Abstract
Turner syndrome (TS) is a chromosomal condition associated with partial or complete absence of the X chromosome that involves characteristic findings in multiple organ systems. In addition to well-known clinical characteristics such as short stature and gonadal failure, TS is also associated with T cell immune alterations and chronic otitis media, suggestive of a possible immune deficiency. Recently, ubiquitously transcribed tetratricopeptide repeat on the X chromosome (UTX), a histone H3 lysine 27 (H3K27) demethylase, has been identified as a downregulated gene in TS immune cells. Importantly, UTX is an X-linked gene that escapes X-chromosome inactivation and thus is haploinsufficient in TS. Mice with T cell-specific UTX deficiency have impaired clearance of chronic viral infection due to decreased frequencies of T follicular helper (Tfh) cells, which are critical for B cell antibody generation. In parallel, TS patients have decreased Tfh frequencies in peripheral blood. Together, these findings suggest that haploinsufficiency of the X-linked UTX gene in TS T cells underlies an immune deficit, which may manifest as increased predisposition to chronic otitis media.
Keywords: Turner syndrome, Epigenetics, UTX, X chromosome, T cell
Introduction
Turner syndrome (TS) is a common chromosomal abnormality, occurring in about 1:4000 live births, which describes phenotypic females with either partial or complete absence of one sex chromosome [1]. TS is named for Dr. Henry Turner who in 1938 described seven women with short stature, sexual immaturity, cubitus valgus, webbed neck, and low posterior hairline [2]. In the mid 1950s, advances in cytogenetic identification allowed the discovery that patients with TS have one normal X chromosome and a missing or structurally altered sex chromosome [3]. In 1965, after analyzing hundreds of karyotypes of patients with various forms of gonadal dysgenesis, Dr. Malcolm Ferguson-Smith proposed that short stature and other TS clinical findings were due to gene deletions from the missing short arm of the X chromosome [4]. This hypothesis was largely validated in subsequent years [5].
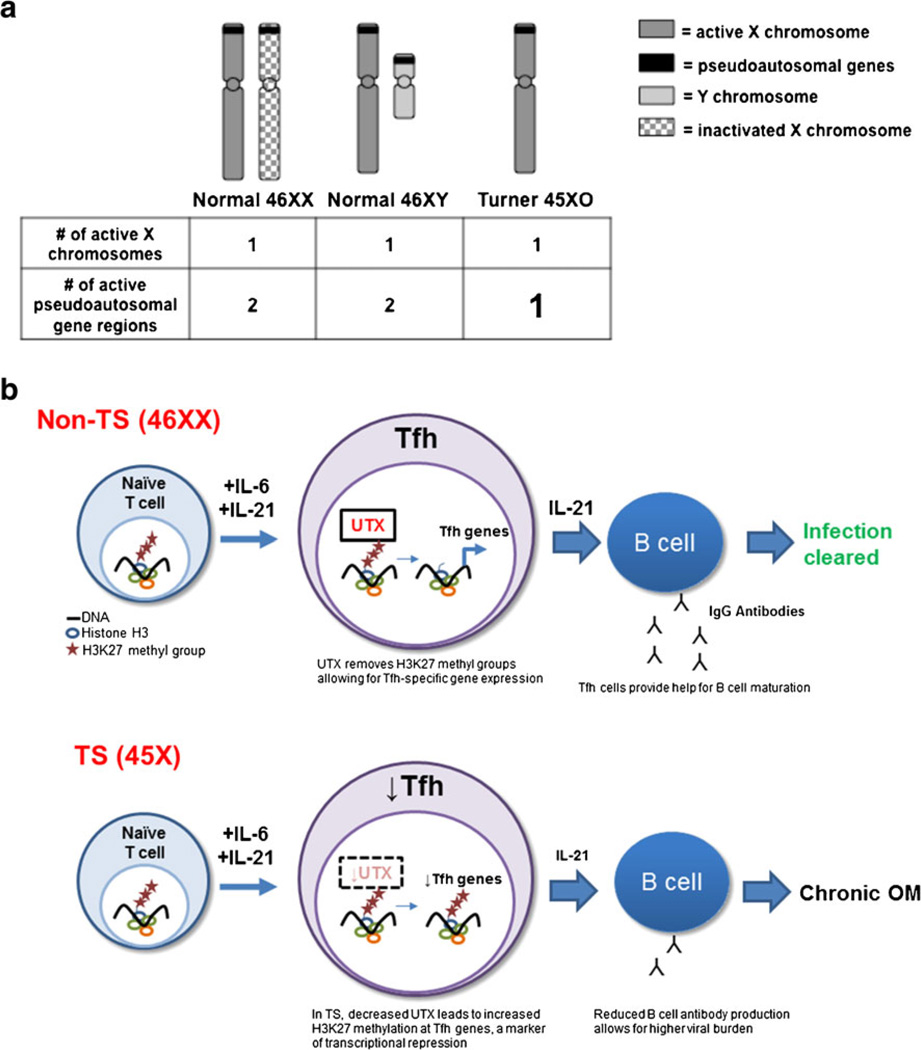
In non-Turner 46 XX females, one copy of the X chromosomes is inactivated to achieve some degree of balanced gene expression between males and females. Thus, absence of one sex chromosome, on the surface, would not be predicted to have any effect. However, X inactivation is incomplete; 15 % of the genes on the silenced X chromosome escape inactivation and are expressed from both chromosomes [6]. Most genes escaping X inactivation are pseudoautosomal genes located on the short arm of the X chromosome and have homologous genes on the Y chromosome. Therefore, most abnormalities seen in TS are thought to be due to haploinsufficiency of genes that are normally expressed by both X chromosomes (Fig. 1a) [1, 6].
Fig. 1.
a TS patients are haploinsufficient in pseudoautosomal genes including UTX. Because pseudoautosomal genes escape inactivation, they are expressed from two copies of the X chromosomes in 46XX females. They are also expressed on the Y chromosome, so they are expressed from two copies in 46XY males. In 45XO TS females, however, they are only expressed from one X chromosome, so they may be haploinsufficient in TS. b Model: UTX deficiency in TS patients predisposes to chronic viral infection due to impaired Tfh differentiation. In 46XX females haplosufficient for UTX, Tfh cells differentiate from naïve CD4+ T cells through UTX-specific H3K27 demethylase activity. H3K27 demethylation results in increased expression of Tfh-specific genes and adequate Tfh help for B cell maturation, anti-viral antibody production, and virus clearance. However, in TS patients and in UTX-deficient mice, UTX haploinsufficiency results in decreased circulating Tfh cells due to H3K27methylation and transcriptional suppression of Tfh-specific genes. Decreased Tfh cells in turn reduces B cell antibody production and prolongs chronic infection
Strong evidence for the concept that pseudoautosomal genes underlie TS findings came from the identification of short stature homeobox-containing gene (SHOX) as the underlying cause of short stature in TS. SHOX is a pseudoautosomal gene that escapes X inactivation and is highly expressed in osteogenic tissue [7, 8]. Mutations in SHOX cause familial short stature in a dominant fashion, suggesting that a quantitative decrease in the SHOX gene product is sufficient to decrease linear growth [9, 10]. Thus, SHOX haploinsufficiency in TS is a major contributor to growth failure in TS [8, 11]. Together, this evidence suggests that the absence of the genetic material from the missing sex chromosome results in TS clinical findings. However, other pseudoautosomal X-linked genes that contribute to the TS phenotype remain to be identified.
A deeper understanding of the genetic underpinning of TS and associated clinical features are needed for the development of better prevention, treatment, and anticipatory guidance strategies for TS patients and their families. An area in need of study is the potential alterations in the TS immune system. This review will discuss the current literature regarding TS and associated immune abnormalities and will highlight the role of a pseudoautosomal gene UTX recently discovered to affect CD4+ T cell differentiation and immune defenses against chronic viral infection.
Turner Syndrome May Be Associated with Immune Abnormalities
TS patients are predisposed to a wide array of clinical findings, including frequent otitis media (OM) (Table 1). The pathogenic mechanism underlying this susceptibility to OM is unclear. One hypothesis is that craniofacial features of TS predispose to chronic OM [12]. Another hypothesis that is not mutually exclusive is that an underlying immune deficiency may be a component of TS and contribute to chronic OM. Consistent with this latter possibility, TS patients have been reported to have immune alterations in T cell and immunoglobulin subsets. These include decreased levels of circulation T and B lymphocytes, reduced levels of serum IgG and IgM, and increased IgA [13–15]. However, other studies did not find major immunological deficiencies in TS subjects [16, 17, 18•]. Thus, more research focused on immune alterations in TS subjects is needed to resolve these discrepancies. A confounder for these analyses is that frequent infections, such as in TS, may cause elevated immune activation compared to non-TS controls. Thus, a careful experimental design which take such confounders into consideration is essential.
Table 1.
Characteristics and comorbidities in Turner syndrome
| Characteristic | Prevalence (%) |
|---|---|
| Growth abnormalities | 90 |
| – Short stature | |
| Reproductive abnormalities | 90 |
| – Ovarian failure | |
| Dermatologic abnormalities | 90 |
| – Multiple pigmented nevi | |
| – Edema of the extremities | |
| – Vitiligo | |
| – Alopecia | |
| Neck abnormalities | 70 |
| – Webbed neck | |
| – Low posterior hairline | |
| Chest abnormalities | 70 |
| – Shield chest | |
| – Wide spaced nipples | |
| Otologic abnormalities | 50 |
| – Otitis media | |
| – Hearing loss | |
| – Low set ears | |
| Renal abnormalities | 50 |
| – Horseshoe kidney | |
| – Renal agenesis | |
| Cardiovascular abnormalities | 50 |
| – Coartaction of the aorta | |
| – Hypertension | |
| – Bicuspid aortic valve | |
| Skeletal abnormalities | 50 |
| – Short 4th metacarpals | |
| – Madelung deformities | |
| – Cubitus valgus | |
| Endocrine abnormalities | 50 |
| – Autoimmune hypothyroidism | |
| – Carbohydrate intolerance | |
| Gastrointestinal abnormalities | 30 |
| – Fatty liver disease | |
| – Celiac disease |
Clinical characteristics of TS patients and their prevalence. Potential immune-mediated findings are highlighted in italics. Characteristics secondary to lymphatic obstruction are in underline. Reference: [44]
TS Patient Immune Cells Express Decreased UTX, a Histone-Modifying Enzyme
To determine whether gene expression in immune cells were altered in TS and also to identify potential pseudoautosomal X-linked genes that contribute to immune alterations, Cook et al. performed a microarray analysis on peripheral blood mononuclear cells (PBMCs) comparing gene expression in control females to TS subjects with confirmed 45X karyotype [19••]. A total of 1169 unique genes showed differential expression in TS PBMCs, including 35 on the X chromosome. In particular, ubiquitously transcribed tetratricopeptide repeat on chromosome X (UTX, encoded by Utx or Kdm6a located at Xp11.3 [20]) was among the top 10 X-linked genes with the largest decrease in expression and the only gene among these candidates that escapes X inactivation [21].
UTX is part of the Jumonji D3 (Jmjd3) family of histone H3 lysine 27 (H3K27) demethylases that epigenetically regulates gene expression (Fig. 1b) [22]. Epigenetic regulation refers to heritable changes in gene expression that do not involve changes in the DNA sequence. Lineage specification is maintained by epigenetic changes that are retained in cells and daughter cells after cell division. For example, chromatin modifications alter the accessibility of genes to transcription factors and RNA polymerases, thus directly impacting gene expression [23]. Chromatin, the condensed packaging of DNA within eukaryotic cells, consists of nucleosomes that contain 146 base pairs of DNA tightly wound around histones. These histones contain amino acid residues exposed around the nucleosome core, or “tails,” that can be biochemically modified at sites of gene promoters and enhancers. These histone modifications are associated with gene expression or gene silencing. Histone H3 lysine 27 trimethylation (H3K27me3) is a transcriptionally repressive modification typically found in heterochromatin or transcriptionally silenced loci. UTX, as a H3K27 demethylase, removes trimethylated and dimethylated groups at H3K27 residues, thus increasing gene expression. UTX is ubiquitously expressed and plays a major role in several cell processes, such as embryonic development [24, 25], cell cycle regulation [26], hematopoiesis [27], and cancer pathogenesis [28, 29]. However, the role of UTX in immune cells was largely unknown.
CD4+ T cells have also been described to undergo epigenetic modifications during T cell differentiation into T helper subsets (e.g., Th1, Th2, Th17, Treg, Tfh) to ensure a heritable gene expression program specific to each subset [23]. A genome-wide study of H3K27 methylation in both naïve and differentiated T cell subsets revealed that upregulation of subset-defining transcription factors, effector molecules, and cytokine was associated with decreased repressive H3K27me3 marks at these gene regions [30]. Although these findings suggested a potential role for UTX in epigenetic regulation of T cell differentiation, whether UTX actually mediated any of these changes was unclear.
UTX Deficiency in T Cells Prevents Tfh Differentiation and Eradication of Chronic Viral Infection
To investigate the role of UTX in T cells in the immune system, Cook et al. turned to genetic mouse models of UTX deficiency. Because UTX knockout mice are embryonic lethal [24, 25], Cook et al. engineered mice with T cell-specific deletion of UTX to determine how decreased UTX may affect T cell function [19••]. Mice with T cell-specific UTX deficiency show normal clearance of acute viral infection but impaired clearance of chronic viral infection. Furthermore, mice that are heterozygous for T cell-specific UTX deficiency show partially attenuated viral loads, suggesting a dose-dependent UTX function in clearance of chronic viral infection [19••].
During chronic viral infection, CD4+ T helper cells play an important role in boosting the CD8+ cytotoxic T cells and B cell-mediated adaptive immune response. Differentiation of CD4+ T cell to the T follicular helper (Tfh) subset, in particular, is critical for generating an appropriate B cell antibody response as revealed by several human genetic immunodeficiencies [31]. T follicular helper cells interact with immature B cells within follicles of secondary lymphoid tissues to promote B cell somatic hypermutation, class switching, and IgG antibody formation [32–35]. In T cell UTX-deficient mice, the impaired immunity to chronic viral infection was associated with decreased Tfh subset differentiation and fewer germinal centers [19••]. As a consequence, B cell IgG production was also impaired [19••]. Interestingly, UTX deficiency in T cells was associated with increased H3K27me3 at genes (e.g., IL6R) important in Tfh differentiation. Therefore, UTX supports Tfh cell differentiation through demethylation of Tfh signature genes, a process required for eliminating chronic viral infection.
TS Patients Have Decreased Tfh Cells
Following infection, otitis-prone children display reduced cytokine-secreting memory T cells and a poor IgG response relative to children not prone to OM, suggesting poor Tfh cell help to B cell antibody responses in children with chronic OM [36]. It was unclear whether TS patients also have an increased predisposition to chronic OM due to decreased Tfh cells. Although T cell UTX deficiency in mice results in fewer Tfh cells, it was not known whether UTX deficiency in TS subject immune cells would translate to decreased Tfh cells. In humans, CD4+CXCR5+ cells in peripheral blood are associated with antibody production, which allows them to serve as a measurable substitute of Tfh [37]. The frequency of CD4+CXCR5+ T cells were reduced by twofold in TS subjects compared to controls [19••]. This suggests that decreased UTX expression in TS patients might increase their predisposition to viral infections due to Tfh cell deficiency and subsequent low antibody levels. Overall, these data suggest that UTX haploinsufficiency in TS patient immune cells has functional consequences: decreased Tfh cells, a subset important in the clearance of chronic OM.
Other Roles for UTX in T Cells
In addition to its effects on Tfh differentiation, UTX is also important for T cell maturation and immune homeostasis. For example, T cell-specific deficiency in both UTX and Jmjd3 (UTX and Jmjd3 double deficiency) prevents thymic egress of T cells resulting in increased numbers of CD4SP mature thymocytes and decreased peripheral T cells [38••]. This effect was associated with increased H3K27me3 at the S1PR1 promoter and a corresponding decrease in surface expression of S1PR1, which is required for mature thymocytes to exit the thymus [38••]. Furthermore, demethylase-defunct UTY failed to rescue CD4SP thymocyte S1PR1 expression in UTX and Jmjd3-deficient males, supporting the notion that H3K27 demethylase activity directly results in S1PR1 expression [38••]. Accumulation of thymic CD4SP thymocytes was not noted in mice with T cell-specific UTX deficiency by itself [19••], suggesting that Jmjd3 may be able to compensate, at least in part, for UTX deficiency during T cell thymic egress.
In addition to its H3K27 demethylase activity, UTX can also regulate gene expression in a demethylase-independent manner through protein interactions with chromatin remodeling complexes, such as Brg1, and transcriptional protein complexes. For example, UTX interacts with Th1-specific transcription factor T-bet and the Brg1-containing SWI/SNF remodeling complex in primary human T cells to upregulate interferon gamma (Ifng) expression [39], independent of its demethylase activity [39]. It is therefore plausible that UTX may play both a demethylase-dependent and independent role in T cell subset differentiation. Indeed, ChIP-seq experiments in UTX-deficient T cells reveal several gene loci for which gene expression is differentially regulated, but no H3K27 methylation changes were detected [19••, 38••]. Further investigation is needed to determine whether gene expression in TS T cells is also regulated by demethylase-independent UTX function.
Conclusions and Future Directions
TS association with chronic OM is well-recognized, but whether immune abnormalities contribute to this association had not been established. Recent gene expression profiling suggests alterations in TS immune cells and, in particular, highlighted reduced T cell expression of UTX, which may lead to decreased Tfh cells in TS. Of note, genetic deficiency of the UTX gene results in the genetic disorder Kabuki syndrome (KS), a rare congenital disorder that is also associated with recurrent OM [19••, 20]. Furthermore, reduced immunoglobulin levels, hypogammaglobulinemia, poor vaccine response, and reduced memory T and B lymphocytes have also been reported in KS [40, 41]. These associations in KS lend support to the role of UTX in regulating T cell development and immune function.
Recent inroads into understanding the TS immune system also provoke a number of questions. First, how does reduced UTX expression affect other immune cells in TS? It is important to note that the role of UTX was studied in a mouse model in which UTX was lacking in T cells. Whether UTX also regulates other immune cell lines, such as B cells, dendritic cells, macrophages, and neutrophils, is still unclear and requires further study. Second, does reduced UTX expression contribute to TS predisposition to autoimmunity? Autoimmune hypothyroidism and celiac disease are strongly associated with TS, and what underlies this predisposition to autoimmunity is controversial. In one study, defective regulatory T cells (Tregs) that fail to inhibit proliferation of effector T cells were described in TS patients [18•]. Another study, however, found no difference in Treg frequencies or function in TS patients [42]. Thus, the mechanism underlying TS predisposition to autoimmunity remains to be defined.
Third, how do other epigenetic alterations regulate immune cells in TS? Recently, altered DNA methylation patterns, another type of epigenetic modification, of multiple autosomal genes involved in bone remodeling, glucose sensitivity, and ovarian function were reported to be altered in TS [43•]. Thus, it is possible that multiple epigenetic mechanisms of gene regulation are dysregulated in TS. Whether changes in DNA methylation impact T cells in TS requires further study.
Finally, the finding of decreased Tfh cells in TS suggests impaired response to viruses and vaccines. How do TS patients respond to commonly administered vaccine? Should TS patients receive a different course of vaccination? These questions are best addressed in the context of vaccine administration so that a specific T cell response can be measured. Additionally, whether decreased immunoglobulins, possibly as a result of decreased Tfh cells, is a clinical feature of TS is currently unclear and deserves further exploration. If a proportion of TS subjects are found to have lower immunoglobulins, treatment with IVIG may be beneficial to these patients. Even though more work needs to be done in the field of immunity and TS, the discovery of UTX as a gene with decreased expression in TS immune cells and the mechanism by which UTX affects Tfh differentiation are important steps toward understanding immune alterations in TS.
Footnotes
This article is part of the Topical Collection on Immune Deficiency and Dysregulation
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest Drs. Thrasher, Hong, Whitmire, and Su declare no conflicts of interest.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major Importance
- 1.Davenport ML. Approach to the patient with Turner syndrome. J Clin Endocrinol Metab. 2010;95(4):1487–1495. doi: 10.1210/jc.2009-0926. [DOI] [PubMed] [Google Scholar]
- 2.Turner HH. A syndrome of infantilism, congenital webbed neck, and cubitus valgus. Endocrinology. 1938;23:566–574. [PubMed] [Google Scholar]
- 3.Ford CE, et al. A sex-chromosome anomaly in a case of gonadal dysgenesis (Turner’s syndrome) Lancet. 1959;1(7075):711–713. doi: 10.1016/s0140-6736(59)91893-8. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson-Smith MA. Karyotype-phenotype correlations in gonadal dysgenesis and their bearing on the pathogenesis of malformations. J Med Genet. 1965;2(2):142–155. doi: 10.1136/jmg.2.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simpson JL. Gonadal dysgenesis and abnormalities of the human sex chromosomes: current status of phenotypic-karyotypic correlations. Birth Defects Orig Artic Ser. 1975;11(4):23–59. [PubMed] [Google Scholar]
- 6.Berletch JB, et al. Genes that escape from X inactivation. Hum Genet. 2011;130(2):237–245. doi: 10.1007/s00439-011-1011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchini A, Rappold G, Schneider KU. SHOX at a glance: from gene to protein. Arch Physiol Biochem. 2007;113(3):116–123. doi: 10.1080/13813450701531201. [DOI] [PubMed] [Google Scholar]
- 8.Blaschke RJ, Rappold GA. SHOX: growth, Leri-Weill and Turner syndromes. Trends Endocrinol Metab. 2000;11(6):227–230. doi: 10.1016/s1043-2760(00)00262-9. [DOI] [PubMed] [Google Scholar]
- 9.Munns CF, et al. Familial growth and skeletal features associated with SHOX haploinsufficiency. J Pediatr Endocrinol Metab. 2003;16(7):987–996. doi: 10.1515/jpem.2003.16.7.987. [DOI] [PubMed] [Google Scholar]
- 10.Rao E, et al. Pseudoautosomal deletions encompassing a novel homeobox gene cause growth failure in idiopathic short stature and Turner syndrome. Nat Genet. 1997;16(1):54–63. doi: 10.1038/ng0597-54. [DOI] [PubMed] [Google Scholar]
- 11.Ellison JW, et al. PHOG, a candidate gene for involvement in the short stature of Turner syndrome. Hum Mol Genet. 1997;6(8):1341–1347. doi: 10.1093/hmg/6.8.1341. [DOI] [PubMed] [Google Scholar]
- 12.Makishima T, et al. Otolaryngologic markers for the early diagnosis of Turner syndrome. Int J Pediatr Otorhinolaryngol. 2009;73(11):1564–1567. doi: 10.1016/j.ijporl.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cacciari E, et al. Serum immunoglobulins and lymphocyte subpopulations derangement in Turner’s syndrome. J Immunogenet. 1981;8(5):337–344. doi: 10.1111/j.1744-313x.1981.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 14.Jensen K, et al. Serum immunoglobulin M, G, and A concentration levels in Turner’s syndrome compared with normal women and men. Hum Genet. 1976;31(3):329–334. doi: 10.1007/BF00270862. [DOI] [PubMed] [Google Scholar]
- 15.Mock, et al. Selective T-cell deficiency in Turner’s syndrome. J Investig Allergol Clin Immunol. 2000;10(5):312–313. [PubMed] [Google Scholar]
- 16.Stenberg AE, et al. Immunological parameters in girls with Turner syndrome. J Negat Results Biomed. 2004;3:6. doi: 10.1186/1477-5751-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rongen-Westerlaken C, et al. Immunologic studies in Turner syndrome before and during treatment with growth hormone. The Dutch Growth Hormone Working Group. J Pediatr. 1991;119(2):268–272. doi: 10.1016/s0022-3476(05)80737-1. [DOI] [PubMed] [Google Scholar]
- 18. Lee YA, et al. CD4+FOXP3+ regulatory T cells exhibit impaired ability to suppress effector T cell proliferation in patients with Turner syndrome. PLoS One. 2015;10(12):e0144549. doi: 10.1371/journal.pone.0144549. This paper suggests that Tregs in TS patients cannot efficiently suppress proliferation of autologous effect T cells. Findings in this paper contradict those reported in Reference 42, and further study is required in this area.
- 19. Cook KD, et al. T follicular helper cell-dependent clearance of a persistent virus infection requires T cell expression of the histone demethylase UTX. Immunity. 2015;43(4):703–714. doi: 10.1016/j.immuni.2015.09.002. Describes the role of UTX in CD4+ T cells and show that UTX is decreased in TS patients providing a mechanismfor immune dysfunctions seen in TS.
- 20.Stagi S, et al. Epigenetic control of the immune system: a lesson from Kabuki syndrome. Immunol Res. 2015 doi: 10.1007/s12026-015-8707-4. [DOI] [PubMed] [Google Scholar]
- 21.Greenfield A, et al. The UTX gene escapes X inactivation in mice and humans. Hum Mol Genet. 1998;7(4):737–742. doi: 10.1093/hmg/7.4.737. [DOI] [PubMed] [Google Scholar]
- 22.Hong S, et al. Identification of JmjC domain-containing UTX and JMJD3 as histone H3 lysine 27 demethylases. Proc Natl Acad Sci U S A. 2007;104(47):18439–18444. doi: 10.1073/pnas.0707292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol. 2009;9(2):91–105. doi: 10.1038/nri2487. [DOI] [PubMed] [Google Scholar]
- 24.Agger K, et al. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature. 2007;449(7163):731–734. doi: 10.1038/nature06145. [DOI] [PubMed] [Google Scholar]
- 25.Shpargel KB, et al. UTX and UTY demonstrate histone demethylase-independent function in mouse embryonic development. PLoS Genet. 2012;8(9):e1002964. doi: 10.1371/journal.pgen.1002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang JK, et al. The histone demethylase UTX enables RB-dependent cell fate control. Genes Dev. 2010;24(4):327–332. doi: 10.1101/gad.1882610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thieme S, et al. The histone demethylase UTX regulates stem cell migration and hematopoiesis. Blood. 2013;121(13):2462–2473. doi: 10.1182/blood-2012-08-452003. [DOI] [PubMed] [Google Scholar]
- 28.van Haaften G, et al. Somatic mutations of the histone H3K27 demethylase gene UTX in human cancer. Nat Genet. 2009;41(5):521–523. doi: 10.1038/ng.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van der Meulen J, et al. The H3K27me3 demethylase UTX is a gender-specific tumor suppressor in T-cell acute lymphoblastic leukemia. Blood. 2015;125(1):13–21. doi: 10.1182/blood-2014-05-577270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei G, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30(1):155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 32.Brooks DG, et al. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J Virol. 2005;79(16):10514–10527. doi: 10.1128/JVI.79.16.10514-10527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fahey LM, et al. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J Exp Med. 2011;208(5):987–999. doi: 10.1084/jem.20101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deenick EK, Ma CS. The regulation and role of T follicular helper cells in immunity. Immunology. 2011;134(4):361–367. doi: 10.1111/j.1365-2567.2011.03487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bauquet AT, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009;10(2):167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma SK, Casey JR, Pichichero ME. Reduced memory CD4+ T-cell generation in the circulation of young children may contribute to the otitis-prone condition. J Infect Dis. 2011;204(4):645–653. doi: 10.1093/infdis/jir340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morita R, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34(1):108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Manna S, et al. Histone H3 Lysine 27 demethylases Jmjd3 and Utx are required for T-cell differentiation. Nat Commun. 2015;6:8152. doi: 10.1038/ncomms9152. This paper shows that double deficiency in UTX and Jmjd3 prevents thymic egress of T cells.
- 39.Miller SA, Mohn SE, Weinmann AS. Jmjd3 and UTX play a demethylase-independent role in chromatin remodeling to regulate T-box family member-dependent gene expression. Mol Cell. 2010;40(4):594–605. doi: 10.1016/j.molcel.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin JL, et al. Immunologic assessment and KMT2D mutation detection in Kabuki syndrome. Clin Genet. 2015;88(3):255–260. doi: 10.1111/cge.12484. [DOI] [PubMed] [Google Scholar]
- 41.Hoffman JD, et al. Immune abnormalities are a frequent manifestation of Kabuki syndrome. Am J Med Genet A. 2005;135(3):278–281. doi: 10.1002/ajmg.a.30722. [DOI] [PubMed] [Google Scholar]
- 42.Su MA, et al. The role of X-linked FOXP3 in the autoimmune susceptibility of Turner Syndrome patients. Clin Immunol (Orlando, Fla) 2009;131(1):139–144. doi: 10.1016/j.clim.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shriram NR, Deepti DD. Evidence for epigenetic alterations in Turner syndrome opens up feasibility of new pharmaceutical interventions. Current Pharmaceutical Design. 2014;20(11):1778–1785. doi: 10.2174/13816128113199990518. An interesting report of altered DNA methylation in TS.
- 44.Lleo A, et al. Autoimmunity and Turner’s syndrome. Autoimmun Rev. 2012;11(6–7):A538–A543. doi: 10.1016/j.autrev.2011.11.015. [DOI] [PubMed] [Google Scholar]