Abstract
The endothelial cells lining the brain capillaries separate the blood from the brain parenchyma. The endothelial monolayer of the brain capillaries serves both as a crucial interface for exchange of nutrients, gases, and metabolites between blood and brain, and as a barrier for neurotoxic components of plasma and xenobiotics. This “blood-brain barrier” function is a major hindrance for drug uptake into the brain parenchyma. Cell culture models, based on either primary cells or immortalized brain endothelial cell lines, have been developed, in order to facilitate in vitro studies of drug transport to the brain and studies of endothelial cell biology and pathophysiology. In this review, we aim to give an overview of established in vitro blood–brain barrier models with a focus on their validation regarding a set of well-established blood–brain barrier characteristics. As an ideal cell culture model of the blood–brain barrier is yet to be developed, we also aim to give an overview of the advantages and drawbacks of the different models described.
Keywords: Blood–brain barrier, endothelium, astrocytes, pericytes, stem cells
Introduction
The blood–brain barrier
The small capillaries of the brain constitute unique morphological and functional units that serve a number of different roles. The capillaries have to supply the nervous tissue with nutrients and oxygen, they have to participate in the maintenance of water and electrolyte balance in the brain interstitial fluid and they must protect the neurons from potentially harmful substances present in the blood. The barrier function of brain capillaries, the blood–brain barrier (BBB), is primarily due to the presence of complex tight junctions and to a specific expression pattern of different solute carriers (SLCs) and ABC-type efflux transporters. The capillaries of the brain are complex structures, consisting of several cell types (see Figure 1). The endothelial cells constitute the capillary wall and thus the actual barrier, but the endothelial cells are surrounded by pericytes (coverage estimated to be ∼30%).1 The endothelial cells and pericytes are surrounded by a basement membrane, and astrocyte endfeet ensheath the abluminal side of the capillaries with a coverage estimated at 99%.1
Figure 1.
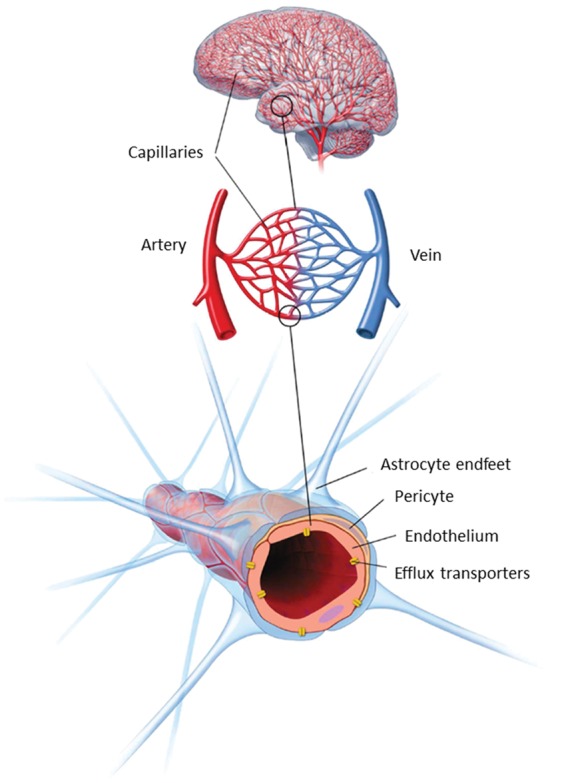
Schematic overview of the structure of the neurovascular unit. The endothelial cells of the brain capillaries are covered (∼30%) with pericytes, embedded in the basement membrane of both endothelial cells and astrocytes. The endothelium and attached pericytes are covered almost completely by a surrounding layer of astrocyte endfeet. Communication between the cell types of the neurovascular unit ensures that the brain endothelium maintains the blood–brain barrier specific phenotype. Modified from Lægemiddelforskning 2015 (http://www.farma.ku.dk/index.php/Laegemiddelforskning-2015/11840/0/).
Both pericytes and astrocytes regulate the phenotype of the endothelium, through mechanisms not yet fully understood but involving cell–cell communication via soluble factors and possibly also direct contact interactions.2,3 The brain capillary endothelial cells (BCEC) and the surrounding accompanying cell types thus constitute the “ neurovascular unit” (NVU), a term reflecting the specialized and unique cellular structure of the brain microvasculature.
There is great interest in generating in vitro models reflecting the properties of the BBB. An ideal in vitro model of the BBB would allow mechanistic studies of BBB tight junctions, transporters, enzymes, macromolecular and immune cell trafficking and signaling and be suitable for rapid screening of BBB permeability for new central nervous system (CNS) drug candidates.
Validation markers for in vitro BBB models
A set of validation markers was chosen to compare the different in vitro models in this review. The markers are shown in Table 1.
Table 1.
Blood–brain barrier validation markers.
| Category | Property | Relevance | Validation | Key references |
|---|---|---|---|---|
| Validation of cell lineage | Monolayer of thin cells with large surface area | All studies | Visualization, F-actin staining | 4,5 |
| Expression of endothelial markers | Von Willebrand’s Factor/PECAM-1 | 6–8 | ||
| Tight junctions | Occludin claudin-5 ZO-1 | Studies of tight junctions – transendothelial transport and uptake studies – Cell polarization | mRNA and protein expression – localization | 9–11 |
| High junctional tightness | TEER and permeability measurements | 12–16 | ||
| Efflux transporters | P-pg | Transendothelial transport and uptake studies – drug delivery to/through the BBB – toxicity | mRNA and protein expression – Cellular uptake or efflux in absence/presence of inhibitors – bi-directional transport studies | 17,18 |
| BCRP | 19–22 | |||
| Mrp | 23–25 | |||
| SLC expression | Glut-1 | Transendothelial transport and uptake studies – drug delivery to/through the BBB. Brain nutrition studies | mRNA and protein expression – Cellular uptake in absence/presence of inhibitors – transendothelial transport studies | 26–28 |
| LAT-1 | 29,30 | |||
| MCT-1 | 31–33 | |||
| Receptor systems | Transferrin receptor | Studies of receptor-mediated transport, brain nutrition studies | mRNA and protein expression – transferrin uptake – transendothelial transport of iron | 34–36 |
| Responsiveness to regulation from NVU cells | Induction by astrocytes | Studies of cell regulation and NVU signalling | Regulation of TEER, P-gp expression and cell morphology | 37–40 |
| Induction by pericytes | Regulation of TEER, proteins involved in vesicular transport | 41,42 |
The markers shown in Table 1 are not a complete set of BBB characteristics. An important issue is that knowledge about the in vivo BBB is still lacking, which makes it difficult to firmly establish the features that an ideal BBB model should possess. Recent studies focusing on the BBB transcriptome and proteome are beginning to accumulate knowledge, which in time may provide a more complete fingerprint of the BBB for the models to mimic.43–51 While no model exactly mimics the in vivo BBB expression of enzymes, transporters, receptors, and structural proteins, they can nevertheless be useful tools. The validation markers chosen in this study have all been shown to have functional importance at the BBB, which makes their expression and function in the model important, at least for studies concerning subjects related to this characteristic.
An important feature of BBB models is high junctional tightness. This is often measured as transendothelial electrical resistance (TEER). TEER obtained by separate groups in separate studies may differ somewhat, not only because of differences in actual junctional tightness but also because of differences in measuring equipment (chopstick electrodes, cup electrodes, impedance measurements), temperature, and handling of the cells during measurements.52 TEER may also be difficult to translate to a functional estimate of tightness, as the tightness of the endothelial monolayer depends both on the composition of the tight junction complexes and on the size of the compound of interest. Validation of functional tightness can also be performed by permeability studies with hydrophilic tracer molecules such as Lucifer yellow (444 Da), sodium fluorescein (376 Da), sucrose (342 Da), or mannitol (180 Da). TEER correlates with permeability for a given small hydrophilic molecule,53–58 but the correlation depends strongly on the size of the molecule and the experimental design (shaking/no shaking, change of medium, sampling during the experiment, single point estimation/steady state calculations). Thus, the optimal characterization of paracellular permeability should include both TEER and tracer flux. Expression and junctional localization of specific tight junction proteins are related characteristics. Tight junctions exist in a range of different tissues and the specific combination, especially of claudins, gives the junctional complex its specific properties.59,60 Claudin-5 has been established as a tightening claudin with high BBB expression, and loss of claudin-5 causes BBB leakage of small molecules.9 Thus, claudin-5 expression is essential in a BBB model if it is to be used for studying transport- or tight junction-related phenomena.
Efflux transporters of the ABC family and SLCs play essential roles in the BBB permeability of small molecules, both endogenous compounds and xenobiotics.2 This makes their expression, correct localisation, and functionality important validation characteristics for a BBB model, at least if the model is to be used for BBB permeability screening, CNS-toxicity studies, pro-drug formulation studies, or studies of nutritional status of the BBB. Validation can be performed via protein or mRNA expression studies, but functional validation with accumulation or bi-directional transport of model substrates and inhibitors should be performed if the model is to be applied in studies where transporters may have a direct influence on the outcome. Macromolecule transport across the BBB is more controversial. Several receptor systems potentially able to mediate transcytosis and thus CNS delivery of ligands or compounds conjugated to ligands have been investigated including insulin receptor, LRP-1, LDL-receptor, leptin receptor, glutathione receptor, diphtheria toxin receptor, and transferrin receptor61–68 (for review of receptor systems applied for brain targeting, see literature69). Of these, the most studied receptor system shown to facilitate CNS delivery of clinically relevant doses in vivo is the transferrin receptor.35,70 LRP-1 has also been utilized to deliver therapeutics across the BBB, for instance by conjugating paclitaxel to the LRP-1 substrate, angiopep-2, which caused a significant increase in the brain uptake and survival of tumor implanted mice.71,72 Similar results have been demonstrated with angiopep-2 coupled to monoclonal antibodies or doxorubicin.73,74 However, controversies exist regarding LRP-1 expression in brain endothelial cells, where some studies have shown that it is mainly found in pericytes,51,75,76 whereas others show expression in endothelial cells.77–79 The transferrin receptor is widely agreed to be highly expressed in brain endothelial cells in vivo, which makes the transferrin receptor a good validation target when setting up a new model.
A brief history of in vitro BBB model development
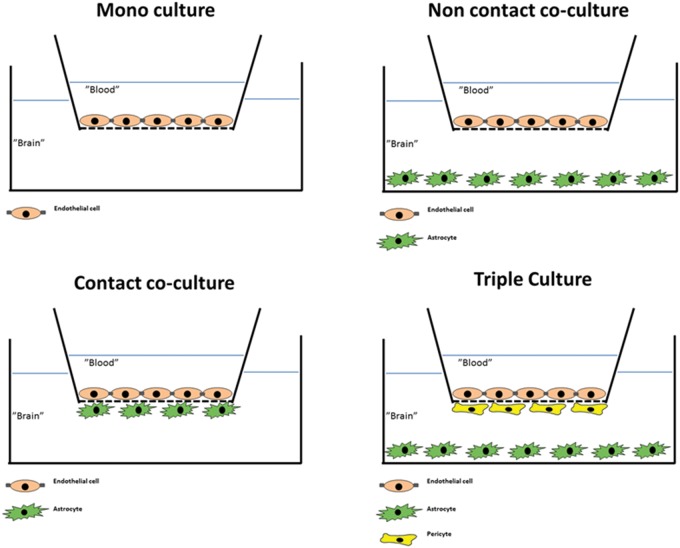
Efforts to generate cell cultures of BCEC started in the early 1970s with isolations of brain capillaries.80,81 A combination of mechanical homogenization of brain tissue and sucrose gradient centrifugations yielded pure and intact brain capillaries, which could be used directly to study BBB properties.81 The isolation techniques have since been modified with the use of filtration steps instead of, or in combination with, centrifugation, and isolated capillaries have been used in a number of functional assays to quantify P-glycoprotein (P-gp) activity and tight junction integrity as well as studying transporter regulation and other properties.24,79,82–84 The methods for isolating brain capillaries were further developed to yield isolation of primary endothelial cells.4,85–87 Isolated brain capillaries were treated with a mixture of enzymes to degrade the basement membranes, remove the pericytes, and release the endothelial cells. These cultures were based solely on endothelial cells without induction by other cells of the neurovascular unit (see Figure 2).
Figure 2.
Commonly used configurations used for culture of brain endothelial cells. Mono-culture: Brain endothelial cells are grown on the upper surface of permeable supports in a two-compartment cell culture system. Media may be added astrocyte-conditioned medium to promote growth and differentiation in the absence of the other cell types of the neurovascular unit. Non-contact co-culture: The endothelial cells are seeded on the upper surface of the support, while astrocytes (or other cell types, often pericytes) are seeded at the bottom of the culture well. This configuration allows for induction of the endothelium by diffusible factors from the “feeder cells” at the bottom of the well, while the insert can be removed after culture for experiments, which can be performed on endothelial cells only. Contact co-culture: Astrocytes (or other cell types) are seeded on the lower surface of the support with endothelial cells on the upper surface. This may allow for direct contact between the opposing cell types. A drawback of the configuration is that the two cell types cannot readily be separated in experimental protocols employing Western blotting or transport and accumulation studies. Triple culture: This configuration includes in its most common form endothelial cells seeded on the upper surface of the support, pericytes seeded on the lower surface, and astrocytes seeded on the bottom of the culture wells. This configuration mimicks the cell arrangement at the neurovascular unit, and allows for interactions between all three cell types. However, due to the different cell types involved, this configuration is also more demanding in terms of workload and experimental skills.
Debault and Cancilla87 reported that co-culture of isolated endothelial cells with C6 glioma cells induced γ-glutamyl transpeptidase activity in the endothelium, which was otherwise lost in culture. Furthermore, Tao et al.88 prepared co-cultures of endothelial cells on coverslips in proximity to an astrocyte cell layer and showed an increase in tight junction length and complexity by freeze fracture studies. Dehouck et al.89 used a co-culture approach in studies with bovine endothelial cells and rat astrocytes seeded on opposite surfaces of permeable membranes in transwell culture inserts (contact-co-culture) (see Figure 2). This caused a tightening of the cell junctions as reflected by an increase in TEER to approximately 660 Ω cm2 as well as better retention of γ-glutamyl transpeptidase activity. The ability of astrocytes to increase TEER in endothelial cell cultures has been demonstrated in numerous later studies, both with contact and non-contact co-cultures (astrocytes seeded on the bottom of the plate below the filter insert) and with mono-cultures of endothelial cells cultured in astrocyte-conditioned medium.5,90–96 More recently, pericytes have been included in some BBB models either as a replacement for astrocytes or in triple culture with astrocytes and endothelial cells.97–103 The endothelial cell/pericyte/astrocyte triple cultures have shown slightly higher TEER values than corresponding endothelial cell/astrocyte co-cultures in studies on rat primary cells, but the exact mechanisms of junctional regulation remain to be established.
The years of model development have resulted in a range of well-established and characterized models run on a routine basis in different laboratories. These are based on pig,104–106 bovine,89,90,107 rat,96,98 and mouse endothelial cells.108–110 These models of non-human origin have provided a wealth of information on the physiology and pathophysiology of the BBB and have allowed very valuable cross-validation between models. Human tissue is difficult to obtain on a regular basis, which has limited the development of primary cultures of human brain endothelial cells and cell-based human models.111–113 However, two different methodological approaches to circumvent this problem have been established. Different groups established and characterized immortalized human brain endothelial cells114,115 and three different groups have published BBB models based on stem cell-derived endothelial cells.116–118
Aim of the review
Many of the models generated during the past 40 years continue to be used in different research groups to analyze several aspects of BBB biology and drug targeting. However, none of the models applied behave in exactly similar ways, and small differences in the way the individual laboratories handle the models can make it a challenge to obtain a clear overview of the benefits and drawbacks of the various in vitro BBB-models.
The aim of this review is to give an updated overview of in vitro models of the BBB, to aid in navigating and interpreting the literature, and in choosing the most practical and appropriate models for particular projects. We have selected a number of commonly used – as well as newly developed models derived from mouse, rat, bovine, porcine, and human endothelial cells, and assessed these against a pre-defined set of BBB validation markers including endothelial phenotype, marker protein expression profile, and function. This may provide a clearer overview of the strengths and weaknesses of commonly applied models and point towards questions still unanswered.
Mouse models – Immortalized and primary mouse brain endothelial cultures
Primary cultures of mouse brain endothelial cells must be freshly isolated prior to experiments and show variation from batch to batch. Coisne et al.109 reported a primary cell co-culture model with mouse endothelial cells and astrocytes, which presented classic BBB characteristics, such as occludin, claudin-3, claudin-5, and P-gp expression. The model had high junctional tightness with TEER averaging almost 800 Ω cm2 and sucrose permeability of 4.5 × 10−6 cm/s (see Table 2). However, TEER measurements of this magnitude are rarely reported in studies on mouse primary endothelial cells, and TEER values of 100–300 Ω cm2 are more commonly reported.42,119–121 Thus, considerable effort has been invested in the generation of immortalized mouse brain endothelial cell lines, which have the advantage of being stable for a number of passages and may yield a large number of endothelial cells with the same genetic and phenotypical characteristics. Furthermore, the established protocols for cell isolation and immortalization can be used to generate BCEC cell lines from genetically modified animals. Work by the Risau group122 resulted in the first mouse brain endothelial cell lines generated by immortalization with Polyoma middle T antigen. bEND.5 and bEND.3 are commercially available cell lines based on this immortalization strategy; however, both cell lines generally display low TEER (around 50 Ω cm2).123–125 Forster et al.108,110,126 generated the alternative cell lines, cEND and cerebEND, from mouse cerebral and cerebellar capillaries, respectively (see Table 2). Both cell lines form monolayers and show spindle-shaped morphology.110,126 The cEND cell line has TEER varying from 300 to 800 Ω cm2 and strong occludin and claudin-5 expression at the tight junctions. The higher electrical resistance of the cEND cell line corresponded to lower permeability of FITC-Dextran 4, 10, 70, and 500 kDa,110 as compared to bEND.3, but functional tightness has not been characterized using small molecule tracers. Both cell lines, cEND and cerebEND, express endothelial cell markers and junctional proteins (Pecam-1, VE-cadherin, claudin-5, occludin, ZO-1) as shown at the mRNA and protein level.110,127 Low levels of claudin-1, claudin-3, and claudin-12 were detected at the mRNA and protein levels in cEND and cerebEND.126,128–130
Table 2.
Mouse in-vitro models of the blood–brain barrier. Morphology, tightness and astrocyte/pericyte induction.
| Model type | Endothelial morphology | Junction Claudins | ∼TEER (Ohm·cm2) (mean values) | Permeability 10−6 (cm/s) | Occludin/ZO proteins | Astrocyte induction | Pericyte induction | Selected key references |
|---|---|---|---|---|---|---|---|---|
| Primary mouse BCEC/astrocyte coculture | Monolayer Spindle shape | 3 and 5 (ICC) | 800 | 4.5 (sucrose) | Occludin (ICC) | – | – | 109 |
| cEND (immortalized mouse cerebral endothelial cells) | Monolayer Spindle shape (serum) Cobblestone (hydrocortisone + insulin) | 5 (ICC,WB), 3 and 12 (WB) low levels of 1 (mRNA) | 300 (serum) to 800 (Hydrocortisone + insulin) | – | Occludin (ICC, WB) | – | – | 110,126,129 |
| cereBEND (immortalized mouse cerebral endothelial cells) | Monolayer Spindle shape | 5 (ICC,WB) low levels of 1,3,12 (mRNA) | 500 | – | Occludin (ICC, WB) ZO1 (ICC, WB) | Slight increase in TEER by coculture with rat glioma C6 cell line | – | 126,130,131 |
: not investigated; ICC: Immunocytochemistry; WB: Western blotting.
Note: The permeability value of the smallest tested compound in the study is given.
cEND cells respond strongly to glucocorticoids by induction of BBB properties. Glucocorticoids induce cytoskeletal rearrangements, regulate tight junction proteins occludin and claudin-5, and cause TEER to increase up to 1000 Ω cm2.110,132–134
The cerebEND model has been further developed and co-cultured with an immortalized rat glial cell line (C6), which caused a slight increase in TEER.130 The presence of P-gp, breast cancer resistance protein (BCRP) and multidrug-resistance protein-4 (Mrp-4) has been demonstrated in cerebEND cells by Western blot and immunofluorescence, but has not been shown in the cEND cell line130 (see Table 3). Functional tests in uptake assays with specific substrates for P-gp (calcein-AM), Mrp-4 (fluo-cAMP), and BCRP (Bodipy-FL-prazosin) showed changes in transporter activity due to oxygen/glucose deprivation (OGD) and due to co-culture of cerebEND cells with C6 astrocytoma.130 Expression of glucose transporter-1 (Glut-1) has been demonstrated in cEND and cerebEND at the mRNA and protein level.126,131
Table 3.
Mouse in-vitro models of the blood–brain barrier. Receptor and transporter expression and function.
| Model type | ABC transporter expression/function | Vectorial net transport of ABC substrates | TFR expression/ function | LAT-1 expression/ function | Glut-1 expression/ function | MCT-1 expression/ function | Selected key references |
|---|---|---|---|---|---|---|---|
| Primary mouse BCEC/astrocyte coculture | P-gp expression (WB) | – | – | – | – | – | 109 |
| cEND (immortalized mouse cerebral endothelial cells) | – | – | – | – | WB | – | 110 |
| cereBEND (Immortalized mouse cerebral endothelial cells) | P-gp, BCRP and Mrp-4 expression (ICC, mRNA, WB) | – | – | – | mRNA, WB (low base levels, upreg. by OGD) | – | 126,130,131 |
: not investigated; ICC: immunocytochemistry; WB: Western blotting.
Note: The permeability value of the smallest tested compound in the study is given.
Both the cEND and the cerebEND cell lines respond to inflammatory stimuli. Treatment of the cEND and cerebEND cells with TNFα resulted in decreased tight junction protein expression and lower TEER.126,133 TNFα induced the expression of inflammatory stress markers including VCAM-1 and ICAM-1 in both cEND and cerebEND.126 Moreover, cEND treated with serum from multiple sclerosis patients showed decrease in occludin, claudin-5, and VE-cadherin levels135 and increased secretion of cytokines and growth factors, such as Ccl12 and Csf3.136
In summary, both in vitro models cEND and cerebEND have proved useful tools in studies of regulation of BBB protein expression under normal and pathophysiological conditions. Both models still need to be characterized regarding the expression and activity of SLC-transporters such as large neutral amino acid transporter (LAT-1) and mono carboxylic acid transporter-1 (MCT-1). Moreover, the effects of co-culture with pericytes on barrier tightness, TJ expression, transporter expression, and general endothelial phenotypic traits need to be further investigated.
Rat models – Mono-, co- and triple cultures of rat BCEC
Rat brains were the first source of BCEC for development of BBB models.85 Contaminating pericytes presented a major problem for primary rat BCEC cultures, and different methods have been employed to increase the purity of the endothelial cultures.137,138 Selection with the P-gp substrate, puromycin, proved successful139 and is now included in the culture protocol in a number of laboratories.55,93,140,141 Puromycin is typically present during the first two to three days of culture. This leads to tighter in vitro models,139,140 which may be caused by a more coherent endothelial monolayer due to the absence of pericytes. Alternatively, the higher resistance may be due to a selection of capillary endothelial cells over endothelial cells from larger microvessels.142 Rat models have been applied in different versions: mono-cultures of endothelial cells have been widely applied, but most recent studies use co-cultures- either endothelial/astrocyte co-cultures or endothelial/astrocyte/pericyte triple cultures. Both purified type-1 astrocytes97 and primary mixed glial cultures96,139 have proven efficient in the induction of a BBB phenotype in rat primary endothelial co-cultures, and models based on astrocyte co-cultures typically present well-characterized BBB models (see below).55,93,141 The triple cultures including pericytes were developed to further mimic the neurovascular unit.97,98 Initially, it was shown that inclusion of pericytes caused a greater differentiation of the brain endothelial cells than astrocyte or pericyte co-culture alone,98 and since then the model has been applied in 13 published papers regarding oxidative stress,143,144 amyloid-ß toxicity,145 and permeability screening146–148 amongst others.
Rat models generally display low to medium TEER, often around 100–300 Ω cm2 depending on the culture method (mono-culture, astrocyte/pericyte co/triple culture, induction with cAMP and/or glucocorticoids).58,96,97,140,141,147–154 However, several studies also report that rat models can reach TEER around 500–800 Ω cm2 under optimal culture conditions.55,93,97,145,155 This translates into permeabilities of sodium fluorescein, Lucifer yellow, and sucrose around 2–19 × 10−6 cm/s in the models displaying lower TEER,96,97,140,141,147,150,156 whereas permeabilities in the range of 0.8–3 × 10−6 cm/s have been reported in the high TEER models55,145,155 (see Table 4).
Table 4.
Rat in-vitro models of the blood–brain barrier. Morphology, tightness and astrocyte/pericyte induction.
| Model type | Endothelial morphology | Junction Claudins | ∼TEER (Ohm · cm2) (mean values) | Permeability 10−6 (cm/s) | Occludin/ZO proteins | Astrocyte induction | Pericyte induction | Selected key references |
|---|---|---|---|---|---|---|---|---|
| Primary rat BCEC/astrocyte co-culture | Monolayer Spindle shape | 5 (ICC,WB) low levels of 12 (mRNA) | 300–600 (Hydrocortisone) | 1.4 (Sucrose) 4.3(Lucifer yellow) | Occludin (ICC, WB) ZO1 (ICC, WB) | Increase in TEER, lowering of Pflourescein | – | 93,96,98, 141,157 |
| Primary rat BCEC/astrocyte/ pericyte triple cultures | Monolayer Spindle shape | 5 (ICC,WB) | 350–723 | 2–4 (flourescein) | Occludin (ICC, WB) ZO1 (ICC, WB) | Increase in TEER, lowering of Pflourescein | Increase in TEER | 97,98, 145,155 |
: not investigated; ICC: immunocytochemistry; WB: Western blotting.
Note: The permeability value of the smallest tested compound in the study is given.
Astrocyte(+); mixed glial culture dominated by astrocytes.
These high TEER-low permeability models have only been achieved using the co- and triple cultures.55,93,97,145,155
The rat models have been shown to express tight junction proteins claudin-5, occludin and ZO-1 in mono-cultures,140,150 astrocyte co-cultures,55,141,151 and triple cultures,97,155 where claudin-5 and ZO-1 protein expression levels are increased relative to mono-cultures.97 Also claudin-1, -3 and -12, and ZO-2 have been shown at either mRNA or protein level.96,157
Data on the rat BBB transcriptome45 and ABC transporters at the rat BBB158 have been obtained on isolated rat brain microvessels and isolated BCEC. Several BBB proteins were found to be down-regulated in mono-culture, notably Glut-1 (39 fold), P-gp (MDR-1A) (14 fold), and transferrin receptor (9 fold).159 Similar down-regulation has been observed in an endothelial/astrocyte co-culture model, where P-gp, transferrin receptor, Mrp-4, and Glut-1 expression levels were largely reduced upon six days of co-culture, whereas expression of BCRP, Mrp-1, and insulin receptor was retained.58 Although down-regulated, expression of a range of ABC transporters including P-gp, BCRP, and at least one isoform of Mrp is still evident at the mRNA level, protein level or both, in rat models using mono-cultures,160 astrocyte-co-cultures,58,96 and triple cultures.97,153 Functional P-gp expression has been well characterized in both astrocyte co-culture models and the triple-culture models.55,93,96,97,141,151 Bi-directional transport studies with rhodamine123 demonstrate vectorial transport favoring the brain-to-blood direction with an efflux ratio of approximately 2.5 in the triple-culture model,97 whereas similar studies in the astrocyte co-culture models have shown efflux ratios around 1.7 for rhodamine 123141 and 6.1 for amprenavir.58 Functional P-gp expression is further demonstrated by apical uptake studies with P-gp substrates showing increased uptake when P-gp inhibitors were co-administered55,96,141,153,156,160 (see Table 5).
Table 5.
Rat in-vitro models of the blood–brain barrier. Receptor and transporter expression and function.
| Model type | ABC transporter expression/function | Vectorial net transport of ABC substrates | TFR expression/ function | LAT-1 expression/ function | Glut-1 expression/ function | MCT-1 expression/ function | Selected key references |
|---|---|---|---|---|---|---|---|
| Primary rat BCEC/astrocyte co-culture | -P-gp (ICC, WB) BCRP, Mrp-3, Mrp-4, Mrp-5 (mRNA) Inhibitor data on uptake for all. | ER of 1.8 for Rhod 123 ER of 6.1 for Amprenavir (inhibited by GF120918) ER of 7.7 for Dantrolene (inhibited by Ko143) | ICC, mRNA, Tf-Cy3 binding | – | mRNA | – | 58,93,96,141, 157,165 |
| Primary rat BCEC/Astrocyte/ Pericyte triple co-culture | P-gp, Mrp-1 (ICC,WB). Inhibitor data on Rhod 123 uptake | ER of 2.5 for Rhod 123 | – | – | ICC, WB | – | 97,98,145 |
: not investigated; ICC: Immunocytochemistry; WB: Western blotting.
Note: The permeability value of the smallest tested compound in the study is given.
The functionality of BCRP on primary rat brain endothelial cells has been shown in the astrocyte co-culture model, both by accumulation assays96 and by bi-directional transport assays,58 in both cases by co-application of the BCRP inhibitor, Ko143.
The expression of glucose and amino acid transporters was demonstrated in primary cultures of brain endothelial cells,97,159,161–163 but few rat BBB culture models have been characterized for SLC transporter functionality. Active glucose uptake was described in primary rat brain endothelial cells, which was positively modulated by n-3 long-chain polyunsaturated fatty acids.162,163 Functional amino acid uptake was also studied in rat primary models.161,164
There could be several reasons for the scarcity of functional studies on SLC transporters in BBB models. Influx transporters, such as Glut-1, are more sensitive to down-regulation by serum-free monolayer culture conditions than efflux pumps.159 Garberg et al.166 suggested that the in vitro BBB models tested were not tight enough to allow estimation of the transcellular component of small molecule transport of glucose and amino acids in permeability assay settings. It remains to be seen whether the newly described and tighter BBB models will be better applicable in uptake and especially in permeability assays for influx transport studies.
An alternative triple-culture model including neurons instead of pericytes has been developed. This showed increased γ-glutamyl transpeptidase activity and slightly increased junctional tightness as compared to an astrocyte-endothelial co-culture model.154 The average TEER of the triple culture was 250–300 Ω cm2, which is still below the TEER reported in the tightest rat models.154
A major advantage of the rat BBB models described above is that syngeneic co-cultures can be established and results obtained on rat BCECs can be correlated with in vivo data in the same species and even strain of rats. The genome and transcriptome of rats are well studied, and a large set of antibodies are available for rat antigens. The development of a complex BBB model, like the triple culture is time-consuming and needs expertise, therefore a patented frozen ready-to-use kit version of the rat endothelial/pericyte/astrocyte model was developed and successfully used in different BBB studies.144,147–149
Bovine models – Astrocyte co-culture models develop high junctional tightness and express efflux transporters
Bovine brains have been used as a source for BCEC since 1983.4 Protocols for the generation of bovine BCEC differ between studies and laboratories, but two approaches dominate:
Size-selective filtering of microvessels followed by culture and use of first passage endothelial cells giving approximately 20–30 million cells per brain.53,56,91,107,167,168
Seeding of undigested microvessels followed by subculture up to passage 7 of endothelial clones sprouting from the capillaries.89,95,169
The subculture of endothelial cell clones expanded the yield of endothelial cells per brain by several fold and lowered contamination from pericytes and non-capillary endothelial cells, thus making the model more suitable for high-throughput studies.90
Most studies apply the bovine brain endothelial cells in contact or non-contact co-culture with astrocytes, but a triple co-culture including endothelial cells, pericytes, and astrocytes has also been developed giving a slight reduction in Lucifer yellow permeability.101
Primary cultures of bovine BCECs display high TEER both in mono-cultures (up to averages around 800 Ω cm2)5,170 and in co-cultures with astrocytes (averages often exceeding 1000 Ω cm2 up to 2500 Ω cm2)53,89–91,107,166,168 with values on single filters up to 3000 Ω cm2.53 This reflects a high expression and junctional localization of claudin-5, ZO-1, and occludin.5,63,90,95,107,171–175 Small molecule permeability is reported in the range of 0.4–15 × 10−6 cm/s depending on the compounds examined, the methods applied, and the TEER of the model5,53,63,–89,91,95,101,107,166,168,171,176–178 (see Table 6).
Table 6.
Bovine in-vitro models of the blood–brain barrier. Morphology, tightness and astrocyte/pericyte induction.
| Model type | Endothelial morphology | Junction Claudins | ∼TEER (Ohm·cm2) (mean values) | Permeability 10−6 (cm/s) | Occludin/ZO proteins | Astrocyte induction | Pericyte induction | Selected key references |
|---|---|---|---|---|---|---|---|---|
| Primary bovine BCEC/rat astrocyte co-culture | Spindle (conventional media) Cobblestone (highly buffered media) | 5 (ICC, WB) 1 (mRNA) | 600–800 (conventional media) 1600 (highly buffered media) | 0.5 (mannitol) | Occludin (ICC) | Increased TEER and P-gp expression in coculture with rat astrocytes Changes in endothelial morphology in contact and non-contact co-culture | – | 91,107, 167,188 |
| Primary bovine BCEC (clonal selection)/ rat astrocyte coculture | Spindle shape | 1 and 5 (ICC) | 800 | 6–12.5 (sucrose) | ZO-1 and Occludin (ICC) | Increased TEER and γ-glutamyl transpeptidase activity in co-culture with rat astrocytes | Slight decrease in PLY when cultured in non-contact co-culture | 89, 90,101 |
| Primary bovine BCEC (clonal selection) monoculture | Spindle shape | 1 and 5 (ICC) | – | 5.8 (sucrose) | ZO-1 and Occludin (ICC) | – | – | 95 |
: not investigated; ICC: immunocytochemistry; WB: Western blotting.
Note: The permeability value of the smallest tested compound in the study is given.
Astrocyte(+); mixed glial culture dominated by astrocytes.
The highly differentiated junctions make the model useful for examination of tight junction modulation and studies of passive permeability of drug compounds.56,149,174,175,179–187
P-gp has been shown to be functionally active in the cultured bovine endothelial cells both by accumulation assays and by bi-directional transport experiments.53,101,167,189–194 Protein expression and functional activity of BCRP and Mrp-1, -4, -5, and -6 have also been demonstrated.25,53,195–197 Mrp -4, -5, and -6 mRNA transcripts were detected in bovine brain endothelial cell mono-cultures and in co-culture with glial cells, with Mrp-6 being up-regulated in co-culture with pericytes.197 The same transcripts were found in endothelial cells in triple culture with pericytes and glial cells101 (see Table 7). Warren et al.158 profiled mRNA expression in a range of ABC transporters compared to human expression levels. The relative expression profiles were comparable between human and bovine brain endothelial cells, although the absolute expression levels varied considerably.
Table 7.
Bovine in-vitro models of the blood–brain barrier. Receptor and transporter expression and function.
| Model type | ABC transporter expression/function | Vectorial net transport of ABC substrates | TFR expression/ function | LAT-1 expression/ function | Glut-1 expression/ function | MCT-1 expression/ function | Selected key references |
|---|---|---|---|---|---|---|---|
| Primary bovine BCEC/rat astrocyte coculture | P-gp, BCRP and Mrp-1 (ICC, mRNA) Inhibitor data on transport | ER of 2.5 for digoxin ER of 4.5 for estrone-3-sulphate ER of 2.4 for etoposide | WB Trans-endothelial transport of holo-transferrin | mRNA | mRNA | – | 53,91,107 |
| Primary Bovine BCEC (clonal selection)/ rat astrocyte coculture | P-gp (WB) Inhibitor data on uptake | ER of 2 for vincristine | Trans-endothelial transport of radiolabelled holo-transferrin | High Pleucine relative to Psucrose | High PGlucose relative to Psucrose | – | 89,90,171,177 |
| Primary bovine BCEC (clonal selection) monoculture- | P-gp (ICC,WB), Mrp-1, -4 and -5 (WB) Inhibitor data on Rhod 123 uptake and quinidine transport | – | – | – | – | – | 95,178 |
: not investigated; ICC: immunocytochemistry; WB: Western blotting.
Note: The permeability value of the smallest tested compound in the study is given.
Bovine BBB models do not always perform well regarding transporter activity and high junctional tightness, and substantial variations occur between and even within laboratories utilizing the models. For instance, many reports have shown bovine BBB models with TEER values in the range of 30–150 Ω cm2 and/or lack of functional activity of both ABC and SLC-transporters known to be present at the BBB in vivo.149,166,173,198,199 Intra-laboratory variations are evident in a series of publications by the group of de Boer where TEER varies from high values around 800 Ω cm2 to around 150–300 Ω cm2,19,187,192,193 using the same model in the same laboratory. This is even clearer in a study by Helms et al.,53 where TEER averages varied from 327 ± 30 Ω cm2 to 2555 ±399 Ω cm2 across model batches within the same study. This emphasizes the need to thoroughly validate the models, especially when setting up a model in a new laboratory.
Bovine models have been applied in several studies investigating receptor-mediated endocytosis or transcytosis across the BBB focusing on the RAGE (receptor for advanced glycation end-products),200 LDL-receptor,63,201 LRP-1,77,202 and the transferrin receptor.65,171,203,204
The bovine models have mostly been applied to study receptor-mediated transcytosis, paracellular permeability, and ABC-mediated efflux, whereas only few studies have characterized SLC transporter expression and function in the model. Rapid transcellular leucine and glucose transport have been demonstrated, which indicated functional expression of LAT-1 and Glut-1.177,205 LAT-1 RNA is highly expressed in freshly isolated bovine brain capillaries and induces tryptophan uptake when expressed in oocytes.29 However, the expression and function of LAT-1 have not been confirmed in the bovine BBB models, beyond mRNA expression being detected with conventional polymerase chain reaction (PCR)107 and indirectly by a non-sodium-dependent, BCH inhibitable leucine uptake206 Other amino acid transporters investigated in the model are the sodium-dependent transporters B(0,+) (SLC6A14)206 and excitatory amino acid transporters-1/-2/-3 (SLC1A1-3),168 which were functionally active with polarized localization at the luminal and abluminal membrane, respectively. Saturable acetic acid transport has been shown in bovine brain endothelial cells, indicative of functional MCT-1 expression.207
BBB models based on primary endothelial cells of bovine origin are labor intensive and reproducibility between and even within labs may be an issue. A simplified model has been developed aiming to circumvent these drawbacks and make the model more suitable for high throughput screening.95 This led to easier establishment and culture, while the resulting model still displayed sucrose and Lucifer yellow permeabilities around 6 × 10−6 cm/s and expression of P-gp, Mrps, and claudin-5.95 The model was further simplified to a “ready-to-use” model, where endothelial cells were passaged to filter plates and frozen.178 This enables shipment of the model to other laboratories without the expertise and routine to establish a BBB model, while maintaining a BBB phenotype comparable to the simplified format mentioned above.
The porcine models – Mono-cultures develop high junctional tightness
Porcine brain endothelial cells (PBEC) were initially isolated by Mischeck et al.208 Two different isolation protocols have been developed and optimized in different labs. One is based on homogenization of entire brain hemispheres (after meninges and secretory regions have been removed) using sterile cutters followed by a dispase digestion. The digested suspension is centrifuged in dextran to separate microvessels from low-density material, and microvessels are incubated with collagenase/dispase to free endothelial cells. These are isolated by centrifugation on a percoll gradient and subcultured for one passage to increase cell yield and purity.105,208,209 The other protocol is based on mechanical homogenization of isolated gray matter followed by size-selective filtering through sequentially smaller nylon mesh (150 and 60 µm) to isolate microvessels. These are digested with collagenase/DNAse/trypsin, and endothelial cells are obtained by culturing microvessel fragments.106,210 Both methods have been used and characterized extensively and, although different, they have some common characteristics. Porcine models generally develop very high TEER in both mono-culture and astrocyte co-culture normally reaching 500 to 1500 Ω cm2.54,92,105,106,210–213 and sometimes up to 2500 Ω cm2.214,215 This is facilitated by removal of serum from the culture medium as well as addition of hydrocortisone.105 The high TEER translates into low permeability of small molecule compounds with sucrose permeabilities ranging from 0.2 to 8 ×10−6 cm/s57,104,106,209,211,214,216 and similar permeability of mannitol54,57,104,209,213 (see Table 8). Comparative studies with mono-cultures versus mono-cultures stimulated with astrocyte-conditioned media and contact or non-contact astrocyte co-cultures demonstrated that astrocytic influence increases junctional tightness, claudin-5 expression, and activity of gamma-glutamyl transpeptidase and alkaline phosphatase.54,92,213,215–217 The effect of pericyte co-culture and astrocyte/pericyte triple culture has also been investigated in porcine models, where slight TEER increases were observed when rat or porcine pericytes were included relative to mono-cultures. However, the inclusion of pericytes in the triple culture model did not cause an additional increase in TEER relative to the endothelial/astrocyte co-culture.213
Table 8.
Porcine in-vitro models of the blood–brain barrier. Morphology, tightness and astrocyte/pericyte induction.
| Model type | Endothelial morphology | Junction Claudins | ∼TEER (Ohm · cm2) (mean values) | Permeability 10−6 (cm/s) | Occludin/ ZO proteins | Astrocyte induction | Pericyte induction | Selected key references |
|---|---|---|---|---|---|---|---|---|
| Primary porcine BCEC (isolation with enzymes) | Cobblestone | 5 (ICC,mRNA) | 250–790 | 6 (sucrose) | Occludin (ICC,mRNA) | Increased TEER and changed morphology by astrocyte co-culture | 54,106,215 | |
| Primary porcine BCEC (isolation including density centrifugation step) | Intermediate | 5 (ICC,WB) | 400–1500 | 0.6–1 (sucrose) 1.8 (mannitol) | ZO1 and Occludin, (ICC,WB) | Increased TEER and claudin-5 expression, decrease in Psucrose and changed morphology by astrocyte co-culture | Decrease in TEER by pericyte co-culture. However increase in TEER by co-culture with bFGF-treated pericytes. Increase in TEER by porcine pericytes in contact-co-culture | 92,104, 213,218–221 |
| Primary porcine BCEC in co-culture with rat astrocytes or astrocyte cell line | Cobblestone in mono-culture, change to spindle in co-culture. Intermediate in both mono and co-culture | 5 (ICC,WB) | 800–1800 | 0.6 (Lucifer yellow) | ZO1 and Occludin (ICC, WB) | Increased TEER and claudin-5 expression, decrease in Psucrose and PLY and changed morphology by astrocyte co-culture | 54,92,215 |
: not investigated; ICC: immunocytochemistry; WB: Western blotting.
Note: The permeability value of the smallest tested compound in the study is given.
PBECs express tight junction proteins such as ZO-1 and -2,92,210,222–225 claudin-5,92,106,214,225 and occludin,92,106,225–230 as determined by real time PCR, Western blotting, confocal- and electron microscopy. The well-differentiated tight junctions of the model make it ideal for examining tight junction expression and modulation, and it has been the model of choice to introduce impedance analysis as a technique to continuously measure TEER in BBB models.231,232
A recent quantitative proteomics comparison of isolated brain capillaries showed that endothelial cells from porcine brain capillaries express a range of BBB-phenotype ABC transporters, with the BCRP:Pgp ratio closer to that of monkey and human than shown by rodent brain capillaries.48 This is reflected in the ABC-transporter expression in porcine models, where P-gp, BCRP, and Mrps-1 and -4 are expressed at the mRNA and protein level.106,215,220,228,229 They mediate polarized transport of P-gp and BCRP substrates228,229,233,234 and limit the accumulation of P-gp, BCRP, and Mrp substrates.106,215,222,235,236 Efflux transporters are thus generally expressed and active in the PBEC models, although subtype specific Mrp functionality including polarization of expression has not been investigated in detail (see Table 9).
Table 9.
Porcine in-vitro models of the blood–brain barrier. Receptor and transporter expression and function.
| Model type | ABC transporter expression/function | Vectorial net transport of ABC substrates | TFR expression/ function | LAT-1 expression/ function | Glut-1 expression/ function | MCT-1 expression/ function | Selected key references |
|---|---|---|---|---|---|---|---|
| Primary porcine BCEC (isolation with enzymes) | P-gp Inhibitor data on uptake and transport BCRP (mRNA) | – | Binding of radiolabeled transferrin | High Pleucine relative to Psucrose | – | – | 106,212, 238,241 |
| Primary porcine BCEC (isolation including density centrifugation step) | P-gp (ICC,mRNA, WB) BCRP (mRNA, WB) Mrp-1 and -4 (mRNA, ICC) Inhibitor data on uptake for all | ER of 2.5 for paclitaxel ER of 4 for Mitoxantrone | – | – | – | – | 104,220,222, 233,234,236 |
| Primary porcine BCEC in coculture with rat astrocytes or astrocyte cell line | P-gp (WB) BCRP (WB) Inhibitor data on uptake for both | – | Uptake of Alexa-555 conjugated human transferrin | – | – | – | 92,215 |
: not investigated; ICC: immunocytochemistry; WB: Western blotting.
Note: The permeability value of the smallest tested compound in the study is given.
SLC expression and function have not been characterized to a great extent in PBEC models. High Glut-1 and some degree of MCT-1 expression have been shown in isolated porcine brain capillaries,48 but their expression or functions have not been characterized. L-Leucine permeability has been shown to be relatively high compared to sucrose (approximately 12 ×10−6 cm/s versus 5 × 10−6 cm/s), which indicates LAT-1 expression, but it was not directly attributed to LAT-1 via inhibition studies or demonstrations of mRNA or protein expression.106 OAT-1 and OAT-3 (SLC22A6 and 22A8) have been shown to be expressed at mRNA and protein level in PBECs, and functional expression was demonstrated as glutaric acid efflux inhibitable by probenecid.237
PBEC models have been used to study macromolecule transport through the BBB, focusing mainly on receptor-mediated transport. Surface expression of transferrin receptor has been shown in PBECs using binding assays with radiolabeled transferrin,238 and PBECs have shown the ability to take up human transferrin labelled with Alexa-555.215 Other receptors investigated include the LDL receptor, the LRP-1, the mannose-6-phosphate receptor, and lactoferrin receptor.64,223,225,239,240 Arylsufatase A has been shown to cross porcine BCEC, without altering the monolayer integrity. Transport was low (around 0.02 % of the applied amount) but to some degree inhibitable by co-administration of mannose-6-phosphate, which indicated receptor-mediated transport via the mannose-6-phosphate receptor.223 Likewise, fusing arylsulfatase A with ApoB, ApoE-I, and ApoE-II caused significant increases in the transcellular transport, indicative of LDL receptor and/or LRP-1-mediated transcytosis.64
The human models – Establishment of models from renewable sources
BBB models based on primary cultured cells from human tissue have been reported (for instance Bernas et al.111). However, human brain tissue is difficult to acquire on a regular basis, which limits the possibilities to establish BBB models based on primary human BCEC. Some commercial vendors offer primary cultures of human brain endothelial cells(for instance Applied Cell Biology Research Institute (Kirkland, WA, USA) as used by Urich et al.242 and ScienCell Research Laboratories (San Diego, CA, USA) as used by Cucullo et al.243), but often with only sparse documentation on the source. Instead efforts have been made to create alternative models based on immortalized brain endothelial cells or human-derived stem cells.114–118 The different human immortalized endothelial cell lines published have different properties. In this review, focus has been given to the hCMEC/D3 cell line, as this is the most widespread and well characterized of the published cell lines.
The human immortalized endothelial cell line hCMEC/D3
Since its generation and initial characterization,114 more than 150 publications have applied and further characterized the hCMEC/D3 cell line, and it is thus a well characterized, easy to use in vitro model of the human BBB (for a recent review see244).
The hTERT/SV40-immortalized hCMEC/D3 clonal cell line is derived from human temporal lobe microvessels isolated from tissue resected during surgery for epilepsy. hCMEC/D3 cells form a contact-inhibited monolayer of elongated cells on collagen type I or type IV. hCMEC/D3 expresses junction-associated IgG-like proteins such as PECAM-1 and JAM-A, adherens and tight junction structural proteins such as VE-cadherin, claudin-3 and -5, and occludin, scaffolding proteins such as ß-catenin and ZO-1 and -2 as well as the cell polarity complex Par-3/Par-6/PKCz, which further contributes to the control of tight junction integrity and apico-basal polarity.114,124,245–251
hCMEC/D3 cell monolayers express the characteristic tight junction proteins of the BBB252; however, the expression level of claudin-5, which is important for junctional tightness, has been reported to be lower than in intact microvessels,242 although optimal culture conditions can improve this. This is reflected by TEER in the range of 30–50 Ω cm2 and permeability for sucrose, mannitol, urea, sodium fluorescein, and Lucifer yellow in the range of 20–90 × 10−6 cm/s were initially reported114,124,245,247,253,254 (see Table 10). Larger molecules have lower permeabilities in the range of 5–13 × 10−6 cm/s for 4 kDa dextrans and 0.2–0.3 × 10−6 cm/s for 70 kDa dextrans.114,247 Hence, the model in its basic state presents a barrier for large molecules, whereas small molecules relatively easily permeate the barrier.
Table 10.
Human in-vitro models of the blood–brain barrier. Morphology, tightness and astrocyte/pericyte induction.
| Model type | Endothelial morphology | Junction Claudins | ∼TEER (Ohm·cm2) (mean values) | Permeability 10−6 (cm/s) | Occludin/ZO proteins | Astrocyte induction | Pericyte induction | Selected key references |
|---|---|---|---|---|---|---|---|---|
| hCMEC3/D3 (immortalized human brain endothelial cells) in monoculture | Intermediate | 1 (mRNA, WB) 3 (ICC, mRNA, WB) 5 (ICC, mRNA, PROT, WB) 12 (ICC, WB) | 40 (standard culture) 200 (with hydrocortisone) | 27.5 (sucrose) 10–57 (fluorescein) 10–26 (LY) 25 (mannitol) | Occludin (mRNA, ICC,WB) ZO1 (ICC,PROT) | Slight increase in TEER by co-culture with human astrocytes | No changes in TEER | 103,114,247 ,248,250–255 |
| hPSC (human pluripotent stem cells) | cobblestone | 5 (ICC, WB) | 250 (monoculture)–700 (astrocyte co-culture) 5350 (pericyte –primed NPC-co-culture) | 0.6 (sucrose) | Occludin (ICC, WB) ZO1 (ICC) | Increase in TEER by co-culture with rat astrocytes and human NPC’s | Increase in TEER by co-culture with human brain pericytes | 118,256 |
| Cord blood-derived endothelial progenitor cells | Cobblestone | 1 (mRNA) 3 (mRNA) 5 (ICC, mRNA, WB) | 70 (monoculture) 160 (pericyte co-culture) | 10–20 (Lucifer yellow) | Occludin (ICC, mRNA, WB) ZO1 (ICC, mRNA) | Decrease in PLucifer yellow by co-culture with rat astrocytes. Increase in protein expression of P-gp, GLUT-1 and occludin | Increase in TEER and decrease in PLucifer yellow by co-culture with bovine brain pericytes | 116,117 |
: not investigated; ICC: immunocytochemistry; WB: Western blotting; PROT: MS-based proteomics; TEER: transendothelial electrical resistance.
Note: The permeability value of the smallest tested compound in the study is given.
Astrocyte(+); mixed glial culture dominated by astrocytes.
The barrier properties are dependent on the culture protocols, and tighter monolayers have been obtained by activating the Wnt/ß-catenin pathway,124 the Wnt/planar cell polarity pathway,245 or nuclear receptors.247 Under these conditions, TEER values above 300 Ω cm2 and Lucifer yellow permeabilities in the range of 10–20 × 10−6 cm/s have been reported.124,247,255 Co-culture with astrocytes and/or pericytes has also been shown to increase TEER, although only to a small degree (from 30 to 60 Ω cm2).103 Another approach has been to subject hCMEC/D3 monolayers to a physiological shear stress (about 5 dyn/cm2) in a microfluidic device, which increased TEER to 120 Ω cm2.257
Thus, a number of studies have shown that the junctional tightness of the hCMEC/D3 model may be improved. Future attempts to improve the tightness of the model should focus on co-culturing pericytes and astrocytes either in 2D103 or 3D258 and/or the presence of shear stress.
One hundred and forty-four SLC transporters have been detected in hCMEC/D3 cells at the transcript level, including SLC2A1 (Glut-1), SLC7A5 (LAT-1), and members of the SLC16 (MCT) family, many of them regulated by cytokines.254,259 In a proteomics study, Glut-1 was shown to be expressed at a level similar to freshly isolated human brain microvessels.252 The same study also revealed high levels of additional influx transporters and receptors, including MCT-1, the insulin receptor, and the transferrin receptor (see Table 11). This study did not detect LAT-1 at the protein level. However, uptake of gabapentin inhibitable by phenylalanine, BCH, and siRNA-mediated LAT-1 knockdown has been reported indicating functional LAT-1 expression in the model.260
Table 11.
Human in-vitro models of the blood–brain barrier. Receptor and transporter expression and function.
| Model type | ABC transporter expression/function | Vectorial net transport of ABC substrates | TFR expression/ function | LAT-1 expression/ function | Glut-1 expression/ function | MCT-1 expression/ function | Selected key references |
|---|---|---|---|---|---|---|---|
| hCMEC3/D3 (immortalized human brain endothelial cells) | P-gp (mRNA, PROT, WB) BCRP (mRNA, PROT, WB) Mrp-1 (mRNA,WB, PROT) Inhibitor data on uptake for all MRP5 (mRNA) | – | PROT | Not detected in proteomics study. However, uptake of gabapentin inhibitable by LAT-1 inhibition has been shown | PROT | PROT | 114,252,260 |
| hPSC (human pluripotent stem cells) | P-gp (ICC, mRNA) BCRP (ICC, mRNA) Mrp-1 (ICC, mRNA) Mrp-2, 4 and -5 (mRNA) Inhibitor data on uptake and transport for all | – | mRNA | mRNA | ICC, mRNA, relatively high PGlucose compared to PSucrose | mRNA | 118,256 |
| Cord blood-derived endothelial progenitor cells | P-gp (ICC, mRNA, WB) Inhibitor data on uptake BCRP (mRNA) Mrp-1, -4 and -5 (mRNA) | – | mRNA | mRNA | mRNA | mRNA | 116,117 |
: not investigated; ICC: immunocytochemistry; WB: Western blotting; PROT: MS-based proteomics; BCRP: breast cancer resistance protein; Mrp: multidrug-resistance protein.
Note: The permeability value of the smallest tested compound in the study is given.
hCMEC/D3 cells express mRNA of 23 ABC efflux transporters, including P-gp, Mrp-4, and BCRP.254,261 P-gp and BCRP expression have further been documented at the protein level,261–263 and P-gp has been shown to be primarily localized at the apical membrane, where it limits apical to basolateral permeability of rhodamine.264 This polarized expression is controlled by the cell polarity complex Par-3/Par-6/PKCz.245
In conclusion, the hCMEC/D3 cell line constitutes an easy to use, thoroughly characterized model of human origin, which appears particularly well suited for drug uptake studies and for unravelling the response of brain endothelium to human pathogens and neuroinflammatory stimuli.265 However, its relatively low junctional tightness under routine culture conditions is still a challenge regarding its use for vectorial transport of small molecule compounds and will require further optimization.
In vitro BBB models generated from human stem cells
Recently, human brain endothelial cells have been derived from stem cell sources including human pluripotent stem cells (hPSCs)118 and human cord blood-derived stem cells of circulating endothelial progenitor and hematopoietic lineages.116,117 These sources could in principle provide renewable and scalable sources for human BBB models.
Human PSCs include both human embryonic stem cells derived from the inner cell mass of human blastocysts266 and induced pluripotent stem cells (iPSCs) obtained from reprogramming somatic cells to a pluripotent state.267,268 BBB-like endothelial monolayers have been obtained with a co-differentiation protocol, in which hPSCs were first cultured in unconditioned media to co-differentiate into a mixture of endothelial cells and neural progenitor cells. This co-differentiation environment is hypothesized to create an embryonic-like brain environment, suitable to induce endothelial cell expression of some key BBB traits.118 Human brain endothelial cells were subsequently subcultured and maintained as virtually pure monolayers on collagen/fibronectin-coated transwell filters or plates.
The resulting hPSC-derived brain cell monolayers develop a restrictive barrier with expression of claudin-5, occludin, and ZO-1 localized to cell–cell contact zones. Monolayers produce baseline TEER values of 250 Ω cm2 but can reach up to 1450 Ω cm2 when co-cultured with rat astrocytes.118 This translates into very low sucrose permeabilities of 0.6 × 10−6 cm/s, similar to the lowest permeabilities reported for bovine and porcine models53,92,104 and far below permeability values reported in primary human models (approximately 170 × 10−6 cm/s269 and below those with hCMEC/D3 (20 × 10−6 cm/s as discussed above) (see Table 10). In the same study, diazepam permeability was around 18 × 10−6 cm/s resulting in a permeability dynamic range (diazepam:sucrose) around 40 fold.118 Glucose permeability across the model was around 3.7 × 10−6 cm/s, approximately seven fold higher than for sucrose, suggestive of functional Glut-1 expression, but this has not been confirmed with functional inhibition studies. Protein expression of P-gp, BCRP and Mrp-1 has been shown with immunocytochemistry, and uptake and transport studies with rhodamine and doxorubicin in combination with ABC transporter inhibitors have shown functional and polarized expression of efflux transporters118,256(see Table 11). Combined, these data suggest downstream utility in drug screening assays, although more validation with a larger set of transporter substrates is required. Likewise, receptor expression and function have not been studied in detail in the model, although a range of receptors including transferrin, insulin, and LDL- receptors have been shown at the mRNA level.118
Alternative human stem cell models based on cord blood-derived stem cells have been developed. These utilize different differentiation protocols, either based on pericyte117 or astrocyte116 co-culture. Both models show endothelial cell phenotype and expression of claudin-5, occludin, and ZO-1. The pericyte co-culture reaches significantly higher junctional tightness than the astrocyte co-culture with a Lucifer yellow permeability around 10 × 10−6 cm/s and TEER around 180 Ω cm2 compared to a Lucifer yellow permeability of 22 × 10−6 cm/s and TEER below 60 Ω cm2 in the astrocyte co-culture (see Table 10). P-gp, BCRP, and Mrp-1, -2, -4, and -5 as well as transferrin receptor and RAGE and a range of SLC transporters including Glut-1 and LAT-1 were found at the mRNA level in pericyte co-cultures.117 Astrocyte co-cultures also showed expression of Glut-1, P-gp and BCRP, and the protein expression levels of Glut-1 and P-gp were found to be up-regulated by the astrocytes116 (see Table 11). As with the hPSC-derived model, the cord blood-derived models still lack validation regarding functional expression of transporters, efflux pumps, and receptors.
The stem cell-derived models offer the opportunity to study the dynamic changes that may occur during BBB development. For example, the current differentiation protocol for the hPSCs recapitulates developmentally relevant in vivo canonical Wnt signaling events between neural progenitor cells and endothelial cells.118,270,271 Similarly, the cord blood-derived endothelial cells are regulated by addition of Wnt3a or Wnt7a, resulting in increased TEER compared to un-stimulated monocultures.117 Furthermore, hPSC-derived brain endothelial cells exhibit significantly increased barrier phenotype in response to retinoic acid (TEER increases up to 2940 ± 800 Ω cm2),256 a hormone implicated in BBB regulation.42,272,273
The stem cell-based models could additionally be used to interrogate other signaling pathways and developmental events such as those with the interacting cells of the NVU.116,117,256 Moreover, with the hPSC system, it would also be possible to model diseased NVU phenotypes using endothelial and neural cells derived from patient-specific iPSCs with diseased genetic backgrounds.274 One caution when using lentivirally reprogrammed iPSCs is that they exhibit random genomic integration of pluripotency factors that could potentially affect the ultimate differentiated phenotype.275 However, hPSC-derived brain endothelial cells have been successfully derived from both human embryonic stem cells118,266 and iPSCs generated by non-integrating methods118,276 to avoid such complications.
In conclusion, the stem cell-derived BBB models represent a promising tool for both mechanistic studies of human brain endothelial cell biology and as a screening tool for CNS-drug permeability studies. However, the models have not yet been extensively characterized, because of the short time period they have been available. Hence, future studies should aim at characterizing these models regarding BBB features as well as validating the reproducibility and “ease of culture” of the models.
Conclusion
Techniques for in vitro culture of brain endothelial cells have been developed continuously over the past 40 years. Endothelial cell cultures have been derived from a number of species, using a variety of isolation and culture methods, which have been optimized for the species in question. This has resulted in a range of in vitro BBB models with different properties, which makes comparisons between different studies and planning of new studies challenging. However, as summarized in this review, the in vitro models have proven to be valuable tools in studies concerning BBB development, physiology, pathophysiology, toxicology, and CNS-drug development. The right choice of model for a study will depend on the research question at hand. Brain endothelial cells of bovine and porcine origin form tight endothelial monolayers with a high transendothelial resistance and are suited for investigations of small molecule transport through the BBB. They display functional efflux transporter activity as well as restrictive tight junctions, resulting in vectorial transport of P-gp and BCRP substrates, and may also be suited for studies of polarized localization of for instance specific receptors or transporters, since the high junctional tightness helps establish good apical:basal polarity. Given that a reliable source of animals is available (abattoir or animal facility), large quantities of endothelial cells can be obtained allowing screening studies. On the other hand, the proteins expressed by bovine and porcine models differ in sequence from their human homologues and this may in some cases translate to differences in affinity and transport rate.50,158 This also poses a challenge when investigating therapeutic antibodies designed to target BBB-expressed proteins, since these are often designed to react with human or mouse and rat homologues. Murine or human endothelial cell culture models may be preferable in these types of studies.
Brain endothelial cell cultures of mouse or rat origin have the advantage of being from species which are thoroughly characterized and are often used as first choice for preclinical studies. While rat and mouse brains are easy to obtain, the generally low yield of endothelial cells from these species has been an obstacle for the routine use of murine endothelial cell models, although quite advanced endothelial cultures can be obtained in dedicated laboratories, e.g. the triple co-culture rat model. Since this model incorporates the three main cell types of the neurovascular unit, it also allows detailed NVU-signaling studies. The establishment and characterization of the immortalized mouse endothelial cell lines such as bEND.3, bEND.5, or cEND can circumvent the problem of low yield of endothelial cells if the cell line has the right characteristics for the given study, but the cell lines have not been widely used so far. Their potential use in preclinical studies does however warrant further attention.
Primary cultures of human brain endothelial cells, reflecting the fully differentiated phenotype, would be ideal for drug development and preclinical studies. It is however difficult to obtain fresh healthy brain tissue on a regular basis. The establishment and characterization of the human immortalized cell line, hCMEC/D3, have given researchers a tool for investigating human brain endothelial cell transporters, receptors, signalling pathways, and metabolism without the issues of availability and variability between isolation batches. The relatively low tightness of the monolayers formed by the hCMEC/D3 cells can be improved by optimizing culture conditions, however not to levels matching the bovine, porcine, or human stem cell-derived models. The hCMEC/D3 cells therefore have some limitations when it comes to vectorial transport studies of small molecules, but may perform well in mechanistic studies of expressed transporters and receptors.
The recent reports describing techniques for the generation of endothelial cell cultures from human stem cells are steps towards a human cell culture model of the brain endothelium. The differentiated endothelial cells form tight monolayers with high electrical resistance and have functional expression of efflux transporters. The human stem cell models are presently being characterized and refined and will, if proven to be easy to handle and reproducible, present great opportunities for researchers in the field.
Open questions and suggestions for future studies
Much progress has been made during the last four decades in the development of in vitro models of the BBB. The field has advanced in parallel with advances in BBB biology and our increased understanding of the roles of the cell types in the neurovascular unit. There are still a lot of open questions within the field of in vitro BBB models, and these cannot be answered without a deeper understanding of the biology of the native barrier/the neurovascular unit. We have outlined some of these below, as an inspiration for future research and as a reminder to those already in the field.
Expression and function of SLC-type uptake transporters
A recent perspectives paper summarizes research trends within the field of SLC-proteins and argues that the field is generally under-studied compared to their biological relevance.277 A similar argument can be made regarding SLCs at the BBB. Traditionally, when characterizing transporter expression in BBB models, the ABC-type efflux transporters have gained most attention. Thus, most models today are well characterized concerning at least P-gp and BCRP expression, whereas it is a common feature of the in vitro BBB models that SLC uptake transporters are relatively uncharacterized, or have low expression levels, as described in the previous sections. It is known that some marketed drugs are transported by SLC-transporters, for instance L-dopa and gabapentin,278 which makes the functional expression of LAT-1 and other SLC transporters important in a BBB model for drug compound screening purposes or for studies regarding regulation of nutrient and micronutrient transporters. Characterization can be performed by a combination of transendothelial transport experiments in combination with substrate and inhibitor profiling, as well as immunocytochemistry showing expression of the transporter in question. Ideally, transporter localization should be confirmed by comparing the localization in intact capillary endothelial cells with the localization in endothelial cells in culture. LAT-1 and Glut-1 are good starting candidates because of their important physiological functions. However, other SLC-transporters may be equally important at the BBB and may have potential as drug targets/transporters. The growing number of studies on the in vivo BBB transcriptome and proteome will assist in directing focus to the SLC transporters of highest significance for future characterization.
The role of other NVU cells, especially pericytes, in BBB models
Pericytes have proven to be essential for the formation of the BBB in vivo,41,42 but the effects of pericytes in vitro vary between BBB models. Results from rat models have shown increased TEER in triple cultures compared to astrocyte-endothelial co-cultures.97 A similar TEER increase was seen in mouse endothelial cells (pericyte co-culture relative to endothelial mono-culture),42 whereas data from pig models have shown reduced TEER in endothelial cells co-cultured with pericytes due to an induction of MMPs.221 The differentiation state of pericytes in vitro was found to be decisive for the effect of co-culture, with pericytes treated with bFGF causing a slightly increased TEER, whereas TGFβ-treated pericytes caused a decrease in TEER.219 However, pericytes were not found to affect tight junction protein expression in vivo, where the main effect of pericytes was to decrease expression of certain genes favoring vascular permeability.42 Thus, the current understanding of pericyte effects in BBB-cell culture models is incomplete. The stem cell models may prove to be effective tools to gain knowledge of signaling effects of pericytes (and other cells of the NVU) and their importance in different stages of BBB induction and maintenance, especially if coupled to detailed transcriptome and proteome analysis, where induction and silencing of individual genes and proteins by the different NVU cells at different development stages can be identified. This kind of knowledge may feed back into the routine use of primary cell cultures to also improve their BBB characteristics. Much of the induction of primary cell models today is dependent on stimulation by cAMP-analogues and steroids (hydrocortisone or dexamethasone). The full effects of these barrier-modulating additives are not known, and the overall BBB characteristics may be better mimicked if barrier-modulating agents can be substituted with induction from NVU cells.
Disease models of the BBB
It is well known that the BBB is a dynamic barrier that changes properties under different conditions. The BBB is affected by different disease states, for example stroke, Alzheimer’s disease, cancer, and multiple sclerosis.279–283 Many in vitro studies on the ischemic BBB have been performed using oxygen-glucose deprived culture conditions and thus quite well-validated models of the BBB during ischemic insults exist.131,143,284–287
Likewise, several models for the BBB under cancer conditions have been developed, for instance by co-culturing BCEC with the glioblastoma cell lines, RG-2 or C6,94,130,155,181,288 and BBB models have been applied to study adhesion and transmigration of metastatic cancer cells.289–293 The BBB changes properties during Alzheimer’s disease, which contributes to- and may even be a leading cause of neurodegeneration.294,295 In vitro BBB models have been extensively applied to investigate changes caused by the Alzheimer’s disease environment and to investigate the ability of the BBB to transport amyloid beta (see reviews296,297). Using cells from rat and mouse models of Alzheimer’s disease, it may be possible to decipher the responses of the BBB during the development of the disease, at the molecular and cellular levels.
Diseases caused by gene-disorders have not been well modelled so far. The human stem cell models may present possibilities to facilitate development of new models from iPSCs isolated from patients with specific CNS-pathologies. Alternatively, mouse and rat models based on endothelial cells isolated from knock-out or transgenic animals may provide useful models for specific disease states, which have been demonstrated with endothelial cells isolated from PPAR-alpha-deficient mice.298
The well-documented changes in BBB properties during different disease states highlight the fact that the BBB should not be considered a static barrier that presents the same obstacle for every disease condition. BBB permeability and drug permeation may change with different pathologies, as is the case of stroke, Alzheimer’s disease and some cancer forms, but in most disease conditions drug permeation remains hindered or even decreases, for instance due to an up-regulation of P-gp as observed in epilepsy.299 Thus, in vitro models mimicking different pathologies should be refined and validated to improve translation of data to the in vivo settings.
Funding
Hans Christian Helms and Birger Brodin wishes to acknowledge the funding received from the Lundbeck Foundation via the project grant “Research Initiatives in Brain Barriers and Drug Delivery” (RIBBDD).
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Roméo Cecchelli is one of the holders of the patent WO 2014/203087 A1: A human blood–brain barrier model derived from stem cells, discussed in the review. Mária A. Deli is a scientific consultant to PharmaCo-Cell Co. Ltd, Japan, and one of the holders of the patent WO2007072953 on the in vitro rat triple BBB model discussed in the review.
Authors’ contributions
Hans Christian Helms and Birger Brodin prepared the outline of the manuscript. Malgorzata Burek and Carola Förster drafted the section “Mouse models – Immortalized and primary mouse brain endothelial cultures.” Maria Deli drafted the section “Rat models – Mono-, co-, and triple cultures of rat BCEC.” Elodie Vandenhaute, Romeo Cecchelli, Hans Christian Helms, and Birger Brodin drafted the section “Bovine models – Astrocyte co-culture models develop high junctional tightness and express efflux transporters.” The section “The porcine models – Mono-cultures develop high junctional tightness” was drafted by N. Joan Abbott and Hans Joachim Galla. Romeo Cecchelli, Pierre-Olivier Couraud, Ignacio A. Romero, Eric V. Shusta, Matthew J. Stebbins, Elodie Vandenhaute, and Babette Weksler drafted the section.” The human models – Establishment of models from renewable sources. Remaining sections and figures were drafted by Hans Christian Helms and Birger Brodin. All authors participated in the feedback and writing process following the initial drafting of the manuscript.
References
- 1.Mathiisen TM, Lehre KP, Danbolt NC, et al. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia 2010; 58: 1094–1103. [DOI] [PubMed] [Google Scholar]
- 2.Abbott NJ. Anatomy and physiology of the blood–brain barriers. In: Hammarlund-Udenaes M, de Lange ECM, Thorne RG. (eds). Drug delivery to the brain, New York, NY: Springer, 2014, pp. 3–21. [Google Scholar]
- 3.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006; 7: 41–53. [DOI] [PubMed] [Google Scholar]
- 4.Bowman PD, Ennis SR, Rarey KE, et al. Brain microvessel endothelial cells in tissue culture: a model for study of blood-brain barrier permeability. Ann Neurol 1983; 14: 396–402. [DOI] [PubMed] [Google Scholar]
- 5.Rubin LL, Hall DE, Porter S, et al. A cell culture model of the blood-brain barrier. J Cell Biol 1991; 115: 1725–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaffe EA, Hoyer LW, Nachman RL. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest 1973; 52: 2757–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorovini-Zis K, Huynh HK. Ultrastructural localization of factor VIII-related antigen in cultured human brain microvessel endothelial cells. J Histochem Cytochem 1992; 40: 689–696. [DOI] [PubMed] [Google Scholar]
- 8.Muller AM, Hermanns MI, Skrzynski C, et al. Expression of the endothelial markers PECAM-1, vWf, and CD34 in vivo and in vitro. Exp Mol Pathol 2002; 72: 221–229. [DOI] [PubMed] [Google Scholar]
- 9.Nitta T, Hata M, Gotoh S, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 2003; 161: 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furuse M, Hirase T, Itoh M, et al. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 1993; 123: 1777–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morita K, Sasaki H, Furuse M, et al. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol 1999; 147: 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crone C, Olesen SP. Electrical resistance of brain microvascular endothelium. Brain Res 1982; 241: 49–55. [DOI] [PubMed] [Google Scholar]
- 13.Butt AM, Jones HC, Abbott NJ. Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J Physiol 1990; 429: 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol 1969; 40: 648–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohno K, Pettigrew KD, Rapoport SI. Lower limits of cerebrovascular permeability to nonelectrolytes in the conscious rat. Am J Physiol 1978; 235: H299–H307. [DOI] [PubMed] [Google Scholar]
- 16.Smith QR, Rapoport SI. Cerebrovascular permeability coefficients to sodium, potassium, and chloride. J Neurochem 1986; 46: 1732–1742. [DOI] [PubMed] [Google Scholar]
- 17.Schinkel AH, Smit JJ, van Tellingen O, et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell 1994; 77: 491–502. [DOI] [PubMed] [Google Scholar]
- 18.Cordon-Cardo C, O'Brien JP, Casals D, et al. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci U S A 1989; 86: 695–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenblatter T, Galla HJ. A new multidrug resistance protein at the blood-brain barrier. Biochem Biophys Res Commun 2002; 293: 1273–1278. [DOI] [PubMed] [Google Scholar]
- 20.Cooray HC, Blackmore CG, Maskell L, et al. Localisation of breast cancer resistance protein in microvessel endothelium of human brain. Neuroreport 2002; 13: 2059–2063. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W, Mojsilovic-Petrovic J, Andrade MF, et al. The expression and functional characterization of ABCG2 in brain endothelial cells and vessels. FASEB J 2003; 17: 2085–2087. [DOI] [PubMed] [Google Scholar]
- 22.Maliepaard M, Scheffer GL, Faneyte IF, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res 2001; 61: 3458–3464. [PubMed] [Google Scholar]
- 23.Leggas M, Adachi M, Scheffer GL, et al. Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol Cell Biol 2004; 24: 7612–7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller DS, Nobmann SN, Gutmann H, et al. Xenobiotic transport across isolated brain microvessels studied by confocal microscopy. Mol Pharmacol 2000; 58: 1357–1367. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Schuetz JD, Elmquist WF, et al. Plasma membrane localization of multidrug resistance-associated protein homologs in brain capillary endothelial cells. J Pharmacol Exp Ther 2004; 311: 449–455. [DOI] [PubMed] [Google Scholar]
- 26.Crone C. Facilitated transfer of glucose from blood into brain tissue. J Physiol 1965; 181: 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nualart F, Godoy A, Reinicke K. Expression of the hexose transporters GLUT1 and GLUT2 during the early development of the human brain. Brain Res 1999; 824: 97–104. [DOI] [PubMed] [Google Scholar]
- 28.Zheng PP, Romme E, van der Spek PJ, et al. Glut1/SLC2A1 is crucial for the development of the blood-brain barrier in vivo. Ann Neurol 2010; 68: 835–844. [DOI] [PubMed] [Google Scholar]
- 29.Boado RJ, Li JY, Nagaya M, et al. Selective expression of the large neutral amino acid transporter at the blood-brain barrier. Proc Natl Acad Sci U S A 1999; 96: 12079–12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oldendorf WH, Szabo J. Amino acid assignment to one of three blood-brain barrier amino acid carriers. Am J Physiol 1976; 230: 94–98. [DOI] [PubMed] [Google Scholar]
- 31.Gerhart DZ, Enerson BE, Zhdankina OY, et al. Expression of monocarboxylate transporter MCT1 by brain endothelium and glia in adult and suckling rats. Am J Physiol 1997; 273: E207–E213. [DOI] [PubMed] [Google Scholar]
- 32.Kido Y, Tamai I, Okamoto M, et al. Functional clarification of MCT1-mediated transport of monocarboxylic acids at the blood-brain barrier using in vitro cultured cells and in vivo BUI studies. Pharm Res 2000; 17: 55–62. [DOI] [PubMed] [Google Scholar]
- 33.Oldendorf WH. Carrier-mediated blood-brain barrier transport of short-chain monocarboxylic organic acids. Am J Physiol 1973; 224: 1450–1453. [DOI] [PubMed] [Google Scholar]
- 34.Jefferies WA, Brandon MR, Hunt SV, et al. Transferrin receptor on endothelium of brain capillaries. Nature 1984; 312: 162–163. [DOI] [PubMed] [Google Scholar]
- 35.Yu YJ, Atwal JK, Zhang Y, et al. Therapeutic bispecific antibodies cross the blood-brain barrier in nonhuman primates. Sci Transl Med 2014; 6: 261ra154. [DOI] [PubMed] [Google Scholar]
- 36.Morris CM, Keith AB, Edwardson JA, et al. Uptake and distribution of iron and transferrin in the adult rat brain. J Neurochem 1992; 59: 300–306. [DOI] [PubMed] [Google Scholar]
- 37.Alvarez JI, Dodelet-Devillers A, Kebir H, et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 2011; 334: 1727–1731. [DOI] [PubMed] [Google Scholar]
- 38.Lee SW, Kim WJ, Choi YK, et al. SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat Med 2003; 9: 900–906. [DOI] [PubMed] [Google Scholar]
- 39.Hayashi Y, Nomura M, Yamagishi S, et al. Induction of various blood-brain barrier properties in non-neural endothelial cells by close apposition to co-cultured astrocytes. Glia 1997; 19: 13–26. [PubMed] [Google Scholar]
- 40.Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature 1987; 325: 253–257. [DOI] [PubMed] [Google Scholar]
- 41.Armulik A, Genove G, Mae M, et al. Pericytes regulate the blood-brain barrier. Nature 2010; 468: 557–561. [DOI] [PubMed] [Google Scholar]
- 42.Daneman R, Zhou L, Kebede AA, et al. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010; 468: 562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daneman R, Zhou L, Agalliu D, et al. The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS One 2010; 5: e13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dauchy S, Dutheil F, Weaver RJ, et al. ABC transporters, cytochromes P450 and their main transcription factors: expression at the human blood-brain barrier. J Neurochem 2008; 107: 1518–1528. [DOI] [PubMed] [Google Scholar]
- 45.Enerson BE, Drewes LR. The rat blood-brain barrier transcriptome. J Cereb Blood Flow Metab 2006; 26: 959–973. [DOI] [PubMed] [Google Scholar]
- 46.Geier EG, Chen EC, Webb A, et al. Profiling solute carrier transporters in the human blood-brain barrier. Clin Pharmacol Ther 2013; 94: 636–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo S, Zhou Y, Xing C, et al. The vasculome of the mouse brain. PLoS One 2012; 7: e52665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kubo Y, Ohtsuki S, Uchida Y, et al. Quantitative determination of luminal and abluminal membrane distributions of transporters in porcine brain capillaries by plasma membrane fractionation and quantitative targeted proteomics. J Pharm Sci 2015; 104: 3060–3068. [DOI] [PubMed] [Google Scholar]
- 49.Shawahna R, Uchida Y, Decleves X, et al. Transcriptomic and quantitative proteomic analysis of transporters and drug metabolizing enzymes in freshly isolated human brain microvessels. Mol Pharm 2011; 8: 1332–1341. [DOI] [PubMed] [Google Scholar]
- 50.Uchida Y, Ohtsuki S, Katsukura Y, et al. Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J Neurochem 2011; 117: 333–345. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Chen K, Sloan SA, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 2014; 34: 11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Srinivasan B, Kolli AR, Esch MB, et al. TEER measurement techniques for in vitro barrier model systems. J Lab Autom 2015; 20: 107–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Helms HC, Hersom M, Kuhlmann LB, et al. An electrically tight in vitro blood-brain barrier model displays net brain-to-blood efflux of substrates for the abc transporters, P-gp, Bcrp and Mrp-1. AAPS J 2014; 16: 1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patabendige A, Skinner RA, Morgan L, et al. A detailed method for preparation of a functional and flexible blood-brain barrier model using porcine brain endothelial cells. Brain Res 2013; 1521: 16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watson PM, Paterson JC, Thom G, et al. Modelling the endothelial blood-CNS barriers: a method for the production of robust in vitro models of the rat blood-brain barrier and blood-spinal cord barrier. BMC Neurosci 2013; 14: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gaillard PJ, de Boer AG. Relationship between permeability status of the blood-brain barrier and in vitro permeability coefficient of a drug. Eur J Pharm Sci 2000; 12: 95–102. [DOI] [PubMed] [Google Scholar]
- 57.Lohmann C, Huwel S, Galla HJ. Predicting blood-brain barrier permeability of drugs: evaluation of different in vitro assays. J Drug Target 2002; 10: 263–276. [DOI] [PubMed] [Google Scholar]
- 58.Liu H, Li Y, Lu S, et al. Temporal expression of transporters and receptors in a rat primary co-culture blood-brain barrier model. Xenobiotica 2014; 44: 941–951. [DOI] [PubMed] [Google Scholar]
- 59.Furuse M. Molecular basis of the core structure of tight junctions. Cold Spring Harb Perspect Biol 2010; 2: a002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krause G, Winkler L, Mueller SL, et al. Structure and function of claudins. Biochim Biophys Acta 2008; 1778: 631–645. [DOI] [PubMed] [Google Scholar]
- 61.Fukuta M, Okada H, Iinuma S, et al. Insulin fragments as a carrier for peptide delivery across the blood-brain barrier. Pharm Res 1994; 11: 1681–1688. [DOI] [PubMed] [Google Scholar]
- 62.Pardridge WM, Kang YS, Buciak JL, et al. Human insulin receptor monoclonal antibody undergoes high affinity binding to human brain capillaries in vitro and rapid transcytosis through the blood-brain barrier in vivo in the primate. Pharm Res 1995; 12: 807–816. [DOI] [PubMed] [Google Scholar]
- 63.Dehouck B, Fenart L, Dehouck MP, et al. A new function for the LDL receptor: transcytosis of LDL across the blood-brain barrier. J Cell Biol 1997; 138: 877–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bockenhoff A, Cramer S, Wolte P, et al. Comparison of five peptide vectors for improved brain delivery of the lysosomal enzyme arylsulfatase A. J Neurosci 2014; 34: 3122–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Visser CC, Voorwinden LH, Crommelin DJ, et al. Characterization and modulation of the transferrin receptor on brain capillary endothelial cells. Pharm Res 2004; 21: 761–769. [DOI] [PubMed] [Google Scholar]
- 66.Tamaru M, Akita H, Fujiwara T, et al. Leptin-derived peptide, a targeting ligand for mouse brain-derived endothelial cells via macropinocytosis. Biochem Biophys Res Commun 2010; 394: 587–592. [DOI] [PubMed] [Google Scholar]
- 67.Gaillard PJ, Brink B, de Boer AG. Diphteria toxin receptor-targeted brain drug delivery. Int Cong Ser 2005; 1277: 185–198. [Google Scholar]
- 68.Rip J, Chen L, Hartman R, et al. Glutathione PEGylated liposomes: pharmacokinetics and delivery of cargo across the blood-brain barrier in rats. J Drug Target 2014; 22: 460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Georgieva JV, Hoekstra D, Zuhorn IS. Smuggling drugs into the brain: an overview of ligands targeting transcytosis for drug delivery across the blood-brain barrier. Pharmaceutics 2014; 6: 557–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Niewoehner J, Bohrmann B, Collin L, et al. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron 2014; 81: 49–60. [DOI] [PubMed] [Google Scholar]
- 71.Regina A, Demeule M, Che C, et al. Antitumour activity of ANG1005, a conjugate between paclitaxel and the new brain delivery vector Angiopep-2. Br J Pharmacol 2008; 155: 185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thomas FC, Taskar K, Rudraraju V, et al. Uptake of ANG1005, a novel paclitaxel derivative, through the blood-brain barrier into brain and experimental brain metastases of breast cancer. Pharm Res 2009; 26: 2486–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Che C, Yang G, Thiot C, et al. New Angiopep-modified doxorubicin (ANG1007) and etoposide (ANG1009) chemotherapeutics with increased brain penetration. J Med Chem 2010; 53: 2814–2824. [DOI] [PubMed] [Google Scholar]
- 74.Regina A, Demeule M, Tripathy S, et al. ANG4043, a novel brain-penetrant peptide-mAb conjugate, is efficacious against HER2-positive intracranial tumors in mice. Mol Cancer Ther 2015; 14: 129–140. [DOI] [PubMed] [Google Scholar]
- 75.Candela P, Saint-Pol J, Kuntz M, et al. In vitro discrimination of the role of LRP1 at the BBB cellular level: focus on brain capillary endothelial cells and brain pericytes. Brain Res 2015; 1594: 15–26. [DOI] [PubMed] [Google Scholar]
- 76.Gosselet F, Candela P, Sevin E, et al. Transcriptional profiles of receptors and transporters involved in brain cholesterol homeostasis at the blood-brain barrier: use of an in vitro model. Brain Res 2009; 1249: 34–42. [DOI] [PubMed] [Google Scholar]
- 77.Demeule M, Currie JC, Bertrand Y, et al. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector angiopep-2. J Neurochem 2008; 106: 1534–1544. [DOI] [PubMed] [Google Scholar]
- 78.Zhao Z, Sagare AP, Ma Q, et al. Central role for PICALM in amyloid-beta blood-brain barrier transcytosis and clearance. Nat Neurosci 2015; 18: 978–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hartz AM, Miller DS, Bauer B. Restoring blood-brain barrier P-glycoprotein reduces brain amyloid-beta in a mouse model of Alzheimer's disease. Mol Pharmacol 2010; 77: 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Joo F, Karnushina I. A procedure for the isolation of capillaries from rat brain. Cytobios 1973; 8: 41–48. [PubMed] [Google Scholar]
- 81.Mrsulja BB, Mrsulja BJ, Fujimoto T, et al. Isolation of brain capillaries: a simplified technique. Brain Res 1976; 110: 361–365. [DOI] [PubMed] [Google Scholar]
- 82.Erdlenbruch B, Alipour M, Fricker G, et al. Alkylglycerol opening of the blood-brain barrier to small and large fluorescence markers in normal and C6 glioma-bearing rats and isolated rat brain capillaries. Br J Pharmacol 2003; 140: 1201–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fricker G, Nobmann S, Miller DS. Permeability of porcine blood brain barrier to somatostatin analogues. Br J Pharmacol 2002; 135: 1308–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hartz AM, Bauer B, Fricker G, et al. Rapid modulation of P-glycoprotein-mediated transport at the blood-brain barrier by tumor necrosis factor-alpha and lipopolysaccharide. Mol Pharmacol 2006; 69: 462–470. [DOI] [PubMed] [Google Scholar]
- 85.Bowman PD, Betz AL, Ar D, et al. Primary culture of capillary endothelium from rat brain. In Vitro 1981; 17: 353–362. [DOI] [PubMed] [Google Scholar]
- 86.DeBault LE, Kahn LE, Frommes SP, et al. Cerebral microvessels and derived cells in tissue culture: isolation and preliminary characterization. In Vitro 1979; 15: 473–487. [DOI] [PubMed] [Google Scholar]
- 87.DeBault LE, Cancilla PA. Gamma-glutamyl transpeptidase in isolated brain endothelial cells: induction by glial cells in vitro. Science 1980; 207: 653–655. [DOI] [PubMed] [Google Scholar]
- 88.Tao-Cheng JH, Nagy Z, Brightman MW. Tight junctions of brain endothelium in vitro are enhanced by astroglia. J Neurosci 1987; 7: 3293–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dehouck MP, Meresse S, Delorme P, et al. An easier, reproducible, and mass-production method to study the blood-brain barrier in vitro. J Neurochem 1990; 54: 1798–1801. [DOI] [PubMed] [Google Scholar]
- 90.Cecchelli R, Dehouck B, Descamps L, et al. In vitro model for evaluating drug transport across the blood-brain barrier. Adv Drug Deliv Rev 1999; 36: 165–178. [DOI] [PubMed] [Google Scholar]
- 91.Gaillard PJ, Voorwinden LH, Nielsen JL, et al. Establishment and functional characterization of an in vitro model of the blood-brain barrier, comprising a co-culture of brain capillary endothelial cells and astrocytes. Eur J Pharm Sci 2001; 12: 215–222. [DOI] [PubMed] [Google Scholar]
- 92.Malina KCK, Cooper I, Teichberg VI. Closing the gap between the in-vivo and in-vitro blood-brain barrier tightness. Brain Research 2009; 1284: 12–21. [DOI] [PubMed] [Google Scholar]
- 93.Abbott NJ, Dolman DE, Drndarski S, et al. An improved in vitro blood-brain barrier model: rat brain endothelial cells co-cultured with astrocytes. Methods Mol Biol 2012; 814: 415–430. [DOI] [PubMed] [Google Scholar]
- 94.Boveri M, Berezowski V, Price A, et al. Induction of blood-brain barrier properties in cultured brain capillary endothelial cells: comparison between primary glial cells and C6 cell line. Glia 2005; 51: 187–198. [DOI] [PubMed] [Google Scholar]
- 95.Culot M, Lundquist S, Vanuxeem D, et al. An in vitro blood-brain barrier model for high throughput (HTS) toxicological screening. Toxicol In Vitro 2008; 22: 799–811. [DOI] [PubMed] [Google Scholar]
- 96.Perriere N, Yousif S, Cazaubon S, et al. A functional in vitro model of rat blood-brain barrier for molecular analysis of efflux transporters. Brain Res 2007; 1150: 1–13. [DOI] [PubMed] [Google Scholar]
- 97.Nakagawa S, Deli MA, Kawaguchi H, et al. A new blood-brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem Int 2009; 54: 253–263. [DOI] [PubMed] [Google Scholar]
- 98.Nakagawa S, Deli MA, Nakao S, et al. Pericytes from brain microvessels strengthen the barrier integrity in primary cultures of rat brain endothelial cells. Cell Mol Neurobiol 2007; 27: 687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dohgu S, Takata F, Yamauchi A, et al. Brain pericytes contribute to the induction and up-regulation of blood-brain barrier functions through transforming growth factor-beta production. Brain Res 2005; 1038: 208–215. [DOI] [PubMed] [Google Scholar]
- 100.Hayashi K, Nakao S, Nakaoke R, et al. Effects of hypoxia on endothelial/pericytic co-culture model of the blood-brain barrier. Regul Pept 2004; 123: 77–83. [DOI] [PubMed] [Google Scholar]
- 101.Vandenhaute E, Dehouck L, Boucau MC, et al. Modelling the neurovascular unit and the blood-brain barrier with the unique function of pericytes. Curr Neurovasc Res 2011; 8: 258–269. [DOI] [PubMed] [Google Scholar]
- 102.Wilhelm I, Fazakas C, Krizbai IA. In vitro models of the blood-brain barrier. Acta Neurobiol Exp (Wars) 2011; 71: 113–128. [DOI] [PubMed] [Google Scholar]
- 103.Hatherell K, Couraud PO, Romero IA, et al. Development of a three-dimensional, all-human in vitro model of the blood-brain barrier using mono-, co-, and tri-cultivation Transwell models. J Neurosci Methods 2011; 199: 223–229. [DOI] [PubMed] [Google Scholar]
- 104.Franke H, Galla HJ, Beuckmann CT. An improved low-permeability in vitro-model of the blood-brain barrier: transport studies on retinoids, sucrose, haloperidol, caffeine and mannitol. Brain Res 1999; 818: 65–71. [DOI] [PubMed] [Google Scholar]
- 105.Hoheisel D, Nitz T, Franke H, et al. Hydrocortisone reinforces the blood-brain properties in a serum free cell culture system. Biochem Biophys Res Commun 1998; 247: 312–315. [PubMed] [Google Scholar]
- 106.Patabendige A, Skinner RA, Abbott NJ. Establishment of a simplified in vitro porcine blood-brain barrier model with high transendothelial electrical resistance. Brain Res 2013; 1521: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Helms HC, Waagepetersen HS, Nielsen CU, et al. Paracellular tightness and claudin-5 expression is increased in the BCEC/astrocyte blood-brain barrier model by increasing media buffer capacity during growth. AAPS J 2010; 12: 759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Burek M, Salvador E, Forster CY. Generation of an immortalized murine brain microvascular endothelial cell line as an in vitro blood brain barrier model. J Vis Exp 2012; 66: e4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Coisne C, Dehouck L, Faveeuw C, et al. Mouse syngenic in vitro blood-brain barrier model: a new tool to examine inflammatory events in cerebral endothelium. Lab Invest 2005; 85: 734–746. [DOI] [PubMed] [Google Scholar]
- 110.Forster C, Silwedel C, Golenhofen N, et al. Occludin as direct target for glucocorticoid-induced improvement of blood-brain barrier properties in a murine in vitro system. J Physiol 2005; 565: 475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bernas MJ, Cardoso FL, Daley SK, et al. Establishment of primary cultures of human brain microvascular endothelial cells to provide an in vitro cellular model of the blood-brain barrier. Nat Protoc 2010; 5: 1265–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Prat A, Biernacki K, Pouly S, et al. Kinin B1 receptor expression and function on human brain endothelial cells. J Neuropathol Exp Neurol 2000; 59: 896–906. [DOI] [PubMed] [Google Scholar]
- 113.Subileau EA, Rezaie P, Davies HA, et al. Expression of chemokines and their receptors by human brain endothelium: implications for multiple sclerosis. J Neuropathol Exp Neurol 2009; 68: 227–240. [DOI] [PubMed] [Google Scholar]
- 114.Weksler BB, Subileau EA, Perriere N, et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J 2005; 19: 1872–1874. [DOI] [PubMed] [Google Scholar]
- 115.Stins MF, Badger J, Sik Kim K. Bacterial invasion and transcytosis in transfected human brain microvascular endothelial cells. Microb Pathog 2001; 30: 19–28. [DOI] [PubMed] [Google Scholar]
- 116.Boyer-Di Ponio J, El-Ayoubi F, Glacial F, et al. Instruction of circulating endothelial progenitors in vitro towards specialized blood-brain barrier and arterial phenotypes. PLoS One 2014; 9: e84179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cecchelli R, Aday S, Sevin E, et al. A stable and reproducible human blood-brain barrier model derived from hematopoietic stem cells. PLoS One 2014; 9: e99733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lippmann ES, Azarin SM, Kay JE, et al. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat Biotechnol 2012; 30: 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stamatovic SM, Keep RF, Kunkel SL, et al. Potential role of MCP-1 in endothelial cell tight junction ‘opening': signaling via Rho and Rho kinase. J Cell Sci 2003; 116: 4615–4628. [DOI] [PubMed] [Google Scholar]
- 120.Weidenfeller C, Schrot S, Zozulya A, et al. Murine brain capillary endothelial cells exhibit improved barrier properties under the influence of hydrocortisone. Brain Res 2005; 1053: 162–174. [DOI] [PubMed] [Google Scholar]
- 121.Deli MA, Abraham CS, Niwa M, et al. N,N-diethyl-2-[4-(phenylmethyl)phenoxy]ethanamine increases the permeability of primary mouse cerebral endothelial cell monolayers. Inflamm Res 2003; 52(Suppl 1): S39–S40. [DOI] [PubMed] [Google Scholar]
- 122.Wagner EF, Risau W. Oncogenes in the study of endothelial cell growth and differentiation. Semin Cancer Biol 1994; 5: 137–145. [PubMed] [Google Scholar]
- 123.Omidi Y, Campbell L, Barar J, et al. Evaluation of the immortalised mouse brain capillary endothelial cell line, b.End3, as an in vitro blood-brain barrier model for drug uptake and transport studies. Brain Res 2003; 990: 95–112. [DOI] [PubMed] [Google Scholar]
- 124.Paolinelli R, Corada M, Ferrarini L, et al. Wnt activation of immortalized brain endothelial cells as a tool for generating a standardized model of the blood brain barrier in vitro. PLoS One 2013; 8: e70233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Steiner O, Coisne C, Engelhardt B, et al. Comparison of immortalized bEnd5 and primary mouse brain microvascular endothelial cells as in vitro blood-brain barrier models for the study of T cell extravasation. J Cereb Blood Flow Metab 2011; 31: 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Silwedel C, Forster C. Differential susceptibility of cerebral and cerebellar murine brain microvascular endothelial cells to loss of barrier properties in response to inflammatory stimuli. J Neuroimmunol 2006; 179: 37–45. [DOI] [PubMed] [Google Scholar]
- 127.Forster C, Waschke J, Burek M, et al. Glucocorticoid effects on mouse microvascular endothelial barrier permeability are brain specific. J Physiol 2006; 573: 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Forster C, Kahles T, Kietz S, et al. Dexamethasone induces the expression of metalloproteinase inhibitor TIMP-1 in the murine cerebral vascular endothelial cell line cEND. J Physiol 2007; 580: 937–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kleinschnitz C, Blecharz K, Kahles T, et al. Glucocorticoid insensitivity at the hypoxic blood-brain barrier can be reversed by inhibition of the proteasome. Stroke 2011; 42: 1081–1089. [DOI] [PubMed] [Google Scholar]
- 130.Neuhaus W, Gaiser F, Mahringer A, et al. The pivotal role of astrocytes in an in vitro stroke model of the blood-brain barrier. Front Cell Neurosci 2014; 8: 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Neuhaus W, Burek M, Djuzenova CS, et al. Addition of NMDA-receptor antagonist MK801 during oxygen/glucose deprivation moderately attenuates the upregulation of glucose uptake after subsequent reoxygenation in brain endothelial cells. Neurosci Lett 2012; 506: 44–49. [DOI] [PubMed] [Google Scholar]
- 132.Harke N, Leers J, Kietz S, et al. Glucocorticoids regulate the human occludin gene through a single imperfect palindromic glucocorticoid response element. Mol Cell Endocrinol 2008; 295: 39–47. [DOI] [PubMed] [Google Scholar]
- 133.Burek M, Forster CY. Cloning and characterization of the murine claudin-5 promoter. Mol Cell Endocrinol 2009; 298: 19–24. [DOI] [PubMed] [Google Scholar]
- 134.Blecharz KG, Drenckhahn D, Forster CY. Glucocorticoids increase VE-cadherin expression and cause cytoskeletal rearrangements in murine brain endothelial cEND cells. J Cereb Blood Flow Metab 2008; 28: 1139–1149. [DOI] [PubMed] [Google Scholar]
- 135.Blecharz KG, Haghikia A, Stasiolek M, et al. Glucocorticoid effects on endothelial barrier function in the murine brain endothelial cell line cEND incubated with sera from patients with multiple sclerosis. Mult Scler 2010; 16: 293–302. [DOI] [PubMed] [Google Scholar]
- 136.Burek M, Haghikia A, Gold R, et al. Differential cytokine release from brain microvascular endothelial cells treated with dexamethasone and multiple sclerosis patient sera. J Steroids Horm Sci 2014; 5: 128. [Google Scholar]
- 137.Abbott NJ, Hughes CC, Revest PA, et al. Development and characterisation of a rat brain capillary endothelial culture: towards an in vitro blood-brain barrier. J Cell Sci 1992; 103(Pt 1): 23–37. [DOI] [PubMed] [Google Scholar]
- 138.Szabo CA, Deli MA, Ngo TK, et al. Production of pure primary rat cerebral endothelial cell culture: a comparison of different methods. Neurobiology (Bp) 1997; 5: 1–16. [PubMed] [Google Scholar]
- 139.Perriere N, Demeuse P, Garcia E, et al. Puromycin-based purification of rat brain capillary endothelial cell cultures. Effect on the expression of blood-brain barrier-specific properties. J Neurochem 2005; 93: 279–289. [DOI] [PubMed] [Google Scholar]
- 140.Calabria AR, Weidenfeller C, Jones AR, et al. Puromycin-purified rat brain microvascular endothelial cell cultures exhibit improved barrier properties in response to glucocorticoid induction. J Neurochem 2006; 97: 922–933. [DOI] [PubMed] [Google Scholar]
- 141.Molino Y, Jabes F, Lacassagne E, et al. Setting-up an in vitro model of rat blood-brain barrier (BBB): a focus on BBB impermeability and receptor-mediated transport. J Vis Exp 2014; 88: e51278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ge S, Song L, Pachter JS. Where is the blood-brain barrier … really? J Neurosci Res 2005; 79: 421–427. [DOI] [PubMed] [Google Scholar]
- 143.Ceruti S, Colombo L, Magni G, et al. Oxygen-glucose deprivation increases the enzymatic activity and the microvesicle-mediated release of ectonucleotidases in the cells composing the blood-brain barrier. Neurochem Int 2011; 59: 259–271. [DOI] [PubMed] [Google Scholar]
- 144.Lukic-Panin V, Deguchi K, Yamashita T, et al. Free radical scavenger edaravone administration protects against tissue plasminogen activator induced oxidative stress and blood brain barrier damage. Curr Neurovasc Res 2010; 7: 319–329. [DOI] [PubMed] [Google Scholar]
- 145.Veszelka S, Toth AE, Walter FR, et al. Docosahexaenoic acid reduces amyloid-beta induced toxicity in cells of the neurovascular unit. J Alzheimers Dis 2013; 36: 487–501. [DOI] [PubMed] [Google Scholar]
- 146.Hellinger E, Veszelka S, Toth AE, et al. Comparison of brain capillary endothelial cell-based and epithelial (MDCK-MDR1, Caco-2, and VB-Caco-2) cell-based surrogate blood-brain barrier penetration models. Eur J Pharm Biopharm 2012; 82: 340–351. [DOI] [PubMed] [Google Scholar]
- 147.Imamura S, Tabuchi M, Kushida H, et al. The blood-brain barrier permeability of geissoschizine methyl ether in Uncaria hook, a galenical constituent of the traditional Japanese medicine yokukansan. Cell Mol Neurobiol 2011; 31: 787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tabuchi M, Imamura S, Kawakami Z, et al. The blood-brain barrier permeability of 18beta-glycyrrhetinic acid, a major metabolite of glycyrrhizin in Glycyrrhiza root, a constituent of the traditional Japanese medicine yokukansan. Cell Mol Neurobiol 2012; 32: 1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bohara M, Kambe Y, Nagayama T, et al. C-type natriuretic peptide modulates permeability of the blood-brain barrier. J Cereb Blood Flow Metab 2014; 34: 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cardoso FL, Kittel A, Veszelka S, et al. Exposure to lipopolysaccharide and/or unconjugated bilirubin impair the integrity and function of brain microvascular endothelial cells. PLoS One 2012; 7: e35919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Garcia-Garcia E, Gil S, Andrieux K, et al. A relevant in vitro rat model for the evaluation of blood-brain barrier translocation of nanoparticles. Cell Mol Life Sci 2005; 62: 1400–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Horai S, Nakagawa S, Tanaka K, et al. Cilostazol strengthens barrier integrity in brain endothelial cells. Cell Mol Neurobiol 2013; 33: 291–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Takata F, Dohgu S, Yamauchi A, et al. In vitro blood-brain barrier models using brain capillary endothelial cells isolated from neonatal and adult rats retain age-related barrier properties. PLoS One 2013; 8: e55166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xue Q, Liu Y, Qi H, et al. A novel brain neurovascular unit model with neurons, astrocytes and microvascular endothelial cells of rat. Int J Biol Sci 2013; 9: 174–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Walter FR, Veszelka S, Pasztoi M, et al. Tesmilifene modifies brain endothelial functions and opens the blood-brain/blood-glioma barrier. J Neurochem 2015; 134: 1040–1054. [DOI] [PubMed] [Google Scholar]
- 156.Kis B, Deli MA, Kobayashi H, et al. Adrenomedullin regulates blood-brain barrier functions in vitro. Neuroreport 2001; 12: 4139–4142. [DOI] [PubMed] [Google Scholar]
- 157.Kis B, Snipes JA, Deli MA, et al. Chronic adrenomedullin treatment improves blood-brain barrier function but has no effects on expression of tight junction proteins. Acta Neurochir Suppl 2003; 86: 565–568. [DOI] [PubMed] [Google Scholar]
- 158.Warren MS, Zerangue N, Woodford K, et al. Comparative gene expression profiles of ABC transporters in brain microvessel endothelial cells and brain in five species including human. Pharmacol Res 2009; 59: 404–413. [DOI] [PubMed] [Google Scholar]
- 159.Calabria AR, Shusta EV. A genomic comparison of in vivo and in vitro brain microvascular endothelial cells. J Cereb Blood Flow Metab 2008; 28: 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Narang VS, Fraga C, Kumar N, et al. Dexamethasone increases expression and activity of multidrug resistance transporters at the rat blood-brain barrier. Am J Physiol Cell Physiol 2008; 295: C440–C450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ichikawa N, Naora K, Hirano H, et al. Isolation and primary culture of rat cerebral microvascular endothelial cells for studying drug transport in vitro. J Pharmacol Toxicol Methods 1996; 36: 45–52. [DOI] [PubMed] [Google Scholar]
- 162.Pifferi F, Jouin M, Alessandri JM, et al. n-3 Fatty acids modulate brain glucose transport in endothelial cells of the blood-brain barrier. Prostaglandins Leukot Essent Fatty Acids 2007; 77: 279–286. [DOI] [PubMed] [Google Scholar]
- 163.Pifferi F, Jouin M, Alessandri JM, et al. n-3 long-chain fatty acids and regulation of glucose transport in two models of rat brain endothelial cells. Neurochem Int 2010; 56: 703–710. [DOI] [PubMed] [Google Scholar]
- 164.Hughes CC, Lantos PL. Uptake of leucine and alanine by cultured cerebral capillary endothelial cells. Brain Res 1989; 480: 126–132. [DOI] [PubMed] [Google Scholar]
- 165.Demeuse P, Kerkhofs A, Struys-Ponsar C, et al. Compartmentalized coculture of rat brain endothelial cells and astrocytes: a syngenic model to study the blood-brain barrier. J Neurosci Methods 2002; 121: 21–31. [DOI] [PubMed] [Google Scholar]
- 166.Garberg P, Ball M, Borg N, et al. In vitro models for the blood-brain barrier. Toxicol In Vitro 2005; 19: 299–334. [DOI] [PubMed] [Google Scholar]
- 167.Gaillard PJ, van der Sandt IC, Voorwinden LH, et al. Astrocytes increase the functional expression of P-glycoprotein in an in vitro model of the blood-brain barrier. Pharm Res 2000; 17: 1198–1205. [DOI] [PubMed] [Google Scholar]
- 168.Helms HC, Madelung R, Waagepetersen HS, et al. In vitro evidence for the brain glutamate efflux hypothesis: brain endothelial cells cocultured with astrocytes display a polarized brain-to-blood transport of glutamate. Glia 2012; 60: 882–893. [DOI] [PubMed] [Google Scholar]
- 169.Meresse S, Dehouck MP, Delorme P, et al. Bovine brain endothelial cells express tight junctions and monoamine oxidase activity in long-term culture. J Neurochem 1989; 53: 1363–1371. [DOI] [PubMed] [Google Scholar]
- 170.Rutten MJ, Hoover RL, Karnovsky MJ. Electrical resistance and macromolecular permeability of brain endothelial monolayer cultures. Brain Res 1987; 425: 301–310. [DOI] [PubMed] [Google Scholar]
- 171.Descamps L, Dehouck MP, Torpier G, et al. Receptor-mediated transcytosis of transferrin through blood-brain barrier endothelial cells. Am J Physiol 1996; 270: H1149–H1158. [DOI] [PubMed] [Google Scholar]
- 172.Fenart L, Casanova A, Dehouck B, et al. Evaluation of effect of charge and lipid coating on ability of 60-nm nanoparticles to cross an in vitro model of the blood-brain barrier. J Pharmacol Exp Ther 1999; 291: 1017–1022. [PubMed] [Google Scholar]
- 173.Wang W, Dentler WL, Borchardt RT. VEGF increases BMEC monolayer permeability by affecting occludin expression and tight junction assembly. Am J Physiol Heart Circ Physiol 2001; 280: H434–H440. [DOI] [PubMed] [Google Scholar]
- 174.Boveri M, Kinsner A, Berezowski V, et al. Highly purified lipoteichoic acid from gram-positive bacteria induces in vitro blood-brain barrier disruption through glia activation: role of pro-inflammatory cytokines and nitric oxide. Neuroscience 2006; 137: 1193–1209. [DOI] [PubMed] [Google Scholar]
- 175.Fauquette W, Amourette C, Dehouck MP, et al. Radiation-induced blood-brain barrier damages: an in vitro study. Brain Res 2012; 1433: 114–126. [DOI] [PubMed] [Google Scholar]
- 176.Dehouck MP, Dehouck B, Schluep C, et al. Drug transport to the brain: comparison between in vitro and in vivo models of the blood-brain barrier. Eur J Pharmaceut Sci 1995; 3: 357–365. [Google Scholar]
- 177.Dehouck MP, Jolliet-Riant P, Bree F, et al. Drug transfer across the blood-brain barrier: correlation between in vitro and in vivo models. J Neurochem 1992; 58: 1790–1797. [DOI] [PubMed] [Google Scholar]
- 178.Vandenhaute E, Sevin E, Hallier-Vanuxeem D, et al. Case study: adapting in vitro blood-brain barrier models for use in early-stage drug discovery. Drug Discov Today 2012; 17: 285–290. [DOI] [PubMed] [Google Scholar]
- 179.Wolburg H, Neuhaus J, Kniesel U, et al. Modulation of tight junction structure in blood-brain barrier endothelial cells. Effects of tissue culture, second messengers and cocultured astrocytes. J Cell Sci 1994; 107(Pt 5): 1347–1357. [DOI] [PubMed] [Google Scholar]
- 180.Guillot FL, Audus KL. Angiotensin peptide regulation of bovine brain microvessel endothelial cell monolayer permeability. J Cardiovasc Pharmacol 1991; 18: 212–218. [DOI] [PubMed] [Google Scholar]
- 181.Raub TJ, Kuentzel SL, Sawada GA. Permeability of bovine brain microvessel endothelial cells in vitro: barrier tightening by a factor released from astroglioma cells. Exp Cell Res 1992; 199: 330–340. [DOI] [PubMed] [Google Scholar]
- 182.Deli MA, Dehouck MP, Abraham CS, et al. Penetration of small molecular weight substances through cultured bovine brain capillary endothelial cell monolayers: the early effects of cyclic adenosine 3',5'-monophosphate. Exp Physiol 1995; 80: 675–678. [DOI] [PubMed] [Google Scholar]
- 183.Deli MA, Dehouck MP, Cecchelli R, et al. Histamine induces a selective albumin permeation through the blood-brain barrier in vitro. Inflamm Res 1995; 44(Suppl 1): S56–S57. [DOI] [PubMed] [Google Scholar]
- 184.Anda T, Yamashita H, Khalid H, et al. Effect of tumor necrosis factor-alpha on the permeability of bovine brain microvessel endothelial cell monolayers. Neurol Res 1997; 19: 369–376. [DOI] [PubMed] [Google Scholar]
- 185.Abbruscato TJ, Davis TP. Combination of hypoxia/aglycemia compromises in vitro blood-brain barrier integrity. J Pharmacol Exp Ther 1999; 289: 668–675. [PubMed] [Google Scholar]
- 186.Mark KS, Miller DW. Increased permeability of primary cultured brain microvessel endothelial cell monolayers following TNF-alpha exposure. Life Sci 1999; 64: 1941–1953. [DOI] [PubMed] [Google Scholar]
- 187.Schaddelee MP, Voorwinden HL, van Tilburg EW, et al. Functional role of adenosine receptor subtypes in the regulation of blood-brain barrier permeability: possible implications for the design of synthetic adenosine derivatives. Eur J Pharm Sci 2003; 19: 13–22. [DOI] [PubMed] [Google Scholar]
- 188.Helms HC, Brodin B. Generation of primary cultures of bovine brain endothelial cells and setup of cocultures with rat astrocytes. Methods Mol Biol 2014; 1135: 365–382. [DOI] [PubMed] [Google Scholar]
- 189.Tsuji A, Terasaki T, Takabatake Y, et al. P-glycoprotein as the drug efflux pump in primary cultured bovine brain capillary endothelial cells. Life Sci 1992; 51: 1427–1437. [DOI] [PubMed] [Google Scholar]
- 190.Fenart L, Buee-Scherrer V, Descamps L, et al. Inhibition of P-glycoprotein: rapid assessment of its implication in blood-brain barrier integrity and drug transport to the brain by an in vitro model of the blood-brain barrier. Pharm Res 1998; 15: 993–1000. [DOI] [PubMed] [Google Scholar]
- 191.Rose JM, Peckham SL, Scism JL, et al. Evaluation of the role of P-glycoprotein in ivermectin uptake by primary cultures of bovine brain microvessel endothelial cells. Neurochem Res 1998; 23: 203–209. [DOI] [PubMed] [Google Scholar]
- 192.van der Sandt IC, Smolders R, Nabulsi L, et al. Active efflux of the 5-HT(1A) receptor agonist flesinoxan via P-glycoprotein at the blood-brain barrier. Eur J Pharm Sci 2001; 14: 81–86. [DOI] [PubMed] [Google Scholar]
- 193.van der Sandt IC, Vos CM, Nabulsi L, et al. Assessment of active transport of HIV protease inhibitors in various cell lines and the in vitro blood–brain barrier. AIDS 2001; 15: 483–91. [DOI] [PubMed] [Google Scholar]
- 194.Perloff MD, von Moltke LL, Fahey JM, et al. Induction of P-glycoprotein expression and activity by ritonavir in bovine brain microvessel endothelial cells. J Pharm Pharmacol 2007; 59: 947–953. [DOI] [PubMed] [Google Scholar]
- 195.Huai-Yun H, Secrest DT, Mark KS, et al. Expression of multidrug resistance-associated protein (MRP) in brain microvessel endothelial cells. Biochem Biophys Res Commun 1998; 243: 816–820. [DOI] [PubMed] [Google Scholar]
- 196.Saint-Pol J, Candela P, Boucau MC, et al. Oxysterols decrease apical-to-basolateral transport of ass peptides via an ABCB1-mediated process in an in vitro Blood-brain barrier model constituted of bovine brain capillary endothelial cells. Brain Res 2013; 1517: 1–15. [DOI] [PubMed] [Google Scholar]
- 197.Berezowski V, Landry C, Dehouck MP, et al. Contribution of glial cells and pericytes to the mRNA profiles of P-glycoprotein and multidrug resistance-associated proteins in an in vitro model of the blood-brain barrier. Brain Res 2004; 1018: 1–9. [DOI] [PubMed] [Google Scholar]
- 198.Anfuso CD, Motta C, Giurdanella G, et al. Endothelial PKCalpha-MAPK/ERK-phospholipase A2 pathway activation as a response of glioma in a triple culture model. A new role for pericytes? Biochimie 2014; 99: 77–87. [DOI] [PubMed] [Google Scholar]
- 199.Hakkarainen JJ, Rilla K, Suhonen M, et al. Re-evaluation of the role of P-glycoprotein in in vitro drug permeability studies with the bovine brain microvessel endothelial cells. Xenobiotica 2014; 44: 283–294. [DOI] [PubMed] [Google Scholar]
- 200.Candela P, Gosselet F, Saint-Pol J, et al. Apical-to-basolateral transport of amyloid-beta peptides through blood-brain barrier cells is mediated by the receptor for advanced glycation end-products and is restricted by P-glycoprotein. J Alzheimers Dis 2010; 22: 849–859. [DOI] [PubMed] [Google Scholar]
- 201.Fillebeen C, Descamps L, Dehouck MP, et al. Receptor-mediated transcytosis of lactoferrin through the blood-brain barrier. J Biol Chem 1999; 274: 7011–7017. [DOI] [PubMed] [Google Scholar]
- 202.Demeule M, Regina A, Che C, et al. Identification and design of peptides as a new drug delivery system for the brain. J Pharmacol Exp Ther 2008; 324: 1064–1072. [DOI] [PubMed] [Google Scholar]
- 203.Chang J, Jallouli Y, Kroubi M, et al. Characterization of endocytosis of transferrin-coated PLGA nanoparticles by the blood-brain barrier. Int J Pharm 2009; 379: 285–292. [DOI] [PubMed] [Google Scholar]
- 204.Raub TJ, Newton CR. Recycling kinetics and transcytosis of transferrin in primary cultures of bovine brain microvessel endothelial cells. J Cell Physiol 1991; 149: 141–151. [DOI] [PubMed] [Google Scholar]
- 205.Audus KL, Borchardt RT. Characterization of an in vitro blood-brain barrier models system for studying drug transport and metabolism. Pharmaceut Res 1986; 3: 81–87. [DOI] [PubMed] [Google Scholar]
- 206.Czeredys M, Mysiorek C, Kulikova N, et al. A polarized localization of amino acid/carnitine transporter B(0,+) (ATB(0,+)) in the blood-brain barrier. Biochem Biophys Res Commun 2008; 376: 267–270. [DOI] [PubMed] [Google Scholar]
- 207.Terasaki T, Takakuwa S, Moritani S, et al. Transport of monocarboxylic acids at the blood-brain barrier: studies with monolayers of primary cultured bovine brain capillary endothelial cells. J Pharmacol Exp Ther 1991; 258: 932–937. [PubMed] [Google Scholar]
- 208.Mischeck U, Meyer J, Galla HJ. Characterization of gamma-glutamyl transpeptidase activity of cultured endothelial cells from porcine brain capillaries. Cell Tissue Res 1989; 256: 221–226. [DOI] [PubMed] [Google Scholar]
- 209.Franke H, Galla H, Beuckmann CT. Primary cultures of brain microvessel endothelial cells: a valid and flexible model to study drug transport through the blood-brain barrier in vitro. Brain Res Brain Res Protoc 2000; 5: 248–256. [DOI] [PubMed] [Google Scholar]
- 210.Schulze C, Smales C, Rubin LL, et al. Lysophosphatidic acid increases tight junction permeability in cultured brain endothelial cells. J Neurochem 1997; 68: 991–1000. [DOI] [PubMed] [Google Scholar]
- 211.Cohen-Kashi-Malina K, Cooper I, Teichberg VI. Mechanisms of glutamate efflux at the blood-brain barrier: involvement of glial cells. J Cereb Blood Flow Metab 2012; 32: 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Skinner RA, Gibson RM, Rothwell NJ, et al. Transport of interleukin-1 across cerebromicrovascular endothelial cells. Br J Pharmacol 2009; 156: 1115–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Thomsen LB, Burkhart A, Moos T. A triple culture model of the blood-brain barrier using porcine brain endothelial cells, astrocytes and pericytes. PLoS One 2015; 10: e0134765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kroll S, El-Gindi J, Thanabalasundaram G, et al. Control of the blood-brain barrier by glucocorticoids and the cells of the neurovascular unit. Ann N Y Acad Sci 2009; 1165: 228–239. [DOI] [PubMed] [Google Scholar]
- 215.Cantrill CA, Skinner RA, Rothwell NJ, et al. An immortalised astrocyte cell line maintains the in vivo phenotype of a primary porcine in vitro blood-brain barrier model. Brain Res 2012; 1479: 17–30. [DOI] [PubMed] [Google Scholar]
- 216.Smith M, Omidi Y, Gumbleton M. Primary porcine brain microvascular endothelial cells: biochemical and functional characterisation as a model for drug transport and targeting. J Drug Target 2007; 15: 253–268. [DOI] [PubMed] [Google Scholar]
- 217.Zhang Y, Li CS, Ye Y, et al. Porcine brain microvessel endothelial cells as an in vitro model to predict in vivo blood-brain barrier permeability. Drug Metab Dispos 2006; 34: 1935–1943. [DOI] [PubMed] [Google Scholar]
- 218.Thanabalasundaram G, El-Gindi J, Lischper M, et al. Methods to assess pericyte-endothelial cell interactions in a coculture model. Methods Mol Biol 2011; 686: 379–399. [DOI] [PubMed] [Google Scholar]
- 219.Thanabalasundaram G, Schneidewind J, Pieper C, et al. The impact of pericytes on the blood-brain barrier integrity depends critically on the pericyte differentiation stage. Int J Biochem Cell Biol 2011; 43: 1284–1293. [DOI] [PubMed] [Google Scholar]
- 220.von Wedel-Parlow M, Wolte P, Galla HJ. Regulation of major efflux transporters under inflammatory conditions at the blood-brain barrier in vitro. J Neurochem 2009; 111: 111–118. [DOI] [PubMed] [Google Scholar]
- 221.Zozulya A, Weidenfeller C, Galla HJ. Pericyte-endothelial cell interaction increases MMP-9 secretion at the blood-brain barrier in vitro. Brain Res 2008; 1189: 1–11. [DOI] [PubMed] [Google Scholar]
- 222.Huwyler J, Drewe J, Klusemann C, et al. Evidence for P-glycoprotein-modulated penetration of morphine-6-glucuronide into brain capillary endothelium. Br J Pharmacol 1996; 118: 1879–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Matthes F, Wolte P, Bockenhoff A, et al. Transport of arylsulfatase A across the blood-brain barrier in vitro. J Biol Chem 2011; 286: 17487–17494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wedel-Parlow Mv, Galla H. A microscopic in vitro study of neutrophil diapedesis across the blood- brain barrier, in Microscopy: Science, Technology, Applications and Education. In: Mendez-Vilas A, Díaz J. (eds). Microscopy: Science, Technology, Applications and Education, The Badajoz, Spain: Formatex, 2010, pp. 1161–1167. [Google Scholar]
- 225.Rempe R, Cramer S, Qiao R, et al. Strategies to overcome the barrier: use of nanoparticles as carriers and modulators of barrier properties. Cell Tissue Res 2014; 355: 717–726. [DOI] [PubMed] [Google Scholar]
- 226.Bornhorst J, Wehe CA, Huwel S, et al. T Impact of manganese on and transfer across blood-brain and blood-cerebrospinal fluid barrier in vitro. J Biol Chem 2012; 287: 17140–17151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Cramer S, Tacke S, Bornhorst J, et al. The influence of silver nanoparticles on the blood-brain and the blood-cerebrospinal fluid barrier in vitro. J Nanomed Nanotechnol 2014; 5: 225. [Google Scholar]
- 228.Lemmen J, Tozakidis IE, Bele P, et al. Constitutive androstane receptor upregulates Abcb1 and Abcg2 at the blood-brain barrier after CITCO activation. Brain Res 2013; 1501: 68–80. [DOI] [PubMed] [Google Scholar]
- 229.Lemmen J, Tozakidis IE, Galla HJ. Pregnane X receptor upregulates ABC-transporter Abcg2 and Abcb1 at the blood-brain barrier. Brain Res 2013; 1491: 1–13. [DOI] [PubMed] [Google Scholar]
- 230.Mulac D, Huwel S, Galla HJ, et al. Permeability of ergot alkaloids across the blood-brain barrier in vitro and influence on the barrier integrity. Mol Nutr Food Res 2012; 56: 475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wegener J, Sieber M, Galla HJ. Impedance analysis of epithelial and endothelial cell monolayers cultured on gold surfaces. J Biochem Biophys Methods 1996; 32: 151–170. [DOI] [PubMed] [Google Scholar]
- 232.Benson K, Cramer S, Galla HJ. Impedance-based cell monitoring: barrier properties and beyond. Fluids Barriers CNS 2013; 10: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Teow HM, Zhou Z, Najlah M, et al. Delivery of paclitaxel across cellular barriers using a dendrimer-based nanocarrier. Int J Pharm 2013; 441: 701–711. [DOI] [PubMed] [Google Scholar]
- 234.Mahringer A, Delzer J, Fricker G. A fluorescence-based in vitro assay for drug interactions with breast cancer resistance protein (BCRP, ABCG2). Eur J Pharm Biopharm 2009; 72: 605–613. [DOI] [PubMed] [Google Scholar]
- 235.Ott M, Fricker G, Bauer B. Pregnane X receptor (PXR) regulates P-glycoprotein at the blood-brain barrier: functional similarities between pig and human PXR. J Pharmacol Exp Ther 2009; 329: 141–149. [DOI] [PubMed] [Google Scholar]
- 236.Gutmann H, Torok M, Fricker G, et al. Modulation of multidrug resistance protein expression in porcine brain capillary endothelial cells in vitro. Drug Metab Dispos 1999; 27: 937–941. [PubMed] [Google Scholar]
- 237.Sauer SW, Opp S, Mahringer A, et al. Glutaric aciduria type I and methylmalonic aciduria: simulation of cerebral import and export of accumulating neurotoxic dicarboxylic acids in in vitro models of the blood-brain barrier and the choroid plexus. Biochim Biophys Acta 2010; 1802: 552–560. [DOI] [PubMed] [Google Scholar]
- 238.van Gelder W, Huijskes-Heins MI, van Dijk JP, et al. Quantification of different transferrin receptor pools in primary cultures of porcine blood-brain barrier endothelial cells. J Neurochem 1995; 64: 2708–2715. [DOI] [PubMed] [Google Scholar]
- 239.Qiao R, Jia Q, Huwel S, et al. Receptor-mediated delivery of magnetic nanoparticles across the blood-brain barrier. ACS Nano 2012; 6: 3304–3310. [DOI] [PubMed] [Google Scholar]
- 240.Hulsermann U, Hoffmann MM, Massing U, et al. Uptake of apolipoprotein E fragment coupled liposomes by cultured brain microvessel endothelial cells and intact brain capillaries. J Drug Target 2009; 17: 610–618. [DOI] [PubMed] [Google Scholar]
- 241.Dickens D, Yusof SR, Abbott NJ, et al. A multi-system approach assessing the interaction of anticonvulsants with P-gp. PLoS One 2013; 8: e64854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Urich E, Lazic SE, Molnos J, et al. Transcriptional profiling of human brain endothelial cells reveals key properties crucial for predictive in vitro blood-brain barrier models. PLoS One 2012; 7: e38149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Cucullo L, Couraud PO, Weksler B, et al. Immortalized human brain endothelial cells and flow-based vascular modeling: a marriage of convenience for rational neurovascular studies. J Cereb Blood Flow Metab 2008; 28: 312–328. [DOI] [PubMed] [Google Scholar]
- 244.Weksler B, Romero IA, Couraud PO. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 2013; 10: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Artus C, Glacial F, Ganeshamoorthy K, et al. The Wnt/planar cell polarity signaling pathway contributes to the integrity of tight junctions in brain endothelial cells. J Cereb Blood Flow Metab 2014; 34: 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Coureuil M, Mikaty G, Miller F, et al. Meningococcal type IV pili recruit the polarity complex to cross the brain endothelium. Science 2009; 325: 83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Forster C, Burek M, Romero IA, et al. Differential effects of hydrocortisone and TNFalpha on tight junction proteins in an in vitro model of the human blood-brain barrier. J Physiol 2008; 586: 1937–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Schrade A, Sade H, Couraud PO, et al. Expression and localization of claudins-3 and -12 in transformed human brain endothelium. Fluids Barriers CNS 2012; 9: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Vu K, Weksler B, Romero I, et al. Immortalized human brain endothelial cell line HCMEC/D3 as a model of the blood-brain barrier facilitates in vitro studies of central nervous system infection by Cryptococcus neoformans. Eukaryot Cell 2009; 8: 1803–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Schreibelt G, Kooij G, Reijerkerk A, et al. Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J 2007; 21: 3666–3676. [DOI] [PubMed] [Google Scholar]
- 251.Tai LM, Holloway KA, Male DK, et al. Amyloid-beta-induced occludin down-regulation and increased permeability in human brain endothelial cells is mediated by MAPK activation. J Cell Mol Med 2010; 14: 1101–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Ohtsuki S, Ikeda C, Uchida Y, et al. Quantitative targeted absolute proteomic analysis of transporters, receptors and junction proteins for validation of human cerebral microvascular endothelial cell line hCMEC/D3 as a human blood-brain barrier model. Mol Pharm 2013; 10: 289–296. [DOI] [PubMed] [Google Scholar]
- 253.Poller B, Gutmann H, Krahenbuhl S, et al. The human brain endothelial cell line hCMEC/D3 as a human blood-brain barrier model for drug transport studies. J Neurochem 2008; 107: 1358–1368. [DOI] [PubMed] [Google Scholar]
- 254.Carl SM, Lindley DJ, Das D, et al. ABC and SLC transporter expression and proton oligopeptide transporter (POT) mediated permeation across the human blood–brain barrier cell line, hCMEC/D3 [corrected]. Mol Pharm 2010; 7: 1057–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Eigenmann DE, Xue G, Kim KS, et al. Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood-brain barrier model for drug permeability studies. Fluids Barriers CNS 2013; 10: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Lippmann ES, Al-Ahmad A, Azarin SM, et al. A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Sci Rep 2014; 4: 4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Griep LM, Wolbers F, de Wagenaar B, et al. BBB on chip: microfluidic platform to mechanically and biochemically modulate blood-brain barrier function. Biomed Microdevices 2013; 15: 145–150. [DOI] [PubMed] [Google Scholar]
- 258.Gromnicova R, Davies HA, Sreekanthreddy P, et al. Glucose-coated gold nanoparticles transfer across human brain endothelium and enter astrocytes in vitro. PLoS One 2013; 8: e81043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Lopez-Ramirez MA, Male DK, Wang C, et al. Cytokine-induced changes in the gene expression profile of a human cerebral microvascular endothelial cell-line, hCMEC/D3. Fluids Barriers CNS 2013; 10: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Dickens D, Webb SD, Antonyuk S, et al. Transport of gabapentin by LAT1 (SLC7A5). Biochem Pharmacol 2013; 85: 1672–1683. [DOI] [PubMed] [Google Scholar]
- 261.Dauchy S, Miller F, Couraud PO, et al. Expression and transcriptional regulation of ABC transporters and cytochromes P450 in hCMEC/D3 human cerebral microvascular endothelial cells. Biochem Pharmacol 2009; 77: 897–909. [DOI] [PubMed] [Google Scholar]
- 262.Poller B, Drewe J, Krahenbuhl S, et al. Regulation of BCRP (ABCG2) and P-glycoprotein (ABCB1) by cytokines in a model of the human blood-brain barrier. Cell Mol Neurobiol 2010; 30: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Durk MR, Chan GN, Campos CR, et al. 1alpha,25-Dihydroxyvitamin D3-liganded vitamin D receptor increases expression and transport activity of P-glycoprotein in isolated rat brain capillaries and human and rat brain microvessel endothelial cells. J Neurochem 2012; 123: 944–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Tai LM, Reddy PS, Lopez-Ramirez MA, et al. Polarized P-glycoprotein expression by the immortalised human brain endothelial cell line, hCMEC/D3, restricts apical-to-basolateral permeability to rhodamine 123. Brain Res 2009; 1292: 14–24. [DOI] [PubMed] [Google Scholar]
- 265.Bernard SC, Simpson N, Join-Lambert O, et al. Pathogenic Neisseria meningitidis utilizes CD147 for vascular colonization. Nat Med 2014; 20: 725–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998; 282: 1145–1147. [DOI] [PubMed] [Google Scholar]
- 267.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131: 861–872. [DOI] [PubMed] [Google Scholar]
- 268.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126: 663–676. [DOI] [PubMed] [Google Scholar]
- 269.Megard I, Garrigues A, Orlowski S, et al. A co-culture-based model of human blood-brain barrier: application to active transport of indinavir and in vivo-in vitro correlation. Brain Res 2002; 927: 153–167. [DOI] [PubMed] [Google Scholar]
- 270.Daneman R, Agalliu D, Zhou L, et al. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A 2009; 106: 641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Stenman JM, Rajagopal J, Carroll TJ, et al. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science 2008; 322: 1247–1250. [DOI] [PubMed] [Google Scholar]
- 272.Mizee MR, Nijland PG, van der Pol SM, et al. Astrocyte-derived retinoic acid: a novel regulator of blood-brain barrier function in multiple sclerosis. Acta Neuropathol 2014; 128: 691–703. [DOI] [PubMed] [Google Scholar]
- 273.Mizee MR, Wooldrik D, Lakeman KA, et al. Retinoic acid induces blood-brain barrier development. J Neurosci 2013; 33: 1660–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Tiscornia G, Vivas EL, Izpisua Belmonte JC. Diseases in a dish: modeling human genetic disorders using induced pluripotent cells. Nat Med 2011; 17: 1570–1576. [DOI] [PubMed] [Google Scholar]
- 275.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007; 318: 1917–1920. [DOI] [PubMed] [Google Scholar]
- 276.Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science 2009; 324: 797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Cesar-Razquin A, Snijder B, Frappier-Brinton T, et al. A call for systematic research on solute carriers. Cell 2015; 162: 478–487. [DOI] [PubMed] [Google Scholar]
- 278.del Amo EM, Urtti A, Yliperttula M. Pharmacokinetic role of L-type amino acid transporters LAT1 and LAT2. Eur J Pharm Sci 2008; 35: 161–174. [DOI] [PubMed] [Google Scholar]
- 279.Knowland D, Arac A, Sekiguchi KJ, et al. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron 2014; 82: 603–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Deeken JF, Loscher W. The blood-brain barrier and cancer: transporters, treatment, and trojan horses. Clin Cancer Res 2007; 13: 1663–1674. [DOI] [PubMed] [Google Scholar]
- 281.Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer's disease. Acta Neuropathol 2009; 118: 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Minagar A, Alexander JS. Blood-brain barrier disruption in multiple sclerosis. Mult Scler 2003; 9: 540–549. [DOI] [PubMed] [Google Scholar]
- 283.Daneman R. The blood-brain barrier in health and disease. Ann Neurol 2012; 72: 648–672. [DOI] [PubMed] [Google Scholar]
- 284.Haseloff RF, Krause E, Bigl M, et al. Differential protein expression in brain capillary endothelial cells induced by hypoxia and posthypoxic reoxygenation. Proteomics 2006; 6: 1803–1809. [DOI] [PubMed] [Google Scholar]
- 285.Kondo T, Kinouchi H, Kawase M, et al. Astroglial cells inhibit the increasing permeability of brain endothelial cell monolayer following hypoxia/reoxygenation. Neurosci Lett 1996; 208: 101–104. [DOI] [PubMed] [Google Scholar]
- 286.Lochhead JJ, McCaffrey G, Quigley CE, et al. Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J Cereb Blood Flow Metab 2010; 30: 1625–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Yang L, Shah KK, Abbruscato TJ. An in vitro model of ischemic stroke. Methods Mol Biol 2012; 814: 451–466. [DOI] [PubMed] [Google Scholar]
- 288.Liu LB, Xue YX, Liu YH, et al. Bradykinin increases blood-tumor barrier permeability by down-regulating the expression levels of ZO-1, occludin, and claudin-5 and rearranging actin cytoskeleton. J Neurosci Res 2008; 86: 1153–1168. [DOI] [PubMed] [Google Scholar]
- 289.Choi YP, Lee JH, Gao MQ, et al. Cancer-associated fibroblast promote transmigration through endothelial brain cells in three-dimensional in vitro models. Int J Cancer 2014; 135: 2024–2033. [DOI] [PubMed] [Google Scholar]
- 290.Fazakas C, Wilhelm I, Nagyoszi P, et al. Transmigration of melanoma cells through the blood-brain barrier: role of endothelial tight junctions and melanoma-released serine proteases. PLoS One 2011; 6: e20758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Lee KY, Kim YJ, Yoo H, et al. Human brain endothelial cell-derived COX-2 facilitates extravasation of breast cancer cells across the blood-brain barrier. Anticancer Res 2011; 31: 4307–4313. [PubMed] [Google Scholar]
- 292.Liu Y, Liu YS, Wu PF, et al. Brain microvascular endothelium induced-annexin A1 secretion contributes to small cell lung cancer brain metastasis. Int J Biochem Cell Biol 2015; 66: 11–19. [DOI] [PubMed] [Google Scholar]
- 293.Rodriguez PL, Jiang S, Fu Y, et al. The proinflammatory peptide substance P promotes blood-brain barrier breaching by breast cancer cells through changes in microvascular endothelial cell tight junctions. Int J Cancer 2014; 134: 1034–1044. [DOI] [PubMed] [Google Scholar]
- 294.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci 2011; 12: 723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Jeynes B, Provias J. The case for blood-brain barrier dysfunction in the pathogenesis of Alzheimer's disease. J Neurosci Res 2011; 89: 22–28. [DOI] [PubMed] [Google Scholar]
- 296.Gosselet F, Saint-Pol J, Candela P, et al. Amyloid-beta peptides, Alzheimer's disease and the blood-brain barrier. Curr Alzheimer Res 2013; 10: 1015–1033. [DOI] [PubMed] [Google Scholar]
- 297.Pflanzner T, Kuhlmann CR, Pietrzik CU. Blood-brain-barrier models for the investigation of transporter- and receptor-mediated amyloid-beta clearance in Alzheimer's disease. Curr Alzheimer Res 2010; 7: 578–590. [DOI] [PubMed] [Google Scholar]
- 298.Mysiorek C, Culot M, Dehouck L, et al. Peroxisome-proliferator-activated receptor-alpha activation protects brain capillary endothelial cells from oxygen-glucose deprivation-induced hyperpermeability in the blood-brain barrier. Curr Neurovasc Res 2009; 6: 181–193. [DOI] [PubMed] [Google Scholar]
- 299.Marchi N, Hallene KL, Kight KM, et al. Significance of MDR1 and multiple drug resistance in refractory human epileptic brain. BMC Med 2004; 2: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]