Abstract
Complement factor H (FH) is an important regulator of the alternative complement pathway. The Y402H polymorphism within the seventh short consensus repeat of FH was recently shown to be associated with age-related macular degeneration, the most common cause of irreversible blindness in the Western world. We examined the effects of this polymorphism on various FH functions. FH purified from sera of age-related macular degeneration patients homozygous for the FH402H variant showed a significantly reduced binding to C-reactive protein (CRP), an acute phase protein, as compared with FH derived from unaffected controls homozygous for the FH402Y variant. Strongly reduced binding to CRP was also observed with a recombinant fragment of FH (short consensus repeat 5–7) containing the same amino acid change. Because the interaction of CRP and FH promotes complement-mediated clearance of cellular debris in a noninflammatory fashion, we propose that the reduced binding of FH402H to CRP could lead to an impaired targeting of FH to cellular debris and a reduction in debris clearance and enhanced inflammation along the macular retinal pigmented epithelium-choroid interface in individuals with age-related macular degeneration.
Age-related macular degeneration (AMD)3 is the most common cause of irreversible blindness in developed countries (1, 2). Early AMD is characterized by the development of hallmark lesions called drusen between the basal surface of the retinal pigmented epithelium (RPE) and Bruch’s membrane. Progression of the disease often leads to atrophy of the RPE and retina, a neovascular response and loss of visual acuity. Drusen contain numerous proteins associated with the complement system, including the membrane attack complex (MAC) (3). By-stander tissue damage caused by aberrant control of the complement pathway has been proposed as one of the primary mechanisms associated with the development of AMD (4, 5). More recent studies have revealed a significant association of AMD with polymorphic variations in the complement factor H (Cfh) gene (6–9). The factor H (FH) protein, a key regulator of the alternative complement pathway, is composed of 20 domains called short consensus repeats (SCRs). As a result of the single nucleotide polymorphism 1277T→C, the tyrosine in position 402 in SCR7 of FH is substituted by a histidine. Individuals homozygous for 402H (genotype CC) have a 5- to 7-fold increased risk for developing AMD than individuals homozygous for 402Y (genotype TT). Heterozygotes (genotype TC) also have an increased risk, but a less significant one (7).
Complement FH promotes dissociation of the alternative pathway C3 convertase and inactivation of C3b to iC3b, thereby inhibiting complement activation. iC3b is a crucial ligand for the macrophage and dendritic cell receptors CD11a-c/18. Factor H binds to complement C3b via interaction sites in SCR domains 1–4, 12–14, and 19–20 (10). These interactions down-modulate complement activation in human plasma and on viable host cell surfaces. Receptors for FH on cell surfaces include negatively charged glycosaminoglycans, phospholipids, and sialic acids. FH has up to three distinct heparin (a model polyanion) binding sites, one of which is located within SCR7 (11, 12).
In addition to heparin and C3b, FH also binds to C-reactive protein (CRP) at two sites, one located at SCR7 and the other in a region spanning between SCR8 and SCR11 (12, 13). Once bound to phosphocholine, CRP activates the classical complement pathway by also binding C1q (14, 15). Subsequent binding of FH to CRP suppresses activation of the alternative complement pathway (13, 16).
An alternatively spliced product of the Cfh gene, FH-like protein 1 (FHL-1), also regulates the alternative complement pathway. It is composed of the first seven SCR domains of FH and four unique amino acids (17) and thus contains the same AMD-associated polymorphism. A group of proteins called FH-related proteins (FHRs) also exists. It consists of five proteins that are structurally similar to FH. Each FHR is a product of its own gene distinct from Cfh FHRs consist of four to nine SCR domains that have a varying degree of homology to those of FH. The homolog of SCR7 is present in all of them except in FHR-4 (17).
Based on existing information, it is logical to speculate that a polymorphism in FH could affect its binding to surface polyanions and/or CRP, or influence the production of FH and/or FHL-1. Therefore, we sought in this investigation to explore the effects of the FH Y402H polymorphism on the FH protein function.
Materials and Methods
Patient and control samples
We collected DNA and serum samples from 118 carefully phenotyped familial AMD cases seen in the Department of Ophthalmology (Helsinki University Hospital, Helsinki, Finland). AMD was verified and graded (large confluent drusen, central geographic atrophy, or neovascular AMD) from fundus photographs and/or angiograms from all patients. A control group that comprised of 71 patients was collected from subjects attending the same hospital for cataract operations. These subjects had no large drusen and no, or minimal, focal pigmentary abnormalities in the macula as verified by fundus photographs and clinical examination by a specialist. All patients were genotyped for the FH 402 polymorphism as described below. For CRP-FH binding studies, a random cohort of 46 AMD patients and 33 control patients was selected out of the larger cohort. The mean age of the AMD cases was 77.0 (range 58.1–92.4) and that of the control subjects was 76.6 (range 69.8–87.5). The study was approved by the Ethics Committee of the Helsinki University Eye and Ear Hospital.
Genotyping
DNA was obtained from peripheral blood samples, amplified by PCR, and sequenced using the forward primer 5′-ctttgttagtaactttagttcg-3′ and the reverse primer 5′-ttagaaagacatgaacatgctagg-3′of the Cfh gene. PCR amplifications were conducted in a 50-µl volume containing 80 ng of genomic DNA, 30 pM of each primer, polymerase buffer, 10 nM of each nucleotide (dNTP), and 0.8 U of Dynazyme polymerase-enzyme (Finnzymes). Sequencing was performed using cycle sequencing with the Big Dye Terminator kit (version 3.1; Applied Biosystems) and reactions were run on an ABI 3730 capillary sequencer according to the manufacturer’s instructions.
Immunohistochemistry
Sections of human eyes were obtained, stained, and analyzed by laser scanning confocal immunofluorescence microscopy as described by Johnson et al. (18).
SDS-PAGE and immunoblotting
To determine whether the FH Y402H polymorphism influenced the molecular nature of serum FH, serum samples from 54 AMD patients and controls either homozygous for FH402Y (genotype TT) or FH402H (genotype CC) or heterozygous for the Y402H polymorphism (genotype TC) were analyzed by immunoblotting using polyclonal and monoclonal (86X and 90X) Abs directed against FH (19). Serum samples from patients and controls were separated on 10% SDS-PAGE gels under nonreducing conditions. The proteins were transferred onto a nitrocellulose membrane and nonspecific binding sites were blocked. Polyclonal goat anti-human FH (Calbiochem) or mAbs and peroxidase-conjugated donkey anti-goat IgG (Jackson ImmunoResearch Laboratories) or peroxidase-conjugated rabbit anti-mouse IgG (Jackson ImmunoResearch Laboratories) were used for the detection of FH and FHRs.
Quantification of CRP
CRP levels were determined using a highly sensitive immunoturbidimetric assay (Orion Diagnostica). CRP concentration was not available for three TT controls and one TC control.
Purification of FH402Y and FH402H
The IgG fraction of goat antiserum to human FH (Quidel) was precipitated with 18% Na2SO4. The precipitate was washed with 18% Na2SO4 in PBS (pH 7.4) and resuspended in sodium carbonate buffer. The precipitate was then coupled to CNBr-activated Sepharose 4B (Amersham Biosciences) according to the manufacturer’s instructions. Sera from 10 controls with the TT genotype were pooled for isolation of FH402Y and sera from 10 patients with the CC genotype were pooled for isolation of and FH402H. Both serum pools were incubated with Sepharose for 1 h and the column was washed with 0.5 M NaCl in PBS. The bound FH proteins were eluted with the chaotropic agent sodium thiocyanate, 3 M, and finally dialyzed against veronal-buffered saline. The purity of the proteins was checked by SDS-PAGE and both silver staining and Western blotting. The concentrations of purified proteins were determined by using absorbance measurements at 280 nm, the BCA Protein Assay (Pierce), and silver staining.
Expression of rFH SCR5-7 and construction of FH SCR5-7402H variants
rFH SCR5-7 constructs were generated as described by Jokiranta et al. (20) using specific primers for SCRs 5 and 7 and pPICZα expression vectors. The Y402H mutation was introduced to the FH SCR5-7 sequence by using the QuikChange MultiSite Mutagenesis kit (Stratagene).
Binding of FH to CRP
Maxisorp microtiter plates (Nunc) were coated with CRP (1 µg/ml; Calbiochem), whose purity was confirmed with silver staining. After blocking, sera (1/1,000) from patients and controls, purified FH proteins, or rSCR5-7 variants were added. The plates were incubated for 1 h at 37°C and washed. Polyclonal goat anti-human FH (1/5,000) was added. Peroxidase-conjugated donkey anti-goat IgG diluted 1/10,000 was used as the secondary Ab. Chromogenic substrate and absorbance measurement at 492 nm were used for detection and results indicated as direct OD values. All the FH-CRP binding experiments were repeated at least twice on duplicate samples.
Binding of FH to heparin
Binding of purified FH proteins (FH402Y and FH402H) and respective SCR5-7 fragments to heparin was analyzed using heparin affinity chromatography in an HPLC system (LaChrom l-7100; Hitachi). Ten micrograms of proteins was diluted in 1/2× PBS and applied to a heparin-Sepharose affinity column (HiTrap; Amersham Biosciences) at a flow rate of 0.5 ml/ min. The column was extensively washed with 0.5× PBS and the bound proteins were eluted using a linear salt gradient ranging from 75 to 500 mM NaCl, in a total volume of 10 ml and at a flow rate of 0.5 ml/min.
Molecular modeling
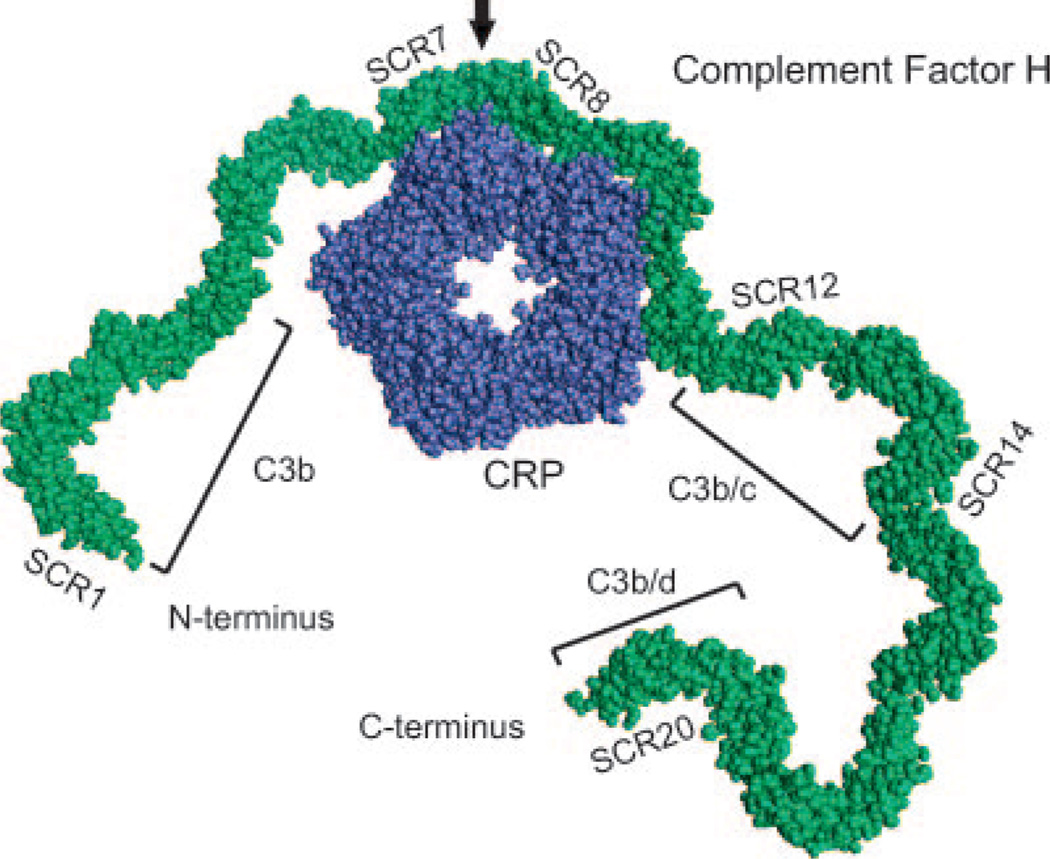
The schematic molecular display model depicting binding between FH and CRP presented in Fig. 4 is based upon the crystal structure of CRP (21) and 10 copies of nuclear magnetic resonance structures of the FH SCR15-16 domain pair as display units (22). The model was created using InsightII 2000 (Accelrys).
FIGURE 4.
A schematic molecular display model depicting binding between FH and CRP based upon the crystal structure of CRP (21) and 10 copies of nuclear magnetic resonance structures of the FH SCR15-16 domain pair as display units (22). The model was constructed using InsightII (Accelrys). SCR7 and SCRs 8-11 are known to be involved in binding to CRP (13). FH-binding sites for C3b, C3b/c, and C3b/d are also indicated (10). The black arrow marks the location of the polymorphic FH amino acid 402.
Statistical analyses
Statistical analyses between more than two groups were conducted using ANOVA. Post hoc comparisons between individual groups were done using the Games-Howell test as the variances of different groups were not homogeneous. Statistical analyses between two groups were done using the two-tailed Student t test.
Results
FH migration patterns and binding to heparin are not affected by Y402H polymorphism
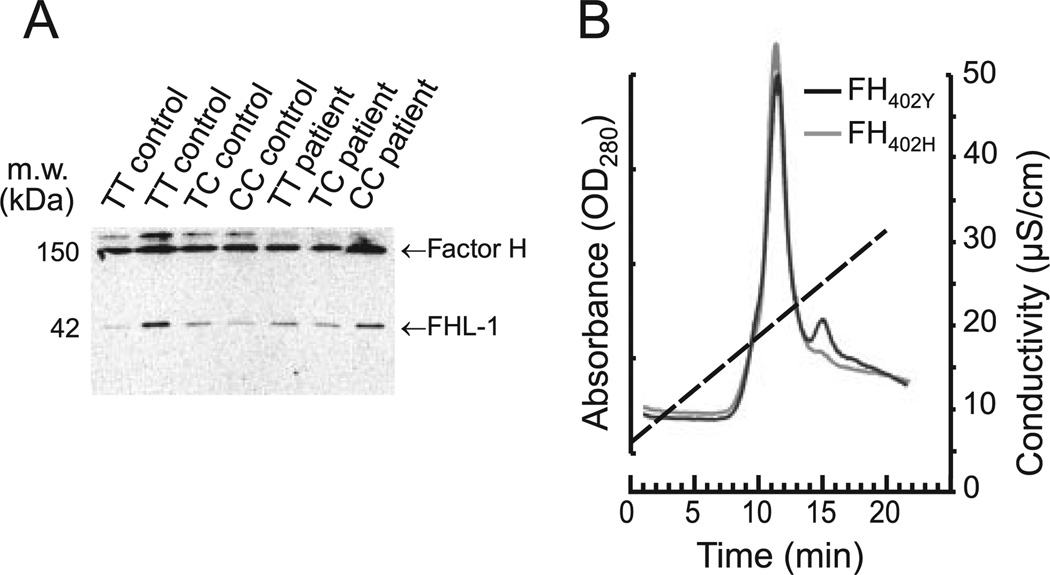
FH proteins in sera of individuals with the three different 402 genotypes (TT, TC, or CC) showed similar migration patterns in SDS-polyacrylamide gels (Fig. 1A). In some serum samples, an extra, more slowly migrating protein band was observed but its presence did not correlate with the FH genotype or disease status. The identity of the band is unknown, but it is commonly seen in FH serum blots. Binding of the two full-length FH variants, FH402Y derived from controls and FH402H derived from patients, to heparin was assessed using heparin-Sepharose affinity chromatography. Both protein variants eluted from the column at the same ionic strength (Fig. 1B), indicating that the Y402H variation does not markedly affect binding of FH to heparin. Similar results were obtained using recombinant fragments of FH SCR5-7 containing either the 402Y or 402H residues.
FIGURE 1.
A, Western blot depicting migration patterns of serum FH and FHL-1 in 1277T→C (Y402H) genotyped AMD patients and controls. Sera were subjected to SDS-PAGE and the separated proteins were blotted onto nitrocellulose and visualized using a mouse mAb (90X) directed against FH. The migration patterns of FH or FHL-1 were similar, irrespective of Cfh genotype or disease state. B, Binding of FH variants to heparin. Ten-microgram samples of purified FH proteins (FH402Y or FH402H) were injected into a heparin-Sepharose column using HPLC. The column was washed and the bound proteins were eluted using a salt gradient (75–500 mM NaCl; 20 min). Both variants eluted at 10 min, suggesting that FH affinity for heparin is not affected by the Y402H variation. Conductivity is represented by the discontinuous line.
Location of CRP and FH in drusen
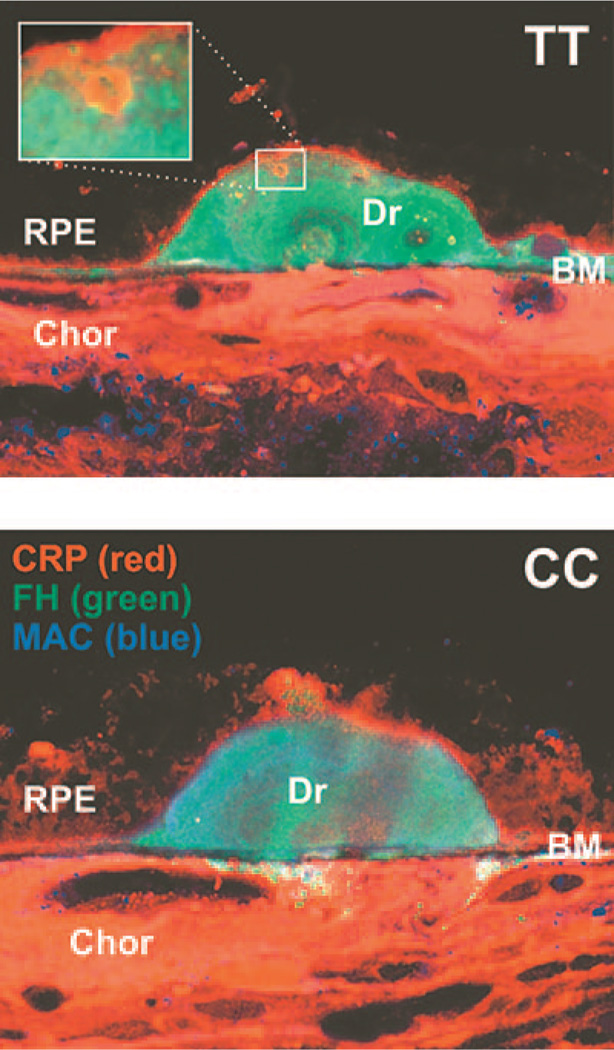
Drusen have been demonstrated to contain complement components. With regard to possible interactions between FH and CRP their expression was analyzed by confocal immunofluorescence microscopy analysis. As shown in two representative cases (Fig. 2), FH was present throughout the drusen in individuals with either the TT or CC genotype. Intriguingly, however, CRP was present in small spheroid particles inside the drusen (Fig. 2, upper panel).
FIGURE 2.
Laser scanning confocal micrograph depicting CRP (stained red) and FH (green) in drusen (Dr) of eyes from AMD patients with either the TT or CC genotype. Drusen are located between the RPE and Bruch’s membrane (BM), which separates choriocapillaris (Chor) from retina. Note distinct spherules staining for CRP in the drusen in the upper panel.
FH402H has reduced binding affinity to CRP
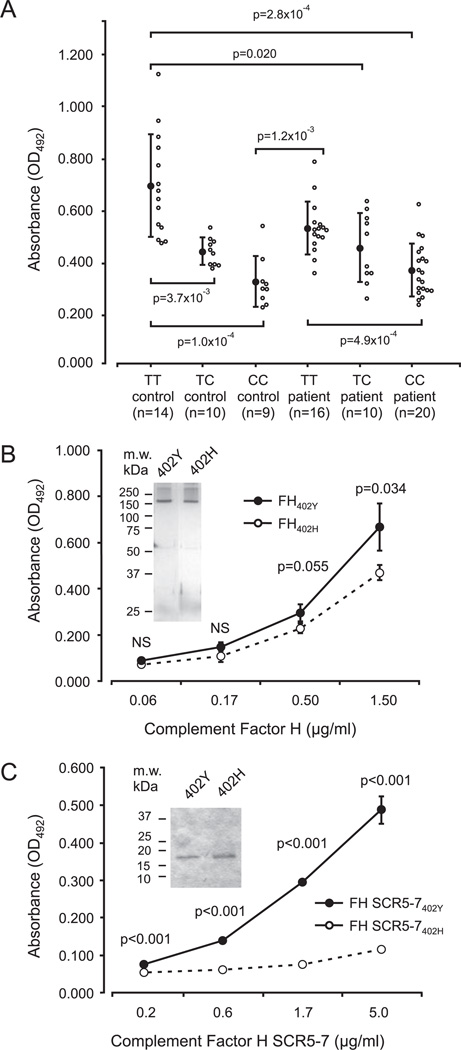
To analyze binding of the different variants of FH to CRP, we set up an ELISA by coating plate wells with CRP and testing binding of serum-derived FH, purified FH, and rFH SCR5-7 fragments to CRP. Significant differences in binding to CRP were found to exist between the different groups (TT controls, TC controls, CC controls, TT patients, TC patients, and CC patients) (F = 15.3, p = 2.9 × 10−10). FH in the sera of AMD patients homozygous for the 402H (CC genotype) exhibited strongly reduced binding to CRP (OD492: 0.369 ± 0.102, n = 20), as compared with patients with the TT genotype (OD492: 0.532 ± 0.102, n = 16, p = 4.9 × 10−4) (Fig. 3A). A similar difference was observed using sera from healthy controls indicating that the functional difference in CRP binding is genotype associated (CC controls: OD492: 0.324 ± 0.098, n = 9, TT controls: OD492: 0.697 ± 0.199, n = 14, p = 1.0 × 10−4) (Fig. 3A). Individuals heterozygous for the C allele (genotype TC) also exhibited reduced binding of serum FH to CRP (Fig. 3A). When data from the patient and control groups were pooled according to genotype, a significant difference in FH binding to CRP was observed (F = 27.4, p = 1.1 × 10−9). This difference was most marked between the CC (OD492: 0.355 ± 0.101, n = 29) and TT genotypes (OD492: 0.609 ± 0.173, n = 30, p = 3.9 × 10−8) and between the TC (OD492: 0.499 ± 0.100, n = 20) and TT genotypes ( p = 4.2 × 10−4) but the difference between CC and TC genotypes was also significant ( p = 6.8 × 10−3). When AMD patient data were pooled regardless of genotype (OD492: 0.445 ± 0.129, n = 46) and compared with pooled control data (OD492: 0.518 ± 0.214, n = 33), no significant difference in FH binding to CRP was observed ( p = 0.062). Similar analyses using full-length FH variants (FH402Y and FH402H) purified from sera were performed to exclude any interfering factors in serum samples. Binding of purified FH402H protein to CRP (OD492: 0.470 ± 0.033) was significantly weaker than that of the FH402Y protein (OD492: 0.668 ± 0.103, p = 0.034) (Fig. 3B). An even more marked difference was observed between the two recombinant fragments of FH in binding to CRP than using the full-length proteins (Fig. 3C). By measuring FH concentrations of the samples, we excluded varying FH levels as the cause for these results (data not shown).
FIGURE 3.
Binding of FH to C-reactive protein as assessed by ELISA. A, Binding of Y402H polymorphic forms of FH in sera to CRP. Patient and control groups of different genotypes are indicated. The absorbance values correspond to the amount of FH bound to microtiter plate-coupled CRP. Each open circle indicates a measurement derived from a single patient or control. The solid circles indicate the average values of all assays in the group and the vertical bars indicate the SDs. Values of p for significant differences are indicated. B, Binding of purified FH variants to CRP. Equal amounts of proteins were used. Each circle is an average of three assays with vertical bars indicating the SDs. Inset, A silver stain of the purified proteins. C, Binding of recombinant fragments of FH SCRs 5-7 to CRP. The two polymorphic forms show a considerable difference in affinity toward CRP. Each circle is an average of four assays with vertical bars indicating the SDs. Inset, A Coomassie stain of the recombinant proteins.
Discussion
Recent studies have provided compelling evidence that inflammation and aberrant regulation of the complement system likely contribute to the etiology of AMD. The presence of complement components, particularly MAC, or C5b-9, in drusen (3) suggests that local regulation of the complement system is dysfunctional and/or that complement-activating material, such as cellular debris or protein aggregates, is accumulating within the sub-RPE region. In the present study, we have examined one potential mechanism which could contribute to AMD pathogenesis. The alternative pathway inhibitor FH was abundantly present in the drusen indicating that it may play a direct role in controlling complement activation by material that has accumulated in the lesions.
We observed that the AMD-associated variant of FH, FH402H, has a lower binding affinity for CRP than the FH402Y variant. Binding of FH to CRP is a critical interaction through which CRP participates in the regulation of complement activation. Drusen in the eyes examined contained spheroid-like particles, which in a TT-genotyped, but not in a CC-genotyped, individual showed costaining for CRP and FH (Fig. 2). In a recent study, CRP was found to be more abundantly expressed in the eyes of AMD patients with FH402H than those with FH402Y (18). CRP, a plate-like pentamer, binds to surface phosphocholine or cholesterol in a Ca2+-dependent manner with each of its five subunits (23). C1q binds to a different region of the pentameric plate via its globular domains (24). Through these interactions, CRP recognizes a target, activates the classical complement pathway, and restricts activation of the complement cascade to the C3 level by binding FH. The domains of FH that mediate binding to CRP lie within SCR7 and within a region that spans SCRs 8-11 (13). A model of the CRP-FH interaction is depicted in Fig. 4.
Our experiments using recombinant fragments comprised of FH SCR5-7 show that 402Y in SCR7 is an important amino acid for the binding of FH to CRP. It could be directly involved in making contact with the CRP molecule on the surface of the SCR7 domain. The Y402H change might thus disrupt the interaction surface with CRP or destabilize the interdomain interface between SCR7 and SCR8 and lead to a conformational change in the whole FH molecule (25, 26). We postulate that an impairment of the FH-CRP interaction leads to an inadequate clearance of eye pigment derived lipofuscin or damaged tissue, debris accumulation, and inflammation (13). This would be the consequence of an inability to locally control activation of the alternative complement pathway (27).
Due to a second CRP-binding site in SCR8-11, the full-length FH seems to be able to somewhat compensate for the reduced binding of SCR7 containing 402H to CRP. This could explain the smaller differences in CRP binding between full-length FH variants than between the rSCR5-7 proteins that lack the second binding site (Fig. 3, B vs C). Because the SCR8-11 binding site is not present in FHL-1, the direct effect of 402Y in binding to CRP might be significant in FHL-1 and important for its function as a local regulator of the alternative complement pathway. FHL-1 consist of SCRs 1-7. Thus, the amino acid 402 in the most C-terminal SCR domain of FHL-1 is more likely directly involved in binding to CRP. Although FHL-1 levels are only about one-tenth of those of FH in plasma, its concentrations can be considerably higher locally and it can be important in controlling local inflammatory processes (17). Based on our recombinant protein data, this function is influenced by the Y402H polymorphism. Another interesting finding is that differences between the CRP-binding affinity of the two FH variants seem to be bigger in assays with serum samples than in assays with purified FH. This could, at least in part, be due to serum FHRs that possibly compete with FH for binding to CRP or C3b. In the case of FHR-1 and FHR-3 deficiency, that has been proposed to be protective against AMD (28, 29), the FH-CRP interaction might be stronger because of a lack of competition by these FHRs for binding to CRP.
The targets for CRP in macular lesions are unknown. Cholesterol has been demonstrated in drusen (30) and is a known ligand for CRP (23). This interaction is highly specific, because not even the closely related epicholesterol is capable of binding to CRP. Other candidate receptors for CRP within the macula might include products of retinal pigment catabolism and/or RPE-derived cell components or protein aggregates in the form of spheroid-like structures as seen in the drusen (Fig. 2).
The production of CRP increases during episodes of inflammation and/or tissue injury (31). When bound to one of its ligands on a surface CRP binds C1q and activates the classical complement pathway. CRP also binds FH, which serves as a cofactor for factor I to inactivate accumulating C3b molecules. The formed iC3b molecules subsequently function as high-affinity ligands for the dendritic cell and macrophage complement receptor CD11b/18, which mediates noninflammatory phagocytosis of opsonized tissue components (13, 32). The same process down-modulates alternative pathway amplification and reduces inflammation and formation of MAC by preventing activation of C5 to the C5a anaphylatoxin and C5b. Formation of iC3b would be less efficient in individuals with the FH402H variant. As such, the inflammation suppressing effects of FH and CRP would be less effective. In consequence, this would lead to a less controlled inflammatory reaction and more tissue damage.
The episodic nature of CRP elevation and related dysregulated complement activation in genetically predisposed individuals is compatible with our knowledge of AMD histopathology. We propose that excessive tissue injury develops, over time, as a result of episodic inflammation and deposition of complement. CRP would normally regulate and target the inflammatory process. This function would be disturbed in individuals with FH402H. CRP levels could become elevated because of infections and/or tissue damage at distant sites and consequences in the eye could be an indirect side effect of a process elsewhere.
In our Finnish study cohort, the presence of the CC genotype carried a 9.7-fold increased risk for AMD (33). Although the binding of FH to CRP is diminished in patients with the CC genotype, not all AMD patients possess this genotype. Other factors are thus also likely to be involved in the pathogenesis of AMD. These might be other polymorphisms in FH itself (e.g., I62V) or in other complement components. This is exemplified by recent findings that variations in genes coding for complement components factor B and C2 are associated with AMD (34), and that a combined FHR-1 and FHR-3 deletion is protective against the disease (28, 29).
In some studies, elevated levels of CRP have been associated with the progression of AMD (35, 36). However, in our analysis the difference in CRP levels between patients and controls ( p = 0.22) or different genotypes (F = 0.51, p = 0.61) was not significant. This result excludes the possibility that varying levels of CRP are the cause for the differences in FH binding to CRP between the different genotypes in this study.
Binding of FH to polyanions is important in the discrimination between alternative pathway activators and nonactivators (37). FH binds to glycosaminoglycans, including heparan sulfate, via its SCR7, SCR9, SCR13, and SCR19-20 domains. The data from this study suggest that compared with CRP binding, it is less likely that a difference in glycosaminoglycan binding between the two proteins is involved in AMD pathogenesis.
In conclusion, we have shown that the polymorphic FH402H variant has a reduced capacity to bind CRP. This could result in excessive inflammation induced by distinct drusen-associated structures (shown in Fig. 2) in individuals who carry this FH variant and develop AMD. Systemic implications of the reduced binding of FH to CRP are also possible and remain to be investigated.
Acknowledgments
We thank our patients for participating in the study and Marja Ikonen (Helsinki, Finland) for collecting the patient samples.
Footnotes
This work was supported by the Academy of Finland, Sigrid Jusélius Foundation, Helsinki University Central Hospital Funds (EVO), Päivikki and Sakari Sohlberg Foundation, Finska Laäkaresaällskapet, The Eye and Tissue Bank Foundation, The Eye Foundation (Helsinki, Finland), Kidneeds Foundation, the National Institutes of Health (EY11515), the American Macular Degeneration Foundation, and the Eye Research Institute.
Abbreviations used in this paper: AMD, age-related macular degeneration; RPE, retinal pigmented epithelium; MAC, membrane attack complex; FH, factor H; SCR, short consensus repeat; CRP, C-reactive protein; FHL-1, complement FH-like protein 1; FHR, FH-related protein.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, Mariotti SP. Global data on visual impairment in the year 2002. Bull. W.H.O. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 2.Congdon N, O’Colmain B, Klaver CC, Klein R, Munoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch. Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 3.Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14:835–846. [PubMed] [Google Scholar]
- 4.Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog. Retin. Eye Res. 2001;20:705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 5.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am. J. Ophthalmol. 2002;134:411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 6.Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, Hageman JL, Stockman HA, Borchardt JD, Gehrs KM, et al. A common haplotype in the complement regulatory gene factor H (HF1/ CFH) predisposes individuals to age-related macular degeneration. Proc. Natl. Acad. Sci. USA. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 9.Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 10.Jokiranta TS, Hellwage J, Koistinen V, Zipfel PF, Meri S. Each of the three binding sites on complement factor H interacts with a distinct site on C3b. J. Biol. Chem. 2000;275:27657–27662. doi: 10.1074/jbc.M002903200. [DOI] [PubMed] [Google Scholar]
- 11.Blackmore TK, Sadlon TA, Ward HM, Lublin DM, Gordon DL. Identification of a heparin binding domain in the seventh short consensus repeat of complement factor H. J. Immunol. 1996;157:5422–5427. [PubMed] [Google Scholar]
- 12.Giannakis E, Jokiranta TS, Male DA, Ranganathan S, Ormsby RJ, Fischetti VA, Mold C, Gordon DL. A common site within factor H SCR 7 responsible for binding heparin, C-reactive protein and streptococcal M protein. Eur. J. Immunol. 2003;33:962–969. doi: 10.1002/eji.200323541. [DOI] [PubMed] [Google Scholar]
- 13.Jarva H, Jokiranta TS, Hellwage J, Zipfel PF, Meri S. Regulation of complement activation by C-reactive protein: targeting the complement inhibitory activity of factor H by an interaction with short consensus repeat domains and 8-11. J. Immunol. 1999;163:3957–3962. [PubMed] [Google Scholar]
- 14.Gewurz H, Mold C, Siegel J, Fiedel B. C-reactive protein and the acute phase response. Adv. Intern. Med. 1982;27:345–372. [PubMed] [Google Scholar]
- 15.Volanakis JE, Narkates AJ. Interaction of C-reactive protein with artificial phosphatidylcholine bilayers and complement. J. Immunol. 1981;126:1820–1825. [PubMed] [Google Scholar]
- 16.Aronen M, Lehto T, Meri S. Regulation of the alternative pathway complement activation by an interaction of C-reactive protein with factor H. Immunol. Infect. Dis. 1993;3:83–87. [Google Scholar]
- 17.Zipfel PF, Jokiranta TS, Hellwage J, Koistinen V, Meri S. The factor H protein family. Immunopharmacology. 1999;42:53–60. doi: 10.1016/s0162-3109(99)00015-6. [DOI] [PubMed] [Google Scholar]
- 18.Johnson PT, Betts KE, Radeke MJ, Hageman GS, Anderson DH, Johnson LV. Individuals homozygous for the age-related macular degeneration risk-conferring variant of complement factor H have elevated levels of CRP in the choroid. Proc. Natl. Acad. Sci. USA. 2006;103:17456–17461. doi: 10.1073/pnas.0606234103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jokiranta TS, Zipfel PF, Hakulinen J, Kuhn S, Pangburn MK, Tamerius JD, Meri S. Analysis of the recognition mechanism of the alternative pathway of complement by monoclonal anti-factor H antibodies: evidence for multiple interactions between H and surface bound C3b. FEBS Lett. 1996;393:297–302. doi: 10.1016/0014-5793(96)00905-2. [DOI] [PubMed] [Google Scholar]
- 20.Jokiranta TS, Jaakola VP, Lehtinen MJ, Parepalo M, Meri S, Goldman A. Structure of complement factor H carboxyl-terminus reveals molecular basis of atypical haemolytic uremic syndrome. EMBO J. 2006;25:1784–1794. doi: 10.1038/sj.emboj.7601052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shrive AK, Cheetham GM, Holden D, Myles DA, Turnell WG, Volanakis JE, Pepys MB, Bloomer AC, Greenhough TJ. Three dimensional structure of human C-reactive protein. Nat. Struct. Biol. 1996;3:346–354. doi: 10.1038/nsb0496-346. [DOI] [PubMed] [Google Scholar]
- 22.Barlow PN, Steinkasserer A, Norman DG, Kieffer B, Wiles AP, Sim RB, Campbell ID. Solution structure of a pair of complement modules by nuclear magnetic resonance. J. Mol. Biol. 1993;232:268–284. doi: 10.1006/jmbi.1993.1381. [DOI] [PubMed] [Google Scholar]
- 23.Taskinen S, Hyvonen M, Kovanen PT, Meri S, Pentikainen MO. C-reactive protein binds to the 3β-OH group of cholesterol in LDL particles. Biochem. Biophys. Res. Commun. 2005;329:1208–1216. doi: 10.1016/j.bbrc.2005.02.091. [DOI] [PubMed] [Google Scholar]
- 24.Gaboriaud C, Juanhuix J, Gruez A, Lacroix M, Darnault C, Pignol D, Verger D, Fontecilla-Camps JC, Arlaud GJ. The crystal structure of the globular head of complement protein C1q provides a basis for its versatile recognition properties. J. Biol. Chem. 2003;278:46974–46982. doi: 10.1074/jbc.M307764200. [DOI] [PubMed] [Google Scholar]
- 25.Lehtinen MJ, Meri S, Jokiranta TS. Interdomain contact regions and angles between adjacent short consensus repeat domains. J. Mol. Biol. 2004;344:1385–1396. doi: 10.1016/j.jmb.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 26.Hourcade D, Holers VM, Atkinson JP. The regulators of complement activation (RCA) gene cluster. Adv. Immunol. 1989;45:381–416. doi: 10.1016/s0065-2776(08)60697-5. [DOI] [PubMed] [Google Scholar]
- 27.Mold C, Gewurz H, Du Clos TW. Regulation of complement activation by C-reactive protein. Immunopharmacology. 1999;42:23–30. doi: 10.1016/s0162-3109(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 28.Hughes AE, Orr N, Esfandiary H, Diaz-Torres M, Goodship T, Chakravarthy U. A common CFH haplotype, with deletion of CFHR1 and CFHR3, is associated with lower risk of age-related macular degeneration. Nat. Genet. 2006;38:1173–1177. doi: 10.1038/ng1890. [DOI] [PubMed] [Google Scholar]
- 29.Hageman GS, Hancox LS, Taiber AJ, Gehrs KM, Anderson DH, Johnson LV, Radeke MJ, Kavanagh D, Richards A, Atkinson J, et al. Extended haplotypes in the complement factor H (CFH) and CFH-related (CFHR) family of genes that protect against age-related macular degeneration: Identification, ethnic distribution and evolutionary implications. Ann. Med. 2006;38:592–604. [PMC free article] [PubMed] [Google Scholar]
- 30. Curcio CA, Millican CL, Bailey T, Kruth HS. Accumulation of cholesterol with age in human Bruch’s membrane. Invest. Ophthalmol. Vis. Sci. 2001;42:265–274. [PubMed] [Google Scholar]
- 31.Pepys MB, Baltz ML. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv. Immunol. 1983;34:141–212. doi: 10.1016/s0065-2776(08)60379-x. [DOI] [PubMed] [Google Scholar]
- 32.Gershov D, Kim S, Brot N, Elkon KB. C-Reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: implications for systemic autoimmunity. J. Exp. Med. 2000;192:1353–1364. doi: 10.1084/jem.192.9.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seitsonen S, Lemmela S, Holopainen J, Tommila P, Ranta P, Kotamies A, Moilanen J, Palosaari T, Kaarniranta K, Meri S, et al. Analysis of variants in the complement factor H, the elongation of very long chain fatty acids-like 4 and the hemicentin 1 genes of age-related macular degeneration in the Finnish population. Mol. Vis. 2006;12:796–801. [PubMed] [Google Scholar]
- 34.Gold B, Merriam JE, Zernant J, Hancox LS, Taiber AJ, Gehrs K, Cramer K, Neel J, Bergeron J, Barile GR, et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat. Genet. 2006;38:458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seddon JM, Gensler G, Milton RC, Klein ML, Rifai N. Association between C-reactive protein and age-related macular degeneration. J. Am. Med. Assoc. 2004;291:704–710. doi: 10.1001/jama.291.6.704. [DOI] [PubMed] [Google Scholar]
- 36.Despriet DD, Klaver CC, Witteman JC, Bergen AA, Kardys I, de Maat MP, Boekhoorn SS, Vingerling JR, Hofman A, Oostra BA, et al. Complement factor H polymorphism, complement activators, and risk of age-related macular degeneration. J. Am. Med. Assoc. 2006;296:301–309. doi: 10.1001/jama.296.3.301. [DOI] [PubMed] [Google Scholar]
- 37.Meri S, Pangburn MK. Discrimination between activators and nonactivators of the alternative pathway of complement: regulation via a sialic acid/polyanion binding site on factor H. Proc. Natl. Acad. Sci. USA. 1990;87:3982–3986. doi: 10.1073/pnas.87.10.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]