroke lesions that encompassed the majority of the area exposed to the light, whereas the short duration group has much smaller lesions. We speculate that the difference in infarction between the two groups represents tissue that was mildly ischemic or had spontaneously reperfused, thus resulting in tissue salvage (penumbra). This has been demonstrated in other studies, where a penumbra like ‘region at risk’ can be induced with the PT model using LLE and intensities.20 In contrast, the standard PT model is well known to induce rapid infarct evolution that lacks penumbra and is exacerbated by ischemia-independent mechanisms including generation of reactive oxygen species and blood–brain barrier breakdown.21,22 This suggests to us that an area of submaximal injury may be necessary to induce ICP elevation at 24 h post-stroke. Alternative explanations are also possible. For example, there could be additional mechanisms blocking ICP elevation in animals with a large unreperfused infarct core. Further studies are required to elucidate the exact mechanism.
Inadvertent occlusion of the AChA during intraluminal MCAo was associated with smaller volume of penumbra 1 h after stroke onset. There are large differences in the volume of penumbral tissue and the rate it is incorporated into the infarct core following occlusion of the MCA in different rat strains.23–26 This is generally thought to be due to differences in the residual blood flow to the ischemic brain via the leptomeningeal collateral circulation.27 We have previously demonstrated that inadvertent occlusion of the AChA, an important internal collateral supply to the MCA results in larger 24-h histological infarct volumes.8 The current analysis indicates that those animals with patent AChA have a larger penumbra.
Our finding of ICP elevation in those with short thread tip lengths (1.5 mm, aiming to keep the AChA patent) but not in those with long-tipped threads (4 mm, aiming to occlude the AChA) strongly suggests that the presence and potentially salvage of penumbra influences ICP elevation. The potential translational significance of this is that the model of pMCAo with good internal collateral supply mimics the recently described scenario of patients with delayed stroke progression. The very similar timing of the two events is suggestive. However, it still remains to be shown that ICP elevation occurs in patients with minor stroke, and definitive proof of ICP elevation directly causing infarct expansion is also still required.
Conclusions
In the current study, we have made two important observations regarding the trigger for the recently described ICP elevation occurring approximately 24 h after MCAo. First, we have shown that it occurs independent of choroid plexus damage. Second, the data indicate that the ICP rise is associated with presence of ischemic penumbra or submaximal tissue injury. Perhaps more remarkably, and in marked contrast to previous thoughts about ICP elevation after stroke, we found that ICP did not rise in animals with larger and more completely evolved infarction. We also found that ICP elevation occurred 24 h after permanent vessel occlusion, but only in the setting of good collaterals. Coupled with our previous data showing that such ICP elevation reduces collateral flow, we suggest that ICP elevation is a possible cause of collateral failure and infarct expansion in patients with stroke-in-progression. Multiple unanswered questions remain, however, these findings suggest that we may be on the cusp of a major change in our understanding of stroke evolution.
Acknowledgements
We would like to thank the Faculty of Health Stores Workshop of the University of Newcastle for manufacture of bespoke surgical and anesthetic equipment.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by a National Health and Medical Research Council Project Grant, APP1033461, the Hunter Medical Research Institute from funds donated by the Greater Building Society and by the National Stroke Foundation (Australia). D. Beard and L. Murtha were both supported by an Australian Postgraduate Award. N. Spratt was supported by a NHMRC career development fellowship, #1035465.
Declaration of conflicting interests
The author(s) declared no conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
DB, CL and DM performed the surgical and experimental components of the study, analyzed and interpreted the data, including statistical analysis and drafted the manuscript. RH, DP conducted histological analysis for the study. RH, DP, LM and NS participated in the design of the study and helped draft the manuscript. DB, CL, DM and NS, conceived the study and participated in its design and coordination. All authors read and approved the final manuscript.
References
- 1.Murtha LA, McLeod DD, McCann SK, et al. Short-duration hypothermia after ischemic stroke prevents delayed intracranial pressure rise. Int J Stroke 2014; 9: 553–559. [DOI] [PubMed] [Google Scholar]
- 2.Murtha LA, McLeod DD, Pepperall D, et al. Intracranial pressure elevation after ischemic stroke in rats: cerebral edema is not the only cause, and short-duration mild hypothermia is a highly effective preventive therapy. J Cereb Blood Flow Metab 2015; 35: 592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burrows G. On disorders of the cerebral circiulation and on the connection between affections of the brain and diseases of the heart, London: Longman, 1846. [PMC free article] [PubMed] [Google Scholar]
- 4.Ennis SR, Keep RF. The effects of cerebral ischemia on the rat choroid plexus. J Cereb Blood Flow Metab 2006; 26: 675–683. [DOI] [PubMed] [Google Scholar]
- 5.Coutts SB, Modi J, Patel SK, et al. CT/CT angiography and MRI findings predict recurrent stroke after transient ischemic attack and minor stroke: results of the prospective CATCH study. Stroke 2012; 43: 1013–1017. [DOI] [PubMed] [Google Scholar]
- 6.Campbell BC, Christensen S, Tress BM, et al. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab 2013; 33: 1168–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beard DJ, McLeod DD, Logan CL, et al. Intracranial pressure elevation reduces flow through collateral vessels and the penetrating arterioles they supply. A possible explanation for ‘collateral failure’ and infarct expansion after ischemic stroke. J Cereb Blood Flow Metab 2015; 35: 861–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLeod DD, Beard DJ, Parsons MW, et al. Inadvertent occlusion of the anterior choroidal artery explains infarct variability in the middle cerebral artery thread occlusion stroke model. PLoS One 2013; 8: e75779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan Y, Wang Y, Yuan F, et al. Effect of suture properties on stability of middle cerebral artery occlusion evaluated by synchrotron radiation angiography. Stroke 2012; 43: 888–891. [DOI] [PubMed] [Google Scholar]
- 10.Kilkenny C, Browne W, Cuthill IC, et al. Animal research: reporting in vivo experiments – the ARRIVE guidelines. J Cereb Blood Flow Metab 2011; 31: 991–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spratt NJ, Fernandez J, Chen M, et al. Modification of the method of thread manufacture improves stroke induction rate and reduces mortality after thread-occlusion of the middle cerebral artery in young or aged rats. J Neurosci Meth 2006; 155: 285–290. [DOI] [PubMed] [Google Scholar]
- 12.McLeod DD, Parsons MW, Levi CR, et al. Establishing a rodent stroke perfusion computed tomography model. Int J Stroke 2011; 6: 284–289. [DOI] [PubMed] [Google Scholar]
- 13.Murtha L, McLeod D and Spratt N. Epidural intracranial pressure measurement in rats using a fiber-optic pressure transducer. J Vis Exp: JoVE 2012. DOI: 10.3791/3689. [DOI] [PMC free article] [PubMed]
- 14.Hiploylee C, Colbourne F. Intracranial pressure measured in freely moving rats for days after intracerebral hemorrhage. Exp Neurol 2014; 255: 49–55. [DOI] [PubMed] [Google Scholar]
- 15.Uldall M, Juhler M, Skjolding AD, et al. A novel method for long-term monitoring of intracranial pressure in rats. J Neurosci Meth 2014; 227: 1–9. [DOI] [PubMed] [Google Scholar]
- 16.McLeod DD, Parsons MW, Hood R, et al. Perfusion computed tomography thresholds defining ischemic penumbra and infarct core: studies in a rat stroke model. Int J Stroke 2015; 10: 553–559. [DOI] [PubMed] [Google Scholar]
- 17.He Z, Yang SH, Naritomi H, et al. Definition of the anterior choroidal artery territory in rats using intraluminal occluding technique. J Neurol Sci 2000; 182: 16–28. [DOI] [PubMed] [Google Scholar]
- 18.Johanson CE, Palm DE, Primiano MJ, et al. Choroid plexus recovery after transient forebrain ischemia: role of growth factors and other repair mechanisms. Cell Mol Neurobiol 2000; 20: 197–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moffat DB. The development of the posterior cerebral artery. J Anat 1961; 95: 485–494. [PMC free article] [PubMed] [Google Scholar]
- 20.Hu X, Brannstrom T, Gu W, et al. A photothrombotic ring stroke model in rats with or without late spontaneous reperfusion in the region at risk. Brain Research 1999; 849: 175–186. [DOI] [PubMed] [Google Scholar]
- 21.Kleinschnitz C, Braeuninger S, Pham M, et al. Blocking of platelets or intrinsic coagulation pathway-driven thrombosis does not prevent cerebral infarctions induced by photothrombosis. Stroke 2008; 39: 1262–1268. [DOI] [PubMed] [Google Scholar]
- 22.Watson BD, Dietrich WD, Busto R, et al. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann Neurol 1985; 17: 497–504. [DOI] [PubMed] [Google Scholar]
- 23.Bardutzky J, Shen Q, Henninger N, et al. Differences in ischemic lesion evolution in different rat strains using diffusion and perfusion imaging. Stroke 2005; 36: 2000–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouts MJ, Tiebosch IA, van der Toorn A, et al. Lesion development and reperfusion benefit in relation to vascular occlusion patterns after embolic stroke in rats. J Cereb Blood Flow Metab 2014; 34: 332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Letourneur A, Roussel S, Toutain J, et al. Impact of genetic and renovascular chronic arterial hypertension on the acute spatiotemporal evolution of the ischemic penumbra: a sequential study with MRI in the rat. J Cereb Blood Flow Metab 2011; 31: 504–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reid E, Graham D, Lopez-Gonzalez MR, et al. Penumbra detection using PWI/DWI mismatch MRI in a rat stroke model with and without comorbidity: comparison of methods. J Cereb Blood Flow Metab 2012; 32: 1765–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riva M, Pappada GB, Papadakis M, et al. Hemodynamic monitoring of intracranial collateral flow predicts tissue and functional outcome in experimental ischemic stroke. Exp Neurol 2012; 233: 815–820. [DOI] [PubMed] [Google Scholar]