Abstract
Purpose
To determine serum vascular endothelial growth factor 165 (VEGF165) levels and the association of the complement factor H gene (CFH) Y402H polymorphism in patients with exudative age-related macular degeneration (AMD) in comparison to unaffected control subjects.
Methods
Sixty-six AMD patients and 66 healthy age- and gender-matched controls were included in this case-control study. The serum VEGF165 was assayed by ELISA (R&D). Genotypes were determined by polymerase chain reaction-restriction fragment length polymorphism analysis. Chisquared tests were used regarding the polymorphism, a t-test regarding the VEGF-levels.
Results
Levels of serum VEGF165 were similar in both groups (p-value = 0.2112). Genotype frequency differed significantly between patients with exudative AMD and the healthy control group (p = 0.003136). The serum VEGF165 levels were similar irrespective of the presence of the CFH Y402H polymorphism (p = 0.4113) and independent of the specific genotype (p = 0.9634).
Conclusion
In the present study exudative AMD is not associated to serum VEGF165 levels; furthermore, our data does not establish a statistical link between VEGF165 and the CFH Y402H polymorphism.
Keywords: Age-related macular degeneration, Complement, Genetics, Neovascular, Vascular endothelial growth factor
Introduction
Age-related macula degeneration (AMD) is a progressive degenerative disorder in the central region of the retina. It is the leading cause of legal blindness in people aged over 55 years in developed countries. Among those patients with severe visual impairment, nearly 90% are caused by exudative AMD, which is a subtype of late-stage AMD found in approximately 20% of all AMD cases.1
Choroidal neovascularization (CNV) is the hallmark of exudative AMD.2 Neovascular stimulus is initiated by increased secretion of vascular endothelial growth factor (VEGF) from retinal pigment epithelium (RPE) cells, preceeded by different inflammatory stimulus including cytokines and other proinflammatory molecules, which in turn causes migration and proliferation of choroidal endothelial cells.3
Vascular endothelial growth factor 165 (VEGF165) appears to be the isoform of VEGF responsible for pathological ocular neovascularization.4-6 VEGF expression is upregulated by a number of growth factors, including epidermal growth factor, transforming growth factor-α and -ß, keratinocyte growth factor, insulin-like growth factor-I, FGF, and platelet-derived growth factor. Thus, a variety of local interactions can modulate local VEGF concentrations.7
Complement components C3a and C5a also induce VEGF expression, which could induce choroidal neovascularization.8 Complement factor H normally inhibits C3 deposition and C5a release after complement activation,9 dysfunctional CFH induces increased production of C3a and C5a.
This study was conducted to determine the difference in levels of serum VEGF165 between patients with exudative AMD and healthy controls. Additionally, we determined the frequency of the CFH Y402H polymorphism in patients with age-related macular degeneration in the Austrian population of Caucasian descent.
Methods
Study Design: Case-Control Study
One hundred thirty-two consecutive patients seen in our department of ophthalmology were included in this study: these included 66 unrelated patients with exudative AMD and 66 control subjects, mostly patients registering for cataract surgery. All patients were aged 55 or older, were of Caucasian origin, and lived in the same geographical area of Austria.
Written informed consent was obtained prior to enrollment. The study was performed in accordance with the Austrian Gene Technology Act, the tenets of the Declaration of Helsinki, and the guidelines of the local ethics committee.
Exudative AMD was diagnosed by ophthalmoscopic fundus examination, optical coherence tomography, and fluorescein/indocyanine angiography. Patients with hereditary diseases, polypoidal choroidal vasculopathy or secondary CNV due to pathologic myopia (>-2 diopters, spherical equivalent), angioid streaks, inflammatory or infectious chorioretinal disease, trauma, or diabetic retinopathy were excluded from the study. AMD patients with previous performed anti-VEGF therapy have been excluded.
All AMD patients enrolled in the present study were classified according to the Age-Related Eye Disease Study system; subgroups were noted (predominantly classic choroidal neovascularization, minimal classic choroidal neovascularization, occult choroidal neovascularization, and retinal angiomatous proliferation) according to the angiographic findings.
Exclusion criteria for the age- and sex-matched controls were evidence of age-related maculopathy (drusen as well as pigmentary changes), macular hemorrhages of any cause. Media opacities resulting in impaired visualization of the macula (cataract grade 3 and 4) was an exclusion criteria for both groups.
Serum VEGF165 Levels
Peripheral venous blood samples were collected according to standard hospital procedure. Red topped tubes were centrifuged at 3000 r/min for 10 min at 4°C and the serum separated and stored at -70°C.
The serum VEGF165 level was determined using a quantitative sandwich enzyme immunoassay technique according to the manufacturer's guidelines (R&D Systems, Inc., Minneapolis, Minnesoa, USA).
A quantitative sandwich enzyme immunoassay technique was used for the quantitative determination of human vascular endothelial growth factor (VEGF165) concentrations according to manufacturer's guidelines. A monoclonal antibody specific for VEGF165 was precoated onto a microplate. Standards and samples were pipetted into the wells and any VEGF165 present was bound by the immobilized antibody. After washing away any unbound substances, an enzyme-linked polyclonal antibody specific for VEGF165 was added to the wells. Following a wash to remove any unbound antibody-enzyme reagent, a substrate solution was added to the wells and color developed in proportion to the amount of VEGF165 bound in the initial step. The color development was stopped and the intensity of the color measured. The recombinant human VEGF standards were measured to form a standard curve. Results were expressed in ng/L.
Genotyping
Genotyping was carried out in a random order by an experienced technician who was masked to the disease status of the samples. An aliquot of 5 mL venous blood from each subject was withdrawn and collected in an EDTA-containing tube. Genomic DNA was isolated from whole blood using a commercial kit (QIA-AMP DNA blood mini kit, Qiagen, Vienna, Austria. The single-nucleotide polymorphisms (Y402H; rs 1061170) located in exon 9 of CFH was genotyped by PCR-directed sequencing using the following primer sequences: 5′-CTT TAG TTC GTC TTC AGT TAT AC-3′ (forward) and 5′-GTC ATC TAT GTT ACT TAG AAA GT-3′ (reverse). PCR products were then purified and sequenced by Single-Read Sequencing reaction (VBC-Biotech, Vienna, Austria).
Statistics
A sample size calculation was performed using an alpha error of 0.05 (5%), a beta error of 0.20 (20%), and a clinical significant difference of 0.18 (18%) to determine the power calculation.
To find differences in groups depending on the polymorphism, Chi-squared tests were observed. If the premises were not fulfilled, Fisher's exact tests were performed.
To find out the height of a VEGF-level depending on AMD or healthy controls, a t-test was computed. Negative values of the titer were replaced by zero, because these are not possible and may occur by incorrect measurements of the instruments used.
Analysis was performed using R 2.4.0 and SAS 9.1. All p-values smaller than 0.01667 were considered to be statistically significant. The critical boundary of 0.0125 results from correction for multiplicity according to Bonferroni due to the number of tests (three tests were performed, 0.05/3 = 0.01667).
Results
In the exudative AMD group, 66 patients were enrolled in this study. Subgroups were noted and classified according to angiographic findings consisting of 16 patients with predominantly classic CNV, 11 patients with minimal classic CNV, 31 patients with occult CNV, and 8 patients with retinal angiomatous proliferation. Sixty-sex healthy controls were age- and gender-matched. The AMD group consisted of 35 (53.0%) females and 31 (47.0%) males, and the mean age was 78.0 ± 7.1 years (range: 56–92 years). The control group consisted of 34 (51.6%) females and 32 (48.4%) males, and the mean age was 76.5 ± 6.5 years (range: 62–94 years).
Regarding the VEGF165 levels, there was no statistically significant difference between the exudative AMD group and the control group (p = 0.2112) (Figure 1). Serum VEGF levels according to the angiographic type of CNV are shown in Table 1.
Figure 1.
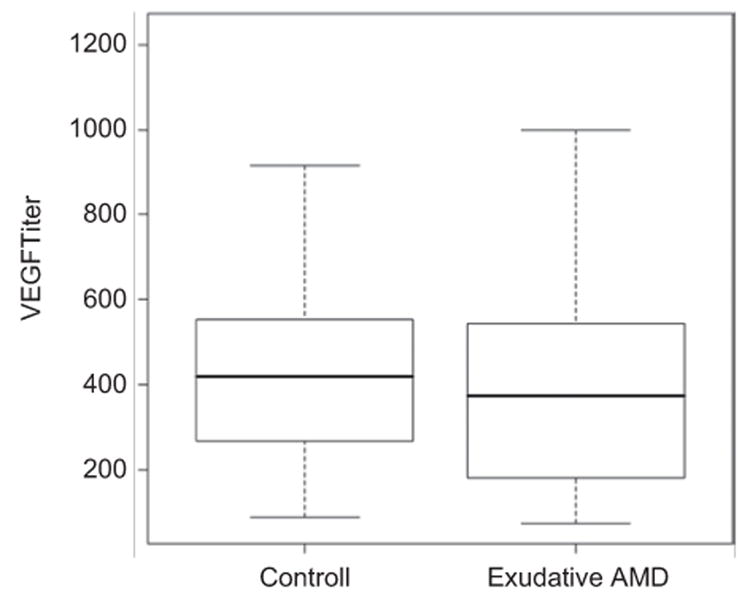
Mean (with standard deviation) VEGF levels (y-axis) in exudative AMD patients compared to the control group (x-axis).
Table 1.
Serum VEGF165 level according to AMD subtypes.
| Exudative AMD subtypes | n | Serum VEGF165 level (ng/L) | |||
|---|---|---|---|---|---|
|
| |||||
| Mean (ng/L) | Min | Max | SD | ||
| Predominantly classic CNV | 16 | 531.89575 | 155.182 | 999.618 | 269.8 |
| Minimally classic CNV | 11 | 324.537273 | 73.828 | 674.201 | 172.4 |
| Occult CNV | 31 | 375.936767 | 71.768 | 1123.194 | 239.4 |
| RAP | 8 | 289.307875 | 98.543 | 613.443 | 177.1 |
CNV = choroidal neovascularisation; RAP = retinal angiomatous proliferation; SD = standard deviation.
Genotype frequency differed significantly between the patients with exudative AMD and the control group (p = 0.003136), (1277TT 21.2%, 1277TC 53.0%, and 1277CC 25.8% in the AMD group; 1277TT 54.5%, 1277TC 43.9%, and 1277CC 15.2% in the control group) (Figure 2).
Figure 2.
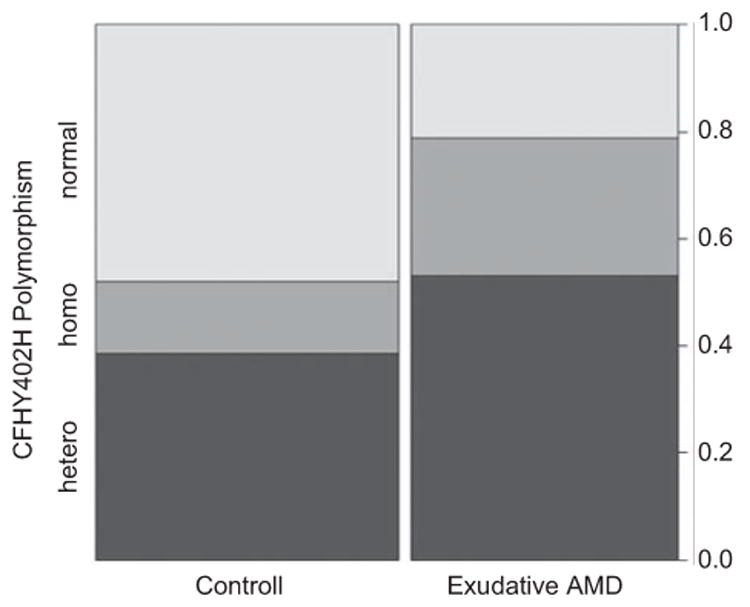
Genotype distribution (y-axis) of the CFH Y402H polymorphism in the exudative AMD patients compared to the control group (x-axis) in percentages.
Genotype distribution according to the angiographic type of CNV is shown in Table 2.
Table 2.
CFH genotype distribution of the complement factor H polymorphism Y402H in different subtypes of exudative AMD.
| Exudative AMD | CFH-polymorphism | ||
|---|---|---|---|
|
| |||
| Normal n (%) | Hetero n (%) | Homo n (%) | |
| Predominately classic CNV | 2 (12.5%YY) | 10 (62.5%YH) | 4 (25.0%HH) |
| Minimally classic CNV | 3 (27.3%YY) | 7 (63.3%YH) | 1 (9.0%HH) |
| Occult CNV | 6 (19.4%YY) | 13 (41.9%YH) | 12 (38.7%HH) |
| RAP | 3 (37.5%YY) | 5 (62.5%YH) | 0 (0%HH) |
CNV = choroidal neovascularisation; RAP = retinal angiomatous proliferation.
The serum VEGF165 levels were similarly irrespective of the presence of the CFH Y402H polymorphism (p = 0.4113) and independent of the specific genotype (p = 0.9634); (Tables 3 and 4).
Table 3.
VEGF165 level according to positive or negative complement factor H polymorphism Y402H.
| Polymorphism | n = 66 | VEGF165 Level (ng/L) | |||
|---|---|---|---|---|---|
|
| |||||
| Mean (ng/L) | Min | Max | SD | ||
| Yes | 52 | 387.401904 | 71.768 | 1123.194 | 242.1 |
| No | 14 | 414.320571 | 88.245 | 825.582 | 239.3 |
SD = Standard Deviation.
Table 4.
VEGF165 level according to genotype distribution of the CFH polymorphism Y402 in patients with exudative AMD.
| Genotype | n = 66 | VEGF165 level (ng/L) | |||
|---|---|---|---|---|---|
|
| |||||
| Mean (ng/L) | Min | Max | SD | ||
| YY (Normal) | 14 | 414.320571 | 88.245 | 825.582 | 239.3 |
| YH (Hetero) | 35 | 362.407229 | 73.828 | 999.618 | 228.3 |
| HH (Homo) | 17 | 438.861529 | 71.768 | 1123.194 | 267.9 |
SD = Standard Deviation.
Discussion
This study was conducted to determine whether differences in levels of serum VEGF165 exist between patients with exudative age-related macular degeneration and healthy controls. Second, the prevalence of the CFH Y402 polymorphism in patients with age-related macular degeneration as compared to a healthy control group in the Austrian population was investigated.
VEGF is a member of the platelet derived growth factor (PDGF) family and plays a central role in the angiogenesis and embryonic vasculogenesis. This growth factor increases microvascular permeability of post capillary venules and capillaries; it is able to stimulate all major functions of endothelial cells needed in angiogenesis—including increased permeability, migration, proliferation and tubeformation.10,11 VEGF messenger RNA has been demonstrated in almost all tissues of the normal eye, most notably in the ciliary body, conjuctiva, RPE/choroids, and the lens.
VEGF selectively stimulates endothelial cells by binding to two receptors, VEGFR-1 and VEGFR-2, which respond in typical fashion to ligand binding by activation of signal transduction cascades. VEGFR-2 is believed to be the principal receptor for VEGF signalling in angiogenesis.7
Alternative splicing of the VEGF gene results in at least four major biologically active human isoforms, containing 121, 165, 189, and 208 amino acids. The isoforms appear to have identical biological activity but differ in molecular weight and affinity to heparin. VEGF165, the predominant isoform in the human eye, is a secreted, heparin-binding, homodimeric, 45-kDa glycoprotein.10–13
VEGF165 is the isoform responsible for pathological ocular neovascularization, whereas VEGF121 appears to be essential for normal retinal vascular function (20–22). Elevated VEGF levels have been found in aquous humor and vitreous of most patients with proliferative diabetic retinopathy and ischemic central retinal vein occlusion, but only rarely in patients without ocular neovascularization.14–16 Immunohistochemical studies of autopsy eyes found VEGF to be elevated in the RPE and choroidal blood vessels of maculas with AMD.17 In subfoveal fibrovascular membranes, the expression of VEGF mRNA was higher in areas with marked inflammatory signs.18,19
In our study serum VEGF165 levels showed no statistically significant difference between the exudative AMD patients and healthy controls (p-value = 0.2112).
In contrast, one previous study showed that AMD patients with active CNV had a significantly higher plasma level of VEGF165 than normal controls.20 The study of Lip et al. showed only a weak association between plasma VEGF165 level and CNV in AMD patients.21
Our study revealed higher levels of serum VEGF165 in patients with predominantly classic CNV, as compared to levels in patients with minimal classic CNV, occult CNV, and RAP. This might be explained by the pathogenesis of the predominantly classic CNV and correlated to the progressive dye-leakage as seen in the early phase of the fluorescein angiogram.
VEGF expression is upregulated by a number of growth factors, including epidermal growth factor, transforming growth factor-α and -ß, keratinocyte growth factor, insulin-like growth factor-I, FGF, and platelet-derived growth factor, so that a variety of local interactions can modulate local VEGF concentrations.7
The route of new vessel growth has been suggested to be in areas of lesser resistance, for example defects in Bruch membrane and RPE irregularities.3
C3a and C5a induce VEGF expression and, as such, can stimulate choroidal neovascularization. The complement factor H inhibits C3 deposition and C5a release after complement activation. The impairment of this regulation in the alternative pathway of the complement activation leads to excessive liberation of different cleavage fragments, such as C3a and C5a, and to the formation of the C5b9 complex, which in turn are able to induce endothelial cell activation and release of growth factors, such VEGF.8,9
Several studies have confirmed an association of the complement factor H (CFH) Y402 gene polymorphism to the risk of AMD.12, 22–29 Our data confirms the higher frequency of the Y402 polymorphism of CFH in exudative AMD patients, as compared to healthy controls (p-value = 0.003136). Regarding the different subgroups of AMD, homozygosity and heterozygosity was found nearly equally in patients with occult CNV. Interestingly, homozcygosity was more prevalent in patients with occult CNV in comparison to those with RAP, predominantly classic CNV, and minimal classic CNV.
Serum VEGF165 levels showed similar results independent of the presence of the CFH polymorphism and independent of the specific genotype distribution in patients with exudative AMD.
In conclusion, our data shows that exudative AMD is not associated to seric VEGF165. These findings underline that AMD is a localized disease with no affect on the circulating serum VEGF165 levels.
Our data confirms that the CFH Y402H polymorphism is a risk factor for exudative age-related macular degeneration with a higher frequency of the Y402 polymorphism in AMD patients.
Acknowledgments
This study was funded through govermental funding—Med. wissenschaftlicher Fond des Buergermeisters der Stadt Wien—BGM 07068.
Footnotes
Declaration of interest: The authors report no conflicts of interest.
References
- 1.Ambatti J. Age-related macular degeneration: Etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48:257–293. doi: 10.1016/s0039-6257(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 2.Spaide RF, Armstrong D, Browne R. Continuing medical education review: choroidal neovascularisation in age-related macular degeneration-what is the cause? Retina. 2003;23:595–614. doi: 10.1097/00006982-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Gass JD. Biomicroscopic and histopathologic considerations regarding the feasibility of surgical excisions of subfoveal neovascular membranes. Am J Ophthalmol. 1994;118:285–298. [PubMed] [Google Scholar]
- 4.Ishida S, Usui T, Yamashiro K, et al. VEGF164-mediated inflammation is required for pathological, but not physiological, ischemia-induced retinal neovascularization. J Exp Med. 2003;198:483–489. doi: 10.1084/jem.20022027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McColm JR, Geisen P, Hartnett ME. VEGF isoforms and their expression after a single episode of hypoxia or repeated fluctuations between hyperoxia and hypoxia: Relevance to clinical ROP. Mol Vis. 2004;10:512–520. [PMC free article] [PubMed] [Google Scholar]
- 6.Yi X, Ogata N, Komada M, et al. Vascular endothelial growth factor expression in choroidal neovascularization in rats. Graefes Arch Clin Exp Ophthalmol. 1997;235:313–319. doi: 10.1007/BF01739641. (1997) [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 8.Nozaki M, Raisler BJ, Sakurai E, et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci U S A. 2006;103:2328–2333. doi: 10.1073/pnas.0408835103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bora PS, Sohn JH, Cruz JM, et al. Role of complement and complement membrane attack complex in laser-induced choroidal neovascularization. J Immunol. 2005;174:491–497. doi: 10.4049/jimmunol.174.1.491. [DOI] [PubMed] [Google Scholar]
- 10.Schlingenmann RO. Role of growth factors and the wound healing response in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2004;242:91–101. doi: 10.1007/s00417-003-0828-0. [DOI] [PubMed] [Google Scholar]
- 11.Schlingenmann RO, Van Hinsbergh V. Role of vascular permeability factor/vascular endothelial growth factor in eye diseases. Br J Ophthalmol. 1997;81:501–512. doi: 10.1136/bjo.81.6.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zareparsi S, Branham KE, Li M, et al. Strong association of the Y402H variant in complement factor H at 1q32 with susceptibility to age-related macular degeneration. Am J Hum Genet. 2005;77:149–153. doi: 10.1086/431426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park JE, Keller GA, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: Differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993;4:1317–1326. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 15.Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118:445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- 16.Frank RN, Amin RH, Eliott D, et al. Basic fibroblast growth factor and vascular endothelial growth factor are present in epiretinal and choroidal neovascular membranes. Am J Ophthalmol. 1996;122:393–403. doi: 10.1016/s0002-9394(14)72066-5. [DOI] [PubMed] [Google Scholar]
- 17.Kliffen M, Sharma HS, Mooy CM, et al. Increased expression of angiogenic growth factors in age related maculopathy. Br J Ophthalmol. 1997;81:54–162. doi: 10.1136/bjo.81.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kvanta A, Algvere PV, Berglin L, et al. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci. 1996;37:1929–1934. [PubMed] [Google Scholar]
- 19.Hattenbach LO, Falk B, Nürnberger F, et al. Detection of inducible nitric oxide synthase and vascular endothelial growth factor in choroidal neovascular membranes. Ophthalmologica. 2002;216:209–214. doi: 10.1159/000059634. [DOI] [PubMed] [Google Scholar]
- 20.Lip PK, Blann AD, Hope-Ross M, et al. Age-related macular degeneration is associated with increased vascular endothelial growth factor, hemorheology, and endothelial dysfunction. Ophthalmology. 2001;108:705–710. doi: 10.1016/s0161-6420(00)00663-1. [DOI] [PubMed] [Google Scholar]
- 21.Tsai DC, Charng MJ, Lee FL, et al. Different plasma levels of vascular endothelial growth factor and nitric oxide between patients with choroidal and retinal neovascularization. Ophthalmologica. 2006;220:246–251. doi: 10.1159/000093079. [DOI] [PubMed] [Google Scholar]
- 22.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards AO, Ritter R, 3rd, Abel KJ, et al. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 24.Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 25.Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magnusson KP, Duan S, Sigurdsson H, et al. CFH Y402H confers similar risk of soft drusen and both forms of advanced AMD. PLoS Med. 2006;3:e5. doi: 10.1371/journal.pmed.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sepp T, Khan JC, Thurlby DA, et al. Complement factor H variant Y402H is a major risk determinant for geographic atrophy and choroidal neovascularization in smokers and nonsmokers. Invest Ophthalmol Vis Sci. 2006;47:536–540. doi: 10.1167/iovs.05-1143. [DOI] [PubMed] [Google Scholar]
- 28.Wegscheider BJ, Weger M, Renner W, et al. Association of complement factor H Y402H gene polymorphism with different subtypes of exudative age-related macular degeneration. Ophthalmology. 2007;114:738–742. doi: 10.1016/j.ophtha.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 29.Steinbrugger I, Haas A, Renner W, et al. Role of the complement component 3 102Gly gene variant in a Central European population with exudative age-related macular degeneration. Spektrum Augenheilkd. 2009;23:353–357. [Google Scholar]


